Abstract
We examined the role of the Extracellular signal Regulated Kinases (ERK) in 1,25 dihydroxyvitamin D (1,25(OH)2D3)-induced gene expression in the differentiated Caco-2 cells. 1,25(OH)2D3-regulated expression of the 25-hydroxyvitamin D, 24 hydroxylase (CYP24) gene (both natural gene and promoter construct) was strongly modulated by altering ERK activity (i.e. reduced by MEK inhibitors and dominant negative (dn) ERK1 and ERK2, activated by Epidermal Growth Factor) but ERK inhibition had no effect on 1,25(OH)2D3-regulated expression of the transient receptor potential cation channel, subfamily V, member 6 (TRPV6). ERK5-mediated phosphorylation of the transcription factor Ets-1 enhanced 1,25(OH)2D3-mediated CYP24 gene transcription in proliferating but not differentiated Caco-2 cells due to reduced levels of ERK5 and Ets-1 (total and phospho-protein levels) in differentiated cells. MEK inhibition reduced 1,25(OH)2D3-induced 3X-VDRE promoter activity but had no impact on the association of vitamin D receptor (VDR) with chromatin suggesting a role for co-activator recruitment in ERK-modulation of vitamin D-regulated CYP24 gene activation. Chromatin immunoprecipitation assays revealed that the ERK1/2 target, mediator 1 (MED1), is recruited to the CYP24, but not the TRPV6, promoter following 1,25(OH)2D3 treatment. MED1 phosphorylation was sensitive to activators and inhibitors of the ERK1/2 signaling and MED1 siRNA reduced 1,25(OH)2D3-regulated human CYP24 promoter activity. This suggests ERK1/2 signaling enhances 1,25(OH)2D3 effects on the CYP24 promoter by MED1-mediated events. Our data show that there are both promoter-specific and cell stage-specific roles for the ERK signaling pathway on 1,25(OH)2D3-mediated gene induction in enterocyte-like Caco-2 cells.
Keywords: CYP24, MAPK, phosphorylation, vitamin D
INTRODUCTION
Several aspects of intestinal function are regulated by 1,25 dihydroxyvitamin D3 (1,25(OH)2D3). It is a classical regulator of the transcellular calcium absorption that occurs in differentiated enterocytes of the proximal small intestine (Bronner et al., 1986), it has anti-cancer effects in the proliferating cells of the colon (Belleli et al., 1992), and it has anti-inflammatory effects that can modulate conditions like inflammatory bowel disease (Froicu et al., 2006). The molecular actions of 1,25(OH)2D3 are mediated through activation of the vitamin D receptor (VDR) leading to increased expression of the genes such as 25-dihydroxyvitamin D3-24-hydroxylase (CYP24), the enzyme that catalyzes the first step in the metabolic inactivation of 1,25 dihydroxyvitamin D3 (1,25(OH)2D3) (Tashiro et al., 2004), and two putative components of transcellular calcium absorption, the calcium binding protein calbindin D9k, and the apical membrane calcium channel transient receptor potential vanilloid type 6 (TRPV6) (Fleet et al., 2002;Song et al., 2003).
Several groups have reported that activation of various signal transduction pathways can influence 1,25(OH)2D3-induced gene regulation. For example, stimulation of protein kinase C (PKC) activity with phorbol esters enhances 1,25(OH)2D3-regulated CYP24 gene expression in the intestinal crypt cell lines IEC-6 (Koyama et al., 1994) and IEC-18 (Armbrecht et al., 2001) and in the kidney cell lines COS-1 and LLC-PK1 (Barletta et al., 2004). Similarly, others have reported that 1,25(OH)2D3-regulated expression of CYP3A4 was impaired by PKC inhibition in proliferating Caco-2 cells (Hara et al., 2002) and that the mitogen activated protein kinase (MAPK) family members ERK 1, 2, and 5 are regulators of 1,25(OH)2D3-mediated CYP24 promoter activity in COS-1 kidney cells (Dwivedi et al., 2002). However, while Narayanan et al. (Narayanan et al., 2004) found that ERK inhibition regulated 1,25(OH)2D3-mediated accumulation of alkaline phosphatase, collagen IA1, and CYP24 mRNA levels in MG-63 bone cells and HeLa cells, the opposite effect was seen in MC3T3-E1 cells. This suggests that the impact of signal transduction pathways on 1,25(OH)2D3 action is cell-type specific.
Here we utilize the differentiated, enterocyte-like Caco-2 cell (Chantret et al., 1988;Pinto et al., 1983) to investigate the role that MAPK/ERK signaling pathways play in 1,25(OH)2D3-mediated gene transcription. We have previously shown that 1,25(OH)2D3 markedly induces expression of calbindin D9k, TRPV6, and CYP24 mRNA in differentiated Caco-2 cells (Fleet et al., 2002), a established model of absorptive intestinal cells that can be used to study 1,25(OH)2D3-regulated calcium absorption (Fleet and Wood, 1999). This study reveals that ERK signaling pathways modulate 1,25(OH)2D3-mediated expression of CYP24 but not TRPV6 mRNA. More detailed analysis showed that ERK1 and ERK2, but not ERK5 signaling enhance 1,25(OH)2D3-mediated CYP24 gene expression in the differentiated Caco-2 cells. We also show that the co-activator mediator 1 (MED1) is likely to be involved in the crosstalk between ERK1/2 signaling and 1,25(OH)2D3 action on the CYP24 gene promoter in differentiated Caco-2 cells.
MATERIALS AND METHODS
Reagents
1α, 25-Dihydroxyvitamin D3 (1,25(OH)2D3) was obtained from BioMol International (Plymouth Meeting, PA) and dissolved in ethanol. Liarozole was from Janssen Research Foundation (Beerse, Belgium) and dissolved in ethanol. PD98059 and U0126 (specific inhibitors for MEK1/2) were obtained from EMD Biosciences, Inc. (San Diego, CA) and dissolved in dimethyl sulfoxide (DMSO). Human recombinant EGF (BD Biosciences, Bedford, MA) was dissolved in sterile water. Rabbit IgG was purchased from Upstate (Charlottsville, VA). Antibodies against total and phosphorylated ERK1/2, total and phosphorylated ERK5 were obtained from Cell Signaling Technology, Inc., (Beverly, MA). The antibody against Ets-1, MED1 (sc-8998) and VDR (sc-1008) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). For western blot of VDR, the 9A7 antibody was from Affinity Bioreagents (Golden, CO). The antibody against the phospho (T38) form of Ets-1 was obtained from Novus Biologicals (Littleton, CO). Calf intestinal phosphatase (CIP) is from Invitrogen (Carlsbad, CA).
Plasmids
The −298 to +74 bp rat CYP24 promoter luciferase construct was from Dr. John Omdahl (University of New Mexico) (Kerry et al., 1996). A CYP24 promoter luciferase constructs containing EBS mutations was generated using the Quikchange XL site-directed mutagenesis kit (Stratagene) following the manufacturer’s instruction. The human CYP24 promoter region from −298 to +74 was amplified from human genomic DNA via PCR with primers P1, 5’-GCTAGCTTCGAAGCACACCCGGTGAA-3’ and P2, 5’-CTCGAGGCTGGAGCACGGGGA GGT-3’. The CYP24 promoter fragment was then cloned upstream from the firefly luciferase reporter gene in the pGL3 basic vector. A reporter gene construct containing three vitamin D response elements (VDRE) from the osteopontin gene, a VP16-VDR-LBD (ligand binding domain) vector, and a Gal4-RXRα-LBD vector were provided by Dr. Sunil Nagpal (Eli Lilly and Company, Indianapolis, IN) (Bettoun et al., 2003). The pFR-Luc plasmid is from Stratagene (La Jolla, CA). Dominant negative mutant constructs of ERK1 (pCEP4-ERK1-K71R) and ERK2 (pCEP4-ERK2-K52R) were provided by Dr. Melanie Cobb (University of Texas Southwest Medical Center, Dallas, TX) (Robbins et al., 1993); the dominant negative mutant of MEK5 was provided by Dr. Jing-Dwan Lee (the Scripps Research Institute, La Jolla, CA) (Kato et al., 1998)
Cell Culture
Caco-2 cells were obtained from American Type Culture Collection (Rockville, MD) at passage 19 and were cultured in high-glucose DMEM supplemented with 1% penicillin/streptomycin, 1 mM sodium pyruvate, 100 µM nonessential amino acids (Life Technologies, Rockville, MD), 50 µg/l gentamycin (Life Technologies), 2 mM L-glutamine, 10 mM HEPES, and 20% (at seeding) or 10% (after confluence) FBS. For studies related to Ets-1 expression and phosphorylation in proliferating cells, cells were seeded in 10% FBS media. Cells were maintained at 37°C in a 5% CO2-95% air atmosphere. The cells were used between passages 24 and 50. We used Caco-2 cells at two different cell stages: proliferating/50% confluent (2 d cells), and differentiated/11 days post-confluent (15 d cells). The differentiation of 15 d cultures was confirmed by PCR detection of the small intestinal differentiation marker calbindin D9k using PCR primers and conditions described previously by our research group {Wang et al., 2004, Am J Physiol}. Treatment solutions were prepared with 0% FBS media in mRNA and protein expression studies and 5% FBS media in calcium transport studies. Serum-free OPTImem medium (Invitrogen, Carlsbad, CA) was used for transfection studies.
Calcium Transport Study
Caco-2 cells were seeded in permeable membrane filter inserts at 2.5 × 105 cells per well (24.5 mm diameter, 0.4 µm, Corning Costar, Cambridge, MA) and maintained in culture for 13 days. Medium was changed every other day until day 13 at which time the cells were exposed to treatment for 48 hours. Calcium transport was measured across Caco-2 cell monolayers as described previously (Fleet et al., 2002). Phenol red movement was assessed to determine the rate of diffusion across the monolayer. Saturable calcium transport (nmol/min/well) was defined as the difference between total transport and diffusional transport as measured by phenol red movement across the monolayer.
