Abstract
High levels of peroxynitrite have been shown to decrease cardiomyocyte contraction through a reduction in phospholamban (PLB) phosphorylation. However, previous reports did not examine the direct effect of peroxynitrite on protein phosphatase activity in the myocardium or the role of specific phosphatases. Here we test the effect of the peroxynitrite donor SIN-1 on protein phosphatase activity in whole heart homogenates, as well as the interaction of PLB with protein phosphatase 1 (PP1) and 2a (PP2a). SIN-1 (200 μmol/L) induced a significant increase in protein phosphatase activity, which was alleviated with the specific PP1/PP2a inhibitor okadaic acid. Conversely, lower concentrations of SIN-1 and the nitric oxide donor spermine NONOate (300 μmol/L) were both without effect on phosphatase activity. We next examined the effect of SIN-1 on the interaction of PLB with PP1 and PP2a using co-immunoprecipitation, since okadaic acid inhibited the effects of SIN-1 in our current and previous studies. SIN-1 significantly increased the interaction of PLB with PP2a, but had no effect on the interaction between PLB and PP1. Urate, a peroxynitrite scavenger, inhibited the effects of SIN-1 on phosphatase activity and the interaction of PLB with PP2a, thus implicating peroxynitrite as the causal species. The results of this study provide further insight into the mechanism through which high levels of peroxynitrite serve to decrease PLB phosphorylation and myocardial contraction. Therefore, peroxynitrite signaling could play a key role in the contractile dysfunction manifested in heart failure where peroxynitrite production and protein phosphatase activity are increased and PLB phosphorylation is decreased.
Keywords: Heart failure, Co-immunoprecipitation, Phosphorylation
INTRODUCTION
The formation of peroxynitrite (ONOO−), the reaction product of nitric oxide (NO.) and superoxide (O2•−), increases during the pathogenesis of heart failure [1,2], and is detrimental to myocardial function [3,4,5]. The peroxynitrite donor SIN-1, has been shown to decrease contraction in isolated cardiomyocytes [6,7]. We recently demonstrated that this peroxynitrite-mediated decrease in cardiomyocyte contraction occurred through a reduction in phospholamban (PLB) Serine16 phosphorylation [7]. PLB is a phosphoprotein which plays a critical role in excitation-contraction coupling by regulating the uptake of Ca2+ into the sarcoplasmic reticulum (SR) [8]. Phosphorylation of PLB Serine16 increases SR Ca2+ uptake, thus increasing SR Ca2+ load, which is a critical determinant of myocardial contractility [9]. Conversely, the dephosphorylation of PLB Serine16 results in the inhibition of SR Ca2+ uptake, which leads to a decrease SR Ca2+ load and myocardial contraction. Protein phosphatase 1 (PP1) and protein phosphatase 2a (PP2a) are the two main phosphatases that dephosphorylate PLB [10]. These phosphatases are specific for phosphorylated serine/threonine residues. Altered protein phosphatase activity was implicated in the reduced PLB Serine16 phosphorylation observed in our previous study [7], but we did not directly examine whether peroxynitrite increases protein phosphatase activity in the myocardium or the role of specific protein phosphatases. Peroxynitrite has been shown to modulate the activity of certain types of protein phosphatases [11,12], but the effect on serine/threonine phosphatase activity has not been examined.
Therefore, the objective of this study is to determine if a high level of peroxynitrite can increase protein phosphatase activity in the myocardium. Thus, we tested whether the peroxynitrite donor SIN-1 increases total protein phosphatase activity in whole heart homogenates and the effect of SIN-1 on the interaction of PLB with PP1 and PP2a. We hypothesize that peroxynitrite will increase protein phosphatase activity and promote the interaction of PP1 and PP2a with PLB.
