Abstract
Peripheral arterial disease is a major health problem and there is a significant need to develop therapies to prevent its progression to claudication and critical limb ischemia. Promising results in rodent models of arterial occlusion have generally failed to predict clinical success and led to questions of their relevance. While sub-optimal models may have contributed to the lack of progress, we suggest that advancement has also been hindered by misconceptions of the human capacity for compensation and the specific vessels which are of primary importance. We present and summarize new and existing data from humans, Ossabaw miniature pigs, and rodents which provide compelling evidence that natural compensation to occlusion of a major artery (i) may completely restore perfusion, (ii) occurs in specific pre-existing small arteries, rather than the distal vasculature, via mechanisms involving flow-mediated dilation and remodeling (iii) is impaired by cardiovascular risk factors which suppress the flow-mediated mechanisms and (iv) can be restored by reversal of endothelial dysfunction. We propose that restoration of the capacity for flow-mediated dilation and remodeling in small arteries represents a largely unexplored potential therapeutic opportunity to enhance compensation for major arterial occlusion and prevent the progression to critical limb ischemia in the peripheral circulation.
Keywords: collateral growth, arterial occlusion, vascular compensation, humans, miniswine, rodents
Peripheral arterial occlusive disease (PAD) is a major health problem. It afflicts ~8 million Americans and is associated with significant morbidity and mortality [44]. The occurrence of this disease is most prevalent in the elderly and in those with major risk factors for vascular disease. Consequently, the incidence of this disease is anticipated to increase dramatically with the aging of the population and the rising prevalence of the metabolic syndrome and progression to type 2 diabetes. Although the majority of patients with PAD are asymptomatic, the disease progresses to claudication and critical limb ischemia which threatens the loss of both limb and life. Thus, there is a significant medical need to develop novel therapies to prevent the progression of peripheral arterial disease [32].
Although basic research efforts in this area have increased dramatically in recent decades, promising results in rodents have generally failed to predict clinical success. This failure has led to questions regarding the ability of small rodent models to reflect disease processes and collateral development in larger species, a call for better models, and a greater understanding of the complex compensation which involves multiple processes, mechanisms, and molecules [10,66,86,100]. While sub-optimal models and simple single-agent therapies may have contributed to the lack of progress, we suggest that advancement has also been hindered by misconceptions of the human capacity for compensation and the specific vessels which are of primary importance. In this review, we cite studies which (i) demonstrate marvelous adaptations to major arterial occlusion in humans which are just as remarkable as those observed in rodents, (ii) indicate that these compensations occur primarily in select pre-existing small arteries rather than the distal microcirculation, (iii) show that the capacity for this compensation is greatly diminished, if not abolished, by the major risk factors for arterial disease, and (iv) suggest that the capacity for compensation may be restored by suppression of risk factors and reversal of endothelial dysfunction. We propose that restoration of the capacity for flow-mediated dilation and remodeling, either alone or in combination with other therapies, represents a largely unexplored potential therapeutic opportunity to enhance compensation for major arterial occlusion and prevent the progression to critical limb ischemia in the peripheral circulation.
CLINICAL OVERVIEW
Clinically, peripheral arterial insufficiency results from acute trauma, thrombosis, or chronic arterial disease, with the latter being the most common. PAD is classified into four major stages with progression from an asymptomatic state to claudication, ischemic rest pain, and ulceration or gangrene. Its prevalence is associated with the primary risk factors for cardiovascular disease and PAD is considered a marker for atherosclerotic disease in other vascular beds. Advancing age is a major factor because occurrence increases from 5% in the general population to ~60% of those ≥85 years [20,61]. Atherosclerotic lesions are often segmental and most commonly localized to proximal portions of the arterial circulation [22]. The most common occlusion site in the peripheral circulation is the superficial femoral artery (58%) followed by the iliac artery (37%) [101]. The most common symptom of peripheral artery disease is claudication and its natural history is mostly benign because of its limited progression to critical limb ischemia. This is based on the clinical observations of the incidence of amputation in claudicants, which is 5% at 5 years [45] and 10% at 10 years [65], as well as the incidence of revascularization which is 18% at 10 years. This means that while much research effort has been focused on developing therapies for critical limb ischemia, there is a substantial need for medical therapies to prevent the progression of peripheral arterial disease from early stages. An understanding of the natural vascular compensation to arterial occlusion in humans is critical in developing such therapies.
TO WHAT EXTENT IS THE HUMAN VASCULATURE CAPABLE OF COMPENSATING FOR MAJOR ARTERIAL OCCLUSION IN THE PERIPHERAL CIRCULATION?
A primary function of the vascular system is to match tissue perfusion with metabolic needs. This is accomplished by a number of mechanisms including metabolic, myogenic, and flow-dependent dilation as well as structural remodeling, which includes changes in both the number and size of vessels. The most severe challenge to the vascular system in accomplishing this task occurs with an acute occlusion of a major artery. Available data and clinical experience reviewed below reveal that, in some humans, the vascular system is capable of providing complete compensation to acute occlusion or ligation of a major artery.
Evidence for Vascular Compensation to Major Arterial Occlusion from Early Treatment of Limb Aneurysms
The concept of a pre-existing collateral circulation and its development was known in the 18th Century and provided the rationale for the ligation of major arteries in the treatment of aneurysms by Anel, Desault, and Hunter [29,75]. Hunter is reported to have performed proximal arterial ligation in five operations for popliteal aneurysms with a 60% success rate. His work demonstrated that ligation of a main artery in human limbs did not always result in life threatening ischemia and gangrene [29,75]. In 1884, Stimson reported to the New York Surgical Society that femoral artery ligation for the treatment of popliteal aneurysm was a viable alternative to rival methods [87]. Consistent with Stimson's statement, Matas, in his 1910 Presidential address to the American Surgical Association, reported on his method to test for the adequacy of the collateral circulation and indicated that this method was important primarily for those who have a compromised cardiovascular system due to aging, disease, or vascular anomalies [59].
Evidence for Complete Vascular Compensation after Ligation for Major Arterial Injuries
Debakey and Simeone reviewed the surgical experience from WWI and WWII and found that, when acute arterial ligation of a major limb artery was necessary after a battlefield wound, the amputation rate due to ischemia and gangrene was high, between ~20 and 70% [23]. However, they noted that in some cases after interruption of a major limb artery the pre-existing circulation was entirely adequate such that no abnormality was apparent a few weeks after ligation.
Additional Evidence from Clinical Practice for Vascular Compensation after Major Arterial Ligation or Occlusion
A complication of renal transplant surgery is that infected arteriotomies can result in massive hemorrhage and necessitate emergency ligation of the common or external iliac artery. In such cases, limb loss is uncommon and early symptoms of arterial insufficiency are rapidly reversed [4,31]. In commenting on the clinical situation, both Schoop [80] and Longland [54] noted that a threat of necrosis subsequent to acute arterial occlusion only occurs for certain sections of large transport arteries. Surgical texts note that arterial occlusion at the most common sites in the lower extremity is relatively well tolerated due to the pre-existing collateral network [8,50,62,68]. Herrmann et al. [40] noted in 1954 that the clinical experience has repeatedly shown that ischemic necrosis does not necessarily occur subsequent to complete obliteration of major arteries and that many middle-aged and elderly patients have viable extremities despite the lack of palpable pulsations. Even with complete occlusion of the terminal aorta and iliac or superficial femoral arteries due to atherosclerotic disease, some patients experience only intermittent claudication – evidence that significant vascular compensation has occurred [22].
HOW DOES THE VASCULATURE COMPENSATE FOR MAJOR ARTERIAL OCCLUSION?
Although the clinical experience and evidence for vascular compensation after major arterial occlusion in some humans is clear, the specific nature of the compensation remains controversial. For example, a recent review in Circulation [86] identified de novo formation versus development from pre-existing vessels as a hotly debated issue related to collateral growth and questioned if extensive collateral trees exist in the human vasculature. While significant variation exists between organs, individuals and species, compelling data exist to demonstrate that the parallel/serial arrangement of the vasculature typically provides pre-existing pathways at the arterial level that can function as a natural bypass or collateral circulation following major arterial occlusions and that compensation is provided by the dilation and enlargement of these pre-existing vessels subsequent to major arterial occlusion.
