Abstract
The association between peripheral arterial disease (PAD) and outcomes has not been studied in a propensity-matched population of community-dwelling older adults. We analyzed the public-use copy of the Cardiovascular Health Study (CHS) datasets to test the hypothesis that baseline PAD is associated with increased all-cause mortality and cardiovascular morbidity. Of the 5795 CHS participants, 5630 had data on baseline ankle-brachial index (ABI) and 767 had PAD defined as ABI <0.9. Propensity scores for PAD were calculated for each participant using 66 baseline covariates and were used to match 679 pairs of participants with and without PAD. Matched Cox regression models were used to estimate associations of PAD with outcomes during a median follow up of 7.5 years. Overall, 55% of matched participants died from all causes during 9958 person-years of follow-up. All-cause mortality occurred in 61% (rate, 8710/100,000 person-years) and 55% (rate, 6503/100,000 person-years of follow up) of participants respectively with and without PAD (matched hazard ratio {HR} when PAD was compared with no-PAD, 1.47; 95% confidence interval {CI}, 1.23–1.76; P<0.0001). Pre-match unadjusted, multivariable-adjusted and propensity-adjusted HR (95% CI) for PAD-associated all-cause mortality were 2.90 (2.61–3.21; P<0.0001), 1.53 (1.36–1.71; P<0.0001) and 1.57 (1.39–1.78; P<0.0001) respectively. Matched HR and 95% CI for PAD for incident heart failure and symptomatic PAD were respectively 1.32 (1.00–1.73; P=0.052) and 3.92 (3.92–7.21; P<0.0001). In conclusion, in a propensity-matched well-balanced population of community-dwelling older adults, baseline PAD was associated with increased all-cause mortality and cardiovascular morbidity.
Keywords: Peripheral artery disease, mortality, propensity score
Peripheral arterial disease (PAD) is a manifestation of systemic atherosclerosis and has been shown to be associated with poor outcomes.1–5 However, most of these studies were based on a small number of select high risk patients and used traditional regression-based risk-adjustments. We used a public-use copy of the Cardiovascular Health Study (CHS) data obtained from the National Heart, Lung and Blood Institute (NHLBI) to test the hypothesis that baseline PAD is associated with increased all-cause mortality and cardiovascular morbidity in a propensity-matched population of community-dwelling older adults.
METHODS
CHS is an NHLBI-funded ongoing, community-based, epidemiologic study of 5888 participants, ≥65 years, from four counties in North Carolina, California, Maryland, and Pittsburgh, Pennsylvania. The details of the rationale, design and implementation of the CHS have been previously reported.6 Briefly, a recruitment of 5201 participants (1989–1990) was supplemented by a second cohort of 687 African-Americans (1992–1993). The objective of the CHS was to study cardiovascular morbidity and mortality in older adults. Of the 5888 original CHS participants, 5795 consented to be included in the de-identified public-use copy of the dataset, and of these 5630 with valid data on ankle-brachial index (ABI) were included in the current analysis.