RNA Isolation and Analysis
Cells were first pretreated with either vehicle (0.1% DMSO) or 50 µM PD98059 or 10 µM U0126 for 30 minutes. The cells were then treated for 2 hours with either vehicle (0.1% ethanol) or 10 nM 1,25(OH)2D3 alone or in combination with one of the inhibitors identified above. For ERK activation, cells were treated with 25 ng/ml EGF or vehicle for 15 minutes and then cells were treated with 10 nM 1,25(OH)2D3 or vehicle for 2 hours before harvest. RNA was isolated using TriReagent (Molecular Research Center Inc., Cincinnati, OH) following the manufacturer’s instructions. Total RNA (2 µg) was reverse transcribed using MMLV reverse transcriptase (Invitrogen). Real time PCR was performed using the BioRad My iQ realtime PCR system and the BioRad SYBR Green supermix (BioRad Laboratories, Hercules, CA). Expression of CYP24, TRPV6, VDR, Ets-1, ESE-1 and ESE-3 genes was determined from the threshold cycle (Ct) value using the method described by Livak et al. (Livak and Schmittgen, 2001). For all genes studied, mRNA expression was normalized to GAPDH mRNA expression, and data were expressed as arbitrary units. The primers used for CYP24 and GAPDH (Klopot et al., 2007), for VDR (Lou et al., 2005), and for Ets-1, ESE-1 and ESE-3 were previously described (Hollenhorst et al., 2004). Primers used for TRPV6 are: Forward primer: TTCCTGCGGGTGGAAGACAGGCA; Reverse primer: ACGCAGGTCTCTCCTCAGGGTC CC. Cycle parameters were 95 °C for 30 s, 56.1 °C (VDR, Ets-1, ESE-1 and ESE-3) or 54°C (CYP24 and GAPDH) or 63°C (TRPV6) for 30s, and 72 °C for 30s.
Transient Transfection and Reporter Gene Assays
Cells seeded in 24-well plates were transfected using Liopofectamine Plus (Invitrogen Corp., Carlsbad, California) following the protocol provided by the manufacturer. Proliferating cells were transfected using a 1:5:5 ratio of DNA:Plus:Lipofectamine, and differentiated cells were transfected using a 1:10:10 ratio of DNA:Plus:Lipofectamine. In the study with PD98059 and U0126, cells were transfected with 300 ng (per well) rat −298 to +74 bp CYP24 luciferase promoter (wild-type and EBS mutant) or VDRE promoter (3X VDRE-luciferase) for 24 hours. Afterwards cells were pre-incubated with the drug inhibitors or vehicle for 30 mins before being induced by vehicle or 100 nM 1,25(OH)2D3 combined with the drugs for an additional 24 hours. In studies with dominant negative mutant plasmids, cells were co-transfected with 500 ng (per well) of plasmids that encode dominant negative mutants of ERK1, ERK2 and MEK5 or control plasmids. After 24 hours cells were treated with vehicle or 100 nM 1,25(OH)2D3 for 24 hours. Renilla luciferase plasmids with appropriate backbone were co-transfected in all experiments for normalization of transfection efficiency. CMV-renilla luciferase plasmid was used for study with rat CYP24 construct; TK-renilla was used for study with the VDRE construct; pRL-null renilla (Promega Corp., Madison, WI) was used for study with dominant negative mutant plasmids. After 24 hours of transfection, cells were treated with 100 nM 1,25(OH)2D3 or vehicle for 24 hours and collected in the 1X passive lysis buffer (Promega Corp., Madison, WI). Luciferase activity in the samples was determined using the Dual-Luciferase Reporter Assay System (Promega Corp., Madison, WI) with a luminometer (TD-20/20, Turner Design). The ratio of firefly/renilla was calculated and used in the study.
Western Blot Analysis
For the study of ERK1/2 and ERK5 phosphorylation differentiated Caco-2 cells were cultured overnight in serum free media and treated with PD98059 (50 µM, 30 min), U0126 (10 µM, 30 min), or EGF (25 ng/ml, 15 min) and their respective vehicles. Cells were lysed in a buffer containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 1% Triton X-100 plus protease inhibitor cocktail (Roche, Indianapolis, IN) and phosphatase inhibitor (Active Motif, Carlsbad, CA). Cell debris was removed by centrifugation at 12,000 RPM for 20 min at 4°C. Proteins (20 µg) were resolved by SDS-PAGE on 12.5% gels, transferred to PVDF membranes and probed with specific antibodies for phosphorylated ERK1/2 and ERK5 according to manufacturer’s protocol. Afterwards the membrane was stripped and reprobed with total ERK1/2, total ERK5 and beta-actin antibodies. Antigen-antibody complexes were detected using the ECL Plus Western blotting detection reagents (Pierce, Rockford, IL). For a comparative study of protein levels between proliferating and differentiated Caco-2 cells, 20 µg protein from cells of each stage was loaded in each lane except for phospho-Ets-1, in which 60 µg protein was loaded in each lane. For study of MED1, 50 µg whole cell lysate was treated with 25 unit of calf intestinal phosphatase (CIP) or vehicle at 37 °C for one hour prior to electrophoresis. 50 µg of protein was resolved by SDS-PAGE on 5% gels and transferred overnight before probing with MED1 antibody. Band intensities of immunoblot were quantified using Total lab TL100 software (Nonlinear Inc, Durham, NC).
VDR Association with Chromatin
To study whether drug inhibitors of kinase pathways inhibit 1,25(OH)2D3-induced movement of VDR to chromatin, differentiated Caco-2 cells were pretreated with either vehicle, 10 µM U0126, or 50 µM PD98059 for 30 min and then incubated with 10 nM 1,25(OH)2D3 for 1 hour in combination with the drugs. Cytoplasmic and chromatin-ssociated fractions were isolated as described previously (Ismail et al., 2004) and immunodetection of VDR protein was carried out using three antibodies: rat anti-chick VDR antibody Ab9A7 (1:2,000 dilution), rabbit anti-rat IgG (1:5,000 dilution, Chemicon, Temecula, CA), and horseradish peroxidase-conjugated anti-rabbit IgG (1:20,000 dilution, Amersham Biosciences, Piscataway, NJ). The membrane was incubated with each antibody for 1 hour at room temperature.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed as described in literature (Meyer et al., 2006) with some modifications. Differentiated Caco-2 cells were fixed with 1.5% formaldehyde at room temperature for 15 minutes. After neutralization with 0.125 M glycine, cells were washed with ice-cold PBS twice and scraped from dishes. Cell nuclei were extracted and then subjected to sonication to produce chromatin fragments from 200 bp to 1000 bp using a Fisher model 60 Sonic Dismembranator (Fisher Scientific, Pittsburgh, PA). The sonicated extract was diluted with dilution buffer (16.7 mM Tris-HCl, pH 8.1; 150 mM NaCl; 0.01% SDS; 1.1% Triton X-100; 1.2 mM EDTA) and precleared for 1 h with 50% slurry of Fastflow protein G (Amersham Biosciences, Piscataway, NJ) at 4°C. Immunoprecipitations were performed overnight with 2 µg rabbit IgG, VDR antibody or MED1 antibody at 4°C. Immune complexes were collected with 50% slurry of protein G for 1 h at 4°C. The protein G with immunocomplexes was washed and the cross-links were reversed overnight in 1% SDS, 0.1 M NaHCO3, and 0.2 M NaCl at 65 degree. DNA fragments were purified using QIAGEN QIAquick Spin Kits (Valencia, CA) and then subjected to PCR using primers reported in the literature to amplify fragments of the proximal human CYP24A1 promoter region (−252 to −51 bp) and human TRPV6 promoter region (−4.3 kb) (Meyer et al., 2006). The PCR products were resolved on 2.5% agarose gels and visualized using ethidium bromide. A small amount of DNA acquired after preclearing (2% of chromatin for an immunoprecipiation) was saved as input to assess the initial presence of the gene fragments.
MED1 RNA interference
Small interfering RNA (siRNA) specific for MED1 (J-004126-10) was purchased from Dharmacon (Lafayette, CO) and a control non-targeting siRNA (D-001210-03-05, Dharmacon) was used as a control. Caco-2 cells seeded at 30–50% in antibiotic free media were transfected with MED1 siRNA or control siRNA at a final concentration of 100 nM using oligofectamine (Invitrogen, Carlsbad, CA). For immunoblot of MED1, cells were harvested 72 hours after siRNA transfection. For reporter gene assays, 48 hours after siRNA transfection, cells were transfected with 300 ng (per well) of a human −298 to +74 bp CYP24 luciferase promoter along with 5 ng of the human VDR expression vection pCR3VDR and 7.5 ng of the control vector PRL-renilla. After 16 hours, cells were treated with 10 nM 1,25(OH)2D3 for 4 hours and cells were harvested for assessment of luciferase assay.
Mammalian Two Hybrid Assay
Mammalian two hybrid assay were used to study the interaction between the ligand binding domains (LBD) of VDR and RXRα. Differentiated 15 d cultures of Caco-2 cells were transfected with 250 ng of Gal4-responsive luciferase reporter vector pFR-Luc, 125 ng of pGal4-RXRα LBD, 125 ng of VP16-VDR LBD and 1 ng of CMV-Renilla vector using Lipofectamine- Plus (5 ul of each reagent per well of a 24 well dish). 24 hours after transfection, cells were treated with vehicle or 100 nM 1,25(OH)2D3 for an additional 24 hours and the luciferase activity in the sample was determined as described in the previous section (Transient transfection and reporter gene assay).
Statistical Analysis
All quantitative data is expressed as the mean ± the standard error of the mean (SEM). Statistical analysis of the data was performed by ANOVA followed by Fisher’s protected LSD (p<0.05). If predicted vs. residual plots showed that the data were not normally distributed, data were log transformed prior to analysis. P-values less than 0.05 were considered statistically significant.
