Summary
NMDA receptor-dependent synaptic modifications, such as long-term potentiation (LTP) and long-term depression (LTD), are essential for brain development and function. LTD occurs mainly by the removal of AMPA receptors from the postsynaptic membrane, but the underlying molecular mechanisms remain unclear. Here we show that activation of caspase-3 via mitochondria is required for LTD and AMPA receptor internalization in hippocampal neurons. LTD and AMPA receptor internalization are blocked by peptide inhibitors of caspase-3 and -9. In hippocampal slices from caspase-3 knockout mice, LTD is abolished whereas LTP remains normal. LTD is also prevented by overexpression of the anti-apoptotic proteins XIAP or Bcl-xL, and by a mutant Akt1 protein that is resistant to caspase-3 proteolysis. NMDA receptor stimulation that induces LTD transiently activates caspase-3 in dendrites, without causing cell death. These data indicate an unexpected causal link between the molecular mechanisms of apoptosis and LTD.
Introduction
Synaptic plasticity is a crucial process that modifies synapses in response to neural activity and experience. Long lasting changes in the structure and function of synapses are important for CNS development and underlie information storage in the brain. LTP and LTD are long-lasting modifications of synapses, particularly well-studied in the CA1 region of the hippocampus. Induction of NMDA receptor-dependent LTD in CA1 (typically by prolonged low frequency stimulation) requires Ca2+ influx and the serine/threonine phosphatases calcineurin/PP2B and PP1 (Malenka and Bear, 2004). Trafficking of AMPA receptors to and away from the synapse determines the abundance of AMPA receptors in the postsynaptic membrane. LTD involves the removal and internalization of AMPA receptors from synapses (Collingridge et al., 2004; Malenka and Bear, 2004; Shepherd and Huganir, 2007). However, the molecular mechanisms underlying LTD, particularly the signaling pathways that couple NMDA receptor stimulation to AMPA receptor internalization, remain largely unknown. Recent studies have implicated the small GTPase Rap and protein kinases p38 and GSK3β in LTD (Peineau et al., 2007; Zhu et al., 2002; Zhu et al., 2005).
Caspases are cysteine proteases with well-established functions in the execution of apoptosis. The caspase-9-caspase-3 cascade is activated by pro-apoptotic molecules such as cytochrome c released from mitochondria, and restrained by cellular IAPs (inhibitor of apoptosis proteins) (Srinivasula and Ashwell, 2008). The release of cytochrome c from mitochondria is inhibited by anti-apoptotic members of the Bcl-2 family of proteins (such as Bcl-xL) and stimulated by pro-apoptotic members (such as BAX and BAK) (Youle and Strasser, 2008).
Interestingly, active caspases are also detected in non-apoptotic cells. In neurons, mitochondria as well as caspases are present in dendrites, axons and pre- and postsynaptic terminals, and there is evidence that caspases can be activated in dendrites, synaptosomes and growth cones (Campbell and Holt, 2003; Gilman and Mattson, 2002; Kuo et al., 2006; Williams et al., 2006; Yuan, 2006). Activated caspases can suppress synaptic currents and caspase-3 can cleave AMPA receptor subunit GluR1 in vitro (Chan et al., 1999; Glazner et al., 2000; Lu et al., 2002). It has been reported that caspases play non-apoptotic roles in the dendritic pruning of Drosophila neurons during development (Kuo et al., 2006; Williams et al., 2006), in the structural remodelling of hippocampal neuron synapses and Xenopus retinal growth cones (Campbell and Holt, 2003; Gilman and Mattson, 2002), and in bird song learning (Huesmann and Clayton, 2006).
Here we report that LTD and AMPA receptor internalization in hippocampal neurons require the activity of caspase-9 and caspase-3/7, and can be blocked by overexpression of anti-apoptotic proteins Bcl-xL and XIAP. Caspase-3 can be transiently activated via the mitochondrial pathway by stimulating NMDA receptors, without causing cell death; and hippocampal slices from caspase-3 knockout mice lose specifically their ability to undergo NMDA receptor-dependent LTD. These findings reveal a critical role of the mitochondrial pathway of caspase activation in synaptic depression.