Baseline PAD, defined by an ABI of <0.9, was present in 767 participants. Baseline seated blood pressure was measured with a random-zero sphygmomanometer.7 Data on socio-demographic, clinical, sub-clinical, and laboratory variables were collected at baseline and have been previously described in details.6,8 Missing values for continuous variables were imputed based on values predicted by age, sex and race. The primary outcome for the current analysis is all-cause mortality. Secondary outcomes included various cardiovascular morbidities. The detail of the process of adjudication of cardiovascular events in CHS is well-documented in the literature.9,10
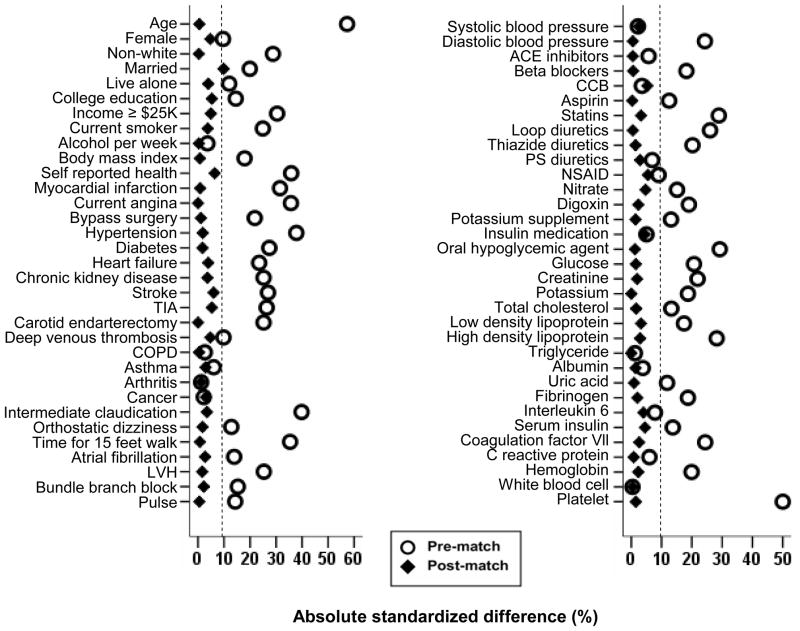
Propensity scores for baseline PAD were estimated for each of the 5630 participants using a non-parsimonious multivariable logistic regression model using 66 baseline covariates displayed in Figure 1.11,12 We matched 679 pairs of participants with and without PAD using a greedy matching protocol described elsewhere.13,14 Absolute standardized differences for all 66 covariates were estimated to assess pre-match imbalances and post-match balances achieved and results were presented as Love plot.13 An absolute standardized differences of 0% indicates no bias, and values <10% suggest inconsequential bias.13,15 Matched Cox proportional hazard analyses were used to determine the association of PAD with outcomes during 7.5 years of median follow up. Homogeneity of the association of PAD and all-cause mortality was assessed by subgroup analyses. All statistical tests were two-sided, and tests with p-value <0.05 were considered significant. SPSS for Windows (Version 15) was used for all data analysis.16
Figure 1.
Love plot for absolute standardized differences for 66 covariates before and after propensity score matching between participants with and without peripheral arterial disease. ACE= angiotensin-converting enzyme; CCB=calcium channel blocker; COPD=chronic obstructive pulmonary disease; LVH= left ventricular hypertrophy; NSAID=non-steroidal anti inflammatory drug; PS=potassium- sparing; TIA=transient ischemic attack
RESULTS
Overall, matched participants had a mean (±SD) age of 76 (±6) years, 54% were women, and 25% were non-whites. Imbalances in baseline characteristics before matching and balances achieved after matching between patients with and without PAD are displayed in Table 1 and Figure 1. After matching, absolute standardized differences for all measured covariates were <10% (most were <5%), suggesting substantial significant covariate balance across the groups (Figure 1). Of the 679 participants with PAD, 666 had mild to moderate PAD (ABI 0.40–0.89) and only 13 had severe PAD (ABI <0.40).
Table 1.
