Abstract
The ability to achieve tumor selective expression of therapeutic genes is an area that needs improvement for cancer gene therapy to be successful. One approach to address this is through the use of promoters that can be controlled by external means, such as hyperthermia. In this regard, we constructed a replication-deficient adenovirus that consists of a mutated herpes simplex virus 1 thymidine kinase (mTK) fused to enhanced green fluorescent protein (EGFP) under the control of the full-length human heat shock (HS) 70b promoter. The virus (AdHSmTK-EGFP) was evaluated both in vitro and in vivo in oral squamous cell carcinoma SCC-9 cells for expression of both mTK and EGFP. The in vitro expression of mTK-EGFP was validated using both 3H-penciclovir and fluorescence-activated cell sorting assays. These studies show that specific expression could be achieved by heating the cells at 41 °C for 1 h, whereas little expression was observed using high doses of virus without hyperthermia. The vector was also evaluated in vivo by direct intratumoral injection into mice bearing SCC-9 xenografts. These studies demonstrated tumor expression of mTK-EGFP after ultrasound heating of the tumors by radioactive biodistribution assays, histology and microPET imaging. These in vivo results, which demonstrate HS-inducible transgene expression using PET imaging, provide a means for noninvasive monitoring of heat-induced gene therapy in local tumors, such as oral squamous cell carcinomas.
Keywords: hyperthermia, adenovirus, PET imaging, heat shock promoter, thymidine kinase
Introduction
More than 850 cancer gene therapy clinical trials have been initiated in the past 20 years using a variety of approaches, including attempts to correct genetic abnormalities, stimulation of antitumor immunity of the host and the introduction of ‘suicide genes’ that can convert a harmless prodrug into a potent cytotoxin for direct tumor cell killing.1 Although these trials have demonstrated the potential efficacy of this strategy, they have not reached their initial expectations of curing cancer. Based on these results, there are many areas where cancer gene therapy can be improved. Some major areas of improvement include the choice of effector genes, the ability to selectively deliver and express these effector genes in tumor cells, and the ability to determine the efficiency of gene transfer as a surrogate for therapeutic outcome.2
Selective expression of the effector can be achieved by either targeting the gene therapy vector to the desired cells (transductional targeting) or transcriptionally limiting expression to the cells. Transductional targeting can either enhance vector delivery to the desired cells or reduce vector delivery to normal organs, whereas transcriptional targeting utilizes selective promoters to control and limit transgene expression to the desired cells.3 Some of these promoters include tyrosinase,4 cyclooxygenase-2,5 human-telomerase6 and carcinoembryonic antigen.7 Another strategy for transcriptional targeting has been the use of promoters that can be externally regulated such as radiation-responsive promoters 8,9 and heat-inducible promoters.10 These promoters have the advantage in that they can be controlled by conformal delivery of radiation and heat, respectively. Our laboratory is interested in the utilization of heat-inducible promoters and evaluating their efficiency for mediating in vivo gene transfer using positron-emission tomographic (PET) imaging.
It has been shown that hyperthermia can be used to induce gene expression after infection with viral vectors. Most of these studies have been conducted using vectors that contain the heat shock (HS) protein 70b promoter to drive the expression of genes such as the herpes simplex virus 1 thymidine kinase (HSV1-TK), interleukin-12 (IL-12), antisense Ku70, β-galactosidase and enhanced green fluorescent protein (EGFP).11–14 These studies have largely focused on the heat-specific expression of these transgenes in cancer cells in vitro with the exception of IL-12 and antisense Ku70, which have been investigated in animal models. One of the goals of this manuscript is to demonstrate that heat-specific transgene expression in vivo can be monitored by PET imaging.
In this study, we have constructed a replication-deficient type 5 adenoviral vector (AdHSmTK-EGFP) encoding a mutant version of HSV1-TK that is driven by the full-length HS promoter. This mutant (mNLS-sr39TK-EGFP) has been previously described15 and consists of HSV1-sr39TK, which has five amino-acid differences from wild-type HSV1-TK,16 a mutated nuclear localization sequence (mNLS) and is fused to EGFP. The AdHSmTK-EGFP was evaluated in human head and neck squamous cell carcinoma SCC-9 cells for mutated herpes simplex virus 1 thymidine kinase (mTK) activity using a tritiated penciclovir assay and for EGFP expression using fluorescence-activated cell sorting (FACS). The vector was then evaluated in mice bearing SCC-9 xenografts to determine if specific mTK-EGFP expression could be achieved after ultrasound heating of the tumor xenografts in biodistribution and microPET imaging assays using radiolabeled substrates. These studies demonstrate that PET imaging can be used to determine the extent of in vivo expression of an effector gene driven by the heat-inducible HS promoter for noninvasive monitoring of therapy.
Materials and methods
Cell line and adenovirus
The oral squamous carcinoma cell line SCC-9 was obtained from the American Type Tissue Culture Center (Manassas, VA) and was maintained in 45% Ham’s F12, 45% Dulbecco’s modified Eagle’s medium (DMEM), 10% heat-inactivated fetal bovine serum (FBS), and 0.4 µg ml−1 hydrocortisone. Ham’s F12 and DMEM were obtained from Invitrogen (Carlsbad, CA), and FBS and hydrocortisone were obtained from Sigma Chemical Company (St Louis, MO). For construction of the AdHSmTK-EGFP, the Gal4 promoter in pGal4-mNLS-sr39TK-EGFP15 was replaced with the full-length human HS protein 70b promoter from p2500-CAT (Stressgen Biotechnologies, Victoria, British Columbia, Canada) via the BglII and HindIII restriction enzyme sites, thus producing HSmTK-EGFP. The HSmTK-EGFP portion was subcloned into pShuttle (Stratagene, La Jolla, CA), which had a bovine growth hormone polyadenylation signal added to the multiple cloning site, via BglII and NotI to create pHSmTK-EGFP. Using this plasmid, recombinant adenoviral plasmid was produced using the AdEasy system (Stratagene). The resultant recombinant Ad plasmid (pAdHSmTK-EGFP) was used to produce purified adenovirus, which was performed by Qbiogene (Montreal, Quebec, Canada). The adenovirus, AdHSmTK-EGFP, was produced at a titer of 3.2 × 1011 pfu ml−1. AdCMVmTK-EGFP was produced as previously described15 at a titer of 5.0 × 109 pfu ml−1.
In vitro 3H-penciclovir assays
The expression of the thymidine kinase portion of AdHSmTK-EGFP was evaluated in a series of in vitro 3H-penciclovir uptake assays. SCC-9 cells were plated in 12-well plates (50 000 cells per well) and infected in triplicate 24 h later with AdHSmTK-EGFP at 50, 100 and 500 plaque forming units (pfu) per cell in serum-free Opti-MEM (Invitrogen). For each assay, a triplicate of uninfected wells served as a negative control. The infections were carried out for 2 h, after which the media was aspirated and replaced with complete media. In the first set of studies, one group of cells was heat shocked for 30, 60, 120 or 240 min at 41 °C by submerging the plates in a water bath, and another set was not heated, either 1, 2 or 3 days following the infection. Following hyperthermia, the plates were returned to the incubator for 1 day. The cells were then washed with phosphate-buffered saline (PBS), and 0.1 µCi of 3H-penciclovir (Moravek Biochemicals, Brea, CA) in 0.5 ml of complete medium was added to the cells. The cells were incubated at 37 °C for 1 h, after which the media was aspirated, the cells washed with PBS, and 1 ml of complete media was added to the cells. The cells were incubated for an additional 1 h at 37 °C to enable washout of nonphosphorylated nucleoside, washed with cold PBS, and 500 µl of 10 mm sodium borate/1% SDS was added to the cells to facilitate cell lysis. Of the lysate, 250 µl was added to scintillation tubes along with 4.5 ml of scintillant, and the radioactivity was counted on a TriCarb2900 Liquid Scintillation Counter (Perkin Elmer, Shelton, CT) to determine the decays per minute (DPM) for each sample. In the second set of studies, the cells were heated for 60 min at 41 °C as described above or not heated 1 day following infection. Following hyperthermia, the plates were returned to the incubator for 1, 2 or 3 days and then assayed for 3H-penciclovir uptake as described. All data were normalized to the milligram of protein per sample using the Pierce BCA Protein Assay Kit (Pierce Biotechnology Inc., Rockford, IL).