Presence of Pre-existing Vessels
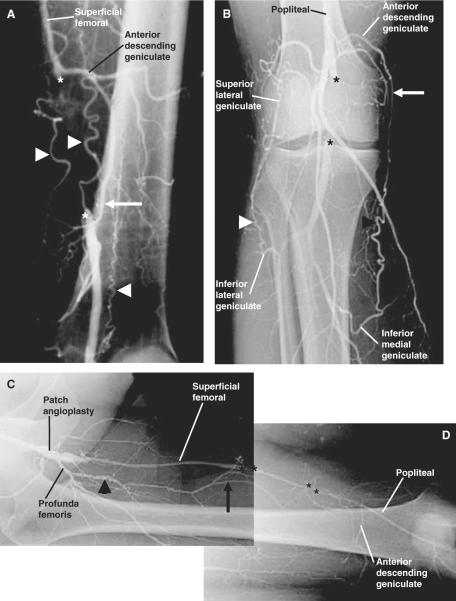
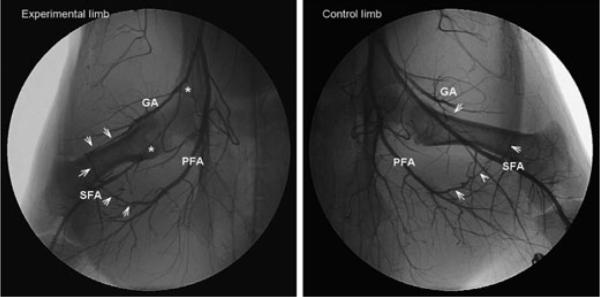
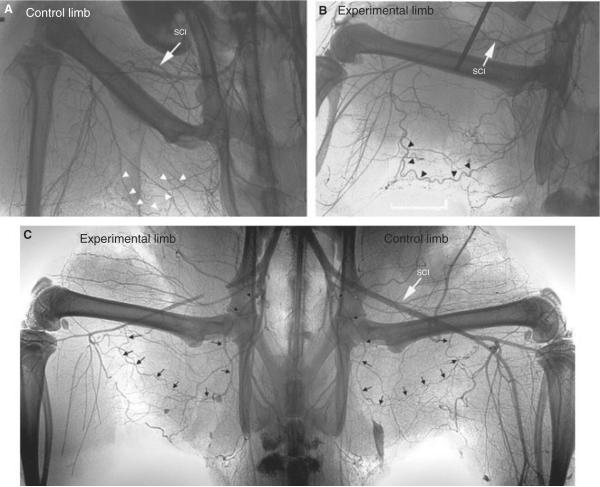
Matas' method to test the efficiency of the collateral circulation prior to aneurismal obliteration provided a functional assessment of pre-existing collaterals [59]. In the Introduction of his 1910 presidential address, Matas noted that “in children and young subjects with normal hearts and blood-vessels, the inherent capacity of the heart and vascular system...is quite sufficient to overcome the resistance of the undeveloped collateral routes, when obstacles are put in the way of the circulation even of the larger central and root trunks.” In 1918, Gray and Lewis presented a human anatomical text which clearly identified anastomoses between large and small arteries and noted that these were the pathways which enlarged as a collateral circulation after arterial ligation [34]. Collateral circulations were described for the major arteries including the thoracic and abdominal aorta, common carotid, external iliac, subclavian, brachial, and femoral arteries. Major pre-existing arterial anastomoses in the femoral-popliteal system include arterial branches from the hypogastric trunk, external iliac, superficial iliac circumflex, and genicular arteries [34,80]. Longland noted the abundance of small arterial anastomoses in skeletal muscle in his address to the Royal College of Surgeons of England in 1953 [54]. Current specialized surgical texts report that the collateral circulation for typical atherosclerotic occlusions in the human lower extremity arises from large arterial branches serving as stems, includes smaller midzone intramuscular arteries, and reenters via a distal major artery, all of which are pre-existing [8,48,50,89]. Human angiograms presented in Figure 1 demonstrate the development of the collateral circulation from pre-existing arteries that are distinct and well-defined in anatomical texts. The importance of these pre-existing vessels is emphasized in reports of arterial injury. In the summary of World War II arterial injuries, Debakey and Simone [23] concluded that the extent to which the collateral circulation was damaged by the injury or debridement was a major determinant of limb survival after major arterial ligation. In a study of civilian lower limb arterial injuries, Hafez et al. [36] identified injuries caused by high-velocity projectiles from firearms as the highest risk for limb loss due to the likelihood of damage to both a major artery and its collateral supply. Angiograms presented in Figures 2, 3 and 4 from miniature swine, rats, and mice after femoral artery ligation demonstrate that major collaterals in these species also develop from pre-existing vessels which form anastomoses between major arteries.
Figure 1.
Angiograms of human lower extremities with arterial occlusion demonstrate that major collaterals arise from pre-existing, named arteries as labeled. Collateral vessels are observed bypassing the site of a superficial femoral artery (SFA) occlusion (A), a popliteal artery occlusion (B), and multiple SFA occlusions (C & D) in three different patients. The occlusion sites are bounded by asterisks. Collateral vessels are identified by arrows and arrowheads with arrowheads denoting regions of greater tortuosity. Note that not all collaterals exhibit significant tortuosity. This demonstration of collateral development from pre-existing small arteries is consistent with reports of the pre-existing collateral circulation published in human anatomy texts since 1918 and in surgery texts and reviews of the human collateral circulation as cited in the text.
Figure 2.
Representative porcine hindlimb angiograms demonstrate major collateral arteries develop from pre-existing small arteries. Studies were performed in 12 adult male Ossabaw miniature swine 4 weeks after ligation of the left superficial femoral artery (SFA). Asterisks in the left panel mark the occluded region in the experimental limb. The angiogram demonstrates that major bypass collaterals, identified by arrowheads, bridge from the gluteal artery (GA) and profunda femoral artery (PFA; deep femoral artery) to reconstitute the distal SFA. Angiograms were reviewed frame by frame to verify that the identified collaterals were responsible for the reconstitution of the distal SFA. Arrowheads in the right panel from a non-occluded control limb demonstrate the pre-existence of collateral paths shown in the left panel. While the center portion of the GA-SFA anastomosis is not seen in the angiogram, its presence was confirmed by dissection. The collateral branches originating from the gluteal artery consistently formed the largest collaterals reconstituting the SFA. An important role for the GA is consistent with the known pre-existing collateral circulation in humans in which the gluteal artery forms major arterial anastomoses [11]. The average diameter of the collaterals at the mid-zone region was 1.1 ± 0.1 mm, versus an average diameter of 3.6 mm for the profunda and 4.9 mm for the common femoral artery. Spatial calibration is identical for both panels.
Figure 3.
Representative published angiograms (used by permission) identify major collateral pathways in the rat hindlimb after femoral artery ligation and demonstrate their pre-existence in control limbs [43,67]. The pathway in the upper set of angiograms [43] involves the perforating artery which connects distally to the popliteal artery. It is initially a major collateral pathway but undergoes regression after 7 days [43]. The lower angiogram [67] shows a more proximal pathway branching into the distal femoral artery. Arrows and arrowheads identify the collateral pathways in the experimental limb and show their pre-existence in the control limbs. The white arrows labeled SCI are added to the images and identify the superficial circumflex iliac artery, which provides a collateral pathway in rats ([55] and Figures 6 and 7) and is a major collateral in other species including humans [34].
Figure 4.
Micrographs from Distasi et al. [24] (used by permission) demonstrate various major collateral pathways in the mouse hindlimb after femoral artery ligation or excision. Vascular casts were made with Microfil after perfusion fixation. For each enlarged collateral path, pre-existing vessels were present in the contralateral control limb as shown in A. Panel B depicts the four major collateral pathways. II, internal iliac artery; FA, femoral artery; PF, profunda femoral artery; S, saphenous artery; P, popliteal artery.