RESULTS
CYP24 inhibition enhances 1,25(OH)2D3 action in differentiated Caco-2 cells
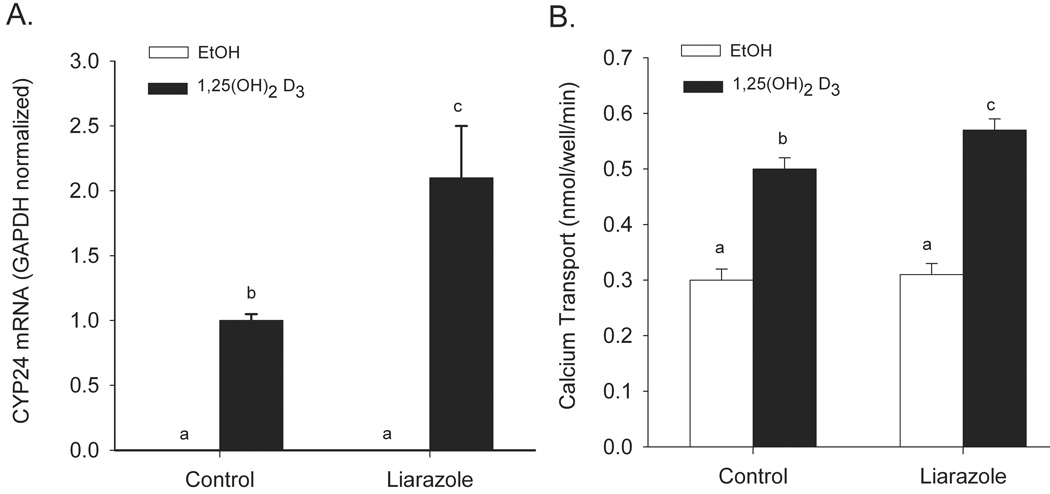
CYP24 enzymatic activity initiates the catabolism of 1,25(OH)2D3 and is a significant regulator of vitamin D action in vivo (Sakaki et al., 2005). In addition, the transcription of the CYP24 gene is highly regulated by 1,25(OH)2D3 (Kerry et al., 1996) and can be used as a sensitive marker of 1,25(OH)2D3-mediated gene activation. Inhibition of 1,25(OH)2D3 degradation by blocking CYP24 enzymatic activity with Liarozole (Ly et al., 1999) increased 1,25(OH)2D3-induced gene expression by 110% (Figure 1A). Similarly, inhibition of CYP24 enzymatic activity enhanced 1,25(OH)2D3–induced transcellular calcium transport by 35% (Figure 1B). These experiments confirm the central role that CYP24-mediated degradation has on 1,25(OH)2D3 action in the differentiated enterocyte-like Caco-2 cells.
Figure 1.
Effect of the cytochrome P450 inhibitor liarazole on 1,25(OH)2D3-regulated CYP24 gene expression (A) and transcellular calcium transport (B) in differentiated Caco-2 cells. (A) Caco-2 cells were treated with vehicle, 10 µM liarozole, 100 nM 1,25(OH)2D3 or the combination of the two drugs for 24 h. (B) Caco-2 cells were treated with vehicle or 10 nM 1,25(OH)2D3 in the presence or absence of 10 µM Liarozole for 48 h. Bars represent the mean ± SEM. (n=9). Values with common letter superscripts are not significantly different (P<0.05).
MEK1/2 inhibition reduces 1,25(OH)2D3-mediated CYP24 gene expression in differentiated Caco-2 cells
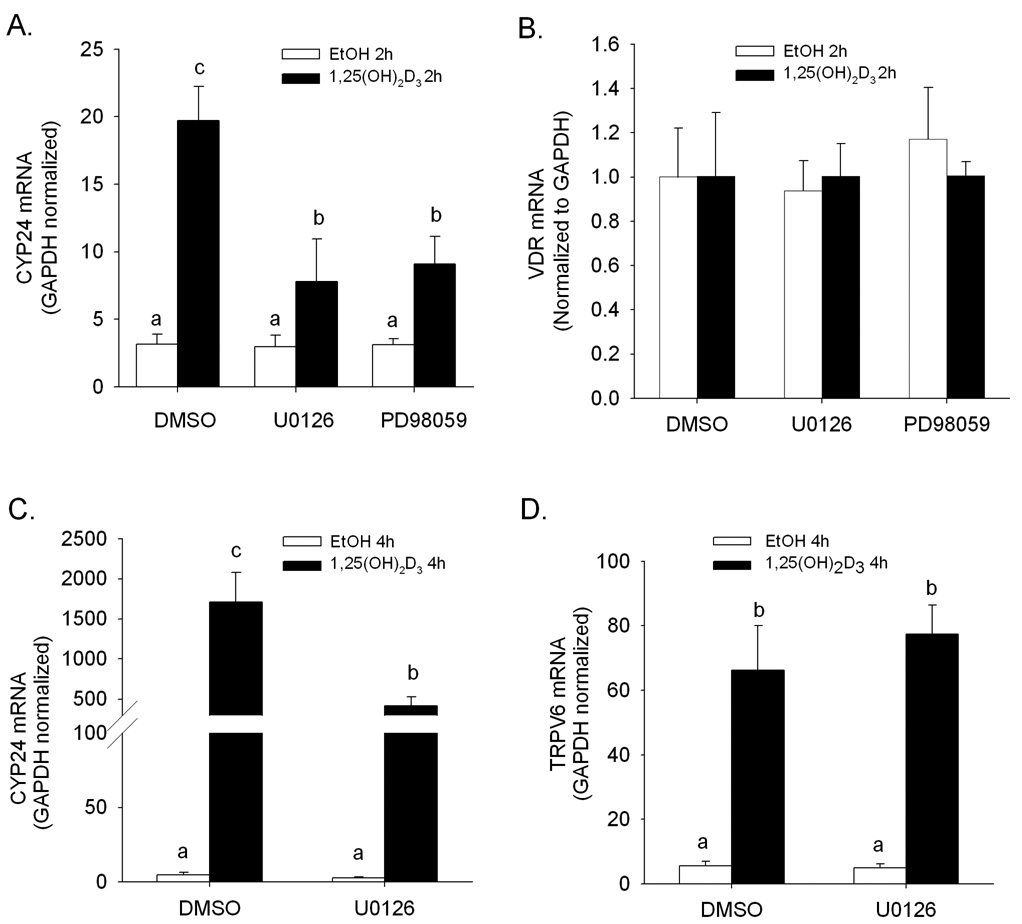
Treatment with the MEK1/2 inhibitors U0126 and PD98059 had no impact on the low, basal expression of CYP24 mRNA in differentiated Caco-2 cells (Figure 2A). However, the accumulation of CYP24 mRNA induced by 1,25(OH)2D3 (2 h, 10 nM) was significantly reduced by U0126 and PD98059. The impact of 1,25(OH)2D3 treatment on TRPV6 mRNA accumulation was not influenced by treatment with U0126 or PD98059 (2-fold in the presence or absence of inhibitor, data not shown). VDR mRNA expression level was not changed by either 1,25(OH)2D3 or the drug inhibitors (Figure 2B).
Figure 2.
Modulation of 1,25(OH)2D3-induced CYP24 and TRPV6 gene expression by MEK1/2 inhibitors in differentiated Caco-2 cells. Differentiated Caco-2 cells were preincubated for 30 minutes with vehicle (DMSO) or inhibitor (10 µM U0126 or 50µM PD98059). Afterwards, vehicle (0.1% ethanol) or 10 nM 1,25(OH)2D3 were combined with inhibitor treatments for an additional 2 h (A, B) or 4 h (C, D). CYP24 (A, C), VDR (B) and TRPV6 (D) mRNA expression levels were normalized to GAPDH levels. Bars represent the mean ± SEM (n = 3). Bars with common letter superscripts are not significantly different (P<0.05). Data in each panel are representative of three independent experiments.
Because TRPV6 gene induction is less vigorous than CYP24 gene induction, we also tested a longer period of 1,25(OH)2D3 treatment (4 h, 10 nM). The inhibitory impact of U0126 on vitamin D-regulated CYP24 mRNA induction that we observed at 2 h was also present under these treatment conditions (Figure 2C). Although TRPV6 mRNA was induced 12-fold after 4 h treatment with 1,25(OH)2D3, we did not observe an inhibitory effect of U0126 on the accumulation of TRPV6 mRNA (Figure 2D).