EXPERIMENTAL PROCEDURES
Preparation of Cardiac Homogenates
Hearts were excised from male CF-1 mice and Langendorff-perfused. For the protein phosphatase assay, hearts were perfused with normal Tyrode solution for 2 minutes and frozen in liquid nitrogen. Hearts were homogenized as previously described [7] using the 10x lysis buffer provided with the protein phosphatase assay kit. Briefly, cardiac tissue was homogenized on ice via three bursts of 5 sec using a PRO250 homogenizer (PRO Scientific, Oxford, CT). Protease inhibitor cocktail (Sigma, St. Louis, MO) was added prior to homogenization. Homogenates were then solublized on ice for 30 min and centrifuged for 10 min at 16,100 g (4°C). The supernatant was then used for the protein phosphatase assay. For co-immunoprecipitation, hearts were perfused with either normal Tyrode solution, SIN-1, or SIN-1+urate for 2 minutes and frozen in liquid nitrogen. Hearts were homogenized as described above except that the protein extraction reagent from the co-immunoprecipitation kit was used. The supernatant was then used for co-immunoprecipitation. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and was approved by the Institutional Laboratory Animal Care and Use Committee.
Protein Phosphatase Activity Assay
Protein phosphatase activity was examined using the Sensolyte Protein Phosphatase Assay Kit (AnaSpec, San Jose, CA). The assay buffer consisted of (in mmol/L): Tris-HCl pH 7.0 (50), Na2EDTA (0.1), 0.01% Brij 35. Cardiac homogenates (100 μg) and experimental treatments (SIN-1, etc.) were added directly to each experimental well (96-well plate), per the instructions. The reaction was then initiated upon addition of 50 μL of the provided pNPP reaction mixture to each experimental well. Total well volume was 100 μL. The protein phosphatase assay was allowed to continue for 60 min at 22°C as recommended by the instructions, after which time the absorbance signal was measured at 405 nm using a BioTek PowerWave XS (Winooski, VT). Enzyme activity (nmol/min) was calculated using the following formula: (V × vol)/(ε × l), where V is the reaction velocity (OD405/min), vol is the reaction volume in liters, ε is the extinction coefficient of pNPP (1.78 × 104 M−1cm−1) and l is the path length of light through the sample in cm (for 100 mL sample, l = 0.5 cm). Enzyme activity was then calculated per mg of protein by dividing the enzyme activity by 0.1 mg.
Co-Immunoprecipitation Analysis
Protein interactions were examined using the ProFound mammalian co-immunoprecipitation kit (Pierce, Rockford, IL). The provided AminoLink Plus gel was coupled to a custom antibody to PLB (Zymed, San Francisco, CA) for 4 hours at 22°C. Cardiac homogenates (500 μg) were then incubated with the coupled gel for 2 hours at 22°C. The purified protein complex was then eluted using 200 μL of the provided elution buffer. Western blot was used to examine PLB interactions, as previously described [7]. Briefly, the co-immunoprecipitation elutant was diluted in Laemmli sample buffer (BioRad, Hercules, CA) and loaded into 15% SDS-polyacrylamide gels along with pre-stained markers. For examination of PP1 and PP2a, samples were boiled for 5 min; for examination of total PLB, samples were not boiled. Following protein separation via electrophoresis for 1 hour at 100 V, gels were transferred to 0.2 mm nitrocellulose membrane at 30 V overnight. Membranes were blocked with 5% dry milk solutions for 2 hours, then agitated with primary antibody diluted in blocking solution for 2 hours. Membranes were probed using either a custom antibody to PLB (Zymed) or PP1α (Cell Signaling, Danvers, MA) or PP2aC (Cell Signaling). Membranes were then rinsed 6 times in TBS for 10 minutes, after which the secondary antibody diluted in blocking solution was added for 45 minutes. Membranes were washed 6 additional times in TBS for 10 minutes. Signals were detected using SuperSignal West Dura substrate (Pierce, Rockford, IL), and captured on Kodak ML film (Sigma). Protein band intensity was quantified using a UVP densitometry system (Upland, CA).
Solutions and Drugs
Normal Tyrode control solution consisted of (in mmol/L): NaCl (140), KCl (4), MgCl2 (1), CaCl2 (1), Glucose (10), and HEPES (5); pH = 7.4 adjusted with NaOH and/or HCl. 3-Morpholinosydnonimine (SIN-1; Alexis, Lausen, Switzerland) was used as a peroxynitrite donor. Urate (Sigma) was used as a peroxynitrite scavenger [13,14]. Okadaic acid (OA; LC Labs, Woburn, MA) was used as a specific inhibitor of PP1/PP2a. Phosphatase Inhibitor Cocktail Set III (PPIC; Calbiochem, La Jolla, CA) was used as a total protein phosphatase inhibitor. Spermine NONOate (Alexis) was used as a nitric oxide donor. All solutions were made fresh on the day of experimentation.