Acute Dilation of Pre-existing Vessels
Blood flow is severely compromised immediately after acute occlusion of a major artery, but it can improve rapidly. This was the basis of the Matas test for collateral efficiency [59]. A hyperemic blush after compression of a major artery indicated an adequate collateral circulation. In Matas' words, “a pink color, no matter how faint, meaning life; a colorless cadaveric pallor meaning death”. Shepherd quantified this response in young healthy humans by measuring calf blood flow by plesthomgraphy before and after acute femoral artery occlusion (compression) [82]. He consistently observed an abrupt and dramatic decrease in calf flow immediately after occlusion to ~15% of the pre-occlusion level followed by near complete restoration of resting flow within ~2 min. Shepherd concluded that the quick recovery in flow after arterial occlusion could result from dilation of the proximal collateral arteries and/or the distal calf vessels. To our knowledge, studies in humans have not been done, nor would such be practical, to determine the relative contribution of collateral and distal vessel dilatation in the improved perfusion after acute occlusion. Available studies in the dog [25,33,46,69,104] and rat [97] demonstrate that distal pressure rises with flow. This distal pressure elevation indicates that the hemodynamic impact of collateral dilation is greater than dilation of the distal vessels. Longland [54] considered the rapid visibility of collaterals in angiograms after femoral artery occlusion in rabbits to be evidence of their dilation, rather than growth, and consistent with the brisk elevation of flow and pressure after femoral artery ligation in dogs.
Chronic Enlargement of Pre-existing Vessels
Longland presented his longitudinal studies of collateral enlargement after femoral artery occlusion in rabbits in the Arris and Gale Lecture at the Royal College of Surgeons of England in 1953 [54]. He reported that within the first half hour after ligation, numerous vessels with diameters from 0.1 to 0.3 mm appeared in the angiogram and formed collateral pathways. The smallest diameters were observed at the mid-zone region. Angiographic filling of these midzone vessels was further increased within a few hours and the mid-zone diameter was observed to be enlarged to 0.2 mm within a few days and ≥0.5 mm at 3 months. By 12 months, regression had occurred as the numerous small collaterals had devolved to relatively few large collaterals. This remains as one of the most thorough and detailed longitudinal studies of collateral growth; only limited longitudinal observations and data are available for humans. Debakey and Simone commented that the collateral circulation could expand quickly, within 6–8 days [23]. Schoop has demonstrated angiographically the progressive enlargement of pre-existing genicular arteries as collaterals from 2 to 8 weeks after femoral artery occlusion in man [80].
While there may be circumstances where new vessels form and become collaterals, this is not the typical or normal compensatory response observed in humans or animals. While there is considerable interest in the development of “new” collaterals, the rationale for believing these to be important when pre-existing anastomoses are available is unclear. Scholz et al. have previously reported that de novo arteriogenesis is not observed after femoral artery ligation in mice nor is it clear that it occurs clinically [79]. This issue is further addressed in two recent reviews which conclude that it is uncertain that “de novo collateral artery growth” occurs and questionable if any vessels so formed would have physiologic relevance [38,39].
IN WHAT VASCULAR SEGMENT DO THE ADAPTATIONS OF PRIMARY HEMODYNAMIC SIGNIFICANCE OCCUR?
Numerous studies exist to indicate that adaptations to arterial occlusion occur throughout the vascular tree from collaterals to capillaries. Many studies have utilized histological or morphological analysis of capillarity, arteriolar density, and the number or size of collateral arteries to assess vascular compensation. But such studies cannot establish the hemodynamic significance of the specific adaptations. Knowledge of the relative importance of adaptations in capillaries, arterioles and collaterals during arterial occlusion is fundamentally important. Unfortunately, only a few studies have quantified the resistance or conductance of various segments acutely and chronically after arterial occlusion. As reviewed below, those studies indicate that the proximal collaterals rather than the distal micro-vessels are the site of primary vascular compensation to arterial occlusion.
Acute Response
Rosenthal and Guyton assessed the dilation of collateral and peripheral vessels in dogs after femoral artery occlusion by measuring changes in conductance [69]. They accomplished this by simultaneously measuring (i) pressure and flow in the anterior tibial artery distal to the site of femoral occlusion and (ii) pressure in the femoral artery proximal to the occlusion. Collateral conductance increased 277 ± 67% during the first 70 seconds following occlusion and an additional 178 ± 66% within an hour. During this same time, peripheral conductance increased <10% from its pre-occlusion level. This observation is consistent with canine and rodent studies cited earlier which reported distal pressure to increase progressively during the first minutes after acute occlusion.
Chronic Response
Eckstein et al. reported hemodynamic studies of collateral function after acute and chronic occlusion of canine femoral, carotid and coronary arteries [25]. While they did not calculate resistance, their measurements of flow and distal pressure in the hindlimb indicate major resistance changes occurred in the collateral circuit. Sanne and Silvertsson [73] measured collateral and microvascular resistances after occlusion of the feline femoral artery in their 1968 study of exercise effects on collateral development. Their data indicate that, under conditions of maximal dilation after acute femoral artery occlusion, the majority (~75%) of the total vascular resistance resided in the collateral vessels. After 5 weeks in both exercise trained and sedentary cats, a significant and substantial decrease was observed for collateral resistance but not for the resistance of the distal vessels [73]. Similar results have been reported for rats and are summarized in Figure 5. With acute femoral artery ligation, 70–80% of total resistance is within the proximal collateral circulation [98,106]. Collateral resistance is decreased ~50% and 70% at 1 and 8 weeks, respectively, after femoral artery ligation [53,98]. During the same time intervals, distal microvascular resistance is unchanged.
Figure 5.
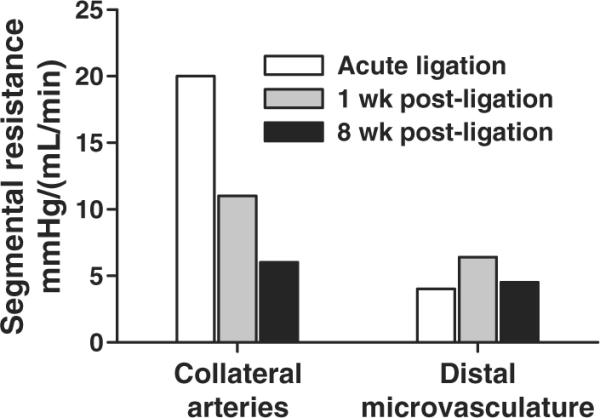
Resistances of the collateral circulation and distal microvasculature after rat femoral artery ligation. The data shown are from Unthank et al. [53,98] for rats and are consistent with other studies in rats [34,106] and cats [73]. The composite data indicate that even after the initial collateral dilation following acute femoral artery occlusion, collateral resistance is 70–80% of the total resistance to the collateral dependent tissue and can decrease by ~70% within weeks. During the same time, no significant decrease in microvascular resistance is observed.
Collaterals as Major Compensators
We consider the cited studies with measurement of distal pressure to provide the best indication of the relative importance of the collateral and microvascular segments in tissue perfusion. Due to the abundance of studies which primarily assess angiogenesis, we review additional studies using different approaches which also provide strong support for the importance of the bypass collaterals. In a longitudinal study of vascular compensation after femoral artery excision in the rabbit hindlimb, Hershey et al. [42] reported an early increase in VEGF levels and capillary density, but perfusion was not increased until significant collateral growth was observed [42]. In a subsequent study by this group [41], VEGF therapy, which doubled capillary density in skeletal muscle in the ischemic limb, had no effect on resting or hyperemic blood flow. Scholz et al. [79] have utilized mice with different capacities for angiogenesis and collateral growth to obtain insight into the importance of these processes in the restoration of perfusion after femoral artery ligation. After femoral artery ligation in various mouse strains, C57BL/6 mice had the greatest improvement in perfusion and BALB/Cs mice the poorest. The C57BL/6 mice also showed the greatest collateral growth but no increase in capillary density was observed. The BALB/Cs mice had the greatest increase in capillary density but the lowest increase in collateral growth. These studies clearly show the importance of collateral growth in the improvement of tissue perfusion.
Available studies of segmental microvascular resistances provide an explanation for the limited effect of increased capillary density on perfusion after arterial occlusion. In normally perfused limbs, intravital micropressure techniques have established that small feeder arteries and the largest arterioles control about 70% of skeletal muscle vascular resistance [5,28,52,111], capillaries ~8–15% [5,28], and venules the remainder. With single, acute ligation of the femoral artery, the composite of these microvascular resistances is reduced to only 20–30% of total resistance [53,98,106]. Consequently, capillaries would be responsible for <10% of total resistance after femoral artery occlusion. In such a situation, a doubling of capillary density would have a minimal effect on perfusion, consistent with Hershey's results [41].