EGF activates MAPK pathways and stimulates 1,25(OH)2D3-induced CYP24 mRNA expression
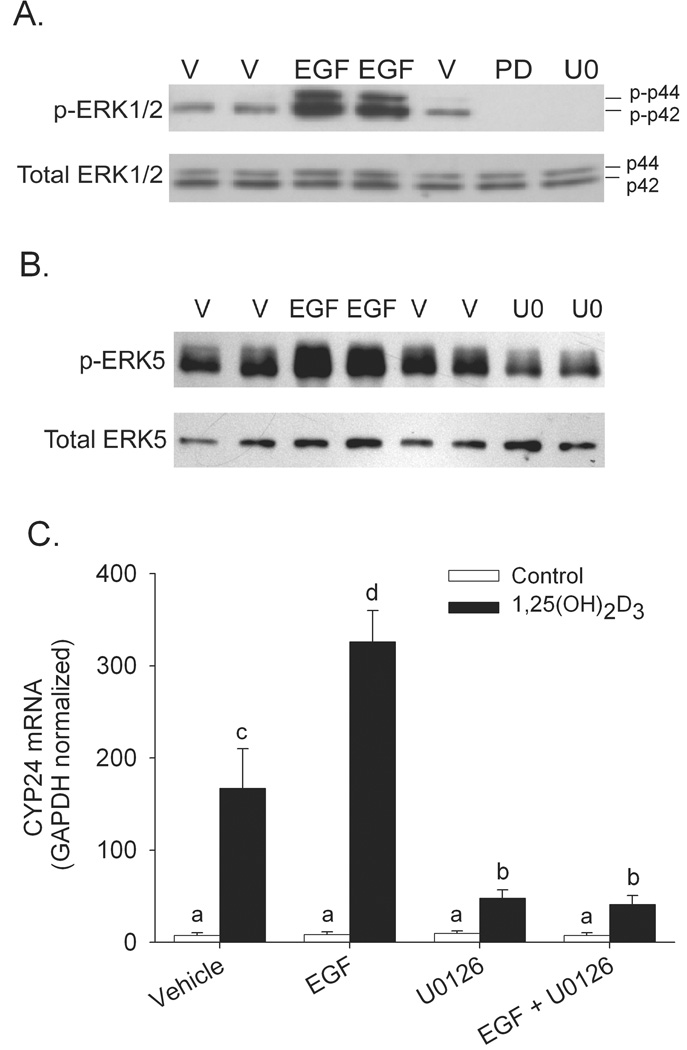
Since our initial studies showed that normal ERK pathway activity is essential for maximal 1,25(OH)2D3-induced CYP24 mRNA expression, we next evaluated whether activation of ERK would enhance this action. Short-term EGF treatment (25 ng/ml, 15 min) is a potent activator of ERK 1 and ERK2 (5-fold increase; Figure 3A) but has only a modest impact on ERK5 phosphorylation (<50% increase; Figure 3B). Consistent with our hypothesis, when cells were preincubated with EGF (25 ng/ml, 15 min), 1,25(OH)2D3-induced CYP24 mRNA expression was enhanced by 72%. U0126 significantly reduced 1,25(OH)2D3-induced CYP24 mRNA expression (by 75%) and also prevented the stimulatory effect of EGF on 1,25(OH)2D3-mediated CYP24 gene expression (Figure 3C).
Figure 3.
Effect of ERK activation by EGF on 1,25(OH)2D3-induced CYP24 gene expression in differentiated Caco-2 cells. Impact of MEK inhibitors (50 µM PD98059, PD; 10 µM U0126, U, 30 min) and EGF (15 min, 25 ng/ml) pretreatment on (A) ERK1/2 or (B) ERK5 phosphorylation. (C) Enhancement of 1,25(OH)2D3-induced CYP24 gene expression by EGF is inhibited by the U0126. Differentiated Caco-2 cells were preincubated with 10 µM U0126 or vehicle for 30 minutes in the presence or absence of EGF (25 ng/ml) for the last 15 min. Afterwards EGF was removed and cells were co-treated with the inhibitor and 10 nM 1,25(OH)2D3 or ethanol vehicle for an additional 2 h. Data are expressed as mean ± SEM. (n=3). Bars with common letter superscripts are not significantly different (P<0.05). Data in each panel are representative of three independent experiments.
1,25(OH)2D3–regulated promoter activity is sensitive to ERK1/2 inhibition in differentiated Caco-2 cells
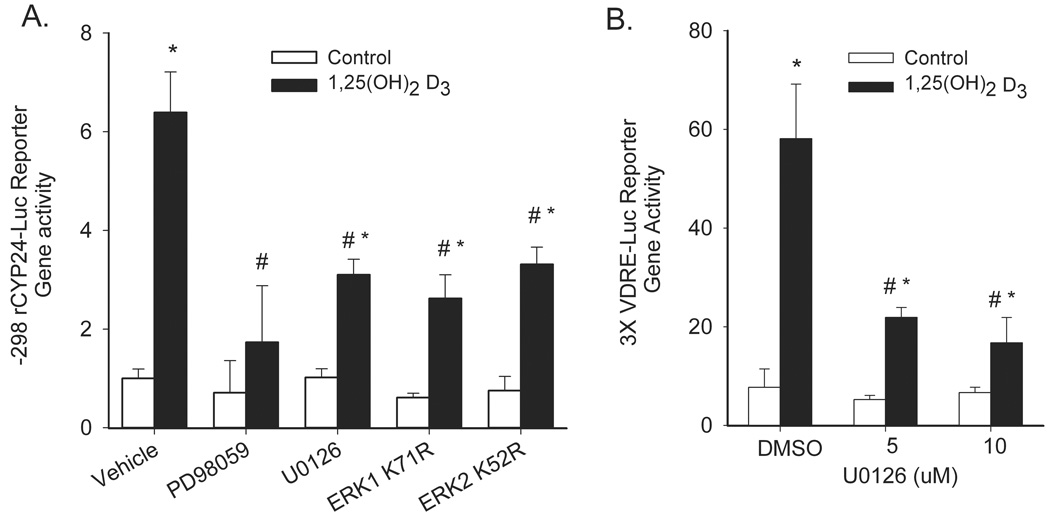
Similar to the effect we saw on CYP24 mRNA accumulation, PD98059 and U0126 treatment inhibited 1,25(OH)2D3-mediated activation of a −298 to +74 bp rat CYP24 promoter reporter gene construct by 62 and 52%, respectively (Figure 4A). In addition, 1,25(OH)2D3-induced CYP24 promoter activity was inhibited by the dominant negative mutants of ERK1 and ERK2 (Figure 4A). Activation of a 3X-VDRE promoter construct by 1,25(OH)2D3 was also significantly inhibited by U0126 treatment (Figure 4B) indicating that the MAPK effect is mediated through this regulatory site and through protein-protein interactions necessary for activation of this DNA binding motif.
Figure 4.
ERK1/2 inhibition reduces 1,25(OH)2D3-induced CYP24 promoter activity in differentiated Caco-2 cells. (A) Differentiated Caco-2 cells were transfected with a −298 to +74 rat CYP24 promoter-luciferase construct along with dominant negative ERK1, ERK2, MEK5, or control plasmids. 24 h after transfection, cells were treated with 100 nM 1,25(OH)2D3 for 24 h before luciferase activity was assayed. For drug inhibitors, cells were preincubated with PD98059 (50 µM), U0126 (10 µM) or vehicle for 30 min prior to the start of the 1,25(OH)2D3 treatment and inhibitor treatment was continued throughout the vitamin D treatment period. (B) Differentiated Caco-2 cells were transfected with a 3X osteopontin VDRE promoter-luciferase construct. After 24 h cells were preincubated with 0, 5, or 10 µM U0126 for 30 min followed by co-treatment with 100 nM 1,25(OH)2D3 for an additional 24 h before luciferase activity was assayed. *, Significantly different from the non-treated control (p < 0.05), #, significantly different from 1,25(OH)2D3 alone (p<0.05). Data in both panels are representative of three independent experiments.
ERK pathway inhibition does not reduce 1,25(OH)2D3-induced association of VDR with chromatin, the CYP24 promoter, or the RXRα
VDR must dimerize with RXR and associate with chromatin to induce transcription (Pike et al., 2007); we examined whether these events were influenced by MEK inhibition. First, we examined whether the ligand-induced interaction between VDR and RXRα is impaired using mammalian two-hybrid assays. 1,25(OH)2D3 treatment significantly induced an interaction between the RXRα LBD and VDR LBD (26 fold). However, U0126 did not reduce this interaction (Figure 5A). Instead, the vitamin D-induced VDR-RXR interaction was significantly increased by co-treatment with 5 µM or 10 µM U0126 (to 43 and 38 fold, respectively).
Figure 5.
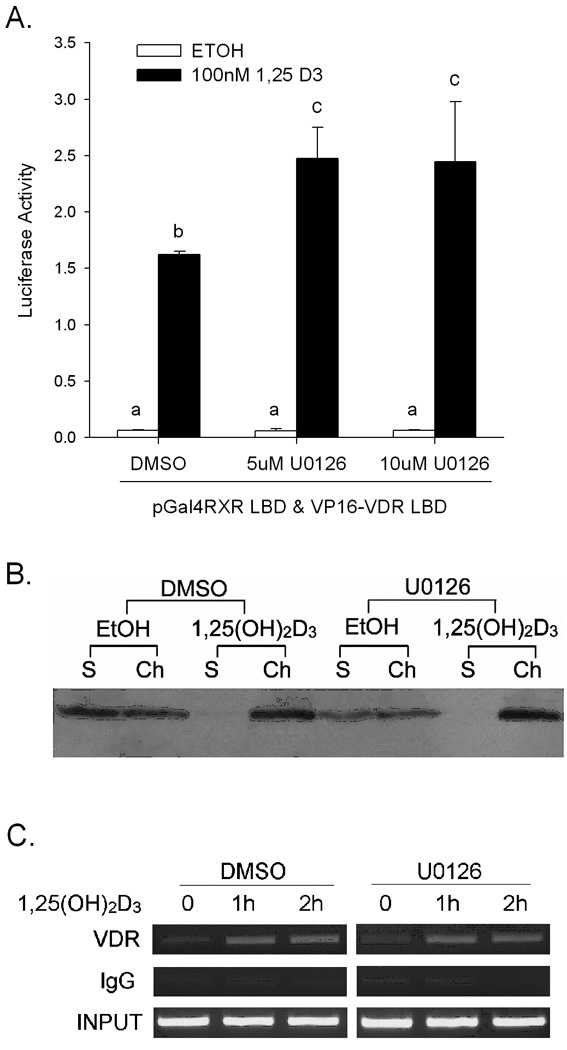
1,25(OH)2D3-induced interactons between VDR and RXRα or DNA are not altered by U0126 in differentiated Caco-2 cells. (A) Two-hybrid assays of vitamin D-induced VDR-RXRα interactions. Differentiated Caco-2 cells were transfected with 250 ng of luciferase reporter vector pFR-Luc, 125 ng of pGal4-RXRα LBD, 125 ng of VP16-VDR LBD and 1 ng of CMV-Renilla. 24 hours after transfection cells were treated with vehicle or 100 nM 1,25(OH)2D3 for 24 hours and the luciferase activity was measured. (B) VDR association with chromatin. Caco-2 cells were preincubated with 10 µM U0126 or vehicle (DMSO) for 30 min, after which cells were co-treated for 1 h with inhibitor and 10 nM 1,25(OH)2D3 or vehicle (ethanol). VDR was detected in soluble (S) and chromatin (Ch) fractions by Western Blot analysis. (C) ChIP analysis of VDR association with the CYP24 promoter. Caco-2 cells were preincubated with 10 µM U0126 or vehicle (DMSO) for 30 minutes, after which cells were co-treated with inhibitor for an additional 1 h or 2 h with 10 nM 1,25(OH)2D3 or vehicle. ChIP assays were performed with 2 µg rabbit IgG or VDR antibody. ChIP-enriched DNA was amplified with primers recognizing the proximal promoter region of CYP24. Data are representative of three independent experiments.