Statistics
Data are presented as the mean±S.E.M. Statistical significance (p<0.05) was determined between groups using an ANOVA (followed by Newman-Keuls test) for multiple groups or a Student’s t-test for two groups.
RESULTS
Effect of SIN-1 on Protein Phosphatase Activity
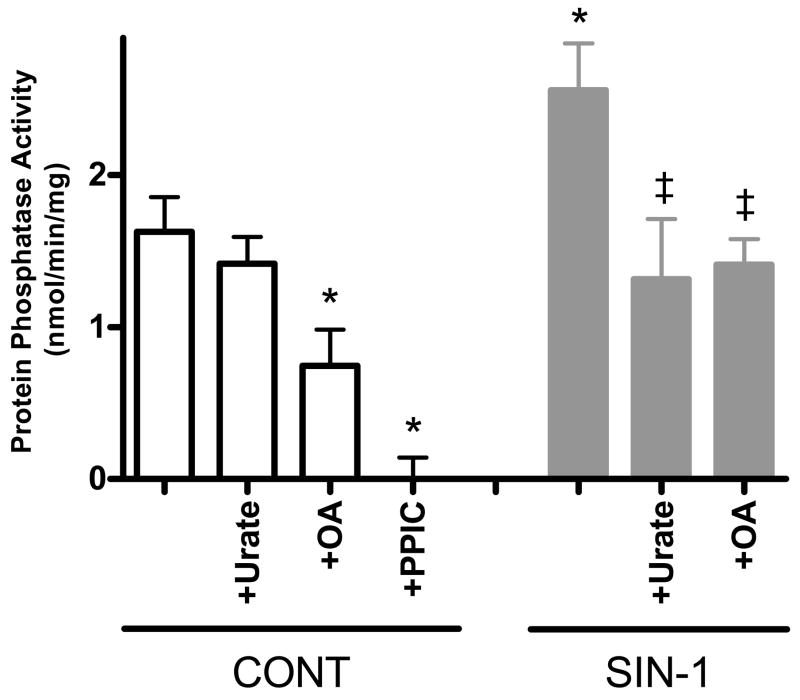
We first examined the effect of SIN-1 on total protein phosphatase activity in whole heart homogenates (Fig. 1). High SIN-1 (200 μmol/L) induced a 57% increase in total protein phosphatase activity (1.6±0.2 vs. 2.6±0.3 nmol/min/mg, p<0.05 vs. Control). This effect was inhibited upon co-incubation with the peroxynitrite scavenger, urate (1.3±0.4 nmol/min/mg, p<0.05 vs. SIN-1), or the specific PP1/PP2a inhibitor, okadaic acid (1.4±0.2 nmol/min/mg, p<0.05 vs. SIN-1). Urate alone had no effect on protein phosphatase activity (1.4±0.2 nmol/min/mg), while okadaic acid alone induced a 54% decrease in protein phosphatase activity (0.7±0.2 nmol/min/mg, p<0.05 vs. Control). This remaining activity is likely due to non-okadaic acid sensitive protein phosphatases. However, total protein phosphatase inhibition with PPIC alleviated all activity (0.0±0.1 nmol/min/mg). The effect of high SIN-1 on protein phosphatase activity can be seen in Fig. 1.
Figure 1. High SIN-1 increases total protein phosphatase activity in whole heart homogenates.
Pooled data (mean±S.E.M.) for total protein phosphatase activity with control (normal Tyrode, clear bars), urate (500 μmol/L), OA (1 μmol/L), PPIC (Na fluoride: 100 μmol/L; Na orthovanadate: 2 μmol/L; Na pyrophosphate, decahydrate: 20 μmol/L; β-glycerophosphate: 20 μmol/L), SIN-1 (200 μmol/L, gray bars), SIN-1+urate (500 μmol/L), and SIN-1+OA (1 μmol/L) treated homogenates. *p<0.05 vs. Control; ‡p<0.05 vs. SIN-1; n = 5–11 hearts/group.
