Abstract
Intranasal exposure of mice to satratoxin G (SG), a macrocyclic trichothecene produced by the indoor air mold Stachybotrys chartarum, selectively induces apoptosis in olfactory sensory neurons (OSNs) of the nose and brain. The purpose of this study was to measure the kinetics of distribution and clearance of SG in the mouse. Following intranasal instillation of female C57B16 mice with SG (500 μg/kg bw), the toxin was detectable from 5 to 60 min in blood and plasma, with the highest concentrations, 30 and 19 ng/ml, respectively, being observed at 5 min. SG clearance from plasma was rapid and followed single-compartment kinetics (t1/2 = 20 min) and differed markedly from that of other tissues. SG concentrations were maximal at 15–30 min in nasal turbinates (480 ng/g), kidney (280 ng/g), lung (250 ng/g), spleen (200 ng/g), liver (140 ng/g), thymus (90 ng/g), heart (70 ng/g), olfactory bulb (14 ng/g), and brain (3 ng/g). The half-lives of SG in the nasal turbinate and thymus were 7.6 and 10.1 h, respectively, whereas in other organs, these ranged from 2.3 to 4.4 h. SG was detectable in feces and urine, but cumulative excretion over 5 days via these routes accounted for less than 0.3% of the total dose administered. Taken together, SG was rapidly taken up from the nose, distributed to tissues involved in respiratory, immune, and neuronal function, and subsequently cleared. However, a significant amount of the toxin was retained in the nasal turbinate, which might contribute to SG’s capacity to evoke OSN death.
Keywords: trichothecene, mycotoxin, Stachybotrys, ELISA, toxicokinetics
Stachybotrys chartarum, a saprophytic black mold that grows on cellulose-based construction materials such as wallboard, ceiling tiles, and wood, has been suggested to be a contributing factor in damp building-related illnesses (Pestka et al., 2008b). Although in vitro and in vivo research suggests that it would be biologically plausible for this fungus to adversely affect humans, establishing a definitive association between S. chartarum and building-related illnesses requires improved understanding of tissue targets, mechanisms, dose-response effects, and the true extent of human exposures (Institute of Medicine, 2004). It is particularly critical to understand how trichothecene mycotoxins elaborated by Stachybotrys might elicit adverse neurologic, respiratory, and immunologic effects in vivo and how such effects might contribute to illnesses reported in individuals residing or working in water-damaged buildings.
Trichothecenes are toxic sesquiterpenoid fungal metabolites that have a common 9, 10 double-bond and a 12, 13 epoxide group but exhibit extensive variation relative to ring oxygenation patterns (Bamburg, 1983). These low–molecular weight (∼200–500 D) mycotoxins interact with the eukaryotic ribosomes (Bamburg, 1983). This interaction results in suppression of polypeptide chain initiation or elongation as well as activation of intracellular stress responses that drive both proinflammatory gene expression and apoptosis (Pestka et al., 2004). Based on in vitro studies in lymphocytes and monocytes, the most toxic of these compounds are the macrocyclic trichothecenes (e.g., satratoxins and roridins) (Pestka and Forsell, 1988; Yang et al., 2000), which possess a cyclic diester or triester ring at C4 and C15 as compared with the less toxic type A and type B groups which contain simpler acyl substituents (Grove, 1988, 1993, 2000). About one third of the S. chartarum isolates from the United States and Europe are members of a chemotype that produces macrocyclic trichothecenes, most notably the satratoxins and roridins (Andersen et al., 2002).
Macrocyclic trichothecenes are detectable in the outer plasmalemma surface and the inner wall layers of Stachybotrys conidiospores (Gregory et al., 2004). Dry spores are readily aerosolized and have a respirable mean aerodynamic diameter of approximately 5 μm (Sorenson et al., 1987; Yike and Dearborn, 2004). In addition, nonviable, fine (≤ 1 μm in diameter) airborne particulates containing satratoxins can be released from cultures (Brasel et al., 2005). It is therefore feasible that spores, fragmented mycelia, and decomposed cellulosic substrates facilitate trichothecene dissemination, delivery, and release to the respiratory tract of individuals in Stachybotrys-contaminated buildings. Trichothecenes have been detected in air samples taken during indoor air quality investigations, suggesting that these compounds can indeed be aerosolized (Bloom et al., 2009; Brasel et al., 2005; Vesper et al., 2000).
A single intranasal instillation of mice with the macrocyclic trichothecenes satratoxin G (SG), isosatratoxin F, or roridin A causes extensive apoptosis with olfactory sensory neurons (OSNs) of the olfactory epithelium and olfactory bulb after 24 h (Islam et al., 2006, 2007). Marked induction of proinflammatory and proapoptotic gene expression occurs in the nasal turbinates prior to the onset of OSN apoptosis. Recent studies in the PC-12 neuronal cell model indicate that SG at concentrations as low as 10 ng/ml induces apoptosis within 48 h (Islam et al., 2008). Prior to and during apoptosis in these cells, SG markedly upregulates expression of the proapoptotic genes that is consistent with observations made in the mouse model (Islam et al., 2006, 2007).
Both prolonged exposure of nasal turbinates to SG and the well-known propensity of OSNs to undergo apoptosis (Cowan and Roskams, 2002) might be contributing factors to selective OSN death. However, critical questions remain regarding absorption, distribution, metabolism, and excretion of SG and other macrocyclic trichothecenes following intranasal exposure. Although conventional chemical analyses have been used to monitor trichothecene absorption and tissue distribution (Barel et al., 1990; Prelusky et al., 1985, 1990), these approaches are relatively insensitive, require extensive sample preparation, and necessitate large amounts of tissue. Radiolabels have also been employed to investigate the toxicokinetics and tissue distribution of type A (eg., T-2 toxin) and type B (e.g., deoxynivalenol) trichothecenes in several animal species (Azcona-Olivera et al., 1995; Barel et al., 1990; Lake et al., 1987; Lun et al., 1989; Meky et al., 2003; Prelusky et al., 1986). Major constraints to using this approach for macrocyclic trichothecenes include: (1) unavailability of radiolabeled macrocylic trichothecenes from commercial sources, (2) difficulties in preparing such compounds with high specific activity, and (3) safety issues associated with handling isotopes in animal facilities and disposal of these materials.