WHAT ARE THE CHARACTERISTICS OF THE VESSELS MOST IMPORTANT IN THE COMPENSATION?
Within the context of major arterial occlusion in limbs and in terms of increasing perfusion, we have presented evidence that the most important vessels are pre-existing arterial branches that form collateral pathways. The emphasis placed on very small vessels (≤50 μ) in recent years has led to considerable confusion regarding the definition and identification of collateral vessels, with some considering all arteries and arterioles in the thigh to be collaterals and others regarding distal vessels and even capillaries as collaterals. Only those vessels which experience an increase in flow subsequent to occlusion function as bypass collaterals. Other vessels experience a decrease in flow and pressure and this may lead to decompensation [16]. It is also important to note, however, that all pre-existing anastomoses which function as collaterals are not equally important and the relative contribution of a specific anastomotic collateral path can vary over time. For example, Figure 6 compares diameters of two arteries which provide a collateral path after femoral artery ligation in rats; the perforating artery (PA) [43,67] and a branch of the superficial circumflex iliac artery (SCI) [55]. At 2 weeks post-ligation, the SCI branch is enlarged relative to control limbs while the PA is not. This is consistent with the observation by Herzog et al. [43] that the PA is enlarged at 1 week after ligation and then regresses. Longland's longitudinal study provides compelling evidence that many vessels begin to enlarge initially after occlusion but only a few remain when the adaptation is finished [54]. Changes in shear stress induce both outward and inward remodeling of small arteries [93,95,96] and can explain the dynamic changes in diameter during chronic adaptation. Resistance to flow is inversely proportional to the fourth power of the radius and directly proportional to length while shear stress is directly proportional to the third power of the radius. Consequently, for an equivalent change in the pressure gradient for flow after arterial occlusion, the largest and shortest pre-existing collateral paths would have the lowest resistance and experience the greatest increase in flow and shear stress. Until equilibrium is reached, the balance of flow between vessels would constantly change as some vessels enlarge and others regress.
Figure 6.

Select enlargement of pre-existing collateral pathways. Both the superficial circumflex iliac (SCI) artery and perforating artery form collateral pathways in the rat after femoral artery ligation. We compared the diameters of both at 2 weeks post-ligation. Collateral paths were established by tissue dissection after perfusion fixation and Microfil casting. The major branch of the SCI and the perforating artery were isolated and diameters determined from cross-sections. The branch of the SCI was significantly enlarged in the experimental limb (>60%) as shown above. In contrast, the perforating artery was enlarged in only three of 12 rats and was reduced in diameter in the remainder (119 ± 20 experimental vs. 237 ± 12 μm control limb, P < 0.001), consistent with the regression previously reported to occur after 1 week [43]. The consistent enlargement of the smaller SCI branch presumably occurs because it provides a shorter path with less resistance than the perforating artery. With enlargement of the SCI branch, blood flow and shear stress are likely reduced in the perforating artery which then undergoes regression (flow-mediated inward remodeling). The decrease in perforating artery diameter is consistent with Schoops observation of the regression of long collaterals in humans [80].
Because of this dynamic process, it is important to identify those collateral vessels with increased flow and shear stress. The ideal of course would be to measure flow and shear, but this is currently not feasible in the limbs of small rodents. Therefore, we review below the characteristics of the primary collaterals which are so important in determining the extent of vascular compensation to major arterial occlusion.
Diameter
In studies of vascular adaptation to arterial occlusion, there has been an emphasis in recent years on vessels ≤50 μm in diameter which presumably undergo 10–20× enlargement [66,71,74,100]. This magnitude of enlargement is not typical of collaterals (Table 1) and this size of vessel would not generally form anastomoses between major arteries, except possibly in the mouse hindlimb. The size of pre-existing anastomoses which become major collaterals is typically >100 μm in rats (Figure 6 and references [43,67]) and increases in size in larger species (Figure 7). In the human leg, the peri-genicular arteries commonly form collaterals [3,34] and their normal diameter averages 2–4 mm [3,72]. Within an individual, the largest diameter and shortest length collaterals have the lowest resistance and make the greatest contribution to perfusion. For example, using data obtained after femoral artery ligation in the rabbit, de Lussanet et al. [56] have concluded that the largest collateral vessels (diameters >0.3 mm) were responsible for nearly 100% of the total blood flow, whereas smaller collaterals (0.1–0.3 mm diameter) contributed little. Thus, it is important to identify the largest collaterals for study within individual species and an absolute size range cannot be specified.
Table 1.
Summary of Literature Search for Studies with Angiograms or Vascular Casting with Reported Diameters of Collaterals and Pre-existing Control Arteries (Hindlimb Collateral Vessel Size and Fold Enlargement)
| First author | Species | Method | Pathway | Duration (weeks) | Initial size (μm) | Final size (μm) | Fold increase |
|---|---|---|---|---|---|---|---|
| Distasi [24] | Mouse | IS | GA | 2 | 57 | 87 | 1.5* |
| Mouse | IS | 2–4 | 59 | 139 | 2.4* | ||
| Scholz [78] | Mouse | CS | GA | 3 | 42 | 71 | 1.7 |
| Chalothorn [13] | Mouse | CS | GA | 3 | 45 | 95 | 2.1* |
| −Angio | PA | 3 | 75 | 150 | 2* | ||
| Chalothorn [14] | Mouse | CS | GA | 3 | 50 | 75 | 1.5* |
| Gruionu [35] | Mouse | IS | GA | 8 | 20 | 40 | 2* |
| Ziegelhoeffer [110] | Mouse | CS | GA | 1 | 39 | 77 | 2* |
| Scholz [76] | Mouse | CS | GA | 1 | 32 | 49 | 1.5* |
| CS | GA | 4 | 32 | 77 | 2.4* | ||
| Buschmann [10] | Mouse | CS | ? | 2 | 10–30 | 160 | 5* |
| Scholz [79] | Mouse | CS | GA | 3 | 36–41 | 76–85 | 2.1–2.4 |
| Herzog [43] | Rats | CC | PA | 2–8 | 140 | 300 | 2.1* |
| Prior [67] | Rat | IV | PA | 3.5 | 230 | 332 | 1.4* |
| Scholz [77] | Rabbit | CS | ? | 2 | 50 | 225 | 4.5* |
| 34 | 50 | 550 | 10* | ||||
| Longland [54] | Rabbit | Angio | Multiple | 12 | 100–300 | 500 | 1.7* |
| Conrad [18] | Dog | CC | Multiple | 11 | 150 | 380 | 2.5* |
| Buschmann [10] | Pig | CS | ? | 2 | 10–30 | 620 | 20* |
CS, cross-sections; IS, in situ after vascular casting; Angio, angiogram; CC, corrosion cast; IV, isolated vessels (pressurized); GA, gracilis artery; PA, perforating artery; ?, not reported.
Estimated from reported diameters.
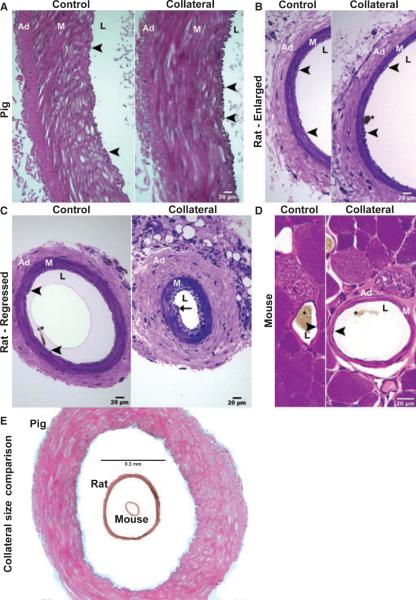
Figure 7.
Cross-sections of major collateral arteries and their controls from miniature pig, rat, and mouse after femoral artery ligation. (A) Comparison of control and collateral porcine arteries demonstrate a remarkable increase in intimal cell number in the collateral. This is characteristic of arteries undergoing flow-mediated outward remodeling and occurs without neointima formation. The same result was observed for the major collaterals in rats (B, branch from superficial circumflex iliac artery) and mice (D). For a regressing collateral pathway identified in the rat (C, perforating artery), neointimal formation (arrow) is apparent, but not an increase in intimal cell number. (E) Images of a mouse and rat collateral are inserted into the lumen of a pig collateral to emphasize the tremendous difference (>10-fold) in vessel size, wall thickness, and distance from inner to outer wall layers. Ad, adventitia; M, media; L, lumen; arrowheads indicate the intima, asterisks denote remnants of Microfil® casting agent in the lumen. Control and collateral artery pairs from all species were imaged at the same magnification.