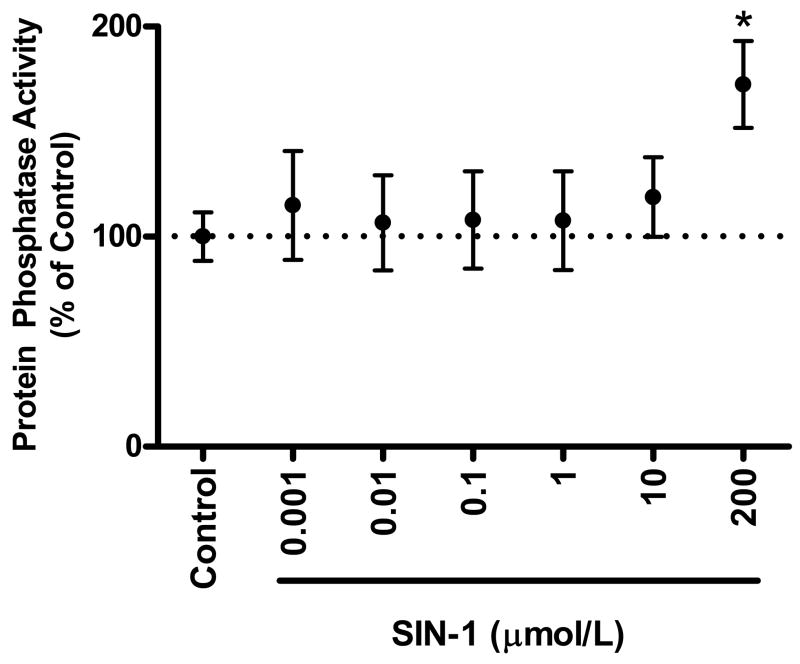
We also examined the effect of lower concentrations of peroxynitrite on protein phosphatase activity (Fig. 2). In a previous study, we demonstrated that 10 μmol/L SIN-1 exerted positive effects on cardiomyocyte contraction [15]. Interestingly, lower concentrations of peroxynitrite were without effect on total protein phosphatase activity (0.001 μmol/L: 1.7±0.4, 0.01 μmol/L: 1.6±0.3, 0.1 μmol/L: 1.6±0.3, 1 μmol/L: 1.6±0.3, 10 μmol/L: 1.8±0.3 nmol/min/mg). The effect of low SIN-1 on protein phosphatase activity can be seen in Fig. 2, which shows the percent of control.
Figure 2. Low concentrations of SIN-1 do not altered total protein phosphatase activity in whole heart homogenates.
Pooled data (mean±S.E.M.) expressed as a % of control for total protein phosphatase activity with control (normal Tyrode) and SIN-1 treated cardiac homogenates. *p<0.05 vs. Control, n = 6–17 hearts/group.
Effect of Spermine NONOate on Protein Phosphatase Activity
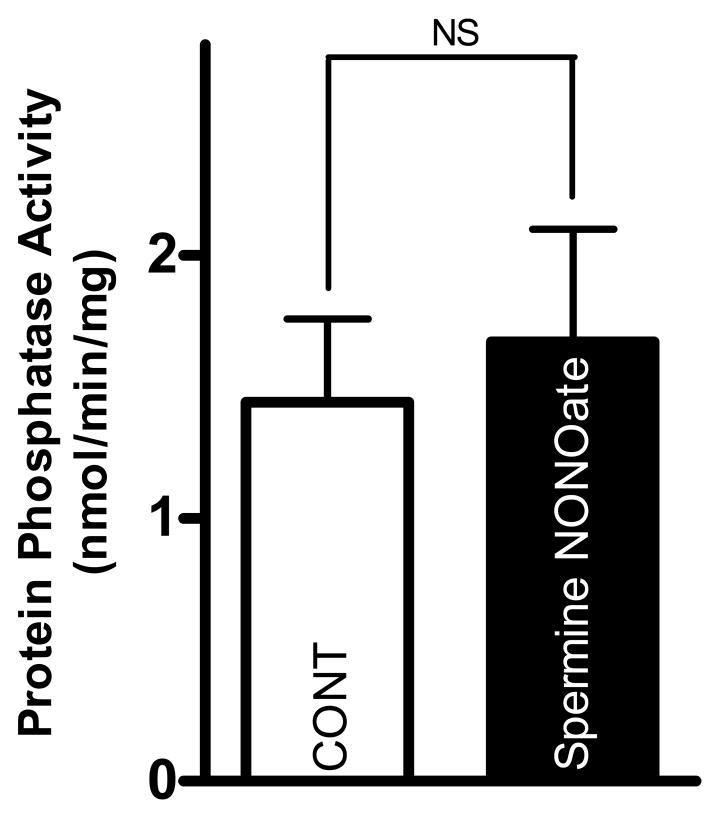
We next examined the effect of the NO donor spermine NONOate (Fig. 3). We previously determined this NO donor to have effects on cardiomyocyte [Ca2+]i-handling [16]. In contrast with the effects of SIN-1, however, spermine NONOate (300 μmol/L) had no effect on total protein phosphatase activity compared to control (1.4±0.3 vs. 1.6±0.4 nmol/min/mg). The effect of spermine NONOate on protein phosphatase activity can be seen in Fig. 3.