Immunochemical assay offers an alternative analytical approach for mycotoxin measurement that is rapid and extremely sensitive but does not necessitate complicated cleanup, evaporation or concentration steps, or large sample amounts (Pestka, 1988). Such assays are now routinely employed for the analysis of mycotoxins in complex food matrices (Schneider et al., 2004). We have recently reported on the use of an ELISA to measure the type B trichothecene deoxynivalenol in mouse tissue (Pestka et al., 2008a). The results indicated that ELISA was comparable with the radioisotope approach for monitoring deoxynivalenol toxicokinetics, with both methods showing that the toxin distributes rapidly throughout the body and is removed during rapid clearance and slower terminal elimination phases. This method was subsequently employed to compare the kinetics and distribution of deoxynivalenol between young and adult mice (Pestka and Amuzie, 2008) and between mice exposed to it orally and intranasally (Amuzie et al., 2008).
Our laboratory has previously generated highly specific SG antisera and a competitive ELISA (Chung et al., 2003) that is amenable to measuring this trichothecene in animal cells, tissues, and excreta. This ELISA has been used successfully to monitor SG association with the ribosome in macrophages and neuronal cells exposed to the toxin in vitro (Bae et al., 2009). The purpose of the present study was to use this ELISA to characterize the kinetics of SG distribution and clearance in mouse tissues and excreta following a single intranasal exposure to the toxin. The results suggested not only that SG can be readily taken up from the nose and distributed throughout the body but that an appreciable amount of the toxin is retained in the nasal turbinate from where it is slowly cleared.
MATERIALS AND METHODS
Chemicals.
All chemicals were of reagent-grade quality or better and purchased from Sigma Chemical Co. (St Louis, MO) unless otherwise described. SG was purified from S. chartarum and identity confirmed by electrospray ionization/collision-induced dissociation tandem mass spectroscopy as previously described (Islam et al., 2009). Concentrated toxin solutions were handled in a fume hood. Toxin-contaminated labware was detoxified by soaking for > 1 h in 100 ml/l sodium hypochlorite (Thompson and Wannemacher, 1986).
Animals.
Female C57BL/6J mice (7–8 weeks) were obtained from Charles River (Portage, MI). Animal handling was conducted in accordance with recommendations established by the National Institutes of Health and were approved by the Michigan State University Institutional Animal Care and Use Committee. Mice were kept in transparent polypropylene cages with stainless steel wire tops and filter covers and acclimated for 1 week prior to onset of different experimental treatments. Environmental conditions included 23°C–25°C, relative humidity of 40–55%, and a 12-h light-dark cycle. Mice were provided food and water ad libitum.
Experimental design.
Mice (n = 35) were anesthetized with 4% halothane and 96% oxygen and then instilled intranasally with SG (500 μg/kg bw) and delivered in 30 μl of pyrogen-free saline (Abbott Laboratories, IL) per mouse. This intranasal SG dose was previously demonstrated to induce robust OSN apoptosis as well as rhinitis and encephalitis in the mouse (Islam et al., 2006). Control mice (n = 5) were instilled with the saline vehicle alone. At various time intervals after SG instillation, groups of mice (n = 5) were deeply anesthetized with an ip injection of 0.1 ml 12% (wt/vol) sodium pentobarbital, and their blood was collected from the caudal vena cava. Following blood collection, mice were euthanized and liver, kidney, spleen, heart, thymus, lung, nasal turbinates, olfactory bulb, and brain excised. Plasma was isolated from blood and stored along with isolated organs at −80°C. For elimination studies, groups of mice (n = 4) were individually housed in Nalgene metabolism cages (MTB-0311; Nunc-Nalgene, Rochester, NY), following intranasal exposure to SG. The design of these cages enabled separate collection of urine and feces that were obtained at 24-h intervals for SG analysis.
Sample preparation and SG ELISA.
A modification of the protocol devised for deoxynivalenol (Pestka et al., 2008a) was employed for preparation of tissue and excreta samples. Briefly, plasma was diluted 1:5 (vol/vol) in 0.05M PBS (pH 7.2), whereas organs were homogenized in PBS (1:5–1:25 [wt/vol]). Urine samples were diluted 1:10 (vol/vol) in PBS. Fecal samples were suspended in PBS at 1:10 (wt/vol) at room temperature for 1 h and then homogenized. Diluted plasma, urine, feces, and organ homogenates were centrifuged at 15,000 × g for 10 min at 4°C. The supernatant fractions were heated to 100°C for 5 min to inactivate endogenous enzymes and precipitate proteins and then centrifuged at 15,000 × g for 10 min at 4°C. This protocol eliminated components from the extract that interfere with the competitive ELISA.
SG was measured in supernatants by a modification of the previously described competitive direct ELISA (Chung et al., 2003). Briefly, SG antiserum (0.1 μg/ml) in PBS was incubated in microtiter plates (100 μl/well) overnight at 4°C. Plates were washed, blocked with 1% (wt/vol) bovine serum album (BSA) in PBS at 25°C for 1 h, and contents aspirated. Equal volumes (50 μl) of SG standard (0–500 ng/ml) and SG-horseradish peroxidase conjugate (50 ng/ml) in 1% (wt/vol) BSA in PBS were mixed and incubated in microtiter plates at 25°C for 1 h. Plates were washed and bound peroxidase determined by incubation with 100 μl/well of K-Blue substrate containing 3,3',5,5'-tetramethylbenzidine (Neogen, Lansing, MI) at 25°C for 0.5 h. The enzyme reaction was stopped by adding 2N sulfuric acid (100 μl/well), and plate was read at 450 nm on an ELISA plate reader (Molecular Devices, Menlo Park, CA). SG concentrations in samples were determined from the standard curve using Softmax software (Molecular Devices). Because SG antibody might potentially cross-react with putative SG metabolites, results were reported as SG equivalents.