However, the size of the collateral could have a significant impact on the remodeling process. Most recent work has been done on small vessels with relatively thin walls which compared with larger vessels would have less matrix, potentially vascular cells with different phenotypes, different ratios of endothelial, smooth muscle and adventitial cells, and greater diffusion distances. The latter could be fundamentally important not only for diffusion of paracrine factors but also for reactive oxygen and nitrogen species which are involved in many of the remodeling processes.
Tortuosity
Some collaterals have multiple undulations and this characteristic appearance is sometimes used to identify collaterals. However, as seen in Figures 1–4, not all collaterals are tortuous and tortuosity is not necessarily a characteristic of a major collateral. Schoop [80] notes that “corkscrew like” collaterals typically have narrow lumens and are found most frequently in younger patients with occlusion of the posterior tibial artery.
Endothelial Response
The endothelium has a requisite role in flow-mediated arterial remodeling, including the transduction of the stimulus and initiation of remodeling by production of signaling molecules including reactive oxygen and nitrogen species [15,21]. Endothelial removal prevents both inward [51] and outward [57] remodeling in response to chronic changes in shear stress. In flow-mediated outward remodeling of large and small arteries, there is a remarkable endothelial response characterized by activation and proliferation which precede outward remodeling [58,85,93,95]. The absence of such a proliferative response is associated with impaired flow-mediated remodeling and collateral growth [84,94]. This known response in arteries experiencing elevated shear has received relatively little attention in the context of hindlimb collateral growth but is observed in major collateral arteries of pigs, rats, and mice [24] after femoral artery occlusion (Figure 7). While some investigators report collateral growth to be associated with intimal hyperplasia, these reports typically involve small arterioles of unknown hemodynamic status [77] or small arteries which undergo regression [43].
WHAT DETERMINES IF SIGNIFICANT NATURAL COMPENSATION WILL OCCUR?
Clinical data clearly establish that some patients can compensate for acute occlusion of a major limb artery and for gradual occlusion by atherosclerotic processes of one or more major arteries, while others with similar pathology experience symptoms and limb loss. Perhaps the most important question in need of an answer is the mechanistic basis for such differences.
Vascular remodeling is an extremely complex process. For flow-mediated remodeling, as occurs in collateral growth, signal transduction [15,21] must occur not only in the endothelial cells exposed to elevated shear but also likely in the vascular smooth muscle cells which experience increased wall circumferential stress subsequent to endothelium-dependent flow-mediated dilation. Transduction in these cells involves ion channels, integrins and focal adhesion, and initiates signaling cascades, transcription factor activation, and gene transcription and translation. Expression of growth promoters and inhibitors, matrix metalloprotease activators and inhibitors, and receptors is altered. Reactive oxygen and nitrogen species levels are also altered and participate in signaling processes, proteolytic degradation and activation or deactivation of enzymes including matrix metalloproteinases. Matrix bound growth factors are released and controlled destruction and deposition of matrix occurs to accommodate outward remodeling. Changes in cellular events occur including migration, homing, proliferation, hypertrophy, and apoptosis. The completion of this dynamic process requires weeks to months and perhaps years. Abnormalities in any of these molecular and cellular processes may suppress and even abolish the capacity for remodeling.
While the specific mechanisms of impairment are mostly unknown, observations cited earlier from the previous and current centuries indicate vascular compensation is impaired in older patients and those with risk factors. Both the progression of peripheral artery disease and levels of inflammatory markers are correlated with impaired flow mediated dilation (endothelial dysfunction) [7]. A negative correlation exists between a pro-inflammatory endothelial phenotype and flow-mediated outward remodeling in human ulnar arteries [99]. Oxidative stress is a common denominator between risk factors and occurs with vascular inflammation and endothelial dysfunction. In the peripheral circulation, the capacity for flow-mediated remodeling of collateral, resistance arteries in animals is abolished under conditions of chronically elevated oxidative stress [63,64,92,94]. While these studies suggest an important role for oxidative stress in the suppression of flow-mediated remodeling, other factors including genetic background [12,37,84] and level of adrenergic activation [26,91] significantly impact collateral growth and function.
The specific anatomic location of the occlusion and the magnitude of the stimulus may also be important factors in determining if complete compensation occurs naturally. With an acute, embolic occlusion of the popliteal artery, there is typically severe underperfusion due to a relatively inefficient collateral circulation consisting of arteries with narrow lumens. But if the tissue survives, these short collaterals can enlarge over years to provide excellent compensation [80]. For an iliac occlusion, the collaterals are larger but must provide flow to a much greater tissue mass such that exercise tolerance may remain limited. The chronological conditions related to an occlusion can also be expected to influence the adaptation [80]. A gradual occlusion due to atherosclerosis will provide the opportunity for collateral enlargement before complete occlusion but may not provide as great a stimulus (shear stress) as an acute embolic occlusion.
CAN THE IMPAIRMENT OF NATURAL COMPENSATION BE REVERSED?
Numerous small clinical trials have attempted to enhance vascular compensation to peripheral arterial occlusion with molecular and cellular therapies. These trials have been based upon results in animals which have shown substantial improvements. Some patients with claudication and critical limb ischemia have experienced remarkable improvement with some therapies including autologous bone marrow-derived mononuclear cells (M.P. Murphy, personal communication). Results in these trials have been inconsistent with only moderate overall effects reported [2,47,66,70]. Such therapies may eventually prove effective, but major questions remain in terms of the optimal stem/progenitor cells or angiogenic/arteriogenic molecule and dosing strategy [47]. Future studies to identify the specific mechanisms responsible for impaired compensation are important to guide the development of novel therapies.
In their study of flow-mediated dilation and outward remodeling of the human ulnar artery, Vita et al. [99] observed a negative correlation between a pro-inflammatory endothelial phenotype and outward remodeling. They speculated that normal outward remodeling may not occur when a pro-inflammatory phenotype exists. Consistent with this perspective, Kinnaird et al. [49] identified abnormal stem/progenitor cell function and impaired responses to growth stimuli as potential mechanisms by which cardiovascular risk factors impair normal collateral development. Our observations that the capacity for flow-mediated outward remodeling in animals can be restored by therapies [49,50,76,78] which reduce oxidative stress and restore normal nitric oxide production [108,109], suggest that patients characterized by abnormal endothelial function might benefit from similar treatments. In support of this concept, recent clinical studies have shown the angiotensin converting enzyme inhibitor ramipril to both improve flow-mediated dilation [30] and provide a very significant and meaningful improvement in pain-free walking time in claudicants [1]. While it is not known if impairment of flow-mediated remodeling can be reversed in humans, especially after decades of disease, this represents a novel therapeutic approach for either primary or adjuvant therapies.
FUTURE DIRECTIONS
There remains an unmet medical need for novel therapies to improve vascular growth and function across the entire spectrum of peripheral arterial disease [32]. Animal studies are needed for both the identification of specific mechanisms responsible for impaired compensation and the development of novel therapies to prevent disease progression and enhance compensation. Previous reviews have identified the failure of many animal studies to predict results in humans. Consequently, future studies need to consider the most appropriate animal models, techniques, and outcomes. While this review has focused on the capacity of the human vasculature to compensate for major arterial occlusion and the identification of the vascular segment that is most important in this compensation, we conclude with specific recommendations that may assist in the design of future studies more relevant to the clinical situation.
Animal Models
The human population afflicted with peripheral artery disease is characterized by advanced age, endothelial dysfunction and elevation of inflammatory markers and oxidative stress. Consequently, it is unclear that studies in young, healthy animals have any relevance to the human condition. Future studies should utilize animal models with cardiovascular risk factors similar to the patient population. Advantages of small and large animal models have been considered by Waters et al. [103]. The ability to assess perfusion and vascular resistances is limited in mouse models, but multiple methods and techniques exist for larger animals [69,73,103]. The differences in the size of vessels and relative proportions of vascular cells are additional reasons to perform large animal studies before advancing to clinical trials.