Figure 3. Spermine NONOate does not alter total protein phosphatase activity.
Pooled data (mean±S.E.M.) for total protein phosphatase activity with control (normal Tyrode, clear bar) and spermine NONOate (300 μmol/L, black bar). p = NS; n = 6 hearts/group.
PLB Co-Immunoprecipitation
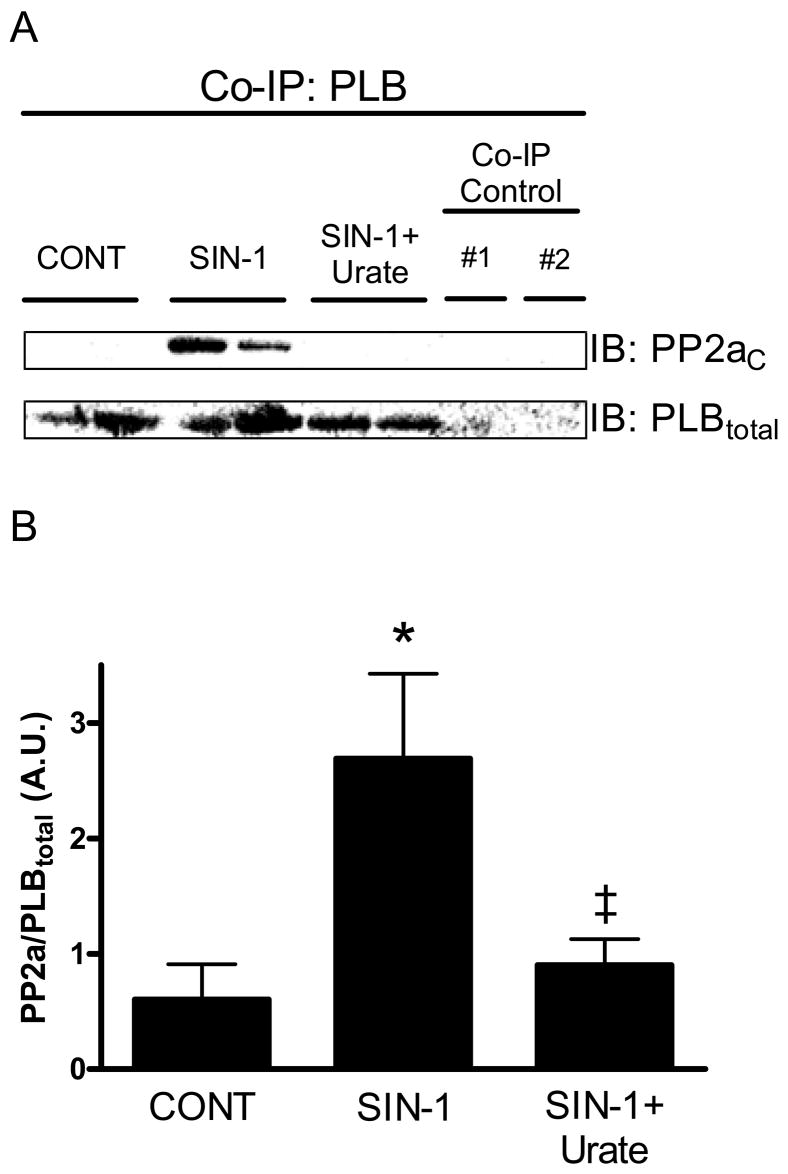
We next examined the effect of SIN-1 on the interaction of PLB with PP1 and PP2a (Fig. 4). High SIN-1 (200 μmol/L) induced a significant increase in the interaction of PLB with PP2a (0.6± 0.3 vs. 2.7±0.7 A.U., p<0.05 vs. Control), as seen in Fig. 4A. This interaction was inhibited upon co-incubation with urate (0.9±0.2 A.U., p<0.05 vs. SIN-1). The summary data for the interaction of PLB with PP2a can be seen in Fig. 4B. We did not observe a significant interaction between PLB and PP1 after treatment with SIN-1 (data not shown). Non-specific binding (PLBtotal, PP2aC, etc.) was not observed in co-immunoprecipitation controls.