The efficacy of the aforementioned sample preparation protocols and SG ELISA was assessed in a spiking study. Tissue homogenates (plasma, spleen, and liver) and diluted feces from unexposed mice were spiked with SG at concentrations equivalent to 25, 100, and 250 ng/g. Following heat processing, SG concentrations were determined by ELISA and recoveries determined (Table 1). Mean recoveries for plasma, spleen, liver, and feces were 91, 102, 67, and 91%, respectively, suggesting that this approach could be used to monitor SG in tissue and excreta following intranasal exposure.
TABLE 1.
SG recoveries from spiked tissue and feces
| SG added | SG recovery |
|||
| Tissue | (ng/g) | (ng/g)a | (%) | Mean recovery (%) |
| Plasma | 25 | 18.1 ± 2.8 | 72.4 | |
| Plasma | 100 | 98.8 ± 7.8 | 98.8 | 91.3 |
| Plasma | 250 | 256.4 ± 5.3 | 102.7 | |
| Spleen | 25 | 22.6 ± 2.8 | 90.4 | |
| Spleen | 100 | 117.0 ± 1.67 | 117.0 | 102.4 |
| Spleen | 250 | 245.8 ± 11.0 | 99.9 | |
| Liver | 25 | 10.3 ± 5.3 | 41.2 | |
| Liver | 100 | 84.7 ± 12.5 | 84.7 | 67.2 |
| Liver | 250 | 189.3 ± 9.3 | 75.7 | |
| Feces | 25 | 21.9 ± 3.8 | 87.6 | |
| Feces | 100 | 87.2 ± 5.3 | 87.2 | 90.9 |
| Feces | 250 | 244.9 ± 14.8 | 98.0 | |
Results reported as mean ± SEM.
Toxicokinetic analysis.
A one-compartment open model (Shargel et al., 2004) was employed to calculate toxicokinetic parameters. SG concentrations in plasma and tissue were fitted to an exponential expression to calculate clearance rates (Li et al., 1997). Because peak SG concentrations in brain and olfactory bulb were very close to the limit of detection and highly variable, toxicokinetic analyses were not conducted in these tissues.
Statistics.
SG concentrations were reported as the mean ± SEM for each group. Data were analyzed using SigmaStat for windows v 3.1, the criterion for significance set at p < 0.05. Differences between two groups were determined by the Student’s t-test, whereas differences between more than two groups were determined by ANOVA with Student-Newman-Keuls post hoc test using SigmaStat v 3.1 (Jandel Scientific, San Rafael, CA).
RESULTS
SG Toxicokinetics in Circulatory Compartment
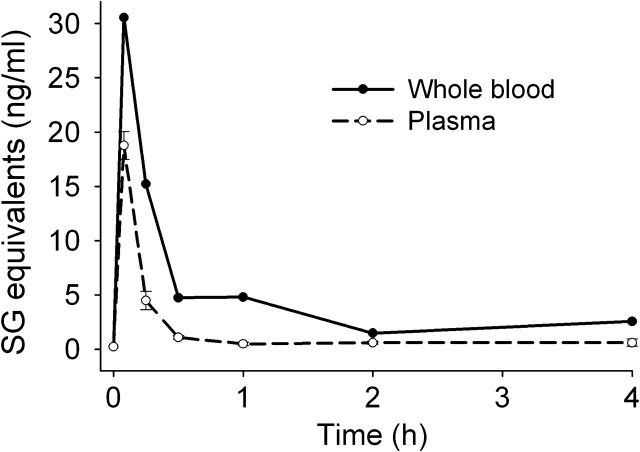
Following a single intranasal exposure to SG (500 μg/kg bw), its toxicokinetics in whole blood and plasma were assessed. The highest SG concentrations in blood (30 ng/ml) and plasma (19 ng/ml) were detected within the first 5 min of dosing, which was the earliest time point measured (Fig. 1). SG concentrations were consistently higher in blood than plasma at all time points. Blood and plasma SG declined rapidly and returned to nearly basal levels at 0.5 and 2 h, respectively. Clearance of SG from plasma followed single-compartment kinetics (t1/2 = 20 min).
FIG. 1.
Kinetics of SG uptake and clearance in blood and plasma. Female C57BL/6J mice (7–8 weeks) were treated with 500 μg/kg bw of SG by intranasal instillation. Blood and plasma were collected at intervals, from 0 h (naive mice) up to 4-h postinstillation. SG concentrations measured in blood and plasma by competitive direct ELISA. Data are mean ± SEM (n =5).
