Abstract
Background:
The decision whether to treat benign skeletal lesions surgically can be difficult to make. The purpose of this study was to validate our previously published method of predicting fracture risk with use of quantitative computed tomography-based structural analysis.
Methods:
We prospectively studied a group of children who presented to a major children's hospital with a benign appendicular skeletal lesion between 2002 and 2007. As in our previous study, the resistance of the affected bone to compressive, bending, and torsional loads was calculated with rigidity analysis performed with the use of serial transaxial quantitative computed tomography data obtained along the length of the bone containing the lesion and from homologous cross sections through the contralateral, normal bone. At each cross section, the ratio of the structural rigidity of the affected bone to that of the normal, contralateral bone was determined.
Results:
Forty-one patients who had not received surgical treatment for the skeletal lesion met the criteria for our study. Thirty-four (83%) of these individuals completed our activity questionnaire at least two years after the quantitative computed tomography-based rigidity analysis. None of the patients for whom no increased fracture risk had been predicted by the rigidity analysis sustained a fracture, even though they had not received surgical treatment.
Conclusions:
Many considerations other than the predicted fracture risk are factored into the decision of whether to treat a benign skeletal lesion. However, this study indicated that quantitative computed tomography-based rigidity analysis is more specific (97% specificity) than criteria based on plain radiographs (12% specificity) for predicting the risk of a pathologic fracture since fracture risk indices based on lesion size alone fail to account for the compensatory remodeling of the host bone that occurs in response to the presence of the lesion in a growing child.
Level of Evidence:
Prognostic Level I. See Instructions to Authors for a complete description of levels of evidence.
Benign skeletal lesions represent a diverse group of pathologic entities that vary greatly in aggressiveness and clinical behavior1,2. After confirming that a lesion is benign, the physician must decide whether the osteolytic defect has weakened the bone sufficiently for a fracture to be imminent. Pathologic fractures of the appendicular skeleton are associated with pain and loss of function3. Thus, preventing these fractures is an important clinical goal.
The optimal treatment for benign bone lesions remains controversial because many factors, such as the type of lesion, anatomic location, size, or rate of growth, must be taken into account. Such treatments may include observation, restricted weight-bearing, bracing, intralesional injection of corticosteroids or demineralized bone matrix with or without bone marrow aspirate, and curettage and packing of the defect with bone graft with or without stabilization of the affected bone with fracture fixation devices to prevent fracture4-21. Two common lesions, unicameral bone cyst and nonossifying fibroma, can heal spontaneously, so the major clinical challenge is deciding whether a given lesion increases the risk that the involved bone will fracture. Reliable methods for assessing the fracture risk associated with a benign bone lesion would be clinically useful in the selection of the most appropriate treatment for a patient.
There are no proven clinical or radiographic guidelines for predicting which children with a benign skeletal lesion are at risk for pathologic fracture. Previous investigators have performed retrospective studies in an attempt to predict the risk of pathologic fracture on the basis of the patient's age, the stage and activity of the lesion, the anatomic site of the lesion, the size of the lesion, and/or the percentage of cortical destruction22-26. However, they failed to identify any single radiographic parameter that accurately predicted the occurrence of a pathologic fracture through any benign bone lesion.
Previously, one of us (B.D.S.) and colleagues demonstrated ex vivo that the force required to fracture a bone with a simulated lytic lesion is proportional to the structural rigidity of the weakest cross section through the bone by calculating the structural rigidities of serial transaxial cross sections through the bone at the site of the simulated lesion27,28. Also, we recently extended our structural mechanics analysis to the in vivo prediction of fracture risk in children and young adults with a variety of benign skeletal neoplasms29. In that diagnostic study, it was demonstrated that the reduction in the load capacity of a bone measured with quantitative computed tomography was more accurate (correct in 97% of cases) for predicting pathologic fracture in children with a benign skeletal lesion than were radiographic guidelines based on the lesion size or the percentage of bone loss (correct in 42% to 61% of cases).