Figure 4. SIN-1 increases the interaction of PLB with PP2a.
A.) Representative western blot (IB) specific for PP2aC and PLBtotal following co-immunoprecipitation (Co-IP) with PLB in cardiac homogenates. Co-IP control #1: crosslinked control gel + homogenate; Co-IP control #2: homogenate (no antibody-coupled AminoLink Plus gel). NOTE: The results from two separate Co-IP experiments from different samples are shown for each experimental condition (CONT, SIN-1, etc.). B.) Pooled densitometry data (mean±S.E.M.) for PP2a band intensity normalized to PLBtotal with control (normal Tyrode), SIN-1 (200 μmol/L), and SIN-1+urate (500 μmol/L). Data displayed as arbitrary units (A.U.). *p<0.05 vs. Control; ‡p<0.05 vs. SIN-1; n = 5–6 hearts/group.
DISCUSSION
We previously demonstrated that a high level of peroxynitrite decreased cardiomyocyte contraction through a reduction in PLB Serine16 phosphorylation [7]. However, in our previous study we did not directly examine the effects of peroxynitrite on protein phosphatase activity in the myocardium. In our current study, we demonstrate for the first time that a high level of peroxynitrite, produced via SIN-1, increases total protein phosphatase activity in the myocardium. Further, this same concentration of peroxynitrite increased the interaction of PLB with PP2a. Thus, the peroxynitrite-induced increase in total protein phosphatase activity observed in our current study correlates directly with the peroxynitrite-induced decrease in PLB Serine16 phosphorylation observed in our previous study [7]. Therefore, high levels of peroxynitrite exert negative effects in cardiomyocytes by increasing protein phosphatase activity, namely PP2a, which leads to the dephosphorylation of PLB Serine16 and reduced cardiomyocyte contraction.
Peroxynitrite Increases Protein Phosphatase Activity
The values that we observed for total protein phosphatase activity under control conditions are consistent with previously published results for mouse cardiac homogenates [17,18]. Treatment with high SIN-1 (200 μmol/L) significantly increased total protein phosphatase activity in cardiac homogenates (Fig. 1). We confirmed peroxynitrite as the causal species using the peroxynitrite scavenger urate, which inhibited the effects of SIN-1. Specific inhibition of PP1 and PP2a with okadaic acid also alleviated the effects of SIN-1, thus implicating these serine/threonine phosphatases. This is consistent with our previous study, in which we demonstrated a 39% decrease in PLB Serine16 with peroxynitrite, that was inhibited by okadaic acid [7]. In the current study, we demonstrate a 57% increase in total protein phosphatase activity in peroxynitrite-treated homogenates. Therefore, this peroxynitrite-induced increase in total protein phosphatase activity correlates directly with the peroxynitrite-induced decrease in PLB Serine16 phosphorylation observed in our previous study [7]. These findings are consistent with previous studies examining protein phosphatase activity and PLB phosphorylation [19,20].