Flow cytometry
For EGFP analysis of AdHSmTK-EGFP infected SCC-9 cells, 5 × 105 cells were infected at 50, 100 and 500 pfu per cell. An uninfected control was also performed in each assay. Infection was allowed to proceed for 1, 2 or 3 days as described above and then the cells were heated at 41 °C for 1 h and collected 1 day later in 1 ml of DMEM plus 5% heat-inactivated FBS. As above, another set of studies was performed where the cells were harvested 1, 2 and 3 days after heating with a 1-day infection. The number of cells expressing EGFP as well as the intensity of the fluorescence was measured on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) in which 50 000 events were counted per sample. The data were analyzed using CellQuest software in which the EGFP mean fluorescent intensity (MFI) was plotted against number of events.
Biodistribution studies
All animal studies were performed under the guidelines for the Care and Use of Research Animals through the Washington University Animal Studies Committee. Female 3- to 4-week old SCID mice (Taconic, Hudson, NY, USA) were implanted subcutaneously on the rear flank with 1 × 107 SCC-9 cells, which had been mixed 1:1 with Matrigel Basement Membrane Matrix (Becton Dickinson). After 18–21 days, the tumors (~400 mg) were directly injected with either 1 × 108 or 1 × 109 pfu of AdHSmTK-EGFP in a volume of 30 µl. One day after injection, the tumor was heated for 1 h at 41 °C using a small animal hyperthermic ultrasound (SAHUS) device. 17,18 Briefly, the mice were anesthetized and placed on a plastic tray that had been fitted with an ultrasound transducer. A temperature-monitoring probe was placed in the tumor, and the transducer and tumor were covered in ultrasound gel. Hyperthermia delivery to the tumor was performed under computer control using software developed in-house, in which the applied acoustical power to the ultrasound transducer was regulated through a proportional-derivative-integral temperature feedback algorithm based on the intratumoral measured temperatures. The temperature was maintained at 41.0 ± 0.3 °C throughout the heating. Following hyperthermia, the animals were allowed to recover for one day. The mice (n = 4–7 per group) were then injected intravenously (i.v.) with 2 µCi of 3H-penciclovir. The animals were killed 4 h after injection, and blood, muscle, liver, kidney, tail and tumors were extracted and weighed. To aid in tissue dissolution for β-counting, 1 ml of hyamine hydroxide (MP Biomedicals, Irvine, CA) was added to the tissue, and the vials were incubated at 65 °C for 18 h. To this, 200 µl of 30% hydrogen peroxide and 50 µl of glacial acetic acid were added, mixed, and the vials were placed back at 65 °C for 30 min. The vials were then cooled to room temperature, scintillant added, incubated in the dark for 30 min, and the samples counted on the scintillation counter. The percent injected dose per gram was calculated based on normalization to a standard dose.
Histology
To determine the EGFP expression ex vivo, tumors were implanted, injected with 1 × 108 of AdCMVmTK-EGFP or 1 × 109 pfu of AdHSmTK-EGFP and heated as above. Two days after Ad injection (one day after heating AdHSmTK-EGFP tumors), the tumors and livers were extracted and frozen in optimal cutting temperature medium (Electron Microscopy Sciences, Hatfield, PA). After freezing, the tissues were cryosectioned into 10 µm slices, fixed with 4% paraformaldehyde and mounted with Prolong Gold antifade mounting medium with 46-diamidino-2-phenyl indole (Invitrogen). Tissue sections were visualized at × 20 magnification using an Olympus Fluoview FV1000 (Center Valley, PA) in which EGFP was visualized using the 488 nm excitation laser.
Imaging studies
SCC-9 cells were implanted, injected with AdHSmTK-EGFP and heated as before, except that for imaging, the tumors were placed on the axillary thorax. 9-(4-[18F]-fluoro-3-hydroxymethylbutyl)guanine (18F-FHBG) was synthesized from (p-anisyldiphenylmethyl)-9-[(4-tosyl)-3-p-anisyldiphenylmethoxy-methylbutyl]guanine, 2,2,2-kry-tofix F[18] in dimethyl sulfoxide, purified on C-18 Cartridge (Waters Corporation, Milford, MA), deprotected, and finally purified using a C-18 column on high-pressure liquid chromatography (Waters Corporation) equipped with a radiodetector (Bioscan Inc., Washington, DC) as described previously.15,19 The mice (n = 3) were injected i.v. with ~100 µCi of 18F-FHBG, anesthetized with 1–2% isoflurane, positioned supine and imaged on a microPET FOCUS 220 (Concorde Microsystems, Knoxville, TN) at 1, 2 and 4 h after injection with a 10 min acquisition time and static OSEM reconstruction. Regions of interest for the tumors were drawn to determine an average activity concentration (nCi cc−1) in the tumors, with the activity being decayed corrected to the time of injection. This value was then multiplied by the mouse weight in grams and then divided by the nCi of the injected dose to give the standard uptake value (SUV) for the tumors.
Statistical analysis
A Student’s two-tailed t-test was employed to analyze the statistical significance for all of the experimental data presented as the mean of independently performed experiments ± s.e.m., with P ≤ 0.05 being considered significant.