Next, the association of VDR protein with chromatin was examined. VDR was found in both the crude chromatin and soluble fractions of the cell and 1,25(OH)2D3 induced a rapid redistribution of VDR to the chromatin fraction (Figure 5B). When cells were preincubated with U0126 prior to 1,25(OH)2D3 treatment, the ligand-induced association of VDR with chromatin was not changed (Figure 5B). Similar results were seen using another MEK inhibitor, PD98059 (data not shown).
Finally, we used a chromatin immunoprecipitation (ChIP) assay to evaluate the effect that MEK inhibition had on VDR binding to the proximal CYP24 promoter containing two active VDREs essential for 1,25(OH)2D3–mediated gene activation (Kerry et al., 1996). While 1,25(OH)2D3 treatment enhanced VDR binding to the CYP24 promoter (Figure 5C), treatment with U0126 did not impair this binding. Similar results were observed for the 1,25(OH)2D3–induced association of RXRα with the CYP24 promoter (data not shown).
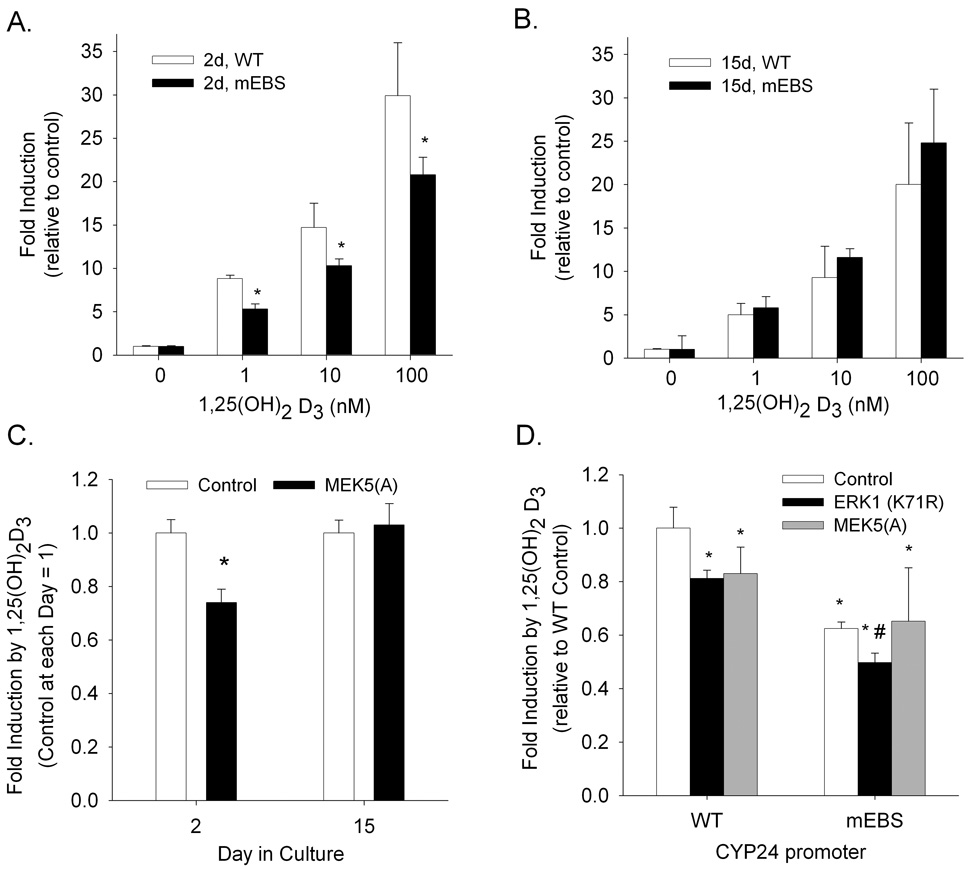
The Ets-1 binding site (EBS) is not important for 1,25(OH)2D3-induced CYP24 promoter activity in differentiated Caco-2 cells
In addition to their effects on ERK1 and ERK2, MEK inhibitors can also affect ERK5 activity (Schweppe et al., 2006). ERK5 activity has previously been shown to regulate 1,25(OH)2D3 action and VDR recruitment to the CYP24 promoter in proliferating COS-1 cells by modulating Ets-1 binding to the promoter (Dwivedi et al., 2000). As a result, we examined the role that ERK5 and the Ets-1 binding site (EBS) have on 1,25(OH)2D3 activation of the CYP24 promoter in both proliferating and differentiated Caco-2 cells. Mutation of the EBS reduced basal expression of the −298 to +74 bp rat CYP24 promoter-luciferase construct by around 50% in both proliferating and differentiated Caco-2 cells (data not shown). 1,25(OH)2D3 treatment induced both wild type and mutant EBS rat CYP24 promoter-luciferase construct activity significantly at both cell stages. However, in proliferating cells, the EBS mutation reduced 1,25(OH)2D3-induced activation of the CYP24 promoter by 30 to 40% compared to the wild type CYP24 promoter (Figure 6A). In contrast, the EBS mutation did not alter 1,25(OH)2D3-induced CYP24 promoter activity in differentiated Caco-2 cells (Figure 6B). Consistent with this, we found that disrupting ERK5 signaling with a dominant negative MEK5 reduced 1,25(OH)2D3-mediated CYP24 promoter activation in proliferating but not differentiated Caco-2 cells (Figure 6C).
Figure 6.
The role of Ets-1 in 1,25(OH)2D3-mediated CYP24 gene regulation is dependent upon the state of Caco-2 cell differentiation. Proliferating (A) and differentiated (B) Caco-2 cells were transfected with a −298 to +74 rat CYP24 promoter luciferase construct containing either a wild type (WT, white bars) or mutant EBS (mEBS, black bars). 24 h later cells were treated in either 0, 1, 10, or 100 nM 1,25(OH)2D3 for an additional 24 h. (C) Proliferating and differentiated Caco-2 cells were transfected with the wild type rat CYP24 promoter construct along with either a control plasmid or a dominant negative MEK5 expression vector. (D) Proliferating Caco-2 cells were transfected with the wild type or mEBS rat CYP24 promoter constructs along with a control plasmid, a dominant negative ERK1, or a dominant negative MEK5 expression vector. 24 h after transfection, cells were treated +/− 100 nM 1,25(OH)2D3 for 24 h and luciferase activity was assayed. In all panels bars represent the mean ± SEM (n=3 per group). In panels (A) and (B) *, significantly different in comparison with wild type value (p < 0.05); in panel (C) *, significantly different from control plasmid (p < 0.05); in panel (D) *, significantly different from wild type vector and control plasmid (p < 0.05); #, significantly different from mEBS vector and control plasmid (p<0.05). Data in all panels are representative of three independent experiments.
We conducted an experiment to test whether ERK1 and ERK5 independently influence 1,25(OH)2D3-induced CYP24 promoter activity in proliferating cells. Both dominant negative ERK1 and dominant negative MEK5 expression blocked activation of the wild type CYP24 promoter by 1,25(OH)2D3 in proliferating cells. When the CYP24 promoter with the mutant EBS was studied, the effect of the dominant negative MEK5 was lost but the inhibition due to the dominant negative ERK1 remained (Figure 6D). This indicates that the impact of ERK1 and ERK5 activity on vitamin D mediated CYP24 gene expression are independent.
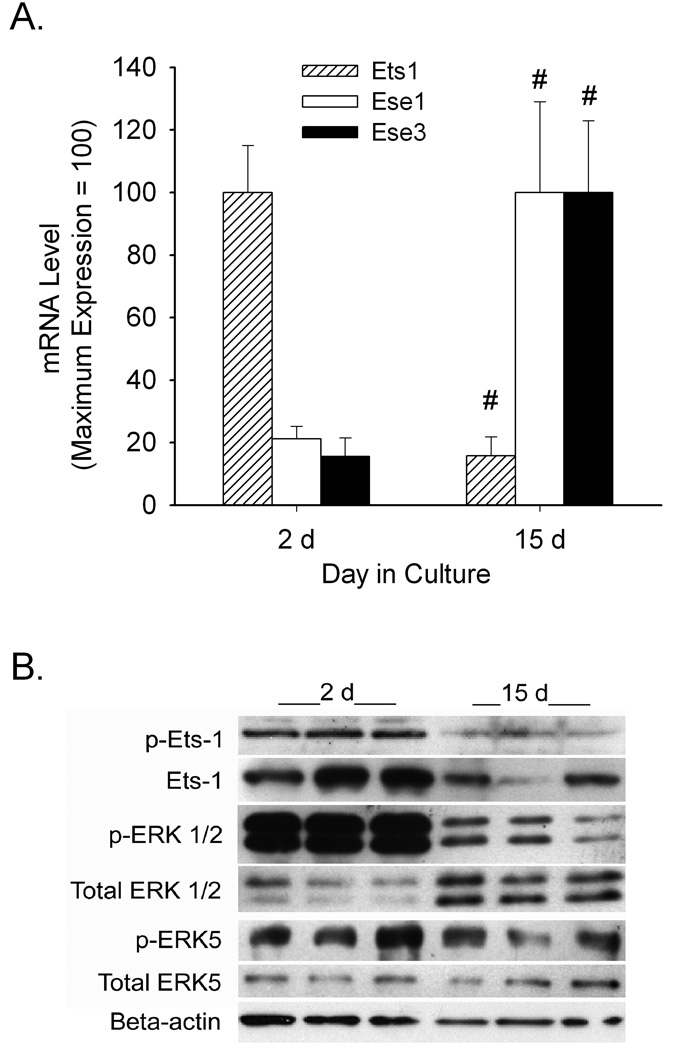
Ets-1 mRNA, protein and phosphorylation state are lower in differentiated Caco-2 cells
To determine why ERK5 regulation of vitamin D-mediated CYP24 gene transcription in differentiated Caco-2 cells was lost we conducted further experiments. The function of the Ets-1 transcription factors depends upon both its level and activation state. When we compared the mRNA expression level of three Ets family members previously shown to be expressed in intestine (Hollenhorst et al., 2004), we found that ESE-1 and ESE-3 mRNA levels increase significantly as the cells differentiated (by 5 to 6-fold) while Ets-1 mRNA expression fell by 85% after differentiation (Figure 7A). Ets-1 undergoes Ras-dependent phosphorylation at threonine 38 (T38) (Yang et al., 1996); Western blot analysis revealed that both total and phospho Ets-1 protein levels were lower in differentiated cells (Figure 7B). This suggests that MAPK signaling is suppressed in differentiated Caco-2 cells. Consistent with this, we found that ERK 1, 2 and 5 phosphorylation levels were lower in differentiated cells compared to proliferating cells (Figure 7B).