SG Distribution in Thoracic Organs
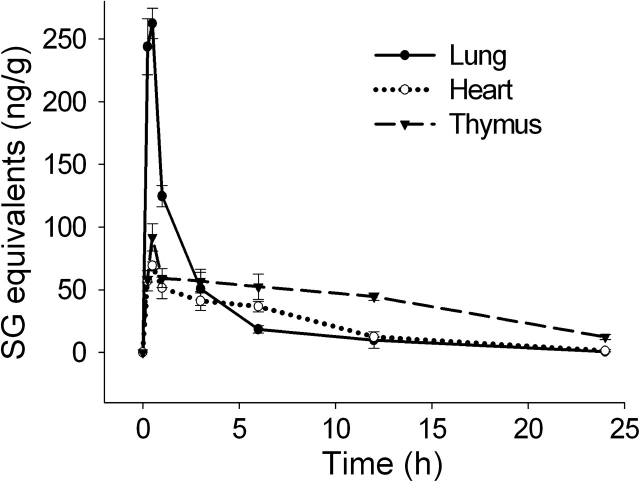
SG content in three thoracic organs, lung, heart, and thymus, were measured to determine extent of toxin distribution to respiratory, cardiovascular, and immune tissue, respectively. Consistent with intranasal instillation, there was rapid uptake of SG in the lung, reaching peak concentrations of approximately 250 ng/g within 0.25–0.5 h (Fig. 2). Maximal SG concentrations of 70 and 90 ng/g were reached within 0.5 h in the heart and thymus, respectively, which were much lower than those in the lung. SG clearances in the lung (t1/2 = 2.8 h) and heart (t1/2 = 4.4 h) were faster than in the thymus (t1/2 = 10.1 h) with the toxin still being detectable in the latter organ after 24 h.
FIG. 2.
Kinetics of SG uptake and clearance in thoracic organs. Mice were treated with SG as described in Figure 1 legend and tissues collected at intervals over 24 h. Lung, heart, and thymus were analyzed for SG by ELISA. Data are mean ± SEM (n = 5).
SG Distribution in Abdominal Organs
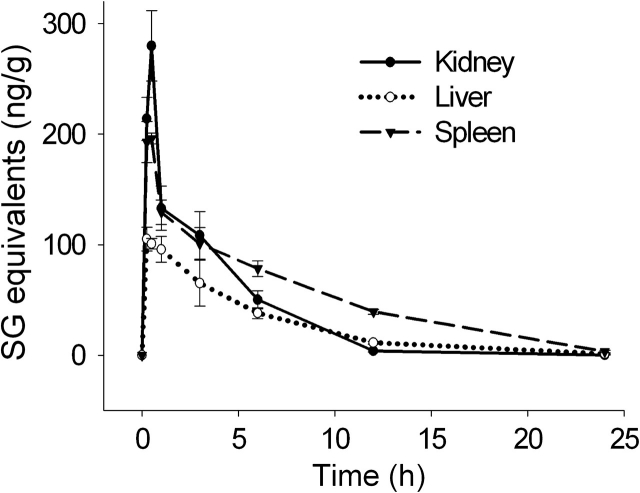
SG concentrations were measured in three abdominal organs, kidney, liver, and spleen, which represent excretory, metabolic, and immune tissue, respectively. Again, maximal SG concentrations were reached in all three organs within 0.5 h (Fig. 3). The kidney exhibited the highest peak concentration, 280 ng/g, whereas the spleen and liver reached 200 and 110 ng/g, respectively. The rank order for clearance rates was kidney (t1/2 = 2.3 h) < liver (t1/2 = 3.7 h) < spleen (t1/2 = 4.3 h).
FIG. 3.
Kinetics of SG uptake and clearance in abdominal organs. Mice were treated with SG as described in Figure 1 legend and tissues collected at intervals over 24 h. Kidney, liver, and spleen were analyzed for SG by ELISA. Data are mean ± SEM (n = 5).
SG Distribution in Nose and Brain
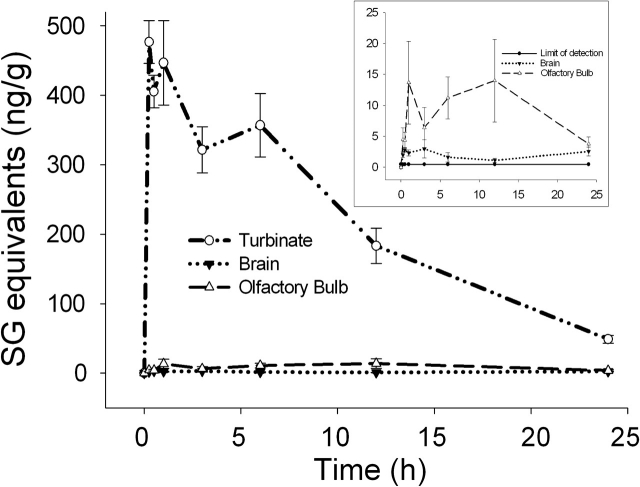
Because SG induces OSN death as well as rhinitis and encephalitis in mice following intranasal instillation (Islam et al., 2006), the toxin’s presence in nasal turbinates, olfactory bulb, and brain was also monitored. Nasal turbinates were found to contain the highest SG levels of all organs analyzed in this study, reaching concentrations of 410–480 ng/g within the first 15–60 min. SG was cleared extremely slowly from nasal turbinates (t1/2 = 7.6 h) as compared with all other tissues (excluding thymus) with 48 ng/g still being detectable after 24 h exposure (Fig. 4). This high SG concentration is consistent with the atrophy of olfactory epithelium, which has been reported for SG-exposed mice.
FIG. 4.
Kinetics and uptake of SG in nasal turbinate and brain. Mice were treated with SG as described in Figure 1 legend and tissues collected at intervals over 24 h. Nasal turbinates, olfactory bulb, and brain were analyzed for SG by ELISA. Data are mean ± SEM (n = 5). SG concentrations are shown for all three organs, whereas inset shows olfactory bulb and brain relative to the assay limit of detection (solid line).
SG was also detectable in the olfactory bulb and brain at concentrations much lower than those in the turbinates or other organs, but still exceeding the assay limit of detection (0.4 ng/g) (Fig. 4, inset). Detection of SG in olfactory bulb and brain suggests that the toxin might indeed reach the brain compartment following intranasal exposure, albeit at much lower concentrations (2–10%) of that observed in other organs.