Baseline characteristics, by peripheral artery disease (PAD)*, before and after propensity score matching
| n (%) or mean (±SD) | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| No PAD (n = 4863) | PAD (n = 767) | p value | No PAD (n = 679) | PAD (n = 679) | p value | |
| Age, years | 73 (±5) | 76 (±6) | <0.0001 | 76 (±6) | 76 (±6) | 0.924 |
| Female | 2,807 (58%) | 406 (53%) | 0.013 | 372 (55%) | 356 (52%) | 0.414 |
| Non-white | 700 (14%) | 198 (26%) | <0.0001 | 172 (25%) | 173 (26%) | 0.950 |
| Married | 3,298 (68%) | 447 (58%) | <0.0001 | 365 (54%) | 398 (59%) | 0.080 |
| Living alone | 583 (12%) | 124 (16%) | 0.002 | 119 (18%) | 109 (16%) | 0.469 |
| College or higher education | 2,132 (44%) | 282 (37%) | <0.0001 | 228 (34%) | 245 (36%) | 0.362 |
| Income ≥ $25 thousand | 1,850 (38%) | 185 (24%) | <0.0001 | 153 (23%) | 167 (25%) | 0.372 |
| Current smoker | 525 (11%) | 151 (20%) | <0.0001 | 135 (20%) | 125 (18%) | 0.491 |
| Alcohol, drinks per week | 2.5 ± 6.3 | 2.3 ± 6.8 | 0.345 | 2.2 ± 6.4 | 2.2 ± 6.8 | 0.963 |
| Body mass index, kg/m2 | 27 (±4) | 26 (±4) | <0.0001 | 26 (±4) | 26 (±4) | 0.902 |
| Self-reported fair to poor general health | 1099 (23%) | 298 (39%) | <0.0001 | 269 (40%) | 248 (37%) | 0.264 |
| Prior myocardial infarction | 392 (8%) | 143 (19%) | <0.0001 | 109 (16%) | 111 (16%) | 0.883 |
| Current angina pectoris | 825 (17%) | 246 (32%) | <0.0001 | 195 (29%) | 195 (29%) | 1.000 |
| Heart failure | 177 (4%) | 72 (9%) | <0.0001 | 47 (7%) | 54 (8%) | 0.469 |
| Hypertension | 2,032 (42%) | 463 (60%) | <0.0001 | 390 (57%) | 396 (58%) | 0.783 |
| Diabetes mellitus | 708 (15%) | 195 (25%) | <0.0001 | 167 (25%) | 162 (24%) | 0.752 |
| Atrial fibrillation by EKG | 110 (2%) | 37 (5%) | <0.0001 | 36 (5%) | 32 (5%) | 0.622 |
| LVH by EKG | 188 (4%) | 79 (10%) | <0.0001 | 56 (8%) | 59 (9%) | 0.771 |