Results
Caspase inhibitors DEVD-FMK and LEHD-FMK block hippocampal LTD but not LTP
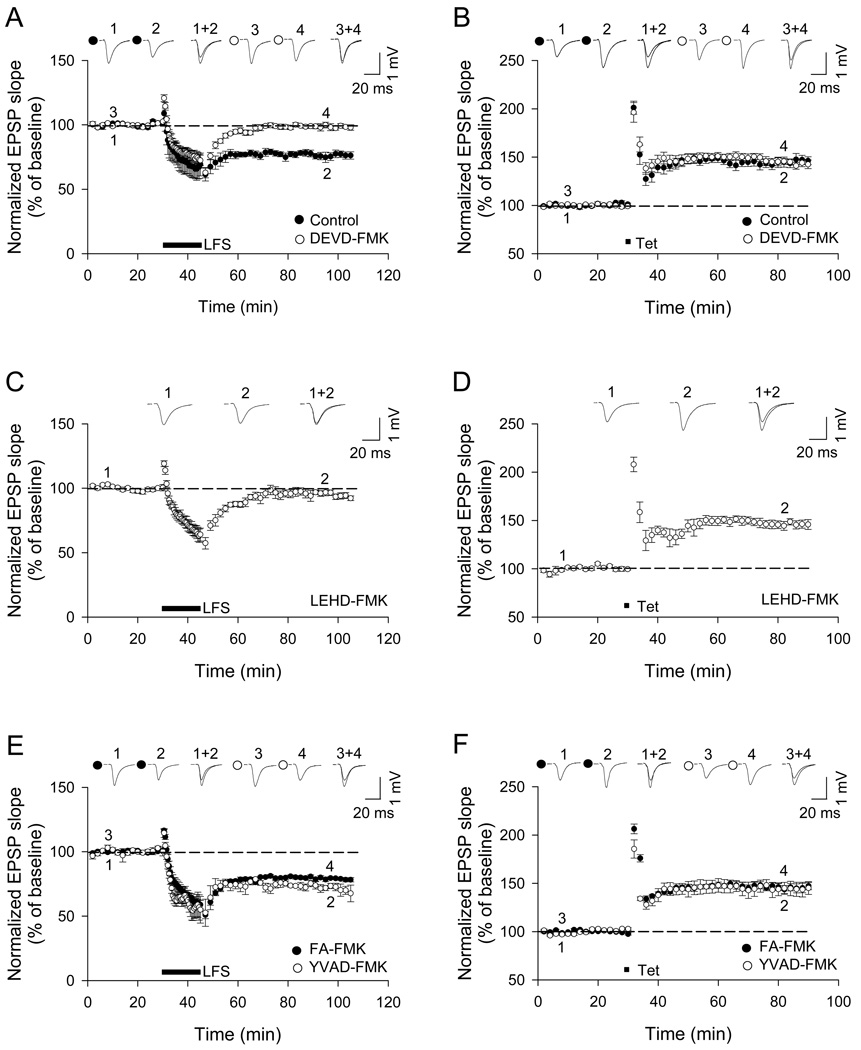
We investigated the role of caspases in synaptic plasticity of Schaffer collateral-CA1 synapses in acute rat hippocampal slices (3–4 weeks age) (Figure 1). In the first series of experiments, slices were incubated with cell-permeant caspase inhibitor peptides: DEVD-FMK (2 µM; an inhibitor with selectivity against caspases-3, -7, -8); LEHD-FMK (2 µM; a caspase-9-preferring inhibitor with less potent activity against caspases-6, -8, -2); YVAD-FMK (5 µM; caspase-1-selective inhibitor); and WEHD-FMK (200 nM; an inhibitor with preference for caspase-1 and caspase-8) (Garcia-Calvo et al., 1998; Talanian et al., 1997; Thornberry et al., 1997). Field excitatory postsynaptic potentials (fEPSPs) were recorded in area CA1 in response to stimulation of Schaffer collateral inputs. Bath application of DEVD-FMK blocked LTD induced by low frequency stimulation (LFS, 1 Hz for 900 sec; 98 ± 2% of baseline in DEVD-FMK; 75 ± 3% of baseline in control untreated slices, p<0.01, n=6, Figure 1A). Importantly, DEVD-FMK had no effect on LTP induced by tetanus (100 pulses at 100 Hz, 4 trains; 142 ± 5% of baseline in DEVD-FMK; 140 ± 5% in control, n=6, Figure 1B), suggesting that the peptide was not non-specifically toxic. A different peptide, LEHD-FMK, also blocked LTD (97 ± 3%, n=6, Figure 1C), without affecting LTP (146 ± 5%, n=6, Figure 1D). Neither LTD nor LTP were affected by YVAD-FMK and WEHD-FMK, similar to the negative control peptide FA-FMK (10 µM; which should not affect caspase activity) (Figure 1E–F; Figure S1A, B). In contrast to DEVD- and LEHD-FMK, bath application of LLY-FMK (5 µM; an inhibitor of calpain) blocked LTP but not LTD (Figure S1C, D). The sensitivity of LTP to a calpain inhibitor is consistent with a previous report (Denny et al., 1990).