Figure 7.
Changes in Ets-1 expression and phosphorylation with Caco-2 cell differentiation. (A) Ets family member mRNA levels in 2 day and 15 day Caco-2 cell cultures. Values expressed relative to the maximum expression value for each target (mean ± SEM, n=3 per group). #, significantly different from 2 day group (p< 0.05). (B) Expression of total and phosphorylated forms of Ets-1, ERK1/2, and ERK5, as well as beta-actin protein levels in 2 day and 15 day Caco-2 cells. Whole cell extracts were examined by Western Blot analysis (60 µg for phosphorylated Ets-1 and 20 µg for all other targets). Data in this figure are representative of three independent experiments.
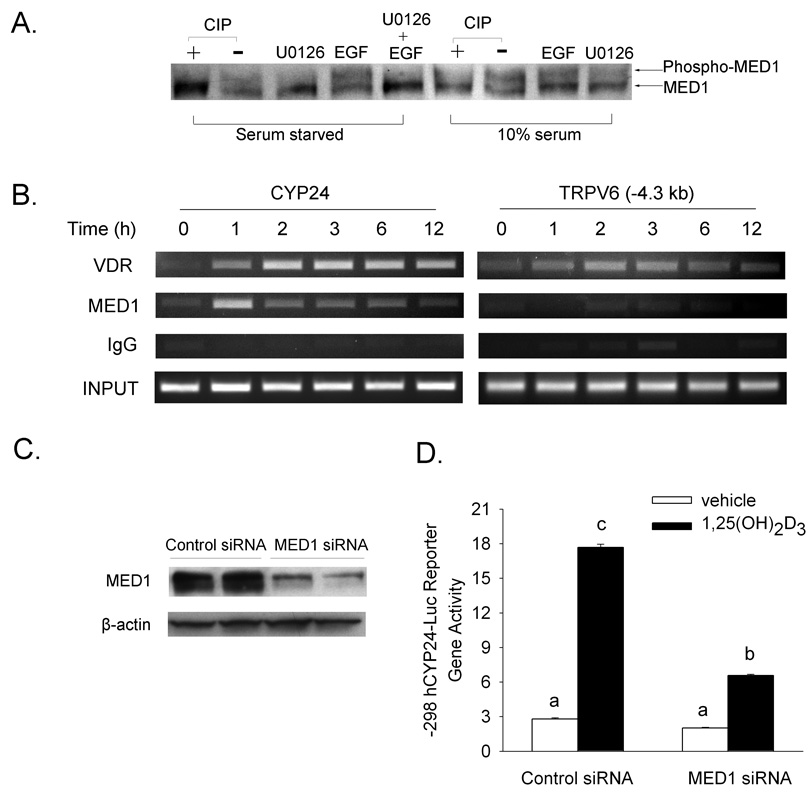
Others have reported that ERK1/2 mediated MED1 phosphorylation enhances ligand-induced transcription of nuclear receptors (Pandey et al., 2005) (Misra et al., 2002). Western blot analysis confirmed that there are two MED1 bands in extracts from Caco-2 cells cultured in serum and that the higher molecular weight band disappears upon treatment of the cell extracts with CIP or after pretreatment of cells with the MEK inhibitor U0126 (Figure 8A). In serum starved cells (where ERK activity is reduced), the phospho MED1 band was reduced but activation of MAPK signaling with short-term EGF pretreatment enhanced MED1 phosphorylation; this effect was inhibited by U0126 (Figure 8A).
Figure 8.
Mediator 1 (MED1) is a phosphoprotein that is essential for 1,25(OH)2D3-regulated CYP24 promoter activity. (A) Phosphorylation of MED1 is modulated by MAPK signaling. Differentiated Caco-2 cells were treated with 10 µM U0126 for 30 min, 100 ng/ml EGF for 15 min or combination of two. Treatments were tested in cells that were either serum-starved overnight or cultured in media supplemented with 10% FBS. Whole cell lysates (50 µg) were treated with 25 unit of CIP, and resolved by 5% SDS-PAGE before probing with MED1 antibody. (B) ChIP analysis shows that MED1 is associated with the CYP24 but not TRPV6 promoter. Differentiated Caco-2 cells were treated with vehicle (EtOH) or 100 nM 1,25(OH)2D3 for the time indicated. ChIP assays were performed with 2 µg rabbit IgG, VDR antibody or MED1 antibody. ChIP enriched DNA was amplified with primers recognizing the promoter region of the CYP24 and TRPV6 (−4.3kb) genes. (C) MED1 siRNA reduces MED1 protein levels in Caco-2 cells. Caco-2 cells were transfected with 100 nM non-targeting control siRNA or MED1 siRNA. 72 h after transfection, 50 µg whole cell extracts were prepared from transfected cells and probed with anti-MED1 and anti-β-actin antibodies. (D) MED1 siRNA reduces 1,25(OH)2D3-regulated CYP24 promoter activity. Caco-2 cells were treated with control or MED1 siRNA for 24 h and then transfected with a human CYP24 promoter-luciferase reporter gene construct. After 16 h cells were treated with vehicle (white bar) or 10 nM 1,25(OH)2D3 (black bar) for 4 h, and harvested for luciferase assays. The data reflect the mean ± SEM (n=3). Bars with common letter superscripts are not significantly different (P<0.05). Data in each panel are representative of three independent experiments.
ChIP assays show that 1,25(OH)2D3 treatment increased the association of VDR with the proximal CYP24 promoter at various time periods (Figure 8B). Like VDR, MED1 was associated with the CYP24 promoter after 1,25(OH)2D3 treatment at all time points, with a peak level at 1 h. Although VDR was also associated with the −4.3 kb (Figure 8B) and −2.1 kb (data not shown) regions of the TRPV6 promoter, there was no significant increase of MED1 association with the TRPV6 promoter at any time point that we examined.
The ChIP experiments indicate a selective recruitment of MED1 to the CYP24 promoter in Caco-2 cells so we examined the requirement of VDR-mediated CYP24 transcription for MED1 using RNA interference to reduce intracellular MED1 levels. MED1 siRNA selectively reduced MED1 by 70% compared with a nonspecific control siRNA (Figure 8C). The loss of MED1 expression in MED1 siRNA treated cells significantly reduced vitamin D dependent induction of the human CYP24 promoter reporter gene activity by 52% compared to control siRNA (Figure 8D).
DISCUSSION
Crosstalk between kinase-mediated signal transduction pathways and 1,25(OH)2D3 regulated gene expression has been described in a variety of cell types and for a number of genes, e.g. CYP24 in IEC-6 (Koyama et al., 1994) IEC-18 (Armbrecht et al.,2001), COS-1, and LLC-PK1 cells (Barletta et al.,2002), CY3A4 in proliferating Caco-2 cells (Yasunami et al., 2004); alkaline phosphatase, collagen IA1, and CYP24 mRNA levels in MG-63 and HeLa cells (Narayanan et al.,2004). Because the mechanism for this crosstalk is not clearly understood, in this paper we have chosen to focus on the modulation of 1,25(OH)2D3 transcriptional activity by MAPK/ERK signaling. Dwivedi et al. have previously published the most complete characterization of this phenomenon in proliferating COS-1 kidney cells (Dwivedi et al., 2000). Their data show that MAPK signaling has effects on 1,25(OH)2D3-regulated CYP24 induction that are mediated through RXRα phosphorylation by the kinases ERK1 and ERK2 and through ERK5-mediated phosphorylation of the transcription factor Ets-1. Our data from proliferating and differentiated Caco-2 cells suggest that the MAPK effect on vitamin D transcriptional activity is more complex.