Results
In vitro 3H-penciclovir assays
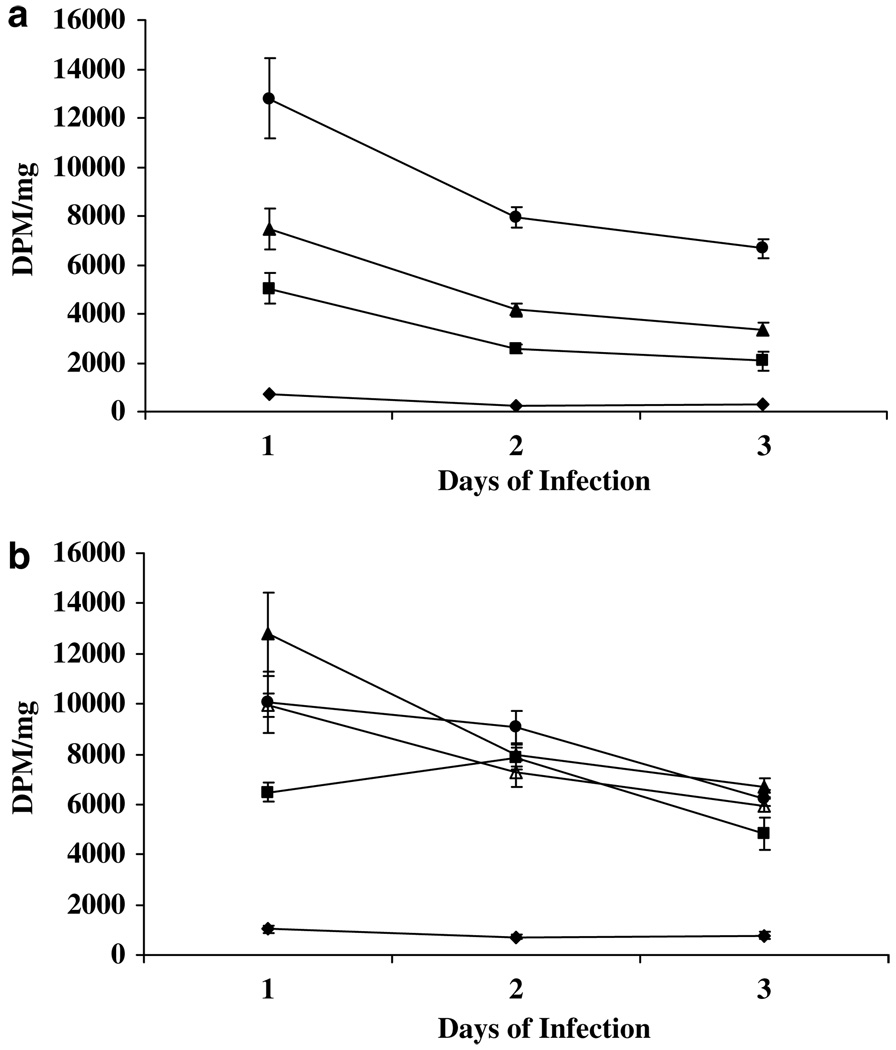
Expression and functional activity of mTK was evaluated at various times after infection or after hyperthermia using various doses of AdHSmTK-EGFP and various heating times at 41 °C. In Figure 1, cells were heated after a 1, 2 or 3-day infection, and then the 3H-penciclovir assay was performed 1 day after heating. Figure 1a shows that increasing amounts of AdHSmTK-EGFP led to significantly greater (P < 0.04) amounts of cell-associated radioactivity with all infection times and that cells infected at 50 pfu per cell had significantly greater (P < 0.01) cell-associated radioactivity than noninfected cells. In addition, the highest cell-associated radioactivity was observed after a 1-day infection, with levels significantly (P < 0.02) decreasing with 2- and 3-day infections. The cell-associated radioactivity in cells infected at 500 pfu per cell and heated at 41 °C for various times is shown in Figure 1b. This shows that there are not significant differences in cell-associated radioactivity when heating for 30, 60, 120 or 240 min, except for a significant decrease when heating for 30 min after a 1 day infection (P < 0.04). Cells that were infected at 500 pfu per cell, but not heated, had significantly less (P < 0.0001) cell-associated radioactivity than cells that were infected and heated. In addition, cells that were infected at 500 pfu per cell, but not heated (1021 ± 140 DPM mg−1), had significantly more uptake than uninfected cells that were not heated (661 ± 90DPM mg−1) (P = 0.05), whereas cells infected at 50 and 100 pfu per cell, but not heated were not significantly greater (data not shown). These data show that a 1-day infection followed by 1 h heating at 41 °C led to the highest expression of mTK-EGFP and that there was a small amount of nonspecific expression of mTK-EGFP after infection without hyperthermia.
Figure 1.
Uptake of 3H-penciclovir in SCC-9 cells infected with AdHSmTK-EGFP as a function of infection time and heating time. Cells were infected with AdHSmTK-EGFP at 0 (diamonds), 50 (squares), 100 (triangles) or 500 (circles) pfu per cell (a). After infection for 1, 2 or 3 days, the cells were heated at 41 °C for 1 h, and uptake and retention of 3H-penciclovir was determined 1 day later. In (b), the cells were infected at 500 pfu per cell for 1, 2 or 3 days. Following infection, the cells were left unheated (diamonds) or heated at 41 °C for 30 (squares), 60 (triangles), 120 (circles) or 240 (open triangles) min. The uptake and retention of 3H-penciclovir was determined 1 day later and presented as the decays per minute (DPM) mg−1 of protein. The data are shown as the mean ± s.e.m. of 2–3 experiments, with each experiment being performed in triplicate.
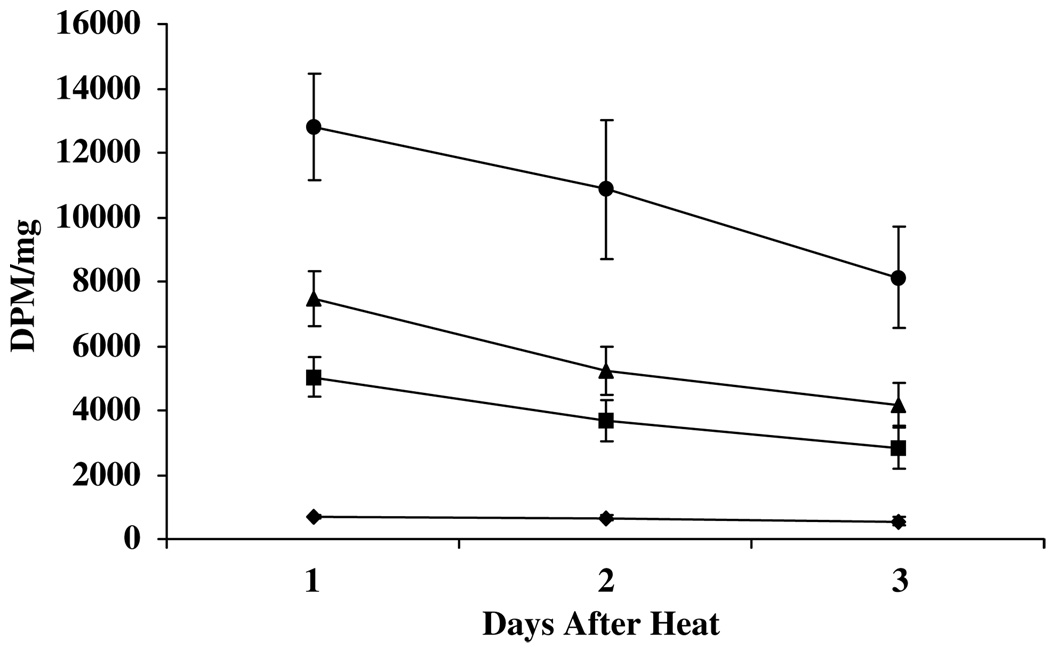
Based on these results, we determined the expression of mTK 1, 2 and 3 days after 1 h of heating at 41 °C in cells that had been infected with AdHSmTK-EGFP for 1 day. This again shows a dose response with increasing amounts of AdHSmTK-EGFP (Figure 2). The greatest amount of cell-associated radioactivity was observed 1 day after heating for all doses of virus, although these values were not significantly greater than 2 days after heating. Overall, these studies show that a 1-day infection followed by 1-h heating at 41 °C led to the highest expression of mTK-EGFP 1 day after hyperthermia. For comparison, the cell-associated radioactivity when the cells were infected with AdHSmTK-EGFP at 100 pfu per cell was 37% of the cell-associated radioactivity when the cells were infected with AdCMVmTK-EGFP at 100 pfu per cell (data not shown). Heating of AdCMVmTK-EGFP-infected cells 1 day after infection at 41 °C for 1 h did not change the cell-associated radioactivity compared to nonheated cells. Infection with AdCMVmTK-EGFP at 500 pfu per cell resulted in significant toxicity that was not observed with AdHSmTK-EGFP.