Experimental models of hindlimb arterial insufficiency
We are not aware of any animal model in which peripheral arterial occlusion occurs naturally and mimics the human condition. Experimentally induced models of peripheral arterial insufficiency and ischemia have included ligation of the aorta [27], iliac and femoral arteries, alone [17,98] or in combination [6] as well as excision of the femoral artery, frequently with the companion vein, or femoral and saphenous arteries [19]. Multi-stage [9,55,81] and gradual occlusion [90,107] models have also been used as well as bilateral ligation [105] and stenosis [60]. Some models induce severe ischemia at rest and others only impaired hyperemic responses or intermittent ischemia. Some obliterate most if not all major pre-existing collateral pathways and observe primarily an angiogenic response. The stimulus level (ischemia or shear stress) undoubtedly varies considerably between models and some are not characterized by a significant inflammatory response in collaterals [90,107]. Every model has advantages and limitations and some will be better suited than others for specific purposes such as assessment of angiogenesis vs. collateral growth, early vs. late stage arterial disease, or intermittent ischemia vs. critical limb ischemia. Investigators are encouraged to consider relevant aspects of human peripheral arterial disease as reviewed above in selecting the most clinically relevant models for their research. These aspects include the location of the occlusion, level of ischemia (intermittent or critical), and the gradual nature of the occlusive process. In addition, it is worth noting that based upon his observations in humans Shepherd [83] concluded that venous occlusion concomitant with arterial occlusion would be expected to compromise collateral development.
Treatment Protocols
The vast majority of studies have administered therapies either before, at the time of, or shortly after surgery and ligation/excision. Surgery alone induces systemic effects that may influence the treatment outcome, and the mechanisms mediating initial responses to acute occlusion may differ from those involved in later stages of adaptation. Therapies which demonstrate significant benefit when administered significantly after surgery and when natural compensation has stabilized, but a perfusion deficit persists, would be better candidates for the clinic than those which produce benefits only when given near the time of arterial occlusion.
Outcomes
In arterial occlusive disease, a primary therapeutic goal is the improvement of tissue perfusion. As skeletal muscle has low resting blood flow, assessment of perfusion under conditions of reactive or functional hyperemia are necessary to adequately assess compensation [103]. Evaluation of collateral and distal vascular resistance is also very useful. Because of the importance of the largest collaterals, the assessment of these vessels is also important. Measurement of resting flow alone and histological assessment of angiogenesis cannot provide firm conclusions for most studies [103].
CONCLUSIONS
Humans, as well as animals, are capable of remarkable natural compensation in response to occlusion of a major artery in the peripheral circulation. Primary compensations are the acute dilation and progressive luminal expansion of small pre-existing arteries which by their anatomical location experience an increase in shear stress and provide a natural bypass. Because adjacent small arteries may experience different degrees of wall shear stress subsequent to occlusion due to differences in diameter and length, their responses may be dissimilar such that some experience expansion and others regression. These compensations (flow-mediated dilation and remodeling) are impaired by risk factors of disease. The restoration of the capacity for flow-mediated dilation and remodeling by improving endothelial function represents a largely unexplored potential therapeutic opportunity. Careful consideration should be given in future studies to selection of the animal and experimental models and, if appropriate, the treatment protocol that would be most relevant to the human population with peripheral arterial disease. Due to significant differences between small and large species in vessel dimensions, relative ratios of vascular cells, and other known species differences, it is highly advisable that promising new therapies should be evaluated in large as well as small animals prior to clinical trials. The Ossabaw pig is characterized by metabolic disorders[88] and peripheral arterial disease[102] and may be a large animal well suited for such studies.
ACKNOWLEDGEMENTS
The authors would like to thank the members of the Sturek Laboratory, the clinical staff at Clarian Health Care System, as well as Randall G. Bills, Jennifer Stashevsky, P. Melanie Pride, and Jon M. Stropes for their expert technical assistance. We acknowledge Keith L. March, MD, PhD for use of the Research Animal Angiography Laboratory of the Indiana Center for Vascular Biology & Medicine. MRD was the recipient of a Translational Research Fellowship from the Indiana University School of Medicine. This work was supported by National Institutes of Health grants HL42898 to JLU, RR013223 and HL062552 to MS, HL092012 to SJM, the Purdue-Indiana University Comparative Medicine Program, and the Fortune-Fry Ultrasound Research Fund of the Department of Cellular & Integrative Physiology at Indiana University School of Medicine.
REFERENCES
- 1.Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Brief communication: ramipril markedly improves walking ability in patients with peripheral arterial disease: a randomized trial. Ann Intern Med. 2006;144:660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- 2.Al Mheid I, Quyyumi AA. Cell therapy in peripheral arterial disease. Angiology. 2008;59:705–716. doi: 10.1177/0003319708321584. [DOI] [PubMed] [Google Scholar]
- 3.Barral X, Salari GR, Toursarkissian B, Favre JP, Gournier JP, Reny P. Bypass to the perigeniculate collateral vessels. A useful technique for limb salvage: preliminary report on 22 patients. J Vasc Surg. 1998;27:928–935. doi: 10.1016/s0741-5214(98)70274-5. [DOI] [PubMed] [Google Scholar]
- 4.Blohme I, Brynger H. Emergency ligation of the external iliac artery. Ann Surg. 1985;201:505–510. doi: 10.1097/00000658-198504000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlen HG, Gore RW, Hutchins PM. Comparison of microvascular pressures in normal and spontaneously hypertensive rats. Microvasc Res. 1977;13:125–130. doi: 10.1016/0026-2862(77)90121-2. [DOI] [PubMed] [Google Scholar]
- 6.Brevetti LS, Paek R, Brady SE, Hoffman JI, Sarkar R, Messina LM. Exercise-induced hyperemia unmasks regional blood flow deficit in experimental hindlimb ischemia. J Surg Res. 2001;98:21–26. doi: 10.1006/jsre.2001.6161. [DOI] [PubMed] [Google Scholar]
- 7.Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 8.Brewster DC. Direct reconstruction for aortoiliac occlusive disease. In: Rutherford RB, editor. Vasc Surg. Elsevier Saunders; Philadelphia, PA: 2005. pp. 1106–1136. [Google Scholar]
- 9.Brown MD, Kelsall CJ, Milkiewicz M, Anderson S, Hudlicka O. A new model of peripheral arterial disease: sustained impairment of nutritive microcirculation and its recovery by chronic electrical stimulation. Microcirculation. 2005;12:373–381. doi: 10.1080/10739680590934817. [DOI] [PubMed] [Google Scholar]
- 10.Buschmann IR, Voskuil M, van Royen N, et al. Invasive and non-invasive evaluation of spontaneous arteriogenesis in a novel porcine model for peripheral arterial obstructive disease. Atherosclerosis. 2003;167:33–43. doi: 10.1016/s0021-9150(02)00389-1. [DOI] [PubMed] [Google Scholar]
- 11.Chait A, Moltz A, Nelson JH., Jr. The collateral arterial circulation in the pelvis: an angiographic study. Am J Roentgenol. 1968;102:392–400. doi: 10.2214/ajr.102.2.392. [DOI] [PubMed] [Google Scholar]
- 12.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 13.Chalothorn D, Moore SM, Zhang H, Sunnarborg SW, Lee DC, Faber JE. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]
- 14.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 15.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 16.Coats P, Hillier C. Differential responses in human subcutaneous and skeletal muscle vascular beds to critical limb ischaemia. Eur J Vasc Endovasc Surg. 2000;19:387–395. doi: 10.1053/ejvs.1999.1023. [DOI] [PubMed] [Google Scholar]