In the model proposed by Dwivedi et al., ERK5 phosphorylates Ets-1 at threonine 38. This increases the association of Ets-1 with a binding site (the EBS) proximal to VDRE1 in the rat CYP24 promoter, leading to formation of a ternary complex of Ets-1 with the VDR and RXR bound to the VDRE (Dwivedi et al., 2000). Our data in proliferating Caco-2 cells is consistent with this model; MEK5 inhibition significantly blunts 1,25(OH)2D3 regulated CYP24 reporter gene activity (Figure 6C and D) and the EBS site is critical for maximal 1,25(OH)2D3 regulated CYP24 promoter activity at this cell stage (Figure 6A). However, in differentiated Caco-2 cells, neither MEK5 inhibition nor EBS mutation reduced CYP24 reporter gene activation by 1,25(OH)2D3 (Figure 6B and C). This suggests that the function of Ets-1 is reduced in differentiated Caco-2 cells and this hypothesis is supported by our observation that both Ets-1 level (mRNA and protein) and T38 phosphorylation state are significantly lower in differentiated Caco-2 cells (Figure 7). Although the level of other intestine-specific Ets-family members increase with enterocyte-like Caco-2 cells differentiation this did not protect 1,25(OH)2D3-regulated CYP24 expression in differentiated cells from the loss of Ets-1, suggesting the roles of the Ets-family members are not interchangeable on the CYP24 promoter. Reduced Ets-1 expression and activity upon differentiation has also been reported during both erythroid and B cell differentiation (Wang et al., 2005;Marziali et al., 2002) but the mechanism for this decline is not known. Higher Ets-1 expression and activity during carcinogenesis or progression of colorectal carcinoma suggests that this is preferentially required for proliferation (Ito et al., 2002;Nakayama et al., 2001). In this context, higher Ets-1 level or activity could serve to reduce overall 1,25(OH)2D3 action by increasing the negative feedback loop (i.e. CYP24 mediated hormone degradation) that protects the proliferating cell from the prodifferentiating actions of 1,25(OH)2D3.
We observed that MEK inhibitors can suppress 1,25(OH)2D3-induced activities of both a mutant EBS-CYP24 promoter and a 3X-VDRE reporter gene. This suggests that ERK1/2 signaling affects transcription through the VDR-RXR heterodimer. Others have demonstrated that the function of VDR and RXRα are modulated by phosphorylation and there are several possible mechanisms by which phosphorylation of these receptors could modulate transcription. First, Dwivedi et al. (Dwivedi et al., 2002) demonstrated that serine 260 (S260) on RXRα is a target of ERK2-mediated phosphorylation and proposed that this event enhances 1,25(OH)2D3-induced CYP24 gene expression by increasing co-activator recruitment. However, in squamous cell carcinoma cells constitutive activation of MAPK signaling by Ras transformation also phosphorylates RXRα at S260 but this inhibits VDR transactivation potential (Solomon et al., 1999) and impairs co-activator recruitment (Macoritto et al., 2008). Other sites in RXRα are also reported to be phosphorylated (S21, S32, T82, Y249) but these also inhibit the transactivation potential of RXRs heterodimeric partners (Bruck et al., 2005;Matsushima-Nishiwaki et al., 2001;Bastien et al., 2002;Lee et al., 2000;Mann et al., 2005).
Another mechanism by which ERK1/2 could influence vitamin D-mediated gene transcription is through VDR phosphorylation. Although VDR phosphorylation at S51 (Hsieh et al., 1993) or S182 (Hsieh et al., 2004) reduces VDR function, phosphorylation at S208 increases VDR-dependent transcriptional activity (Jurutka et al., 1996). This is a plausible mechanism but our data show that MAPK signaling is not influencing either VDR-RXR heterodimerization (by 2-hybrid assay) or binding of VDR to chromatin (Figure 5). This suggests that the impact of MAPK on vitamin D-mediated transcription may be mediated through recruitment of essential transcriptional co-activators. Consistent with this, others have reported that inhibition of phosphorylation with okadaic acid enhanced the interactions between VDR and DRIP205 (now called mediator 1 or MED1) (Barletta et al., 2002) and that phosphorylation of VDR at S208 can enhance the interaction between VDR and MED1 (Arriagada et al., 2007). However, these observations do not explain why TRPV6 induction by 1,25(OH)2D3 is not influenced by MEK inhibition.
Meyer et al. (Meyer et al., 2007) reported that while the MED1 is recruited to the CYP24 promoter in the intestine of 1,25(OH)2D3-injected mice, it is not recruited to the VDREs at −4.3 kb and −2.1 kb in the TRPV6 promoter. Our data confirm and expand this observation in differentiated Caco-2 cells (Figure 8B). MED1 is thought to be essential for transcription by acting as a bridge between transcription factors and RNA polymerase II (Zhang et al., 2005). Recent studies with MED1 siRNA show that MED1 is required for estrogen receptor and thyroid receptor function on target genes (Zhang et al., 2005; Pandey et al., 2005). Consistent with these findings, our study with MED1 siRNA show that MED1 is also important for 1,25(OH)2D3–mediated CYP24 promoter activity in Caco-2 cells. Pandey et al.(Pandey et al., 2005) have shown that ERK1/2 activation increases MED1 phosphorylation leading to MED1 stabilization and increased thyroid hormone-dependent transcription in HeLa cells. In addition, two ERK2 sites have previously been identified in MED1 (threonine 1017 and 1444) and phosphorylation at these sites enhances PPARγ transactivation potential even though PPARγ-MED1 interactions were not affected (Misra et al., 2002). This suggests that for genes that depend upon MED1 for transcriptional activation, MED1 phosphorylation promotes formation of the mediator complex and recruitment of RNA polymerase II. While it is not clear how the VDREs in TRPV6 mediate 1,25(OH)2D3-induced gene regulation without MED1, the lack of MED1 recruitment to this promoter explains why MAPK inhibition has no influence on the 1,25(OH)2D3-induced expression of TRPV6. TRPV6 is not the only gene that is regulated by 1,25(OH)2D3 that does not have an absolute requirement for MED1; 1,25(OH)2D3 treatment in ROS 17/2.8 cells does not induce a rapid recruitment of MED1 to osteocalcin promoter region (Carvallo et al., 2008).
In summary, our data further refine our understanding of how the activity of ERK signal transduction pathways modulate 1,25(OH)2D3-mediated gene expression. We demonstrate that ERK5 signaling is the primary activator of Ets-1 activity in proliferating Caco-2 cells, thus accounting for the critical importance of the EBS on 1,25(OH)2D3-mediated CYP24 gene expression in that cell stage. In contrast, regulation of CYP24 gene expression through the EBS is less important in differentiated Caco-2 cells due to a combination of reduced Ets-1 protein levels, reduced ERK5 signaling, and reduced Ets-1 phosphorylation. Our data also suggest that ERK1/2 signaling on 1,25(OH)2D3-mediated CYP24 gene regulation is through MED1 phosphorylation and the subsequent recruitment of RNA polymerase II to the CYP24 promoter. However, since induction of the TRPV6 promoter by 1,25(OH)2D3 is independent of MED1, it is not affected by modulation of ERK1/2 signaling. In conclusion, our data show that the interplay between ERK signaling and 1,25(OH)2D3-mediated gene expression is not only specific to the cell type and state of differentiation but also to the structure and function of the gene promoter examined.
ACKNOWLEDGEMENTS
We thank Dr. Wesley J. Pike and Dr. Mark. B. Meyer (University of Wisconsin, Madison) for their help optimizing the chromatin immunoprecipitation technique.
Contract grant sponsor: National Institutes of Health; Contract grant number: DK054111 (to J.C.F.).
Contract grant sponsor: United States Department of Agriculture, Agricultural Research Service; Contract grant Number: 58-1950-4-401 (to R.W.).