| Transient ischemic attack | 235 (5%) | 93 (12%) | <0.0001 | 65 (10%) | 76 (11%) | 0.374 |
| Stroke | 156 (3%) | 75 (10%) | <0.0001 | 48 (7%) | 59 (9%) | 0.270 |
| Intermittent claudication | 34 (0.7%) | 70 (9.1%) | <0.0001 | 31 (5%) | 36 (5%) | 0.534 |
| Chronic obstructive pulmonary disease | 631 (13%) | 93 (12%) | 0.562 | 85 (13%) | 84 (12%) | 0.935 |
| Chronic kidney disease | 956 (20%) | 234 (31%) | <0.0001 | 200 (30%) | 189 (28%) | 0.548 |
| Cancer | 697 (14%) | 104 (14%) | 0.617 | 97 (14%) | 90 (13%) | 0.637 |
| Medications | ||||||
| ACE inhibitors | 344 (7%) | 100 (13%) | <0.0001 | 84 (12%) | 79 (11.6%) | 0.677 |
| Beta blockers | 610 (13%) | 112 (15%) | 0.117 | 100 (15%) | 102 (15%) | 0.879 |
| Calcium channel blockers | 594 (12%) | 163 (21%) | <0.0001 | 136 (20%) | 129 (19%) | 0.633 |
| Aspirin | 162 (3%) | 48 (6%) | <0.0001 | 41 (6%) | 34 (5%) | 0.409 |
| Statin | 98 (2%) | 25 (3.3%) | 0.033 | 26 (4%) | 21 (3%) | 0.463 |
| Loop diuretics | 296 (6%) | 87 (11%) | <0.0001 | 70 (10%) | 66 (10%) | 0.719 |
| Thiazide diuretics | 514 (11%) | 111 (15%) | 0.002 | 91 (13%) | 93 (14%) | 0.874 |
| Potassium sparing diuretics | 39 (0.8%) | 9 (1.2%) | 0.290 | 7 (1%) | 8 (1%) | 0.803 |
| NSAID | 454 (12%) | 237 (16%) | <0.0001 | 88 (13%) | 88 (13%) | 1.000 |
| Pulse, beats per minute | 68 ± 11 | 69 ± 12 | <0.0001 | 69± 12 | 69 ± 12 | 0.925 |
| Average blood pressure (mm Hg) | ||||||
| Systolic | 135 ± 21 | 146 ± 23 | <0.0001 | 144 ± 24 | 145 ± 23 | 0.787 |
| Diastolic | 71 ± 11 | 71 ± 13 | 0.912 | 71 ± 12 | 71 ± 13 | 0.936 |
| Serum creatinine, mg/dL | 0.95 ± 0.35 | 1.08 ± 0.57 | <0.0001 | 1.06 ± 0.49 | 1.06 ± 0.52 | 0.828 |
| Serum glucose, mg/dL | 109 ± 34 | 118 ± 46 | <0.0001 | 117 ± 43 | 116 ± 45 | 0.789 |
| Serum insulin, mcU/ml | 15 ± 23 | 20 ± 27 | <0.0001 | 21 ± 38 | 21 ± 45 | 0.956 |
| Serum potassium, mEq/L | 4.16 ± 0.37 | 4.18 ± 0.41 | 0.177 | 4.19 ± 0.39 | 4.18 ±0.41 | 0.422 |