Using the biomechanical guidelines derived from our previous study29, we conducted the present prospective clinical study to evaluate the specificity of quantitative computed tomography-based structural rigidity analysis for predicting the risk of pathologic fracture over time in a cohort in children with a benign skeletal lesion. We hypothesized that the specificity of our quantitative computed tomography method for predicting fracture risk would be greater than that of radiographic methods currently in use.
Materials and Methods
Study Cohort
After approval was obtained from the committee of clinical investigations at our institution, fifty-eight children and young adults were identified as candidates for our study through a review of the medical records of patients with a benign bone lesion who had undergone quantitative computed tomography-based biomechanical analysis of their fracture risk between 2002 and 2007 at our institution. The inclusion criteria were (1) a benign osteolytic lesion involving the appendicular skeleton, (2) no increased fracture risk predicted by quantitative computed tomography-based rigidity analysis and/or criteria based on plain radiographs, and (3) more than two years of follow-up if the patient had not been surgically treated to prevent a fracture. Patients were excluded from the study if they had a malignant tumor. The patients who met the inclusion criteria and had not been surgically treated were asked to complete an activity questionnaire (see Appendix) that evaluated fracture occurrence and the level of activity as rated on the University of California at Los Angeles (UCLA) physical activity scale30. Patients who had had surgical treatment of the lesion (aspiration and injection, curettage, and/or allograft bone packing) were excluded from calculations of the specificity of the imaging but were asked for qualitative data by means of a treatment questionnaire regarding the reasons they chose treatment (see Appendix).
Study Questionnaire
Potential participants were mailed an introductory letter (see Appendix). Along with the letter, the patients who had received surgical treatment were mailed the treatment questionnaire and those who had not received surgical treatment were mailed the activity questionnaire. Two weeks after the mailing, patients who had not yet returned the completed activity or treatment questionnaire were called to determine if they would consent to participate in the study. At that time, any patients who consented were asked the questions on the activity or treatment questionnaire. Informed consent was obtained from all patients (or from their parents if they were less than eighteen years old) who responded to our questionnaires. When a child was too young to do so, the parents responded to the questionnaires.
Fracture Risk Prediction
The risk of a pathologic fracture is determined both by the strength of the bone and by the loads applied to it31. A fracture is likely when the applied load on the bone during a specific activity exceeds the load-bearing capacity of the bone. The load-bearing demands on the bone depend on the patient's size, weight, activity level, and loading regimen. The loads applied to the bone may be dramatically reduced if a patient's activities are limited by pain due to the skeletal lesion. The load-bearing capacity depends on the structural properties of the bone, which are determined by the material properties of the bone tissue, the anatomic site of the lesion, the geometry of both the lesion and the host bone, and the aggressiveness of the lesion. The increased fragility associated with these radiolucent lesions suggests that the strength of the bone tissue surrounding the lesion is degraded and/or the stresses generated within the bone during loading are increased because of changes in bone geometry. Any method for predicting fracture risk must be able to measure both changes in the material properties of bone tissue and changes in bone geometry induced by the neoplasm. Structural rigidity quantifies the resistance of a bone to axial, bending, or twisting forces; it is the product of a material property (i.e., modulus of elasticity, E, or shear modulus, G) and a cross-sectional geometric property (i.e., area, A; moment of inertia, I; or polar moment of inertia, J) that quantifies how the bone tissue is arranged in space relative to a bending or twisting axis32. For a bone containing an osteolytic lesion, the axial (EA), bending (EI), and torsional (GJ) rigidity integrates the size, shape, and location of the lesion and the material and geometric properties of the host bone for specific cross sections through the bone. The least rigid section through the bone governs the mechanical behavior of the entire bone.
The bone-tissue modulus is a function of the bone-mineral density33,34. The bone geometry can be represented by the cross-sectional area and the moment of inertia35. The moment of inertia quantifies how the bone tissue is distributed in space around its central axis; it varies directly with the fourth power of the distance of the bone tissue relative to a specific bending axis so that the resistance of the bone to bending dramatically increases as bone tissue is distributed away from that bending axis. Therefore, the cortical expansion induced by a bone cyst may partly compensate for the mechanical effect of the lesion itself. Structural mechanics analysis and concepts from composite beam theory can be used to predict the load-bearing capacity of a bone with a lytic lesion since bone fails at a constant strain independent of bone density36,37.