SG Clearance via Feces and Urine
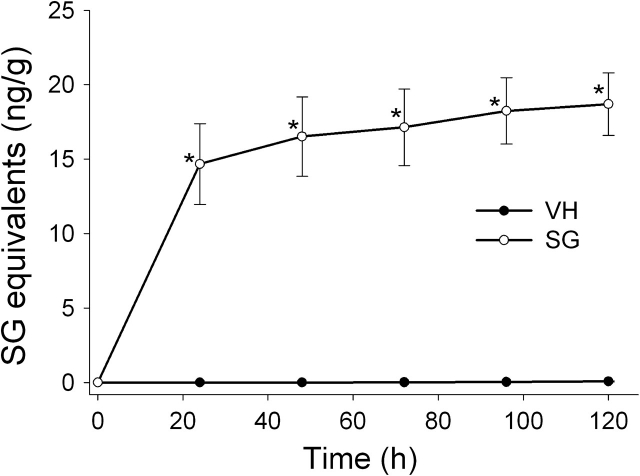
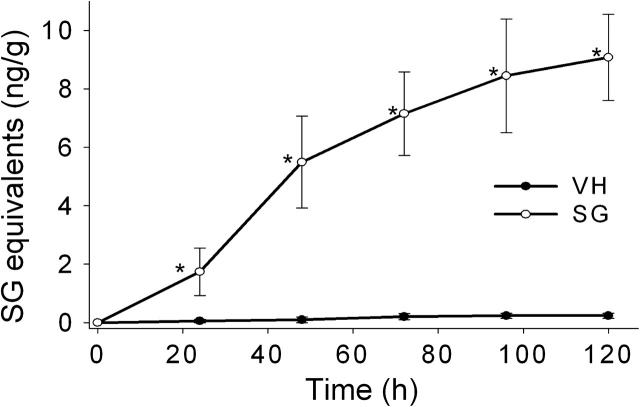
Cumulative excretion of SG in feces and urine was monitored over 5 days. Approximately 80% of total SG fecal excretion occurred within the first 24 h of exposure, with little SG being excreted thereafter (Fig. 5). Approximately 9, 61, 79, and 93% of total urinary SG excreted was detectable at 1, 2, 3, and 4 days, respectively (Fig. 6). Total fecal and urinary excretion of SG (29 ng) accounted for only a small fraction (0.3%) of the total exposure dose (≈10 μg for a 20 g mouse).
FIG. 5.
Cumulative excretion of SG in feces. Mice were treated with SG as described in Figure 1 legend and housed individually in metabolic cages for 5 days. Feces was collected at 24 h intervals, diluted, homogenized, and supernatants assayed for SG by competitive direct ELISA. Data are mean ± SEM (n = 4). Asterisks indicate significantly higher than non-SG–exposed mice at corresponding time (p < 0.05).
FIG. 6.
Cumulative excretion of SG in urine. Mice were treated with SG as described in Figure 5 legend. Urine was collected in metabolic cages at 24 h intervals, diluted, centrifuged, and supernatants assayed for SG by ELISA. Data are mean ± SEM (n = 4). Asterisks indicate significantly higher than non-SG–exposed mice at corresponding time (p < 0.05).
DISCUSSION
Stachybotrys and its macrocyclic trichothecenes have been suggested to contribute etiologically to adverse neurologic, immune, and respiratory effects in individuals exposed to these agents in water-damaged buildings. Substantial deposition (> 50%) of either very large (> 5 μm in particle diameter; like a fungal spore) and very small particles (< 10 nm in diameter; nanoparticles) occurs in the nasal airways of humans and laboratory animals following nasal inhalation (Cheng et al., 1997a,b, 1990; Yeh and Schum, 1980). Modeling studies confirm that large S. chartarum fragments (≥ 5 μm) would deposit in the mouse nose (Cho et al., 2005). Accordingly, the nasal airway is likely to be an important exposure site and target for Stachybotry and its toxins.
The results presented here are the first to report on tissue absorption, distribution, and clearance of a macrocyclic trichothecene delivered via the nasal tract in an experimental animal. The low SG levels observed in blood and plasma as well as its rapid clearance from plasma in the mouse likely result from efficient redistribution from the circulatory compartment to various organs. These data further indicate that (1) SG is distributed to many tissues within 30 min of intranasal instillation, (2) an organ rank order for SG peak concentrations being nasal turbinates > kidney > lung > spleen > thymus > heart > olfactory bulb > brain, (3) SG had an exceptionally long half-life in nasal turbinates as compared with other tissues, and (4) immunochemically detectable toxin was excreted via both the fecal and urinary tract excretory routes.
The rapid clearance of SG from blood of intranasally instilled mice observed here was consistent with an earlier study in which rat pups were intratracheally instilled with S. chartarum spores containing an equivalent 100 μg of SG/kg bw (Yike and Dearborn, 2004). In that study, it was observed that approximately 5% of the dose was recoverable in bronchiolar lavage fluid. Toxin recovery in this fluid decreased to 0.34% after 30 min and declined rapidly thereafter. Although 0.2% of SG was recoverable in ethanol extracts of whole blood of those pups immediately after exposure, the toxin was undetectable after 15 min. These findings thus established that SG can be rapidly released from S. chartarum spores into tissues of exposed animals. Although a different species and airway administration route was used, these data are consistent with our observations that SG is detectable in blood for only a relatively short time.
Because the nose is an inhaled air entry portal, it is a key target for toxicity by airborne contaminants with the surface epithelial lining being subject to injury (Harkema, 1991). The high SG concentrations and relatively slow clearance from the nasal compartment observed here are particularly critical findings because acute intranasal exposure of mice to SG specifically induces OSN apoptosis with atrophy of the olfactory epithelium (Islam et al., 2006). Time-course studies using a single instillation of mice with 500 μg/kg bw SG, the same dose employed here, indicate that maximum atrophy of the olfactory epithelium occurs at 3 days post-instillation (PI). Exposure to lower doses (100 μg/kg bw) for five consecutive days resulted in similar apoptosis and atrophy, suggesting that, over the short term, these effects are cumulative.