| Total cholesterol, mg/dL | 210 ± 38 | 216 ± 43 | <0.0001 | 214 ± 41 | 214 ± 42 | 0.795 |
| Triglyceride, mg/dL | 138 ± 75 | 145 ± 85 | 0.015 | 148 ± 95 | 143 ± 79 | 0.315 |
| Albumin, g/dL | 3.99 ± 0.29 | 3.98 ± 0.29 | 0.077 | 3.99 ± 0.29 | 3.98 ± 0.29 | 0.592 |
| Fibrinogen, mg/dL | 317 ± 72 | 337 ± 84 | <0.0001 | 334 ± 81 | 334 ± 83 | 0.932 |
| C reactive protein, mg/dL | 4.5 ± 8 | 6.1 ± 9 | <0.0001 | 5.9 ± 11 | 5.9 ± 9 | 0.917 |
| Hemoglobin, gram/dL | 14.03 ± 1.40 | 13.94 ± 1.50 | 0.132 | 13.96 ± 1.81 | 13.97 ± 1.49 | 0.926 |
| White blood cells, 103/μL | 6.22 ± 1.93 | 6.82 ± 2.88 | <0.0001 | 6.59 ± 2.35 | 6.59 ± 1.87 | 0.927 |
| Platelets, 103/μL | 244 ± 64 | 245 ± 80 | 0.530 | 243 ± 68 | 245 ± 82 | 0.639 |
PAD was defined as ankle brachial index <0.9
ACE=angiotensin converting enzyme, EKG=electrocardiography, LVH=left ventricular hypertrophy, MI=myocardial infarction, NSAID=non-steroidal anti-inflammatory drug
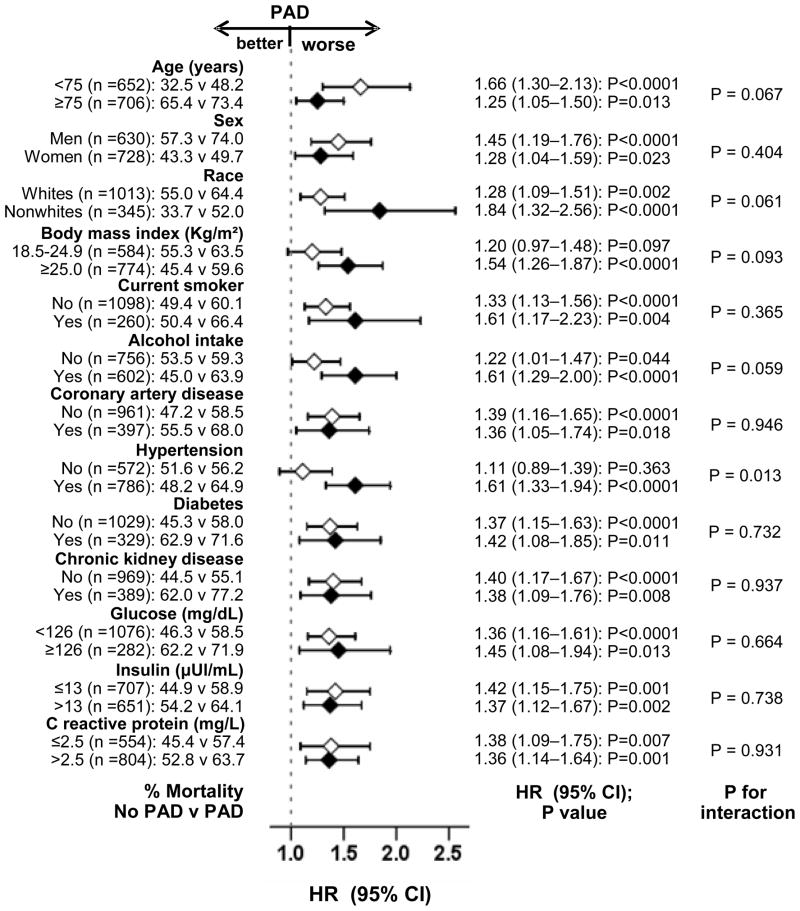
During 9958 person-years of follow-up 753 (55%) participants died from all causes. All-cause mortality occurred in 61% (rate, 8710/100,000 person-years) and 50% (rate, 6503/100,000 person-years of follow up) of participants respectively with and without PAD (matched HR, 1.47; 95% CI, 1.23–1.76; P<0.0001; Figure 2 and Table 2). When ABI was used as a continuous variable, every one-tenth of an increase in ABI (e.g. from 0.7 to 0.8 or from 1.3 to 1.4) was associated with a decreased all-cause mortality (HR, 0.94; 95% CI, 0.91–0.97; P<0.0001). The association between PAD and all-cause mortality was homogeneous across a wide spectrum of participants (Figure 3). Associations of PAD with other outcomes in the matched cohort are displayed in Table 2.
Figure 2.
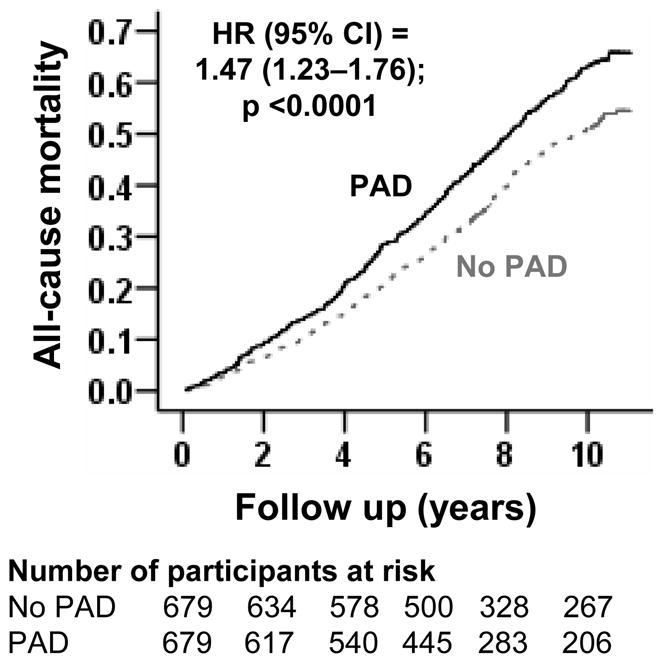
Kaplan-Meier plot for all-cause mortality by peripheral arterial disease (PAD)
Table 2.