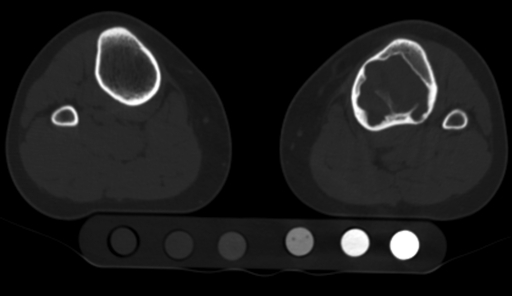
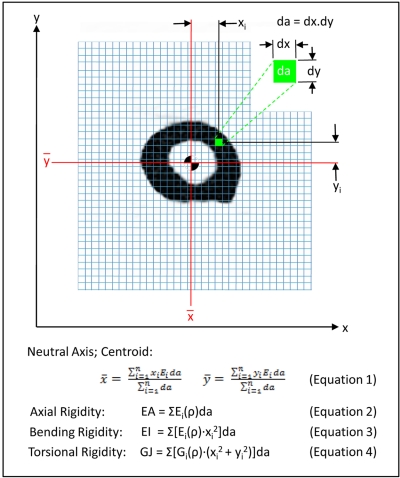
Both structural analysis with quantitative computed tomography and radiographic analysis were performed as previously described27-29,38, and they are further detailed in the Appendix. Briefly, the defect and the adjacent bone in the affected limb and homologous regions in the contralateral, normal limb of each patient were scanned with a high-speed helical quantitative computed tomography scanner (GE Highlight Advantage; GE Medical Systems, Waukesha, Wisconsin) with use of sequential, transaxial, cross-sectional slices (3.0 mm thick and spaced 3.0 mm apart) from 2.5 cm proximal to 2.5 cm distal to the margins of the defect. The typical field of view resulted in an in-plane resolution of approximately 0.5 to 0.7 mm/pixel. The modulus of elasticity for each pixel in the image was calculated from the apparent density with use of empirically derived constitutive relationships for cancellous and cortical bone33,34. Although bone is composed of mineral, organic matrix, and water, computed tomography primarily reflects the attenuation signature of the mineral phase. Therefore, the density measured with quantitative computed tomography approximates the density of the mineral phase, or the ash density (ρash, in grams per cubic centimeter). Six solid hydroxyapatite phantoms (Computerized Imaging Reference Systems, Norfolk, Virginia) of known mineral densities (0.003, 0.078, 0.178, 0.538, 1.048, and 1.597 g/cm3) were placed in the same image field of view (Fig. 1). Linear regression between the attenuation coefficient and the corresponding ash densities for the phantom chambers was performed for each examination. The regression equations were used to convert the gray level of the quantitative computed tomography image (in Hounsfield units) into an equivalent bone density for each voxel element forming the image39. For each transaxial computed tomography image, the axial rigidity (EA), bending rigidity (EI), and torsional rigidity (GJ) were calculated by summing the modulus-weighted area of each pixel of the bone section by its position relative to the centroid of the bone (Fig. 2). The ratio of the structural rigidities of the affected bone relative to the normal, contralateral bone was determined at homologous cross sections to express the relative reduction in the load-carrying capacity of the affected bone. To account for a “worst-case” scenario, the minimum principal moments of inertia were used when these ratios were calculated. Pathologic fracture was predicted if the ratio for EA, EI, or GJ was 65% or less29.
Fig. 1.
Lower-extremity computed tomography, with a hydroxyapatite phantom, showed a nonossifying fibroma with destruction of 61% of the cortex. The left tibia was predicted to be at risk for fracture with standard radiographic methods but not with quantitative computed tomography-based rigidity analysis. This patient was not treated surgically and had not sustained a fracture over a six-year period.
Fig. 2.