Figure 2.
Uptake of 3H-penciclovir in SCC-9 cells infected with AdHSmTK-EGFP as a function of time after heating. Cells were infected with AdHSmTK-EGFP at 0 (diamonds), 50 (squares), 100 (triangles) or 500 (circles) pfu per cell. After infection for 1 day, the cells were heated at 41 °C for 1 h, and uptake and retention of 3H-penciclovir was determined 1, 2 and 3 days later. The data are shown as the mean ± s.e.m. of 2–3 experiments, with each experiment being performed in triplicate.
Flow cytometry
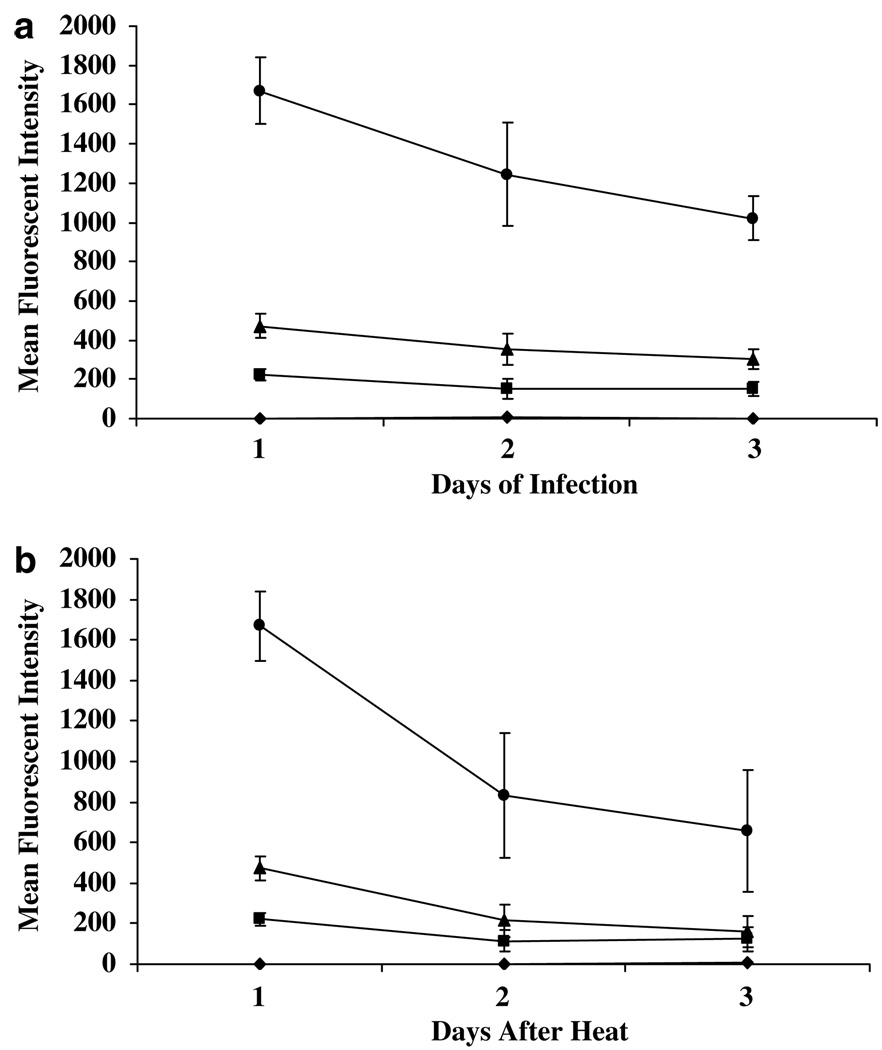
We independently confirmed the expression of mTK-EGFP using flow cytometry to detect EGFP. These studies confirmed the increasing expression of mTK-EGFP with increasing doses of AdHSmTK-EGFP (Figure 3a) as observed in the 3H-penciclovir assays. Also, the highest expression was observed 1 day after hyperthermia following an infection for 1 day (Figure 3b). There was a statistically significant (P < 0.0005) increase in the MFI for cells infected at 500 pfu per cell without heating (MFI = 35.5) compared to uninfected cells (MFI = 3.1; data not shown). This shows that there is an 11-fold increase in expression of mTK-EGFP at this pfu per cell without heating compared to background, but that this is still 6-fold less than infection at 50 pfu per cell with hyperthermia.
Figure 3.
Expression of mTK-EGFP as determined by fluorescence-activated cell sorting (FACS) analysis of SCC-9 cells infected with AdHSmTK-EGFP. (a) Cells were infected with AdHSmTK-EGFP at 0 (diamonds), 50 (squares), 100 (triangles) or 500 (circles) pfu per cell. After infection for 1, 2 or 3 days, the cells were heated at 41 °C for 1 h, and expression of mTK-EGFP was determined by FACS analysis 1 day later. (b) Cells were again infected with AdHSmTK-EGFP at 0 (diamonds), 50 (squares), 100 (triangles) or 500 (circles) pfu per cell. After infection for 1 day, the cells were heated at 41 °C for 1 h, and expression of mTK-EGFP was determined by FACS analysis 1, 2 and 3 days later. The data are shown as the mean ± s.e.m. of 4–6 independent experiments, with each experiment being performed as a single point.
Biodistribution studies
Tumor uptake of 3H-penciclovir was not significantly different between the negative control tumors (Opti-MEM, heat only, and 1 × 109 pfu of AdHSmTK-EGFP without heat), but these tumors were significantly lower (P < 0.05) than tumors injected with the positive control AdCMVmTK-EGFP (1 × 108 pfu) or AdHSmTK-EGFP (1 × 108 and heated at 41 °C for 1 h) (Table 1). The tumor uptake of 3H-penciclovir from AdHSmTK-EGFP (1 × 109 and heated at 41 °C for 1 h) injected tumors was significantly greater than tumors that received heat only or 1 × 109 pfu of AdHSmTK-EGFP without heat, but not greater than Opti-MEM tumors. An increase in the tumor uptake of 3H-penciclovir was observed when injecting 1 × 109 pfu AdHSmTK-EGFP versus 1 × 108, although the increase did not reach significance. AdCMVmTK-EGFP (1 × 108 pfu) served as a positive control and was greater than the heated 1 × 108 and 1 × 109 pfu AdHSmTK-EGFP injected tumors, but the difference did not reach significance. A biodistribution performed at 1 h after injection of 3H-penciclovir did not show a difference between tumors injected with Opti-MEM (2.59 ± 0.20) and tumors injected with 1 × 108 pfu of AdHSmTK-EGFP either heated (2.57 ± 0.28) or not heated (2.80 ± 0.23; data not shown). It is likely that differences were not observed at this time point because the nonphosphorylated substrate did not clear sufficiently from the control tumors. It is not clear why the liver uptake of 3H-penciclovir was significantly lower (P < 0.04) for the 1 × 109 no heat group compared to the other negative control groups (Opti-MEM or heat only). Because of this, the liver uptakes of 3H-penciclovir were significantly greater (P < 0.05) between the heated AdHSmTK-EGFP groups and the 1 × 109 no heat group, but were not significantly greater than the other negative control groups (Opti-MEM or heat only). The liver uptake of 3H-penciclovir was significantly greater (P < 0.01) in mice injected with AdCMVmTK-EGFP than in the liver of all other groups. Blood, muscle and kidney did not show significant differences in 3H-penciclovir uptake between any of the negative groups and the AdHSmTK-EGFP groups that were heated. These studies show that specific in vivo expression of mTK can be observed in tumors injected with AdHSmTK-EGFP and heated at 41 °C for 1 h. In addition, it is observed that even upon intratumoral injection of the adenovirus, some virus gets into the systemic circulation to infect the liver, resulting in high 3H-penciclovir uptake in tumors injected with AdCMVmTK-EGFP. This high liver expression is not observed upon injection of AdHSmTK-EGFP, where expression should be limited to the area of heating.