ABBREVIATIONS
- 1α,25 (OH)2D3
1α, 25 dihydroxyvitamin D3
- ChIP
chromatin immunoprecipitation
- CIP
calf intestinal phosphatase
- CYP24
25-hydroxyvitamin D3 24-hydroxylase
- DMEM
Dulbecco's Modified Eagle Medium
- DRIP
Vitamin D Receptor-Interacting Protein
- EBS
Ets-1 binding site
- EGF
Epidermal Growth Factor
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3̀ phophate dehydrogenase
- JNK
Jun N-terminal kinase
- LBD
ligand binding domains
- MAPK
mitogen activated protein kinase
- MED1
mediator 1
- PKC
protein kinase C
- RT-PCR
reverse transcriptase polymerase chain reaction
- RXR
retinoid X receptor
- siRNA
small interfering RNA
- TRPV6
transient receptor vanelloid family member 6
- VDR
vitamin D receptor
- VDRE
vitamin D response element
Reference List
- Armbrecht HJ, Boltz MA, Hodam TL, Kumar VB. Differential responsiveness of intestinal epithelial cells to 1,25- dihydroxyvitamin D3--role of protein kinase C. J Endocrinol. 2001;169:145–151. doi: 10.1677/joe.0.1690145. [DOI] [PubMed] [Google Scholar]
- Arriagada G, Paredes R, Olate J, van Wijnen A, Lian JB, Stein GS, Stein JL, Onate S, Montecino M. Phosphorylation at serine 208 of the 1 alpha,25-dihydroxy Vitamin D3 receptor modulates the interaction with transcriptional coactivators. J Steroid Biochem Mol Biol. 2007;103:425–429. doi: 10.1016/j.jsbmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta F, Dhawan P, Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am J Physiol Endocrinol Metab. 2004;286:E598–E608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- Barletta F, Freedman LP, Christakos S. Enhancement of VDR-mediated transcription by phosphorylation: correlation with increased interaction between the VDR and DRIP205, a subunit of the VDR-interacting protein coactivator complex. Mol Endocrinol. 2002;16:301–314. doi: 10.1210/mend.16.2.0764. [DOI] [PubMed] [Google Scholar]
- Bastien J, Adam-Stitah S, Plassat JL, Chambon P, Rochette-Egly C. The phosphorylation site located in the A region of retinoic X receptor alpha is required for the antiproliferative effect of retinoic acid (RA) and the activation of RA target genes in F9 cells. J Biol Chem. 2002;277:28683–28689. doi: 10.1074/jbc.M203623200. [DOI] [PubMed] [Google Scholar]
- Belleli A, Shany S, Levy J, Guberman R, Lamprecht SA. A protective role of 1,25-dihydroxyvitamin D3 in chemically induced rat colon carcinogenesis. Carcinogenesis. 1992;13:2293–2298. doi: 10.1093/carcin/13.12.2293. [DOI] [PubMed] [Google Scholar]
- Bettoun DJ, Burris TP, Houck KA, Buck DW, Stayrook KR, Khalifa B, Lu J, Chin WW, Nagpal S. Retinoid X Receptor is a Non-Silent Major Contributor to Vitamin D Receptor-Mediated Transcriptional Activation. Mol Endocrinol. 2003;17:2320–2328. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- Bruck N, Bastien J, Bour G, Tarrade A, Plassat JL, Bauer A, Adam-Stitah S, Rochette-Egly C. Phosphorylation of the retinoid x receptor at the omega loop, modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell Signal. 2005;17:1229–1239. doi: 10.1016/j.cellsig.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Carvallo L, Henriquez B, Paredes R, Olate J, Onate S, van Wijnen AJ, Lian JB, Stein GS, Stein JL, Montecino M. 1 alpha,25-dihydroxy vitamin D-3-Enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1 alpha,25-dihydroxy vitamin D-3 Receptor-SRC-1 coactivator complex. J Cell Physiol. 2008;214:740–749. doi: 10.1002/jcp.21267. [DOI] [PubMed] [Google Scholar]
- Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48:1936–1942. [PubMed] [Google Scholar]
- Dwivedi PP, Hii CS, Ferrante A, Tan J, Der CJ, Omdahl JL, Morris HA, May BK. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem. 2002;277:29643–29653. doi: 10.1074/jbc.M204561200. [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Omdahl JL, Kola I, Hume DK, May BK. Regulation of rat cytochrome P450C24 (CYP24) gene expression - Evidence for functional cooperation of Ras-activated Ets transcription factors with the vitamin D receptor in 1,25-dihydroxyvitamin D-3-mediated induction. J Biol Chem. 2000;275:47–55. doi: 10.1074/jbc.275.1.47. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Wood RJ. Specific 1,25(OH)2D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–G964. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Yasunami Y, Adachi T. Alteration of cellular phosphorylation state affects vitamin D receptor-mediated CYP3A4 mRNA induction in Caco-2 cells. Biochem Biophys Res Commun. 2002;296:182–188. doi: 10.1016/s0006-291x(02)00860-4. [DOI] [PubMed] [Google Scholar]
- Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J-C, Jurutka PW, Nakajima S, Galligan MA, Haussler CA, Shimizu Y, Shimizu N, Whitfield GK, Haussler MA. Phosphorylation of the human vitamin D receptor by protein kinase C. Biochemical and functional evaluatin of the serine 51 recognition site. J Biol Chem. 1993;268:15118–15126. [PubMed] [Google Scholar]
- Hsieh JC, Dang HT, Galligan MA, Whitfield GK, Haussler CA, Jurutka PW, Haussler MR. Phosphorylation of human vitamin D receptor serine-182 by PKA suppresses 1,25(OH)2D3-dependent transactivation. Biochem Biophys Res Commun. 2004;324:801–809. doi: 10.1016/j.bbrc.2004.09.139. [DOI] [PubMed] [Google Scholar]
- Ismail A, Nguyen CV, Ahene A, Fleet JC, Uskokovic MR, Peleg S. Effect of Cellular Environment on the Selective Activation of the Vitamin D Receptor by 1{alpha},25-dihydroxyvitamin D3 and its Analog 1{alpha}-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26-9228) Mol Endocrinol. 2004;18:874–887. doi: 10.1210/me.2003-0310. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takeda T, Okada M, Matsuura N. Expression of ets-1 and ets-2 in colonic neoplasms. Anticancer Res. 2002;22:1581–1584. [PubMed] [Google Scholar]
- Jurutka PW, Hsieh JC, Nakajima S, Haussler CA, Whitfield GK, Haussler MR. Human vitamin D receptor phophorylation by casein kinase II at ser-208 potentiates transcriptional activation. Proc Natl Acad Sci USA. 1996;93:3519–3524. doi: 10.1073/pnas.93.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- Klopot A, Hance KW, Peleg S, Barsony J, Fleet JC. Nucleo-cytoplasmic cycling of the vitamin D receptor in the enterocyte-like cell line, Caco-2. J Cell Biochem. 2007;100:617–628. doi: 10.1002/jcb.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Inaba M, Nishizawa Y, Ohno S, Morii H. Protein kinase C is involved in 24-hydroxylase gene expression induced by 1,25 (OH)2D3 in rat intestinal epithelial cells. J Cell Biochem. 1994;55:230–240. doi: 10.1002/jcb.240550210. [DOI] [PubMed] [Google Scholar]
- Lee HY, Suh YA, Robinson MJ, Clifford JL, Hong WK, Woodgett JR, Cobb MH, Mangelsdorf DJ, Kurie JM. Stress pathway activation induces phosphorylation of retinoid X receptor. J Biol Chem. 2000;275:32193–32199. doi: 10.1074/jbc.M005490200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lou YR, Nazarova N, Talonpoika R, Tuohimaa P. 5alpha-dihydrotestosterone inhibits 1alpha,25-dihydroxyvitamin D3-induced expression of CYP24 in human prostate cancer cells. Prostate. 2005;63:222–230. doi: 10.1002/pros.20189. [DOI] [PubMed] [Google Scholar]
- Ly LH, Zhao ZY, Holloway L, Feldman D. Liarazole acts synergistically with 1a,25-dihydroxyvitamin D3 to inhibit growth of DU145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140:2071–2076. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- Macoritto M, Nguyen-Yamamoto L, Huang DC, Samuel S, Yang XF, Wang TT, White JH, Kremer R. Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands. J Biol Chem. 2008;283:4943–4956. doi: 10.1074/jbc.M707517200. [DOI] [PubMed] [Google Scholar]
- Mann KK, Padovani AM, Guo Q, Colosimo AL, Lee HY, Kurie JM, Miller WH., Jr Arsenic trioxide inhibits nuclear receptor function via SEK1/JNK-mediated RXRalpha phosphorylation. J Clin Invest. 2005;115:2924–2933. doi: 10.1172/JCI23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marziali G, Perrotti E, Ilari R, Lulli V, Coccia EM, Moret R, Kuhn LC, Testa U, Battistini A. Role of Ets-1 in transcriptional regulation of transferrin receptor and erythroid differentiation. Oncogene. 2002;21:7933–7944. doi: 10.1038/sj.onc.1205925. [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Okuno M, Adachi S, Sano T, Akita K, Moriwaki H, Friedman SL, Kojima S. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 2001;61:7675–7682. [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- Misra P, Owuor ED, Li W, Yu S, Qi C, Meyer K, Zhu YJ, Rao MS, Kong AN, Reddy JK. Phosphorylation of transcriptional coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP). Stimulation of transcriptional regulation by mitogen-activated protein kinase. J Biol Chem. 2002;277:48745–48754. doi: 10.1074/jbc.M208829200. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Ito M, Ohtsuru A, Naito S, Sekine I. Expression of the ets-1 proto-oncogene in human colorectal carcinoma. Mod Pathol. 2001;14:415–422. doi: 10.1038/modpathol.3880328. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Tovar SV, Falzon M, Weigel NL. The functional consequences of cross talk between the vitamin D receptor and ERK signaling pathways are cell specific. J Biol Chem. 2004;278:47298–47310. doi: 10.1074/jbc.M404101200. [DOI] [PubMed] [Google Scholar]
- Pandey PK, Udayakumar TS, Lin XJ, Sharma D, Shapiro PS, Fondell JD. Activation of TRAP/Mediator subunit TRAP220/Med1 is regulated by mitogen-activated protein kinase-dependent phosphorylation. Mol Cell Biol. 2005;25:10695–10710. doi: 10.1128/MCB.25.24.10695-10710.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike JW, Zella LA, Meyer MB, Fretz JA, Kim S. Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J Bone Miner Res. 2007;22 Suppl 2:V16–V19. doi: 10.1359/jbmr.07s207. [DOI] [PubMed] [Google Scholar]
- Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadow N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffer K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- Robbins DJ, Zhen EH, Cheng MG, Xu SC, Vanderbilt CA, Ebert D, Garcia C, Dang A, Cobb MH. Regulation and Properties of Extracellular Signal-Regulated Protein Kinase-1, Kinase-2, and Kinase-3. J Am Soc Nephrol. 1993;4:1104–1110. doi: 10.1681/ASN.V451104. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- Schweppe RE, Cheung TH, Ahn NG. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J Biol Chem. 2006;281:20993–21003. doi: 10.1074/jbc.M604208200. [DOI] [PubMed] [Google Scholar]
- Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3- dependent signal transduction by phosphorylating human retinoid X receptor alpha. J Clin Invest. 1999;103:1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Abe T, Oue N, Yasui W, Ryoji M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol Cell Endocrinol. 2004;226:27–32. doi: 10.1016/j.mce.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang D, John SA, Clements JL, Percy DH, Barton KP, Garrett-Sinha LA. Ets-1 deficiency leads to altered B cell differentiation, hyperresponsiveness to TLR9 and autoimmune disease. Int Immunol. 2005;17:1179–1191. doi: 10.1093/intimm/dxh295. [DOI] [PubMed] [Google Scholar]
- Wang XN, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D-3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–260. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BS, Hauser CA, Henkel G, Colman MS, VanBeveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunami Y, Hara H, Iwamura T, Kataoka T, Adachi T. C-jun N-terminal kinase modulates 1,25-dihydroxyvitamin D3-induced cytochrome P450 3A4 gene expression. Drug Metab Dispos. 2004;32:685–688. doi: 10.1124/dmd.32.7.685. [DOI] [PubMed] [Google Scholar]
- Zhang XT, Krutchinsky A, Fukuda A, Chen W, Yamamura S, Chalt BT, Roeder RG. MED1/TRAP220 exists predominantly in a TRAP/mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription. Mol Cell Biol. 2005;19:89–100. doi: 10.1016/j.molcel.2005.05.015. [DOI] [PubMed] [Google Scholar]