Axial (EA), bending (EI), and torsional (GJ) rigidity were calculated, with use of the indicated algorithms, from transaxial computed tomography images of the affected and contralateral, unaffected bone. The ratio of the rigidity of the affected bone normalized by that of the contralateral limb at a homologous cross section was calculated. The bone was predicted to be at risk of fracture if EA, EI, or GJ was ≤65% of that on the contralateral side. da = pixel size.
According to the criteria based on the plain radiographs, a skeletal lesion was considered to be at increased risk of fracture if the defect length was ≥3.3 cm, the defect width was ≥2.5 cm, or there was involvement of ≥50% of the cortex3,22,23,26,35,40-47 as measured on anteroposterior or lateral views.
Statistical Analysis
Specificity values were calculated with use of the standard formula to determine the diagnostic performances of radiographic and quantitative computed tomography parameters, and 95% confidence intervals were calculated according to the efficient-score method, corrected for continuity48. These values are reported in the form of proportions with the confidence interval.
Source of Funding
This study was funded by National Institutes of Health Grant R01 CA 40211-13 and the Whitaker Foundation; funding was used for the development of the quantitative computed tomography structural analysis model of fracture risk prediction. The Children's Orthopedic Surgery Foundation supported the research fellowship for Natalie L. Leong.
Results
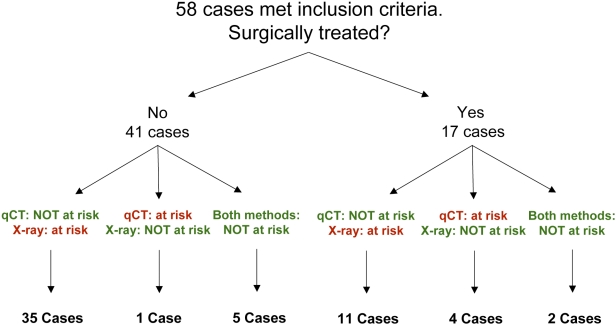
Fifty-eight patients met the inclusion criteria for this study. Thirty-eight were male and twenty were female, and their ages ranged from six to nineteen years (mean and standard deviation, 12.7 ± 3.1 years) at the time that the quantitative computed tomography and plain radiography analyses were performed to predict fracture risk. The lesions included nonossifying fibromas (thirty-six), unicameral bone cysts (eleven), fibrous dysplasia (five), chondromas (three), neurofibromatosis (one), interosseous ganglion (one), and aneurysmal bone cyst (one). The lesions were located in the femur (twenty-nine), tibia (twenty-six), fibula (two), and humerus (one). Forty-one patients received no treatment for the bone lesion, and these patients were followed for two to six years (mean, 3.9 ± 1.5 years). Seventeen patients were treated surgically with (1) intralesional injection of corticosteroids with or without bone matrix and with or without bone marrow aspirate or (2) curettage and packing with allograft bone. After providing informed consent, forty-nine (84%) of the patients (thirty-four who had not been treated and fifteen who had been treated; see Appendix) completed the activity or treatment questionnaire. Of the patients who had not been treated, six could not be contacted and one refused to participate in the study. Also, two patients who had been treated did not return their treatment questionnaire. There were no significant differences in age, sex, lesion site, or lesion type between those who completed the activity or treatment questionnaire and those who did not.
Of the forty-one patients who received no treatment, thirty-six had a disagreement between the fracture risk prediction based on the plain radiographs and that based on the quantitative computed tomography rigidity analysis: thirty-five patients were predicted to be at risk for a fracture on the basis of the plain radiographs but not on the basis of the quantitative computed tomography rigidity analysis, and the converse was true for one patient. Five patients who did not receive surgical treatment had had concordant predictions that there was no increased fracture risk (Fig. 3).
Fig. 3.
Fracture prediction and treatment status of the patient cohort. Seventeen patients were treated surgically while forty-one patients were not. Fifteen of the seventeen patients who were treated surgically responded to our treatment questionnaire regarding the reasons why they elected to have surgery. Of the forty-one patients who underwent surgery, we obtained follow-up information from thirty-four (83%), six could not be contacted, and one refused to participate in the study. qCT = quantitative computed tomography-based structural rigidity analysis, and X-ray = plain radiography.