Table 1.
Biodistribution of 3H-penciclovir in mice bearing SCC-9 tumors (% ID/g ± s.e.m.; n = 4–7)
| Tissue | Opti-MEM | CMV | Heat only | 1 × 109 No heat | 1 × 108 pfu | 1 × 109 pfu |
|---|---|---|---|---|---|---|
| Blood | 0.98 ± 0.22 | 0.56 ± 0.05 | 0.49 ± 0.13 | 0.38 ± 0.09 | 0.86 ± 0.16 | 1.04 ± 0.21 |
| Muscle | 0.42 ± 0.07 | 0.37 ± 0.05 | 0.42 ± 0.11 | 0.28 ± 0.03 | 0.53 ± 0.08 | 0.76 ± 0.26 |
| Liver | 1.07 ± 0.11 | 14.57 ± 2.10 | 0.78 ± 0.04 | 0.63 ± 0.02 | 1.21 ± 0.09 | 1.24 ± 0.24 |
| Kidney | 0.79 ± 0.08 | 0.68 ± 0.07 | 0.56 ± 0.06 | 0.49 ± 0.07 | 0.79 ± 0.08 | 1.02 ± 0.24 |
| Tumor | 0.48 ± 0.05 | 1.68 ± 0.25 | 0.40 ± 0.02 | 0.41 ± 0.05 | 1.03 ± 0.14 | 1.22 ± 0.28 |
Tumors were injected with Opti-MEM, AdCMVmTK-EGFP (CMV; 1 × 108 pfu), or AdHSmTK-EGFP (1 × 108 and 1 × 109 pfu). Twenty-four hours later, some tumors were heated (heat only, 1 × 108 pfu, 1 × 109 pfu) at 41 °C for 1 h, while others were not (Opti-MEM, CMV, 1 × 109 no heat). Twenty-four hours later, mice were injected i.v. with 3H-penciclovir and killed at 4 h.
Histology
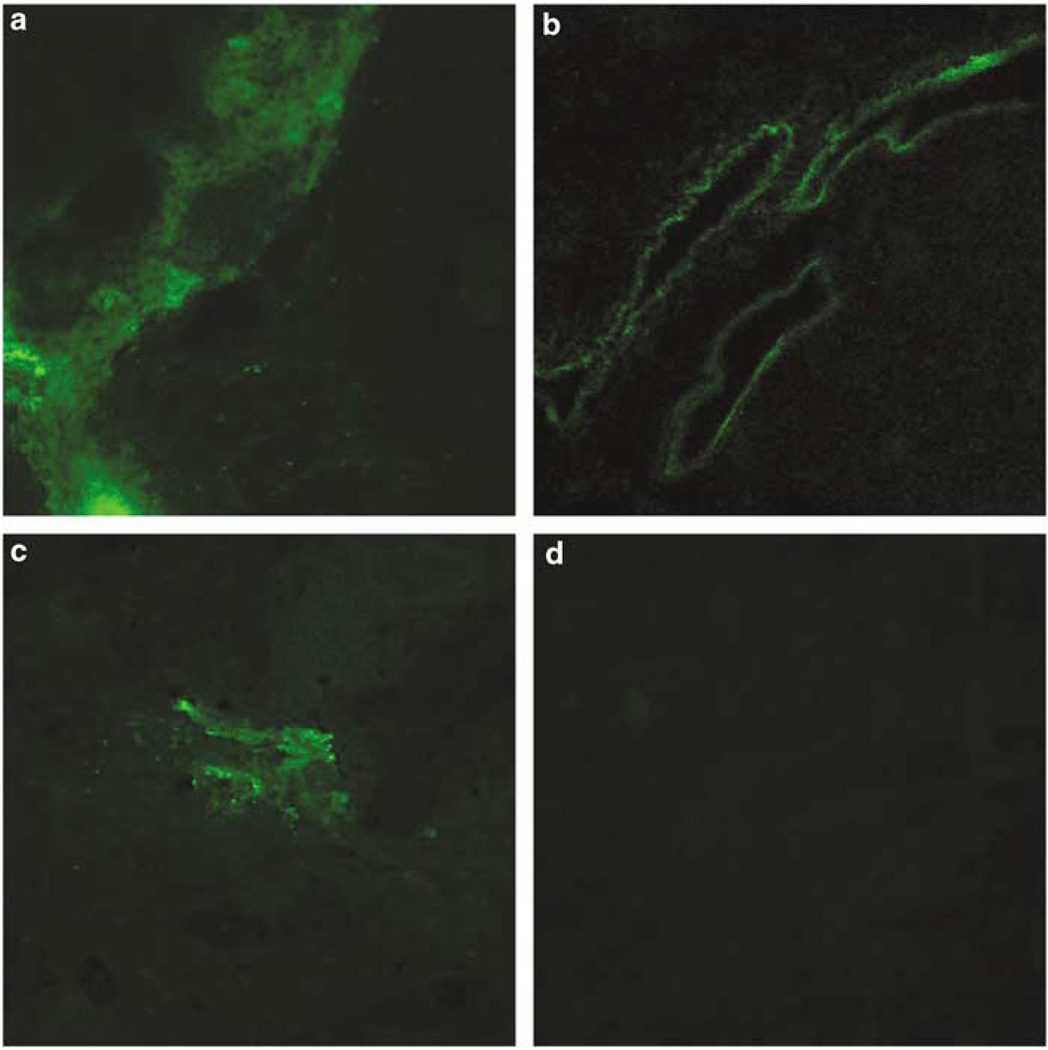
Ex vivo EGFP fluorescence images of tumors (a, c) and livers (b, d) from mice in which tumors were directly injected with 1 × 108 pfu of AdCMVmTK-EGFP (a, b) or 1 × 109 pfu of AdHSmTK-EGFP (c, d) and heated are shown in Figure 4. Tumors that were injected with 1 × 109 pfu of AdHSmTK-EGFP and not heated (data not shown) confirm the biodistribution results that there is no apparent expression of mTK-EGFP in the tumor or liver. Upon heating, mTK-EGFP expression is observed in the tumors (c) whereas there is still no apparent expression of mTK-EGFP in the liver (d). This is in contrast to injection of a lower dose of AdCMVmTK-EGFP, which shows good expression of mTK-EGFP in both the tumor (a) and liver (b). These studies along with the biodistribution studies imply that hepatic toxicity should be reduced when conducting therapy experiments in this model.
Figure 4.
Representative histological sections of SCC-9 tumors (a, c) and livers (b, d) from the same mice that were injected intratumorally with 1 × 108 pfu of AdCMVmTK-EGFP (a, b) or 1 × 109 pfu of AdHSmTK-EGFP and heated (c, d) at 41 °C for 1 h. Tumor and liver sections were mounted and visualized at × 20 magnification in which enhanced green fluorescent protein (EGFP) was visualized using the 488 nm excitation laser.