- 17.Conrad M. Effects of therapy on maximal walking time following femoral ligation in the rat. Circ Res. 1977;41:775–778. doi: 10.1161/01.res.41.6.775. [DOI] [PubMed] [Google Scholar]
- 18.Conrad MC, Anderson JL, Garrett JB. Chronic collateral growth after femoral artery occlusion in the dog. J Appl Physiol. 1971;31:550–555. doi: 10.1152/jappl.1971.31.4.550. [DOI] [PubMed] [Google Scholar]
- 19.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 20.Criqui M, Fronek A, Barrett-Connor E, Klauber M, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 21.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg. 1985;201:115–131. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debakey ME, Simeone FA. Battle injuries of the arteries in World War II: an analysis of 2471 cases. Ann Surg. 1946;123:534–579. [PubMed] [Google Scholar]
- 24.Distasi MR, Case J, Ziegler MA, et al. Suppressed hindlimb perfusion in Rac2−/− and Nox2−/− mice does not result from impaired collateral growth. Am J Physiol Heart Circ Physiol. 2009;296:H877–H886. doi: 10.1152/ajpheart.00772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckstein RW, Gregg DE, Pritchard WH. The magnitude and time of development of the collateral circulation in occluded femoral, carotid and coronary arteries. Am J Physiol. 1941;132:351–361. [Google Scholar]
- 26.Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE. Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol. 2005;289:H744–H753. doi: 10.1152/ajpheart.00129.2005. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez LA, Caride VJ, Twickler J, Galardy RE. Renin-angiotensin and development of collateral circulation after renal ischemia. Am J Physiol Heart Circ Physiol. 1982;243:H869–H875. doi: 10.1152/ajpheart.1982.243.6.H869. [DOI] [PubMed] [Google Scholar]
- 28.Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol. 1975;228:791–796. doi: 10.1152/ajplegacy.1975.228.3.791. [DOI] [PubMed] [Google Scholar]
- 29.Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl. 2007;89:466–471. doi: 10.1308/003588407X183472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghiadoni L, Versari D, Magagna A, et al. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. J Hypertens. 2007;25:361–366. doi: 10.1097/HJH.0b013e3280115901. [DOI] [PubMed] [Google Scholar]
- 31.Gorey TF, Bulkley GB, Spees EK, Jr, Sterioff S. Iliac artery ligation: the relative paucity of ischemic sequelae in renal transplant patients. Ann Surg. 1979;190:753–757. doi: 10.1097/00000658-197912000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gornik HL. Rethinking the morbidity of peripheral arterial disease and the “normal” ankle-brachial index. J Am Coll Cardiol. 2009;53:1063–1064. doi: 10.1016/j.jacc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Graham AJ. The effect of tetraethylammonium bromide on the return of blood-pressure in the femoral artery distal to an acute occlusion. Br J Surg. 1951;38:519–522. doi: 10.1002/bjs.18003815217. [DOI] [PubMed] [Google Scholar]
- 34.Gray H, Lewis WH. Anatomy of the Human Body. Lea & Febiger; Philadelphia, PA: 1918. p. 1396. [Google Scholar]
- 35.Gruionu G, Hoying JB, Pries AR, Secomb TW. Structural remodeling of mouse gracilis artery after chronic alteration in blood supply. Am J Physiol Heart Circ Physiol. 2005;288:H2047–H2054. doi: 10.1152/ajpheart.00496.2004. [DOI] [PubMed] [Google Scholar]
- 36.Hafez HM, Woolgar J, Robbs JV. Lower extremity arterial injury: results of 550 cases and review of risk factors associated with limb loss. J Vasc Surg. 2001;33:1212–1219. doi: 10.1067/mva.2001.113982. [DOI] [PubMed] [Google Scholar]
- 37.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann LG, Cranley JJ, Preuninger RM. Importance of collateral circulation in obliterative arterial disease of the lower extremities. Geriatrics. 1954;9:1–7. [PubMed] [Google Scholar]
- 41.Hershey JC, Baskin EP, Corcoran HA, et al. Vascular endothelial growth factor stimulates angiogenesis without improving collateral blood flow following hindlimb ischemia in rabbits. Heart Vessels. 2003;18:142–149. doi: 10.1007/s00380-003-0694-z. [DOI] [PubMed] [Google Scholar]
- 42.Hershey JC, Baskin EP, Glass JD, et al. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 43.Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol. 2002;283:H2012–H2020. doi: 10.1152/ajpheart.00257.2002. [DOI] [PubMed] [Google Scholar]
- 44.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 45.Imparato AM, Kim GE, Davidson T, Crowley JG. Intermittent claudication: its natural course. Surgery. 1975;78:795–799. [PubMed] [Google Scholar]
- 46.John HT, Warren R. The stimulus to collateral circulation. Surgery. 1961;49:14–24. [PubMed] [Google Scholar]
- 47.Kalka C, Baumgartner I. Gene and stem cell therapy in peripheral arterial occlusive disease. Vasc Med. 2008;13:157–172. doi: 10.1177/1358863x08088616. [DOI] [PubMed] [Google Scholar]
- 48.Kalman PG. Profundaplasty: isolated and adjunctive applications. In: Rutherford RB, editor. Vasc Surg. Elsevier Saunders; Philadelphia, PA: 2005. pp. 1174–1180. [Google Scholar]
- 49.Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res. 2008;78:257–264. doi: 10.1093/cvr/cvm116. [DOI] [PubMed] [Google Scholar]
- 50.Kohler TR. Hemodynamics of arterial occlusive disease. In: Strandness DE, Van Breda A, editors. Vascular Diseases: Surgical and Interventional Therapy. Churchill Livingstone; New York: 1994. pp. 65–72. [Google Scholar]
- 51.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science (Washington) 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 52.Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. J Appl Physiol. 1994;76:1512–1519. doi: 10.1152/jappl.1994.76.4.1512. [DOI] [PubMed] [Google Scholar]
- 53.Lash JM, Nixon JC, Unthank JL. Exercise training effects on collateral and microvascular resistances in rat model of arterial insufficiency. Am J Physiol Heart Circ Physiol. 1995;268:H125–H137. doi: 10.1152/ajpheart.1995.268.1.H125. [DOI] [PubMed] [Google Scholar]
- 54.Longland CJ. The collateral circulation of the limb. Ann R Coll Surg Engl. 1953;13:161–176. [PMC free article] [PubMed] [Google Scholar]
- 55.Lundberg G, Luo F, Blegen H, Kalin B, Wahlberg E. A rat model for severe limb ischemia at rest. Eur Surg Res. 2003;35:430–438. doi: 10.1159/000072228. [DOI] [PubMed] [Google Scholar]
- 56.de Lussanet QG, van Golde JC, Beets-Tan RG, et al. Magnetic resonance angiography of collateral vessel growth in a rabbit femoral artery ligation model. NMR Biomed. 2006;19:77–83. doi: 10.1002/nbm.1003. [DOI] [PubMed] [Google Scholar]
- 57.Masuda H, Kawamura K, Sugiyama T, Kamiya A. Effects of endothelial denudation in flow-induced arterial dilatation. Front Med Biol Eng. 1993;5:57–62. [PubMed] [Google Scholar]
- 58.Masuda H, Kawamura K, Tohda K, Shozawa T, Sageshima M, Kamiya A. Increase in endothelial cell density before artery enlargement in flow-loaded canine carotid artery. Atherosclerosis. 1989;9:812–823. doi: 10.1161/01.atv.9.6.812. [DOI] [PubMed] [Google Scholar]
- 59.Matas R. I. Testing the efficiency of the collateral circulation as a preliminary to the occlusion of the great surgical arteries. Ann Surg. 1911;53:1–43. doi: 10.1097/00000658-191101000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathien GM, Terjung RL. Influence of training following bilateral stenosis of the femoral artery in rats. Am J Physiol. 1986;250:H1050–H1059. doi: 10.1152/ajpheart.1986.250.6.H1050. [DOI] [PubMed] [Google Scholar]
- 61.Meijer WT, Grobbee DE, Hunink MGM, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Arch Intern Med. 2000;160:2934–2938. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 62.Menard MT, Belkin M. Femoropopliteal and tibial occlusive disease. In: Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Upchurch GR, editors. Greenfield's Surgery: Scientific Principles and Practice. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 1648–1661. [Google Scholar]
- 63.Miller SJ, Coppinger BJ, Zhou X, Unthank JL. Antioxidants reverse age-related collateral growth impairment. J Vasc Res. 2009;47:108–114. doi: 10.1159/000235965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol. 2007;292:H2523–H2531. doi: 10.1152/ajpheart.01296.2006. [DOI] [PubMed] [Google Scholar]