The specificity of the prediction methods in our study was determined in the cohort of thirty-four patients who had not been treated surgically and who had completed the activity questionnaire. In this group, no pathologic fractures occurred through any osteolytic lesion. Most patients reported high levels of physical activity that were unchanged from their activity level prior to the diagnosis of the lesion; twenty patients reported no change, eight were less active, and six were more active than before the diagnosis. The mean UCLA activity score (and standard deviation) of those who completed the activity questionnaire was 8.9 ± 1.7 out of a maximum of 10 points. Twenty-six of these patients reported that they currently were participating in impact sports.
Overall, the specificity of the quantitative computed tomography-based rigidity analysis was 97% (95% confidence interval = 83% to 100%), with the method correctly predicting that a bone containing an osteolytic lesion would not fracture when the patient engaged in activities of daily living. In comparison, the criteria based on the plain radiographs had a specificity of only 12% (95% confidence interval = 4% to 28%) for the prediction that a bone containing an osteolytic lesion would not fracture. This difference is significant (α = 0.05).
The osteolytic defect was treated surgically in seventeen patients irrespective of the fracture risk predictions. Using a t test, we determined that there was no significant difference between the treated and untreated groups in terms of the percentage of the affected cross-sectional area. The fracture risk predictions were discordant for fifteen of these patients. For eleven of them, the quantitative computed tomography-based rigidity analysis predicted no increased fracture risk whereas the criteria based on the plain radiographs predicted an increased fracture risk; the converse was true for four patients. For two patients, neither method predicted that the affected bone was at increased fracture risk.
Of the seventeen surgically treated patients, fifteen completed our questionnaire about the decision-making process involved in electing to have surgery even though the lesion was determined to be not associated with a risk of fracture with quantitative computed tomography or plain radiography, or both. Of the fifteen patients who responded to our questionnaire, fourteen reported that they made their decision because they thought that the surgeon favored the procedure, eleven were worried that the affected bone might break, ten did not want to have restrictions on physical activity, ten were worried that the tumor might get worse, eight were experiencing pain caused by the tumor, and two did not want to have regular follow-up visits to monitor the lesion.
Discussion
Current radiographic guidelines for predicting fracture risk in children with a benign skeletal lesion are neither sensitive nor specific29. In our previous study29, we applied receiver operating characteristic analysis to a cohort of patients with a benign skeletal lesion retrospectively to establish thresholds for the axial (EA), bending (EI), and torsional (GJ) rigidity calculated from transaxial computed tomography images through the affected bone and the contralateral, normal bone. A 35% reduction in structural rigidity discriminated fracture from non-fracture cases with 100% sensitivity and 94% specificity29. In the present study, these thresholds were applied prospectively to a cohort of children with a benign skeletal lesion. We were unable to study the natural history of these benign osteolytic lesions because of ethical considerations; most surgeons felt obligated to treat a bone lesion if the quantitative computed tomography-based rigidity analysis predicted that the affected bone was at increased risk for fracture. Therefore, we could not evaluate the sensitivity of the quantitative computed tomography-based fracture-risk predictions. However, we could prospectively evaluate the specificity of these predictions as compared with that of criteria predicated on the size of the lesion as measured on plain radiographs. Of forty-one patients in whom the affected bone was predicted to be at high risk for fracture on the basis of the lesion size but not predicted to be at risk for fracture on the basis of the reduction in the rigidity of the affected bone, thirty avoided a surgical intervention that would most likely have been unnecessary. None of the affected bones that had been predicted not to fracture fractured during our 130 person-years of follow-up. One patient in whom the bone was predicted to be at increased risk for fracture on the basis of the quantitative computed tomography-based rigidity analysis, but who was not treated, did not sustain a fracture (lowering the specificity to 97%).