Imaging studies
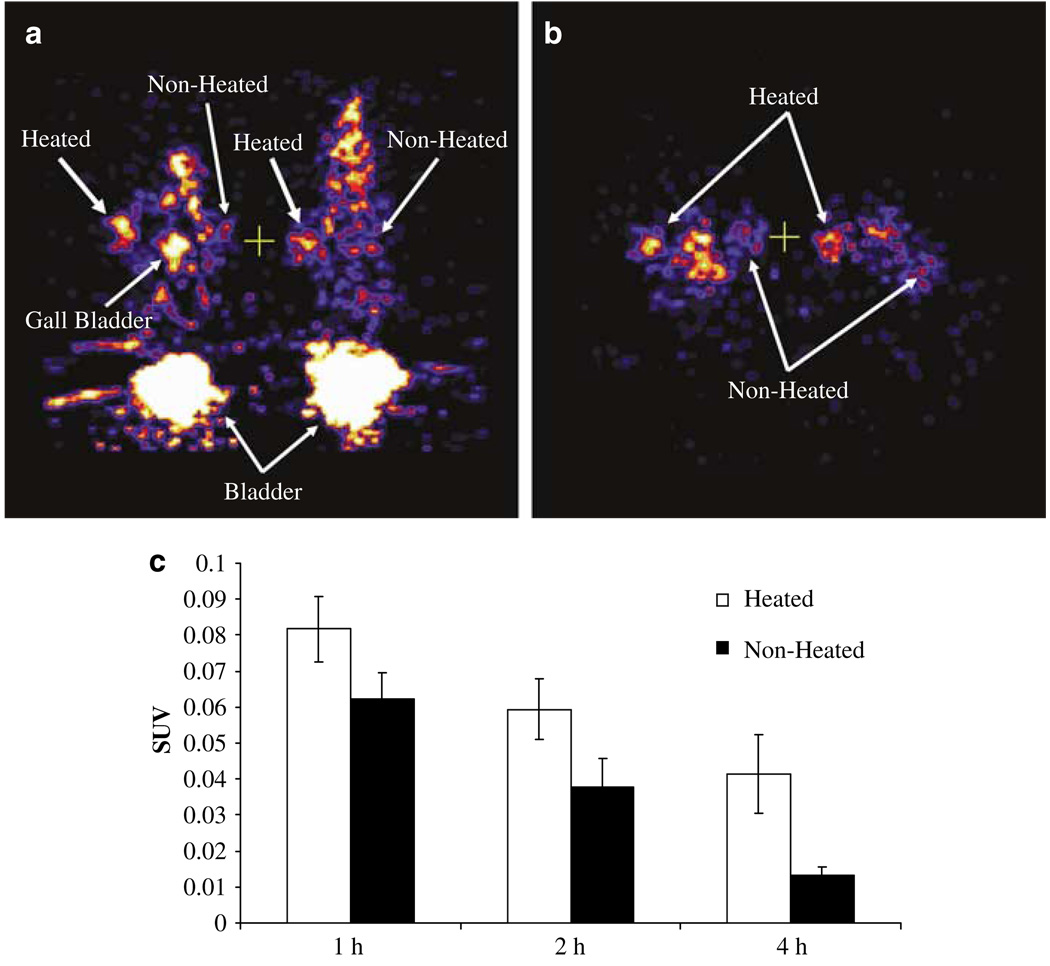
Small animal PET images of 18F-FHBG at 4 h after injection are shown in Figure 5. This figure shows a representative coronal (a) and transaxial (b) PET image of mice bearing two SCC-9 xenografts in the axillary thorax. Each tumor was injected with 1 × 109 pfu of AdHSmTK-EGFP, but only the tumors on the left were heated at 41 °C for 1 h. These images show good uptake and retention of 18F-FHBG in tumors that were heated whereas the tumors that were not heated demonstrated less uptake. As in previous studies with 18F-FHBG, the gall bladder and radioactive clearance through the bladder is observed in the coronal images (a) whereas the liver is not observed.15 The chemical differences between penciclovir and FHBG might impact the nonspecific background trapping by endogenous human TK and washout rates of the nonphosphorylated compounds, thus accounting for the difference in liver uptake between the biodistribution and imaging studies. The SUVs that were obtained from the microPET images of the left and right tumors at 1, 2 and 4 h after 18F-FHBG injection are shown in Figure 5c. This shows that there was significantly greater (P < 0.02) uptake and retention of 18F-FHBG in tumors that were heated compared to tumors that were not heated at 4 h after injection, whereas the difference between heated and nonheated did not reach significance at 1 or 2 h. These studies demonstrate that heat-induced expression of mTK can be monitored noninvasively through PET imaging with 18F-FHBG.
Figure 5.
Representative coronal (a) and transaxial (b) microPET images of mice bearing two SCC-9 xenografts in the axillary thorax 4 h after administration of 18F-FHBG. The mice were injected intratumorally with 1 × 109 pfu of AdHSmTK-EGFP 48 h before the imaging session, and the tumors on the left were heated at 41 °C for 1 h 1 day before imaging. Standard uptake values (SUVs) were determined from the microPET data for the heated and nonheated tumors at 1, 2, and 4 h after injection of 18F-FHBG (c). The SUV data is presented as the mean ± s.e.m. for each tumor (n = 3).
Discussion
HSV1-TK is an enzyme that has been widely studied for use in suicide gene therapy as well as a reporter gene for noninvasive imaging of gene transfer.1,20 One of the drawbacks in using HSV1-TK for suicide gene therapy with constitutively active promoters is the expression of the enzyme in nontarget tissues. This can be observed in Table 1, where mTK-EGFP is expressed in the liver of mice as evidenced by high 3H-penciclovir uptake even after a local, intratumoral injection of an adenoviral vector that uses the CMV promoter. Therefore, use of a promoter that can restrict the expression of the suicide gene to the tissue of choice should reduce the toxicity associated with normal tissue expression of the enzyme when conducting therapy studies. In these studies, we have used the HS 70b promoter to drive specific expression of mTK-EGFP. The goal of these studies was to evaluate the time course and specificity of mTK-EGFP expression in vitro and apply these parameters in vivo to demonstrate that PET imaging can be used to monitor in vivo expression and therefore guide future therapy studies.
Hyperthermia is probably the most potent radio-sensitizer known to date21 and has demonstrated a significant increase in complete remissions and disease free survival when combined with radiotherapy for the treatment of head and neck squamous cell carcinomas (HNSCC) in phase III clinical trials.22,23 Tumor temperatures greater than 42 °C are difficult to achieve in the clinic, whereas temperatures between 40.5 and 41.5 °C can be achieved for 90% of the tumor.24,25 For these reasons, we chose to evaluate AdHSmTK-EGFP in the context of HNSCC with the SCC-9 cells heated at a clinically relevant temperature of 41 °C. In addition, future therapy studies with this suicide gene approach may be enhanced by combining with radiotherapy and hyperthermia.
Most studies that have used hyperthermia to regulate gene expression via the HS promoter have been conducted in vitro.10 Blackburn et al. demonstrated the heat-specific expression of a cytosine deaminase (CD)/TK fusion protein in vitro using a replication-deficient adenovirus and the HS promoter.26 Similarly, Brade et al.12 showed HS-mediated expression of the same fusion protein and β-galactosidase in breast cancer cells after heating to 43 °C. Borrelli et al.11 investigated the time course of EGFP expression after infection of human prostate cancer cells with an adenovirus and heating at 41 °C. Lee et al.27,28 showed that the use of a selectively replicating adenovirus resulted in higher expression of the CD/TK fusion protein after heating at 41 °C compared to a nonreplicating adenovirus. These studies show that heat-specific expression of a target protein can be achieved in vitro using the HS promoter in the context of adenoviral vectors.