In direct contrast to those effects observed with high SIN-1, lower concentrations of SIN-1 (0.001–10 μmol/L SIN-1) were without effect on total protein phosphatase activity (Fig. 2), despite the increase in cardiomyocyte contraction observed in our previous study [15]. The NO donor spermine NONOate (300 μmol/L) was also without effect on total protein phosphatase activity (Fig. 3). At this concentration, spermine NONOate was previously determined to have effects on cardiomyocyte [Ca2+]i-handling [16]. However, this lack of effect on protein phosphatase activity is consistent with a previous report, which demonstrated that two different nitric oxide donors, NOR-3 and sodium nitroprusside, also had little effect on the activity of PP1 or PP2a [21]. This provides clear evidence that the effects of peroxynitrite on protein phosphatase activity are exclusive to high levels of peroxynitrite and distinct from those observed with NO.
Peroxynitrite Increases the Interaction of PLB and PP2a
The interaction of PLB with PP2a was significantly increased with high SIN-1 (Fig. 3). Peroxynitrite was confirmed as the causal species as urate inhibited this SIN-1-induced interaction. A significant interaction between PLB and PP1 was not detected with high SIN-1. Although this result does not agree with our initial hypothesis, this is not surprising given that PP2a is more sensitive to okadaic acid than PP1 [22]. Okadaic acid inhibited the effects of peroxynitrite on protein phosphatase activity, as well as the peroxynitrite-induced decrease in cardiomyocyte contraction and PLB Serine16 phosphorylation observed in our previous publication [7]. However, we likely did not observe a complete dephosphorylation of PLB Serine16 (39% decrease) in our previous study [7] because PP2a does not appear to be the main protein phosphatase in the cardiomyocyte that dephosphorylates PLB [10]. Therefore, peroxynitrite increases the interaction between PLB and PP2a, thus providing a mechanism for the peroxynitrite-induced decrease in PLB Serine16 phosphorylation and the subsequent reduction in myocyte contraction.
The production of peroxynitrite has been shown to increase during the pathogenesis of numerous cardiac disorders, including heart failure [1,2,5]. This is likely due to the expression of inducible nitric oxide synthase (NOS2) [5,23]. PP2a activity is also increased in heart failure [20], while PLB Serine16 phosphorylation is decreased [24,25,26]. Therefore, the results contained herein, coupled with the results of our previous studies, provide a mechanism for part of the myocardial dysfunction observed in heart failure. That is, increased peroxynitrite production leads to the activation of PP2a, which serves to decrease PLB Serine16 phosphorylation, and ultimately leads to alterations in myocardial [Ca2+]i-handling and decreased myocardial contraction. Thus, peroxynitrite signaling could play a key role in the contractile dysfunction manifested in heart failure. However, it is also possible for peroxynitrite and PP2a to target other proteins within the cardiomyocyte. Additional targets for PP2a include the L-type Ca2+ channel, troponin I, and the ryanodine receptor [27,28]. We previously demonstrated that NOS2 expression depresses ryanodine receptor function through a cGMP-independent signaling pathway, perhaps via peroxynitrite formation [29]. Further, peroxynitrite has been shown to directly inactivate SERCA2a [30,31], but this occurs via direct nitration and does not involve PP1 or PP2a. Therefore, peroxynitrite could potentially target additional excitation-contraction coupling proteins.
In conclusion, high levels of peroxynitrite serve to decrease PLB Serine16 phosphorylation in cardiomyocytes by increasing protein phosphatase activity and promoting the interaction of PLB with PP2a. Thus, cardiomyocyte contraction is ultimately reduced through the production of high levels of peroxynitrite.