In addition to the quantitative computed tomography-based fracture-risk prediction, there were many considerations that factored into the decision regarding whether to treat a benign skeletal lesion surgically, such as the presence of pain and the patient's concerns about leaving the lesion untreated or restricting physical activity. Quantitative computed tomography-based rigidity analysis should not be used as an absolute guide for the decision whether to treat a benign bone lesion, but rather it should be utilized as an objective tool that can help a patient and a physician to come to the best decision, which also depends on the patient's lifestyle and individual preferences.
A limitation of our computed tomography-based technique is that it exposes the patient to relatively large doses of radiation, so that repeated measurements are less desirable in children. We are currently developing a quantitative magnetic resonance imaging-based method of analysis49 that eliminates exposure to radiation. Furthermore, newer multidetector helical computed tomography scanners expose patients to much lower doses of radiation. Other limitations of our computed tomography-based analysis are that computed tomography scanners are not as readily available as standard radiographic units, the application of our algorithm requires sophisticated knowledge of image-analysis software to properly align virtual images of right and left bone pairs, and the interpretation of the results requires a background in structural mechanics. Some of these issues can be addressed by disseminating our software to interested parties or by allowing clinicians to send computed tomography images by means of the Internet, over secure lines, to be analyzed by our research group.
In summary, this prospective clinical study indicates that computed tomography-based structural analysis of homologous regions of affected and normal bones provides a highly specific method for predicting fracture risk in children with a variety of benign neoplasms involving the skeleton. This analysis is much more specific than the often-cited criterion of a lesion involving ≥50% of the cortex as seen on plain radiographs. That criterion is associated with large errors in the estimation of the load-bearing capacity of the affected bone, since fracture risk indices based on lesion size alone fail to account for the compensatory remodeling of the host bone that occurs in response to the presence of the lesion in a growing child. Computed tomography-based structural analysis enables the orthopaedic surgeon to quantify the mechanical properties of the bone at a particular site and to determine if there is a sufficient reduction in the load-carrying capacity of the bone to merit surgical intervention.
Appendix
The activity questionnaire, the treatment questionnaire, the introductory letter sent to the patients, the details of the biomechanical analysis, and a table listing the clinical features of all patients in the study are available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Supplementary Material
Acknowledgments
Note: The authors thank Sara Swaim and Patricia Connell for their assistance and contributions to this study.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Whitaker Foundation and the National Institutes of Health (R01 CA 40211-13) and less than $10,000 from the Children's Orthopedic Surgery Foundation. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Aboulafia AJ, Kennon RE, Jelinek JS. Begnign bone tumors of childhood. J Am Acad Orthop Surg. 1999;7:377-88 [DOI] [PubMed] [Google Scholar]
- 2.Dormans JP, Flynn JM. Pathologic fractures associated with tumors and unique conditions of the musculoskeletal system. : Beaty JH, Kasser JR, Rockwood and Wilkins’ fractures in children. Vol 3 5th ed Philadelphia: Lippincott Williams and Wilkins; 2001. p 139-240 [Google Scholar]