The temporal expression of mTK-EGFP in SCC-9 cells was evaluated using a 3H-penciclovir assay to determine functional mTK activity (Figures 1 and 2) as well as by flow cytometry for EGFP fluorescence (Figure 3). Both of these methods showed that the highest expression was achieved 1 day after heating cells for 1 h at 41 °C that had been infected with AdHSmTK-EGFP 1 day earlier. This is in agreement with Borrelli et al.11 that showed the best expression of EGFP was 1 day after heating at 41 °C with a 12–24 h infection. However, they showed that a 2 and 4 h HS was superior to a 1 h HS, whereas we did not observe a significant difference between any of these heating times. Borrelli et al. also reported some level of uninduced EGFP expression when infecting at 120 pfu per cell or greater. Similarly, we observed expression of mTK-EGFP using either assay at 500 pfu per cell without heating. Overall, these studies determined the timing for infection and heating to achieve good mTK-EGFP expression that could then be used for in vivo evaluation. Importantly, expression was not observed without heating unless high doses of AdHSmTK-EGFP were used.
The expression of mTK-EGFP was also evaluated in vivo in mice bearing subcutaneous SCC-9 xenografts that were directly injected with AdHSmTK-EGFP (Table 1; Figure 4). The biodistribution study (Table 1) shows specific tumor uptake of 3H-penciclovir after injection of AdHSmTK-EGFP and heating at 41 °C. Other in vivo studies utilizing the HS promoter have evaluated β-galactosidase, antisense Ku70 and IL-12. Brade et al.12 showed high expression of β-galactosidase in HeLa tumors directly injected with vector and heated to 44 °C, whereas minimal expression was observed in unheated tumors and in liver. Li et al.14 demonstrated significant tumor growth delay in mice bearing FSa-II tumors that were directly injected with an antisense Ku70 adenovirus, heated at 42.5 °C for 30 min and irradiated.14 The Dewhirst group has extensive experience evaluating IL-12 in mice and cats.13,29–32 These studies show that heat induced IL-12 can inhibit tumor growth in mice either alone or in combination with radiation and also have an antiangiogenic effect. In addition, a phase I study in cats demonstrated local production of IL-12 after intratumoral injection of vector that limited systemic toxicity.32
Our ex vivo imaging shows tumor expression of EGFP that is focal in nature after intratumoral injection of AdCMVmTK-EGFP or AdHSmTK-EGFP that was heated (Figure 4). This focal expression is limited to ~10–15% of the tumor volume and is consistent with previous studies that show limited expression of transgenes after direct intratumoral injection of adenoviral vectors.12,33 Mouse studies by the Dewhirst group were similar to ours in that a significant amount of vector leaked from the target site resulting in high liver expression when a constitutive promoter was used whereas no apparent liver expression was observed when the HS promoter was used.30 Our studies show that liver expression of mTK-EGFP is limited to sites around blood vessels after intratumoral injection of 1 × 108 pfu of AdCMVmTK-EGFP compared to a more homogenous expression of GFP in the liver after intratumoral injection of 3 × 108 pfu of AdCMVGFP in their mouse model. Although there is no apparent liver expression of mTK-EGFP after intratumoral injection of AdHSmTK-EGFP and heating as assayed by fluorescence, there is a trend for higher 3H-penciclovir uptake in the liver of these groups compared to the negative control groups implying that there may be low levels of expression. Systemic heating was not previously observed when using this SAHUS device in a different mouse model as systemic temperature readings varied by a maximum of 0.6 °C and the temperature just outside of the ultrasound field showed less than 1.0 °C variation from baseline.17 Therefore, we do not anticipate expression of mTK-EGFP in the liver resulting from a systemic heating effect, but this may need to be investigated in more depth.
Although others have demonstrated the utility of site-specific expression of an effector gene in vivo following HS, this is the first study to monitor in vivo expression noninvasively. Figure 5 shows that microPET imaging can be used to detect mTK-EGFP expression in tumors injected with vector and heated at 41 °C for 1 h. Significant differences were not observed between heated and nonheated tumors at 1 (similar to the 3H-penciclovir biodistribution study) and 2 h after injection of the 18F-FHBG substrate, but were observed 4 h after injection. This implies that at least 4 h is needed for the nonphosphorylated substrate to be cleared from the nonheated tumors and provide a differential with the heated, mTK-expressing tumors. In general, this differs from other studies where imaging is performed 1–2 h after injection of 18F-FHBG.34 One possible explanation is that different cells have different nucleoside transport mechanisms that may change the time course for optimal cell uptake and subsequent release of the radioactive substrate. In a recent review, Li et al.35 discuss the potential of microPET imaging for noninvasively monitoring the current state of combined hyperthermia and gene therapy studies. This review shows how microPET imaging can be used to monitor the improvement in viral vector distribution after a mild HS 6 h after administration of virus. This indicates that the focal expression observed in our in vivo results may be improved by applying heat to tumors 6–12 h after viral administration instead of 24 h.
In conclusion, we evaluated AdHSmTK-EGFP in vitro and in vivo to demonstrate the specificity of protein expression after heating at 41 °C. The in vitro studies show the specificity and time course of expression, although limited expression was observed at high viral doses without heating. The in vivo studies demonstrate heat-specific tumor uptake of radiolabeled TK substrates and the ability to noninvasively image this uptake with PET. Overall, these studies provide the proof-of-principle for using PET imaging to monitor heat-specific induction of TK gene therapy.
Acknowledgements
We thank Erin Jackson and Julie Prior for their assistance with the histology studies, Sharon Bloch for her assistance with confocal microscopy, and Scott Harpstrite for his assistance in the production of 18F-FHBG. We are also grateful to Nicole Fettig, Dawn Werner, Margaret Morris, Jerrel Rutlin and Lori Strong for performing the biodistribution studies, the microPET imaging, and the microPET data analysis. This work was supported by NCI Grants R21 CA106587 (BER), P50 CA94056 (DPW), and R01 CA63121 (EGM).