Peripheral artery disease (PAD)* and outcomes in the matched cohort
| Outcomes | Rate, per 100,000 person-years (Events/total follow up years) |
Absolute rate difference** (per 100,000 person-years) | Matched hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| No PAD (n = 679) | PAD (n = 679) | ||||
| All-cause mortality | 6503(337/5182) | 8710 (416/4776) | + 2207 | 1.47 (1.23–1.76) | <0.0001 |
| Incident symptomatic PAD | 527(27/5124) | 1447 (65/4492) | + 920 | 3.92 (2.13–7.21) | <0.0001 |
| Incident heart failure | 2971(145/4880) | 4152 (180/4335) | + 1181 | 1.32 (1.00–1.73) | 0.052 |
| Incident acute myocardial infarction | 1423(71/4989) | 1995 (91/4561) | + 572 | 1.26 (0.88–1.80) | 0.206 |
| Incident angina pectoris | 2064(100/4845) | 2581 (114/4417) | + 517 | 1.18 (0.85–1.63) | 0.321 |
| Incident stroke | 1860(92/4946) | 2401 (108/4498) | + 541 | 1.35 (0.96–1.89) | 0.088 |
| Incident transient ischemic attack | 510(26/5094) | 514 (24/4670) | + 4 | 1.00 (0.54–1.86) | 1.000 |
PAD was defined as ankle brachial index <0.9
Absolute rate differences were calculated by subtracting the rates of events in the non-PAD group from those in the PAD group (before values were rounded)
Figure 3.
Hazard ratio (HR) and 95% confidence interval (CI) for all-cause mortality associated with peripheral artery disease (PAD) in subgroups of patients. ACE=angiotensin-converting enzyme; HF=heart failure
In the pre-match cohort of 5630 participants, 2001 (36%) died from all causes, with 63% and 31% respectively in those with and without PAD (unadjusted HR, 2.90; 95% CI, 2.61–3.21; P<0.0001). Multivariable-adjusted and propensity-adjusted hazard ratios for all-cause mortality were respectively 1.53 (95% CI, 1.36–1.71; P<0.0001) and 1.57 (95% CI, 1.39–1.78; P<0.0001; Table 3). Associations of PAD with other outcomes in the pre-match cohort are displayed in Table 3.
Table 3.
Peripheral artery disease* and outcomes in the pre-match cohort
| Outcomes | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| All-cause mortality | ||
| Unadjusted | 2.90 (2.61–3.21) | <0.0001 |
| Propensity-adjusted | 1.57 (1.39–1.78) | <0.0001 |
| Multivariable-adjusted | 1.53 (1.36–1.71) | <0.0001 |
| Incident symptomatic peripheral artery disease | ||
| Unadjusted | 6.40 (4.73–8.65) | <0.0001 |
| Propensity-adjusted | 3.78 (2.63–5.42) | <0.0001 |
| Multivariable-adjusted | 3.94 (2.84–5.46) | <0.0001 |
| Incident heart failure | ||
| Unadjusted | 2.83 (2.43–3.31) | <0.0001 |
| Propensity-adjusted | 1.59 (1.32–1.91) | <0.0001 |
| Multivariable-adjusted | 1.49 (1.26–1.71) | <0.0001 |
| Incident acute myocardial infarction | ||
| Unadjusted | 2.18 (1.75–2.72) | <0.0001 |
| Propensity-adjusted | 1.65 (1.28–2.13) | <0.0001 |
| Multivariable-adjusted | 1.72 (1.35–2.17) | <0.0001 |
| Incident stroke | 1.35 (0.96–1.89) | 0.088 |
| Unadjusted | 2.12 (1.74–2.58) | <0.0001 |
| Propensity-adjusted | 1.36 (1.08–1.71) | 0.009 |
| Multivariable-adjusted | 1.30 (1.06–1.60) | 0.013 |
Peripheral artery disease was defined as ankle brachial index <0.9
Absolute rate differences were calculated by subtracting the rates of new-onset heart failure in the high serum uric acid group from the rate of new-onset heart failure in the normal serum uric acid group (before values were rounded)
DISCUSSION
The findings from the current analysis demonstrate that the prevalence of PAD in community dwelling older adults was relatively high, that PAD was mostly mild to moderate in severity and asymptomatic in nature, and that baseline PAD was associated with all-cause mortality and cardiovascular morbidity. To the best of our knowledge, this is the first demonstration of such associations in a large population of propensity matched, community dwelling older adults in which participants with and without PAD were well balanced in 66 measured baseline covariates.