- 3.Adler CP. Solid aneurysmal bone cyst with pathologic bone fracture. Skeletal Radiol. 1995;24:214-6 [DOI] [PubMed] [Google Scholar]
- 4.Gitelis S, Wilkins R, Conrad EU., 2nd Benign bone tumors. Instr Course Lect. 1996;45:425-46 [PubMed] [Google Scholar]
- 5.Bitzan P, Windhager R, Lang S, Richling B, Kotz R. [Incidence of recurrence of aneurysmal bone cysts following surgical treatment and adjuvant therapy with phenol]. Z Orthop Ihre Grenzgeb. 1995;133:422-8 German [PubMed] [Google Scholar]
- 6.Bunting R, Lamont-Havers W, Schweon D, Kliman A. Pathologic fracture risk in rehabilitation of patients with bony metastases. Clin Orthop Relat Res. 1985;192:222-7 [PubMed] [Google Scholar]
- 7.Burgers AM, Taminiau AH. Simple bone cysts treated by multiple drill-holes. Acta Orthop Scand. 1997;68:86. [DOI] [PubMed] [Google Scholar]
- 8.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26:615-25 [DOI] [PubMed] [Google Scholar]
- 9.Caffey J. On fibrous defects in cortical walls of growing tubular bones: their radiologic appearance, structure, prevalence, natural course, and diagnostic significance. Adv Pediatr. 1955;7:13-51 [PubMed] [Google Scholar]
- 10.Campanacci M, Capanna R. Unicameral bone cysts. Round table discussion. Treatment comparison of steroid injection versus surgery. : Uhthoff HK, editor Current concepts of diagnosis and treatment of bone and soft tissue tumors. New York: Springer; 1984. p 321-7 [Google Scholar]
- 11.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25-36 [PubMed] [Google Scholar]
- 12.Capanna R, Dal Monte A, Gitelis S, Campanacci M. The natural history of unicameral bone cyst after steroid injection. Clin Orthop Relat Res. 1982;166:204-11 [PubMed] [Google Scholar]
- 13.Delloye C, De Nayer P, Malghem J, Noel H. Induced healing of aneurysmal bone cysts by demineralized bone particles. A report of two cases. Arch Orthop Trauma Surg. 1996;115:141-5 [DOI] [PubMed] [Google Scholar]
- 14.Drennan DB, Maylahn DJ, Fahey JJ. Fractures through large non-ossifying fibromas. Clin Orthop Relat Res. 1974;103:82-8 [DOI] [PubMed] [Google Scholar]
- 15.Easley ME, Kneisl JS. Pathologic fractures through nonossifying fibromas: is prophylactic treatment warranted? J Pediatr Orthop. 1997;17:808-13 [PubMed] [Google Scholar]
- 16.Fidler M. Prophylactic internal fixation of secondary neoplastic deposits in long bones. Br Med J. 1973;1:341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia Filho RJ, Dos Santos JB, Korukian M, Laredo Filho J. Conservative treatment of solitary bone cysts—a study of 55 patients. Rev Paul Med. 1992;110:131-7 [PubMed] [Google Scholar]
- 18.Freeby JA, Reinus WR, Wilson AJ. Quantitative analysis of the plain radiographic appearance of aneurysmal bone cysts. Invest Radiol. 1995;30:433-9 [DOI] [PubMed] [Google Scholar]
- 19.Galasko CS. Letter: the fate of simple bone cysts which fracture. Clin Orthop Relat Res. 1974;101:302-4 [PubMed] [Google Scholar]
- 20.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671-2 [DOI] [PubMed] [Google Scholar]
- 21.Goel AR, Kriger J, Bronfman R, Lauf E. Unicameral bone cysts: treatment with methylprednisone acetate injections. J Foot Ankle Surg. 1994;33:6-15 [PubMed] [Google Scholar]
- 22.Arata MA, Peterson HA, Dahlin DC. Pathological fractures through non-ossifying fibromas. Review of the Mayo Clinic experience. J Bone Joint Surg Am. 1981;63:980-8 [PubMed] [Google Scholar]
- 23.Ahn JI, Park JS. Pathological fractures secondary to unicameral bone cysts. Int Orthop. 1994;18:20-2 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Takagi K, Kitagawa T, Harada M. Microdensity of solitary bone cyst after steroid injection. J Pediatr Orthop. 1988;8:566-8 [DOI] [PubMed] [Google Scholar]
- 25.Neer CS, Francis KC, Johnston AD, Kiernan HA., Jr Current concepts on the treatment of solitary unicameral bone cyst. Clin Orthop Relat Res. 1973;97:40-51 [DOI] [PubMed] [Google Scholar]