SG-induced OSN apoptosis is dose dependent with the no-effect and lowest-effect doses at 24-h postinstillation being 5 and 25 μg/kg bw SG, respectively (Islam et al., 2006) with the latter dose being equivalent to 2.5 × 107 S. chartarum spores/kg bw (Pestka et al., 2008b). Furthermore, elevated expression of the proapoptotic genes Fas, FasL, p75NGFR, p53, Bax, caspase-3, and caspase-activated DNase in the ethmoid turbinate olfactory epithelium corresponds to OSN apoptosis. SG also induces an acute, neutrophilic rhinitis within 24 h PI that corresponds to elevated proinflammatory cytokine and chemokine mRNA expression in the nasal turbinates. Accordingly, both prolonged exposure of nasal turbinates to SG and the propensity of OSNs to undergo apoptosis might be contributing factors to selective OSN death.
In addition to OSN apoptosis in olfactory epithelium, marked atrophy of the olfactory neuronal and glomerular layers of the olfactory bulb are also detectable along with mild neutrophilic encephalitis in mice exposed to SG (Islam et al., 2006). The detection of SG in the olfactory bulb and the brain observed here, albeit at very low concentrations, is thus another critical observation. The capacity of macrocyclic trichothecenes to selectively target neurons in the nose and olfactory bulb of the brain of mice is particularly intriguing because olfactory function loss often occurs with early stages of neurodegenerative illnesses such as Parkinson’s and Alzheimer’s diseases (Demarquay et al., 2007; Hawkes, 2003; Takeda et al., 2007).
Macrocyclic trichothecenes are immunosuppressive and can interfere with both macrophage and lymphocyte function (Pestka et al., 2004). It was therefore notable that SG clearance from thymus was relatively slow compared with other organs. The predilection of SG for this immune organ might reflect its ongoing rapid proliferation and this high ribosome content. Macrocyclic and other trichothecenes are well known to be toxic to thymocytes in vitro and in vivo (Islam et al., 1998; Miura et al., 1998; Poapolathep et al., 2004; Sorenson et al., 1987; Sugita-Konishi et al., 1994). It will be important to determine whether the relatively slow clearance of SG from the thymus contributes to interference with innate or acquired immune function.
A final critical observation here was that, using previously described physiologically based kinetic parameters (Brown et al., 1997), total SG body burden was estimated to be 1.4 μg/mouse, accounting for only 14% of the administered dose (10 μg/mouse). Furthermore, only a very small fraction of total SG dose (0.3%) was detectable in the total feces and urine collected over 5 days. It might be speculated that SG was metabolized by phase I and/or phase II enzymes yielding products that did not cross-react in the ELISA. SG concentrations in liver were much lower than other organs that might indeed reflect such metabolic conversions. In support of this contention, the type A trichothecene T-2 toxin undergoes hydrolysis at the C4 and C15 positions in the liver to yield T-2 triol, a conversion carried out in part by hepatic carboxylesterase (Wu et al., 2010). Analogous hydrolysis of SG would yield the less toxic metabolite verrucarol (Jarvis et al., 1984), which is essentially undetectable in the ELISA employed here (Chung et al., 2003). Spiking studies reported here consistently indicated SG recovery from liver homogenates were lower than spleen, plasma, and feces (Table 1), an effect that could result from SG hydrolysis. Intriguingly, the non-olfactory nasal mucosa of rodents expresses carboxylesterase (Nikula et al., 1995; Olson et al., 1993), suggesting that the nose could also be a major site of SG metabolism. Further investigation of the metabolism and detoxification of SG by the liver, nasal mucosa, and other tissues is therefore warranted.
Taken together, the sample preparation method and ELISA described herein offer a simplified strategy that can be used to determine how factors such as dose, species, life stage, gender, genetic background, and route/duration of exposure might impact SG uptake and clearance in experimental animals. The data presented here indicate that, following intranasal instillation in the mouse, SG is rapidly distributed to numerous tissues including those involved in neurologic, respiratory, and immune function, all of which are putatively associated with damp-building–related illnesses. While the high initial SG concentrations in the nasal compartment are explainable because of the exposure route employed, the reasons for slow clearance from this very sensitive site are not intuitively obvious and require further study perhaps using isotopically labeled compounds.
FUNDING
Michigan State University Respiratory Research Initiative; the National Institute for Environmental Health Sciences (Michigan State University Foundation Strategic Partnership Grant and Public Health Service Grant ES03358 to J.J.P.).
Acknowledgments
We thank Jack Harkema, Lori Bramble, Evelyne Mbandi, Sarah Godbehere, and Mary Rosner. Animal studies were conducted in accordance with National Institutes of Health guidelines as overseen by the All University Committee on Animal Use and Care at Michigan State University.