The observed associations between PAD and poor outcomes can be explained by a direct effect of PAD, a confounding by measured covariates such comorbidities associated with PAD, or a confounding by an unmeasured covariate. PAD is a manifestation of systemic atherosclerosis and as such patients with PAD are more likely to have other manifestations of atherosclerosis.17 In fact, before matching, more participants with PAD had coronary artery disease, hypertension, diabetes, heart failure and stroke (Table 1). However, after matching, participants with and without PAD were well-balanced in 66 measured baseline covariates including all of the above cardiovascular comorbidities and risk factors. Therefore, the increased PAD-associated mortality observed in the current analysis may not be explained by differences in baseline prevalence of atherosclerotic diseases. However, it is possible that atherosclerosis was more widespread and severe in those with PAD than in those without. Among patients undergoing coronary angiography for suspected coronary artery disease, compared to those without PAD, those with PAD had a higher prevalence of obstructive coronary artery disease including left main and 3- or 4-vessel disease.18 It is also possible that atherosclerosis in participants with PAD progressed at much faster rate than in those without PAD. Therefore, increased PAD-associated mortality may have mediated via atherosclerotic diseases in other vascular beds.
Except for incident symptomatic PAD, among our matched participants, PAD was not associated with incident cardiovascular morbidity. However, directions of these associations were generally positive. The lack of statistically significant associations was likely due to the small sample size of the matched cohort. Further, propensity matching generally provides more conservative estimates than those obtained from multivariable-adjusted or propensity-adjusted models. In fact, the multivariable-adjusted or propensity-adjusted associations of PAD with all incident cardiovascular morbidity were significant in our pre-match cohort. This suggests that increased PAD-associated mortality was at least in part mediated via increased atherosclerotic cardiovascular morbidity. Further, the incident cardiovascular morbidity does not include fatal cases, and it is possible that those with PAD may have experienced more fatal cardiovascular atherosclerotic events.
To the best of our knowledge this is the first report of an association of PAD with all-cause mortality and cardiovascular morbidity in a propensity-matched population of community-dwelling older adults. There are several important clinical and public health implications of these findings. These findings suggest that although PAD is a marker of systemic atherosclerosis, its presence may indicate more severe and widespread atherosclerosis and an eventual association with increased mortality. This is important as PAD was asymptomatic in over 90% of community-dwelling older adults, and thus may be considered a silent killer often unappreciated by clinicians and public health experts. Asymptomatic PAD is known to be associated with poorer functional performance, poorer quality of life, and more adverse calf muscle characteristics compared with persons with intermittent claudication.19 However, our findings suggest that it also has an independent association with all-cause mortality and major cardiovascular morbidity. These findings also highlight the importance of prevention, early detection and treatment of PAD in older adults.