References
- 1.McCormick F. Cancer gene therapy: fringe of cutting edge? Nat Rev Cancer. 2001;1:130–141. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto M, Curiel DT. Cancer gene therapy. Technol Cancer Res Treat. 2005;4:315–330. doi: 10.1177/153303460500400402. [DOI] [PubMed] [Google Scholar]
- 3.Rein DT, Breidenbach M, Curiel DT. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006;2:137–143. doi: 10.2217/14796694.2.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siders WM, Halloran PJ, Fenton RG. Melanoma-specific cytotoxicity induced by a tyrosinase promoter-enhancer/herpes simplex virus thymidine kinase adenovirus. Cancer Gene Ther. 1998;5:281–291. [PubMed] [Google Scholar]
- 5.Yamamoto M, Alemany R, Adachi Y, Grizzle WE, Curiel DT. Characterization of the cyclooxyygenase-2 promoter in an adenoviral vector and its application for the mitigation of toxicity in suicide gene therapy of gastrointestinal cancers. Mol Ther. 2001;3:385–394. doi: 10.1006/mthe.2001.0275. [DOI] [PubMed] [Google Scholar]
- 6.Uchino J, Takayama K, Harada A, Kawakami Y, Inoue H, Curiel DT, et al. Infectivity enhanced, hTERT promoter-based conditionally replicative adenoviruses are useful for SCLC treatment. Cancer Gene Ther. 2005;12:737–748. doi: 10.1038/sj.cgt.7700838. [DOI] [PubMed] [Google Scholar]
- 7.Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Therapy. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- 8.Greco O, Powell TM, Marples B, Joiner MC, Scott SD. Gene therapy vectors containing CArG elements from the Egr1 gene are activated by neutron irradiation, cisplatin and doxorubicin. Cancer Gene Ther. 2005;12:655–662. doi: 10.1038/sj.cgt.7700834. [DOI] [PubMed] [Google Scholar]
- 9.Yamini B, Yu X, Gillespie GY, Kufe DW, Weichselbaum RR. Transcriptional targeting of adenovirally delivered tumor necrosis factor alpha by temozolomide in experimental glioblastoma. Cancer Res. 2004;64:6381–6384. doi: 10.1158/0008-5472.CAN-04-2117. [DOI] [PubMed] [Google Scholar]
- 10.Li CY, Dewhirst MW. Hyperthermia-regulated immunogene therapy. Int J Hyperthermia. 2002;18:586–596. doi: 10.1080/0265673021000017082. [DOI] [PubMed] [Google Scholar]
- 11.Borrelli MJ, Schoenherr DM, Wong A, Bernock LJ, Corry PM. Heat-activated transgene expression from adenovirus vectors infected into human prostate cancer cells. Cancer Res. 2001;61:1113–1121. [PubMed] [Google Scholar]
- 12.Brade AM, Ngo D, Szmitko P, Li PX, Liu FF, Klamut HJ. Heat-directed gene targeting of adenoviral vectors to tumor cells. Cancer Gene Ther. 2000;7:1566–1574. doi: 10.1038/sj.cgt.7700267. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, Hu JK, Lohr F, Zhang L, Braun R, Lanzen J, et al. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res. 2000;60:3435–3439. [PubMed] [Google Scholar]
- 14.Li GC, He F, Shao X, Urano M, Shen L, Kim D, et al. Adenovirus-mediated heat-activated antisense Ku70 expression radiosensitizes tumor cells in vitro and in vivo. Cancer Res. 2003;63:3268–3274. [PubMed] [Google Scholar]
- 15.Luker GD, Sharma V, Pica CM, Dahlheimer JL, Li W, Ochesky J, et al. Noninvasive imaging of protein-protein interactions in living animals. Proc Natl Acad Sci USA. 2002;99:6961–6966. doi: 10.1073/pnas.092022399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black ME, Newcomb TG, Wilson HM, Loeb LA. Creation of drug-specific herpes simplexvirus type 1 thymidine kinase mutants for gene therapy. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AK, Moros E, Novak P, Straube W, Zeug A, Locke JE, et al. MicroPET-compatible, small animal hyperthermia ultrasound system (SAHUS) for sustainable, collimated and controlled hyperthermia of subcutaneously implanted tumors. Int J Hyperthermia. 2003;20:32–44. doi: 10.1080/02656730310001609326. [DOI] [PubMed] [Google Scholar]
- 18.Novák P, Moros EG, Parry JJ, Rogers BE, Myerson RJ, Zeug A, et al. Experience with a small animal hyperthermia ultrasound system (SAHUS): report on 83 tumours. Phys Med Biol. 2005;50:5127–5139. doi: 10.1088/0031-9155/50/21/012. [DOI] [PubMed] [Google Scholar]
- 19.Luker GD, Sharma V, Piwnica-Worms D. Visualizing protein-protein interactions in living animals. Methods. 2003;29:110–122. doi: 10.1016/s1046-2023(02)00285-2. [DOI] [PubMed] [Google Scholar]
- 20.Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7:5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 22.Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia. 1990;6:479–486. doi: 10.3109/02656739009140944. [DOI] [PubMed] [Google Scholar]
- 23.Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–169. doi: 10.1016/0360-3016(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 24.Myerson RJ, Straube WL, Moros EG, Emami BN, Lee HK, Perez CA, et al. Simultaneous superficial hyperthermia and external radiotherapy: report of thermal dosimetry and tolerance to treatment. Int J Hyperthermia. 1999;15:251–266. doi: 10.1080/026567399285639. [DOI] [PubMed] [Google Scholar]
- 25.Oleson JR, Dewhirst MW, Harrelson JM, Leopold KA, Samulski TV, Tso CY. Tumor temperature distributions predict hyperthermia effect. Int J Radiat Oncol Biol Phys. 1989;16:559–570. doi: 10.1016/0360-3016(89)90472-0. [DOI] [PubMed] [Google Scholar]
- 26.Blackburn RV, Galoforo SS, Corry PM, Lee YJ. Adenoviral-mediated transfer of heat-inducible double suicide gene into prostate carcinoma cells. Cancer Res. 1998;58:1358–1362. [PubMed] [Google Scholar]
- 27.Lee YJ, Galoforo SS, Battle P, Lee H, Corry PM, Jessup JM. Replicating adenoviral vector-mediated transfer of a heat-inducible suicide gene for gene therapy. Cancer Gene Ther. 2001;8:397–404. doi: 10.1038/sj.cgt.7700310. [DOI] [PubMed] [Google Scholar]
- 28.Lee YJ, Lee H, Borrelli MJ. Gene transfer into human prostate adenocarcinoma cells with an adenoviral vector: Hyperthermia enhances a double suicide gene expression, cytotoxicity and radiotoxicity. Cancer Gene Ther. 2002;9:267–274. doi: 10.1038/sj.cgt.7700433. [DOI] [PubMed] [Google Scholar]
- 29.Lohr F, Hu K, Huang Q, Zhang L, Samulski TV, Dewhirst MW, et al. Enhancement of radiotherapy by hyperthermia-regulated gene therapy. Int J Radiat Oncol Biol Phys. 2000;48:1513–1518. doi: 10.1016/s0360-3016(00)00788-4. [DOI] [PubMed] [Google Scholar]
- 30.Lohr F, Huang Q, Hu K, Dewhirst MW, Li CY. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin Cancer Res. 2001;7:3625–3628. [PubMed] [Google Scholar]
- 31.Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang X, et al. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int J Hyperthermia. 2006;22:587–606. doi: 10.1080/02656730600983063. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui F, Li CY, Larue SM, Poulson JM, Avery PR, Pruitt AF, et al. A phase I trial of hyperthermia-induced inter-leukin-12 gene therapy in spontaneously arising feline soft tissue sarcomas. Mol Cancer Ther. 2007;6:380–389. doi: 10.1158/1535-7163.MCT-06-0342. [DOI] [PubMed] [Google Scholar]
- 33.Zinn KR, Buchsbaum DJ, Chaudhuri T, Mountz JM, Kirkman RL, Rogers BE. Noninvasive monitoring of gene transfer using a reporter receptor imaged with a high affinity peptide radiolabeled with 99mTc or 188Re. J Nucl Med. 2000;41:887–895. [PubMed] [Google Scholar]
- 34.Yaghoubi SS, Gambhir SS. PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]-FHBG. Nat Protoc. 2006;1:3069–3075. doi: 10.1038/nprot.2006.459. [DOI] [PubMed] [Google Scholar]
- 35.Li GC, He F, Ling CC. Hyperthermia and gene therapy: potential use of microPET imaging. Int J Hyperthermia. 2006;22:215–221. doi: 10.1080/02656730600784677. [DOI] [PubMed] [Google Scholar]