References
- Amuzie CJ, Harkema JR, Pestka JJ. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: comparison of nasal vs. oral exposure. Toxicology. 2008;248:39–44. doi: 10.1016/j.tox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Andersen B, Nielsen KF, Jarvis BB. Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia. 2002;94:392–403. [PubMed] [Google Scholar]
- Azcona-Olivera JI, Ouyang Y, Murtha J, Chu FS, Pestka JJ. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995;133:109–120. doi: 10.1006/taap.1995.1132. [DOI] [PubMed] [Google Scholar]
- Bae HK, Shinozuka J, Islam Z, Pestka JJ. Satratoxin G interaction with 40S and 60S ribosomal subunits precedes apoptosis in the macrophage. Toxicol. Appl. Pharmacol. 2009;237:137–145. doi: 10.1016/j.taap.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR. Biological and biochemical actions of trichothecene mycotoxins. Prog. Mol. Subcell. Biol. 1983;8:41–110. [Google Scholar]
- Barel S, Yagen B, Bialer M. Pharmacokinetics of the trichothecene mycotoxin verrucarol in dogs. J. Pharm. Sci. 1990;79:548–551. doi: 10.1002/jps.2600790619. [DOI] [PubMed] [Google Scholar]
- Bloom E, Nyman E, Must A, Pehrson C, Larsson L. Molds and mycotoxins in indoor environments–a survey in water-damaged buildings. J. Occup. Environ. Hyg. 2009;6:671–678. doi: 10.1080/15459620903252053. [DOI] [PubMed] [Google Scholar]
- Brasel TL, Douglas DR, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl. Environ. Microbiol. 2005;71:114–122. doi: 10.1128/AEM.71.1.114-122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Cheng YS, Yeh HC, Swift DL. An experimental method for measuring aerosol deposition efficiency in the human oral airway. Am. Ind. Hyg. Assoc. J. 1997a;58:207–213. doi: 10.1080/15428119791012856. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Cheng YS, Yeh HC, Swift DL. Measurements of airway dimensions and calculation of mass transfer characteristics of the human oral passage. J. Biomech. Eng. 1997b;119:476–482. doi: 10.1115/1.2798296. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Hansen GK, Su YF, Yeh HC, Morgan KT. Deposition of ultrafine aerosols in rat nasal molds. Toxicol. Appl. Pharmacol. 1990;106:222–233. doi: 10.1016/0041-008x(90)90242-m. [DOI] [PubMed] [Google Scholar]
- Cho S-H, Seo S-C, Schmechel D, Grinshpun SS, Reponen T. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos. Environ. 2005;39:5454–5465. [Google Scholar]
- Chung YJ, Jarvis BB, Tak H, Pestka JJ. Immunochemical assay for satratoxin G and other macrocyclic trichothecenes associated with indoor air contamination by Stachybotrys chartarum. Toxicol. Mech. Methods. 2003;13:247–252. doi: 10.1080/713857196. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Roskams AJ. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc. Res. Tech. 2002;58:204–215. doi: 10.1002/jemt.10150. [DOI] [PubMed] [Google Scholar]
- Demarquay G, Ryviln P, Royet JP. Olfaction and neurological diseases: a review of the literature. Rev. Neurol. 2007;163:155–167. doi: 10.1016/s0035-3787(07)90387-2. [DOI] [PubMed] [Google Scholar]
- Gregory L, Pestka JJ, Dearborn DG, Rand TG. Localization of satratoxin-G in Stachybotrys chartarum spores and spore-impacted mouse lung using immunocytochemistry. Toxicol. Pathol. 2004;32:26–34. doi: 10.1080/01926230490260790. [DOI] [PubMed] [Google Scholar]
- Grove JF. Non-macrocyclic trichothecenes. Nat. Prod. Rep. 1988;5:187–209. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- Grove JF. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993;10:429–448. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- Grove JF. Non-macrocyclic trichothecenes. Part 2. Prog. Chem. Org. Nat. Prod. 2000;69:1–70. [Google Scholar]
- Harkema JR. Comparative aspects of nasal airway anatomy: relevance to inhalation toxicology. Toxicol. Pathol. 1991;19 doi: 10.1177/0192623391019004-102. (Pt 1), 321–336. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov. Disord. 2003;18:364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Damp Indoor Spaces and Health. Washington, DC: National Academies Press; 2004. pp. 1–355. [PubMed] [Google Scholar]
- Islam Z, Amuzie CJ, Harkema JR, Pestka JJ. Neurotoxicity and inflammation in the nasal airways of mice exposed to the macrocyclic trichothecene mycotoxin roridin A: kinetics and potentiation by bacterial lipopolysaccharide co-exposure. Toxicol. Sci. 2007;98:526–541. doi: 10.1093/toxsci/kfm102. [DOI] [PubMed] [Google Scholar]
- Islam Z, Harkema JR, Pestka JJ. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006;114:1099–1107. doi: 10.1289/ehp.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Hegg CC, Bae HK, Pestka JJ. Satratoxin G-induced apoptosis in PC-12 neuronal cells is mediated by PKR and caspase independent. Toxicol. Sci. 2008;105:142–152. doi: 10.1093/toxsci/kfn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Nagase M, Yoshizawa T, Yamauchi K, Sakato N. T-2 toxin induces thymic apoptosis in vivo in mice. Toxicol. Appl. Pharmacol. 1998;148:205–214. doi: 10.1006/taap.1997.8338. [DOI] [PubMed] [Google Scholar]
- Islam Z, Shinozuka J, Harkema JR, Pestka JJ. Purification and comparative neurotoxicity of the trichothecenes satratioxin G and roridin L2 from Stachybotrys chartarum. J. Toxicol. Environ. Health A. 2009;72:1242–1251. doi: 10.1080/15287390903129234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BB, Yatawara CS, Greene SL, Vrudhula VM. Production of Verrucarol. Appl. Environ. Microbiol. 1984;48:673–674. doi: 10.1128/aem.48.3.673-674.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake BG, Phillips JC, Walters DG, Bayley DL, Cook MW, Thomas LV, Gilbert J, Startin JR, Baldwin NC, Bycroft BW. Studies on the metabolism of deoxynivalenol in the rat. Food Chem. Toxicol. 1987;25:589–592. doi: 10.1016/0278-6915(87)90019-6. [DOI] [PubMed] [Google Scholar]