- 26.Kaelin AJ, MacEwen GD. Unicameral bone cysts. Natural history and the risk of fracture. Int Orthop. 1989;13:275-82 [DOI] [PubMed] [Google Scholar]
- 27.Whealan KM, Kwak SD, Tedrow JR, Inoue K, Snyder BD. Noninvasive imaging predicts failure load of the spine with simulated osteolytic defects. J Bone Joint Surg Am. 2000;82:1240-51 [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Cabe GD, Tedrow JR, Hipp JA, Snyder BD. Failure of trabecular bone with simulated lytic defects can be predicted non-invasively by structural analysis. J Orthop Res. 2004;22:479-86 [DOI] [PubMed] [Google Scholar]
- 29.Snyder BD, Hauser-Kara DA, Hipp JA, Zurakowski D, Hecht AC, Gebhardt MC. Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg Am. 2006;88:55-70 [DOI] [PubMed] [Google Scholar]
- 30.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228-41 [PubMed] [Google Scholar]
- 31.Hayes WC. Biomechanics of cortical and trabecular bone: implications for assessment of fracture risk. : Mow VC, Hayes WC, Basic orthopaedic biomechanics. New York: Raven Press; 1991. p93-142 [Google Scholar]
- 32.Lai WM, Rubin D, Krempl E. Introduction to continuum mechanics. 3rd ed Boston: Butterworth-Heinemann; 1993 [Google Scholar]
- 33.Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21:155-68 [DOI] [PubMed] [Google Scholar]
- 34.Snyder SM, Schneider E. Estimation of mechanical properties of cortical bone by computed tomography. J Orthop Res. 1991;9:422-31 [DOI] [PubMed] [Google Scholar]
- 35.Thompson RC., Jr Impending fracture associated with bone destruction. Orthopedics. 1992;15:547-50 [DOI] [PubMed] [Google Scholar]
- 36.Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. J Biomech. 1998;31:601-8 [DOI] [PubMed] [Google Scholar]
- 37.Turner CH. Yield behavior of bovine cancellous bone. J Biomech Eng. 1989;111:256-60 Erratum in: J Biomech Eng. 1989;111:335 [DOI] [PubMed] [Google Scholar]
- 38.Snyder BD, Cordio MA, Nazarian A, Kwak SD, Chang DJ, Entezari V, Zurakowski D, Parker LM. Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res. 2009;15:7676-83 [DOI] [PubMed] [Google Scholar]
- 39.Goodsitt MM, Rosenthal DI. Quantitative computed tomography scanning for measurement of bone and bone marrow fat content. A comparison of single- and dual-energy techniques using a solid synthetic phantom. Invest Radiol. 1987;22:799-810 [DOI] [PubMed] [Google Scholar]
- 40.Hipp JA, Springfield DS, Hayes WC. Predicting pathologic fracture risk in the management of metastatic bone defects. Clin Orthop Relat Res. 1995;312:120-35 [PubMed] [Google Scholar]
- 41.Beals RK, Lawton GD, Snell WE. Prophylactic internal fixation of the femur in metastatic breast cancer. Cancer. 1971;28:1350-4 [DOI] [PubMed] [Google Scholar]
- 42.Fidler M. Incidence of fracture through metastases in long bones. Acta Orthop Scand. 1981;52:623-7 [DOI] [PubMed] [Google Scholar]
- 43.Parrish FF, Murray JA. Surgical treatment for secondary neoplastic fractures. A retrospective study of ninety-six patients. J Bone Joint Surg Am. 1970;52:665-86 [PubMed] [Google Scholar]
- 44.Wilkins RM, Sim FH, Springfield DS. Metastatic disease of the femur. Orthopedics. 1992;15:621-30 [DOI] [PubMed] [Google Scholar]
- 45.Keene JS, Sellinger DS, McBeath AA, Engber WD. Metastatic breast cancer in the femur. A search for the lesion at risk of fracture. Clin Orthop Relat Res. 1986;203:282-8 [PubMed] [Google Scholar]
- 46.Zickel RE, Mouradian WH. Intramedullary fixation of pathological fractures and lesions of the subtrochanteric region of the femur. J Bone Joint Surg Am. 1976;58:1061-6 [PubMed] [Google Scholar]
- 47.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-64 [PubMed] [Google Scholar]
- 48.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857-72 [DOI] [PubMed] [Google Scholar]
- 49.Hong J, Hipp JA, Mulkern RV, Jaramillo D, Snyder BD. Magnetic resonance imaging measurements of bone density and cross-sectional geometry. Calcif Tissue Int. 2000;66:74-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.