- 65.Muluk SC, Muluk VS, Kelley ME, et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001;33:251–257. doi: 10.1067/mva.2001.112210. discussion 257–258. [DOI] [PubMed] [Google Scholar]
- 66.van Oostrom MC, van Oostrom O, Quax PHA, Verhaar MC, Hoefer IE. Insights into mechanisms behind arteriogenesis: what does the future hold? J Leukoc Biol. 2008;84:1379–1391. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- 67.Prior BM, Lloyd PG, Ren J, et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 68.Reddy DJ, Shepard AD. Aortoiliac Disease. In: Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Upchurch GR, editors. Greenfield's Surgery: Scientific Principles and Practice. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 1634–1647. [Google Scholar]
- 69.Rosenthal SL, Guyton AC. Hemodynamics of collateral vasodilatation following femoral artery occlusion in anesthetized dogs. Circ Res. 1968;23:239–248. doi: 10.1161/01.res.23.2.239. [DOI] [PubMed] [Google Scholar]
- 70.Rowlands TE, Donnelly R. Medical therapy for intermittent claudication. Eur J Vasc Endovasc Surg. 2007;34:314–321. doi: 10.1016/j.ejvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.van Royen N, Piek JJ, Schaper W, Bode C, Buschmann I. Arteriogenesis: mechanisms and modulation of collateral artery development. J Nucl Cardiol. 2001;8:687–693. doi: 10.1067/mnc.2001.118924. [DOI] [PubMed] [Google Scholar]
- 72.Salaria H, Atkinson R. Anatomic study of the middle genicular artery. J Orthop Surg (Hong Kong) 2008;16:47–49. doi: 10.1177/230949900801600112. [DOI] [PubMed] [Google Scholar]
- 73.Sanne H, Sivertsson R. The effect of exercise on the development of collateral circulation after experimental occlusion of the femoral artery in the cat. Acta Physiol Scand. 1968;73:257–263. doi: 10.1111/j.1748-1716.1968.tb04104.x. [DOI] [PubMed] [Google Scholar]
- 74.Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Cardiovasc Res. 1999;43:835–837. doi: 10.1016/s0008-6363(99)00191-1. [DOI] [PubMed] [Google Scholar]
- 75.Schechter DC, Bergan JJ. Popliteal aneurysm: a celebration of the bicentennial of John Hunter's operation. Ann Vasc Surg. 1986;1:118–126. doi: 10.1016/S0890-5096(06)60712-7. [DOI] [PubMed] [Google Scholar]
- 76.Scholz D, Elsaesser H, Sauer A, et al. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF)−/− mice. J Mol Cell Cardiol. 2003;35:177–184. doi: 10.1016/s0022-2828(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 77.Scholz D, Ito W, Fleming I, et al. Ultrastructure and molecular histology of rabbit hindlimb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 78.Scholz D, Schaper W. Enhanced arteriogenesis in mice overexpressing erythropoietin. Cell Tissue Res. 2006;324:395–401. doi: 10.1007/s00441-005-0072-5. [DOI] [PubMed] [Google Scholar]
- 79.Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 80.Schoop W. Limb collaterals. In: Schaper W, Schaper J, editors. Collateral Circulation: Heart, Brain, Kidneys, Limbs. Kluwer Academic Publishers; Boston, MA: 1993. pp. 317–327. [Google Scholar]
- 81.Seifert FC, Banker M, Lane B, Bagge U, Anagnostopoulos CE. An evaluation of resting arterial ischemia models in the rat hind limb. J Cardiovasc Surg (Torino) 1985;26:502–508. [PubMed] [Google Scholar]
- 82.Shepherd JT. The effect of acute occlusion of the femoral artery on the blood supply to the calf of the leg before and after release of sympathetic vasomotor tone. Clin Sci. 1950;9:355–365. [PubMed] [Google Scholar]
- 83.Shepherd JT. The blood flow through the calf of the leg during acute occlusion of the femoral artery and vein. Circulation. 1952;6:281–285. doi: 10.1161/01.cir.6.2.281. [DOI] [PubMed] [Google Scholar]
- 84.Sheridan KM, Ferguson MJ, Distasi MR, et al. Impact of genetic background and aging on mesenteric collateral growth capacity in Fischer 344, Brown Norway, and Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2007;293:H3498–H3505. doi: 10.1152/ajpheart.00040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sho E, Komatsu M, Sho M, et al. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol. 2003;75:1–11. doi: 10.1016/s0014-4800(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 86.Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 87.Stimson LA. III. An inquiry into the use of the ligature in the treatment of aneurysm. Ann Surg. 1885;1:13–25. [PMC free article] [PubMed] [Google Scholar]
- 88.Sturek M, Wenzel J, Byrd JP, et al. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swindle M, editor. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. CRC Press; Boca Raton, FL: 2007. pp. 397–402. [Google Scholar]
- 89.Sumner DS, Zierler RE. Vascular physiology: essential hemodynamic principles. In: Rutherford RB, editor. Vasc Surg. Elsevier Saunders; Philadelphia, PA: 2005. pp. 75–123. [Google Scholar]
- 90.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 91.Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol. 2008;586:1649. doi: 10.1113/jphysiol.2007.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tuttle JL, Hahn TL, Sanders BM, et al. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol. 2002;283:H146–H155. doi: 10.1152/ajpheart.00766.2001. [DOI] [PubMed] [Google Scholar]
- 93.Tuttle JL, Nachreiner RD, Bhuller AS, et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 94.Tuttle JL, Sanders BM, Burkhart HM, et al. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–351. doi: 10.1038/sj.mn.7800151. [DOI] [PubMed] [Google Scholar]
- 95.Unthank JL, Fath SW, Burkhart HM, Miller SC, Dalsing MC. Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circ Res. 1996;79:1015–1023. doi: 10.1161/01.res.79.5.1015. [DOI] [PubMed] [Google Scholar]
- 96.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol. 1996;271:H914–H923. doi: 10.1152/ajpheart.1996.271.3.H914. [DOI] [PubMed] [Google Scholar]
- 97.Unthank JL, Nixon JC, Dalsing MC. Acute compensation to abrupt occlusion of rat femoral artery is prevented by NO synthase inhibitors. Am J Physiol. 1994;267:H2523–H2530. doi: 10.1152/ajpheart.1994.267.6.H2523. [DOI] [PubMed] [Google Scholar]
- 98.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol. 1995;79:73–82. doi: 10.1152/jappl.1995.79.1.73. [DOI] [PubMed] [Google Scholar]
- 99.Vita JA, Holbrook M, Palmisano J, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117:3126–3133. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J Vasc Surg. 2003;38:198–203. doi: 10.1016/s0741-5214(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 101.Walden R, Adar R, Rubinstein ZJ, Bass A. Distribution and symmetry of arteriosclerotic lesions of the lower extremities: an arteriographic study of 200 limbs. Cardiovasc Intervent Radiol. 1985;8:180–182. doi: 10.1007/BF02552893. [DOI] [PubMed] [Google Scholar]
- 102.Wang HW, Langohr IM, Sturek M, Cheng JX. Imaging and quantitative analysis of atherosclerotic lesions by CARS-based multimodal nonlinear optical microscopy. Arterioscler Thromb Vasc Biol. 2009;29:1342–1348. doi: 10.1161/ATVBAHA.109.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol. 2004;97:773–780. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- 104.Winblad JN, Reemtsma K, Vernhet JL, Laville LP, Creech O. Etiologic mechanisms in the development of collateral circulation. Surgery. 1959;45:105–117. [PubMed] [Google Scholar]
- 105.Yang HT, Ogilvie RW, Terjung RL. Training increases collateral-dependent muscle blood flow in aged rats. Am J Physiol Heart Circ Physiol. 1995;268:H1174–H1180. doi: 10.1152/ajpheart.1995.268.3.H1174. [DOI] [PubMed] [Google Scholar]
- 106.Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise training produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol Heart Circ Physiol. 2002;282:H301–H310. doi: 10.1152/ajpheart.00160.2001. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y, Tang G, Yan J, et al. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J Vasc Surg. 2008;48:1546–1558. doi: 10.1016/j.jvs.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H Oxidase derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1008–H1016. doi: 10.1152/ajpheart.00114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol. 2009;297:H2227–H2233. doi: 10.1152/ajpheart.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 111.Zweifach BW, Kovalcheck S, DeLano F, Chen P. Micropressure-flow relationships in a skeletal muscle of Spontaneously Hypertensive Rats. Hypertension. 1981;3:601–614. doi: 10.1161/01.hyp.3.5.601. [DOI] [PubMed] [Google Scholar]