Several limitations of our study must be acknowledged. We had no data on the extent and severity of PAD. Large-vessel, but not small-vessel PAD has been shown to be associated with increased mortality.3,20 This may have underestimated the association of PAD and mortality in our study. Patients without PAD at baseline may have developed PAD during follow up. This regression dilution may also have underestimated the PAD-mortality association observed in our study.21 We were able to match 89% of participants with PAD and any effects due to loss of participants during matching are likely to be minimal. Further, we were able to replicate our key findings using traditional risk-adjustment approaches in the pre-match cohort. Confounding due to unmeasured covariate is possible. However, for an unmeasured covariate to become a confounder it must be near-perfect predictor of mortality, must not be strongly correlated with any of the 66 covariates used in our study, and also be associated with PAD. In conclusion, we demonstrate that among community-dwelling older adults, PAD is an asymptomatic disease associated with high prevalence of other atherosclerotic cardiovascular comorbidities. However, when all of these differences were balanced after rigorous propensity matching, baseline PAD was independently associated with increased mortality. Ankle-brachial index should be routinely assessed in community-dwelling older adults to identify those with PAD, and these patients should be appropriately treated.22,23
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
“The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the CHS Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiu JH, Topol EJ, Whitlow PL, Hsu AP, Tuzcu EM, Franco I, Moliterno DJ. Peripheral vascular disease and one-year mortality following percutaneous coronary revascularization. Am J Cardiol. 2003;92:582–583. doi: 10.1016/s0002-9149(03)00726-4. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH. Systemic atherosclerosis risk and the mandate for intervention in atherosclerotic peripheral arterial disease. Am J Cardiol. 2001;88:43J–47J. doi: 10.1016/s0002-9149(01)01881-1. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985;72:768–773. doi: 10.1161/01.cir.72.4.768. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Luo Y, Xu Y, Yang J, Zheng L, Hasimu B, Yu J, Hu D. Risk factors of peripheral arterial disease and relationship between low ankle - brachial index and mortality from all-cause and cardiovascular disease in Chinese patients with type 2 diabetes. Circ J. 2007;71:377–381. doi: 10.1253/circj.71.377. [DOI] [PubMed] [Google Scholar]
- 5.Thatipelli MR, Pellikka PA, McBane RD, Rooke TW, Rosales GA, Hodge D, Herges RM, Wysokinski WE. Prognostic value of ankle-brachial index and dobutamine stress echocardiography for cardiovascular morbidity and all-cause mortality in patients with peripheral arterial disease. J Vasc Surg. 2007;46:62–70. doi: 10.1016/j.jvs.2007.03.022. discussion 70. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O’Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 8.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 10.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 13.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 16.SPSS. SPSS for Windows, Rel. 15. Chicago, IL: SPSS Inc., Chicago, IL; 2008. [Google Scholar]
- 17.Ness J, Aronow WS, Newkirk E, McDanel D. Prevalence of symptomatic peripheral arterial disease, modifiable risk factors, and appropriate use of drugs in the treatment of peripheral arterial disease in older persons seen in a university general medicine clinic. J Gerontol A Biol Sci Med Sci. 2005;60:255–257. doi: 10.1093/gerona/60.2.255. [DOI] [PubMed] [Google Scholar]
- 18.Sukhija R, Yalamanchili K, Aronow WS. Prevalence of left main coronary artery disease, of three- or four-vessel coronary artery disease, and of obstructive coronary artery disease in patients with and without peripheral arterial disease undergoing coronary angiography for suspected coronary artery disease. Am J Cardiol. 2003;92:304–305. doi: 10.1016/s0002-9149(03)00632-5. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, Green D, Sufit R, Hoff F, Nishida T, Sharma L, Pearce WH, Schneider JR, Criqui MH. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criqui MH, Browner D, Fronek A, Klauber MR, Coughlin SS, Barrett-Connor E, Gabriel S. Peripheral arterial disease in large vessels is epidemiologically distinct from small vessel disease. An analysis of risk factors. Am J Epidemiol. 1989;129:1110–1119. doi: 10.1093/oxfordjournals.aje.a115233. [DOI] [PubMed] [Google Scholar]
- 21.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 22.Aronow WS. Drug treatment of peripheral arterial disease in the elderly. Drugs Aging. 2006;23:1–12. doi: 10.2165/00002512-200623010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]