- Li S, Marquardt RR, Frohlich AA, Vitti TG, Crow G. Pharmacokinetics of ochratoxin A and its metabolites in rats. Toxicol. Appl. Pharmacol. 1997;145:82–90. doi: 10.1006/taap.1997.8155. [DOI] [PubMed] [Google Scholar]
- Lun AK, Moran ET, Jr, Young LG, McMillan EG. Absorption and elimination of an oral dose of 3H-deoxynivalenol in colostomized and intact chickens. Bull. Environ. Contam. Toxicol. 1989;42:919–925. doi: 10.1007/BF01701636. [DOI] [PubMed] [Google Scholar]
- Meky FA, Turner PC, Ashcroft AE, Miller JD, Qiao YL, Roth MJ, Wild CP. Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem. Toxicol. 2003;41:265–273. doi: 10.1016/s0278-6915(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Miura K, Nakajima Y, Yamanaka N, Terao K, Shibato T, Ishino S. Induction of apoptosis with fusarenon-X in mouse thymocytes. Toxicology. 1998;127:195–206. doi: 10.1016/s0300-483x(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Nikula KJ, Novak RF, Chang IY, Dahl AR, Kracko DA, Zangar RC, Kim SG, Lewis JL. Induction of nasal carboxylesterase in F344 rats following inhalation exposure to pyridine. Drug Metab. Dispos. 1995;23:529–535. [PubMed] [Google Scholar]
- Olson MJ, Martin JL, LaRosa AC, Brady AN, Pohl LR. Immunohistochemical localization of carboxylesterase in the nasal mucosa of rats. J. Histochem. Cytochem. 1993;41:307–311. doi: 10.1177/41.2.8419465. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Enhanced surveillance of foodborne mycotoxins by immunochemical assay. J Assoc. Off. Anal. Chem. 1988;71:1075–1081. [PubMed] [Google Scholar]
- Pestka JJ, Amuzie CJ. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food Chem. Toxicol. 2008;46:2826–2831. doi: 10.1016/j.fct.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Forsell JH. Inhibition of human lymphocyte transformation by the macrocyclic trichothecenes roridin A and verrucarin A. Toxicol. Lett. 1988;41:215–222. doi: 10.1016/0378-4274(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Islam Z, Amuzie CJ. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 2008a;178:83–87. doi: 10.1016/j.toxlet.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol. Sci. 2008b;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR, Moon Y, Chung YJ. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Poapolathep A, Kumagai S, Suzuki H, Doi K. Development of early apoptosis and changes in T-cell subsets in mouse thymocyte primary cultures treated with nivalenol. Exp. Mol. Pathol. 2004;77:149–152. doi: 10.1016/j.yexmp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hamilton RM, Trenholm HL, Miller JD. Tissue distribution and excretion of radioactivity following administration of 14C-labeled deoxynivalenol to White Leghorn hens. Fundam. Appl. Toxicol. 1986;7:635–645. doi: 10.1016/0272-0590(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hartin KE, Trenholm HL. Distribution of deoxynivalenol in cerebral spinal fluid following administration to swine and sheep. J. Environ. Sci. Health B. 1990;25:395–413. doi: 10.1080/03601239009372697. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Veira DM, Trenholm HL. Plasma pharmacokinetics of the mycotoxin deoxynivalenol following oral and intravenous administration to sheep. J. Environ. Sci. Health B. 1985;20:603–624. doi: 10.1080/03601238509372499. [DOI] [PubMed] [Google Scholar]
- Schneider E, Curtui V, Seidler C, Dietrich R, Usleber E, Martlbauer E. Rapid methods for deoxynivalenol and other trichothecenes. Toxicol. Lett. 2004;153:113–121. doi: 10.1016/j.toxlet.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Shargel L, Wu-pong S, Yu ABC. Applied Biopharmaceutics and Pharmacokinetics. New York: McGraw-Hill Medical; 2004. [Google Scholar]
- Sorenson WG, Frazer DG, Jarvis BB, Simpson J, Robinson VA. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 1987;53:1370–1375. doi: 10.1128/aem.53.6.1370-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita-Konishi Y, Kumagai S, Mizuochi T. The cytotoxicity of macrocyclic trichothecenes, roridin A and verrucarin A, on murine T-cells is reduced by Ia-negative splenic adherent cells. Toxicon. 1994;32:1051–1057. doi: 10.1016/0041-0101(94)90389-1. [DOI] [PubMed] [Google Scholar]
- Takeda A, Kikuchi A, Matsuzaki-Kobayashi M, Sugeno N, Itoyama Y. Olfactory dysfunction in Parkinson's disease. J. Neurol. 2007;254:2–7. [Google Scholar]
- Thompson WL, Wannemacher RW., Jr Structure-function relationships of 12,13-epoxytrichothecene mycotoxins in cell culture: comparison to whole animal lethality. Toxicon. 1986;24:985–994. doi: 10.1016/0041-0101(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Vesper S, Dearborn DG, Yike I, Allan T, Sobolewski J, Hinkley SF, Jarvis BB, Haugland RA. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: quantitative assessment before, during, and after remediation. J. Urban Health. 2000;77:68–85. doi: 10.1007/BF02350963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Dohnal V, Huang L, Kuca K, Yuan Z. Metabolic pathways of trichothecenes. Drug Metab. Rev. 2010;42:250–267. doi: 10.1080/03602530903125807. [DOI] [PubMed] [Google Scholar]
- Yang GH, Jarvis BB, Chung YJ, Pestka JJ. Apoptosis induction by the satratoxins and other trichothecene mycotoxins: relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Schum GM. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 1980;42:461–480. doi: 10.1007/BF02460796. [DOI] [PubMed] [Google Scholar]
- Yike I, Dearborn DG. Pulmonary effects of Stachybotrys chartarum in animal studies. Adv. Appl. Microbiol. 2004;55:241–273. doi: 10.1016/S0065-2164(04)55009-8. [DOI] [PubMed] [Google Scholar]