Acknowledgments
Supported by the American Heart Association (Pre-doctoral Fellowship 0715159B, MJK; Scientist Development Grant 0735079N, JPD), and the National Institutes of Health (K02HL094692, R01HL079283, MTZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res. 2001;49:798–807. doi: 10.1016/s0008-6363(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–98. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez BL, Liu GL, Christopher TA, Ma XL. Peroxynitrite, the product of nitric oxide and superoxide, causes myocardial injury in the isolated perfused rat heart. Coron Artery Dis. 1997;8:149–53. doi: 10.1097/00019501-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ferdinandy P, Panas D, Schulz R. Peroxynitrite contributes to spontaneous loss of cardiac efficiency in isolated working rat hearts. Am J Physiol. 1999;276:H1861–7. doi: 10.1152/ajpheart.1999.276.6.H1861. [DOI] [PubMed] [Google Scholar]
- 5.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–7. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 6.Stojanovic MO, Ziolo MT, Wahler GM, Wolska BM. Anti-adrenergic effects of nitric oxide donor SIN-1 in rat cardiac myocytes. Am J Physiol Cell Physiol. 2001;281:C342–9. doi: 10.1152/ajpcell.2001.281.1.C342. [DOI] [PubMed] [Google Scholar]
- 7.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77:353–61. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta -agonists. J Biol Chem. 2000;275:38938–43. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 9.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–9. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 10.MacDougall LK, Jones LR, Cohen P. Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur J Biochem. 1991;196:725–34. doi: 10.1111/j.1432-1033.1991.tb15871.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopez CJ, Qayyum I, Mishra OP, Delivoria-Papadopoulos M. Effect of nitration on protein tyrosine phosphatase and protein phosphatase activity in neuronal cell membranes of newborn piglets. Neurosci Lett. 2005;386:78–81. doi: 10.1016/j.neulet.2005.04.089. [DOI] [PubMed] [Google Scholar]
- 12.Kucherenko Y, Browning J, Tattersall A, Ellory JC, Gibson JS. Effect of peroxynitrite on passive K+ transport in human red blood cells. Cell Physiol Biochem. 2005;15:271–80. doi: 10.1159/000087237. [DOI] [PubMed] [Google Scholar]
- 13.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–9. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 14.Trackey JL, Uliasz TF, Hewett SJ. SIN-1-induced cytotoxicity in mixed cortical cell culture: peroxynitrite-dependent and -independent induction of excitotoxic cell death. J Neurochem. 2001;79:445–55. doi: 10.1046/j.1471-4159.2001.00584.x. [DOI] [PubMed] [Google Scholar]
- 15.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Biphasic effect of SIN-1 is reliant upon cardiomyocyte contractile state. Free Radic Biol Med. 2008;45:73–80. doi: 10.1016/j.freeradbiomed.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2295–303. doi: 10.1152/ajpheart.2001.281.6.H2295. [DOI] [PubMed] [Google Scholar]
- 17.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–54. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 18.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–35. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, Hanske G, Schmitz W, Neumann J. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem. 2004;279:40827–34. doi: 10.1074/jbc.M405770200. [DOI] [PubMed] [Google Scholar]
- 20.Boknik P, Fockenbrock M, Herzig S, Knapp J, Linck B, Luss H, Muller FU, Muller T, Schmitz W, Schroder F, Neumann J. Protein phosphatase activity is increased in a rat model of long-term beta-adrenergic stimulation. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:222–31. doi: 10.1007/s002100000283. [DOI] [PubMed] [Google Scholar]
- 21.Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch Biochem Biophys. 2002;404:271–8. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 22.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 23.Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation. 2004;109:1886–91. doi: 10.1161/01.CIR.0000124231.98250.A8. [DOI] [PubMed] [Google Scholar]
- 24.Bartel S, Stein B, Eschenhagen T, Mende U, Neumann J, Schmitz W, Krause EG, Karczewski P, Scholz H. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol Cell Biochem. 1996;157:171–9. doi: 10.1007/BF00227896. [DOI] [PubMed] [Google Scholar]
- 25.Sande JB, Sjaastad I, Hoen IB, Bokenes J, Tonnessen T, Holt E, Lunde PK, Christensen G. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc Res. 2002;53:382–91. doi: 10.1016/s0008-6363(01)00489-8. [DOI] [PubMed] [Google Scholar]
- 26.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31:479–91. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 27.Neumann J, Boknik P, Herzig S, Schmitz W, Scholz H, Gupta RC, Watanabe AM. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am J Physiol. 1993;265:H257–66. doi: 10.1152/ajpheart.1993.265.1.H257. [DOI] [PubMed] [Google Scholar]
- 28.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552:109–18. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation. 2001;104:2961–6. doi: 10.1161/hc4901.100379. [DOI] [PubMed] [Google Scholar]
- 30.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–81. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 31.Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, Haworth RA. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005;111:988–95. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]