Figure 1.
Caspase inhibitors DEVD- and LEHD-FMK block LTD in CA1 hippocampal neurons. Acute hippocampal slices (from 3–4 week old rats) were incubated with caspase inhibitors for 2 hr before recording (DEVD-FMK, 2 µM; LEHD-FMK, 2 µM; FA-FMK, 10 µM; YVAD-FMK, 5 µM). Extracellular field EPSPs were evoked by stimulating Schaffer collateral – CA1 synapses. (A and B) Effect of DEVD-FMK on LTD and LTP, induced by low frequency stimulation (LFS) and tetanic stimulation (Tet) respectively (control, n=6; DEVD-FMK, n=6). (C and D) Effect of LEHD-FMK on LTD and LTP (n=6). (E and F) Effect of YVAD-FMK or negative control peptide FA-FMK on LTD and LTP (n=6). Pooled data and example traces are shown in all panels. Symbols and error bars indicate mean ± SEM. See also Figure S1.
We measured basal fEPSP slope before and after the application of 2 µM DEVD-FMK, and found no significant change after 2 hr exposure (Figure S1E). Thus the caspase inhibitor blocked LTD without affecting basal synaptic transmission over the time course of LTD experiments. The fact that DEVD- and LEHD-FMK specifically blocked LTD without affecting LTP or basal synaptic strength argues against non-specific effects of these peptides on synaptic transmission. DEVD-, LEHD-, YVAD- and WEHD-FMK show differential potency against individual caspases (Garcia-Calvo et al., 1998; Talanian et al., 1997; Thornberry et al., 1997), and we only observed that DEVD- and LEHD-FMK, but not YVAD- and WEHD-FMK, blocked LTD. We thus inferred that a subset of caspases (most likely caspase-3/7 and caspase-9) is required for induction of LTD, but not LTP.
Bcl-xL and XIAP block LTD but not LTP in hippocampal slice cultures
Because the peptide inhibitors show limited selectivity for individual caspases, we additionally used molecular approaches to inhibit caspases. We overexpressed caspase-inhibitor proteins XIAP, CrmA, and anti-apoptotic protein Bcl-xL in CA1 neurons of organotypic hippocampal slice cultures. XIAP (X-chromosome-linked inhibitory apoptosis protein) is an inhibitor of caspase-3, -7 and -9 (Deveraux et al., 1998; Deveraux et al., 1997; Roy et al., 1997). An N-terminally truncated form of XIAP containing the third Bir domain of XIAP (XIAP-Bir3) specifically inhibits caspase-9, which is an upstream activator of caspase-3 (Wilkinson et al., 2004). XIAP-Bir1,2, an XIAP construct containing the N-terminal two Bir domains, specifically inhibits caspase-3/7 (Chai et al., 2001; Riedl et al., 2001; Sun et al., 2000). Bcl-xL is an anti-apoptotic Bcl-2 family protein (Kuwana and Newmeyer, 2003) that prevents mitochondrial release of pro-apoptotic factors, thereby blocking activation of caspase-9 and caspase-3. CrmA (a cowpox serpin) is a selective inhibitor of caspase-1, -5, and -8 (Garcia-Calvo et al., 1998).
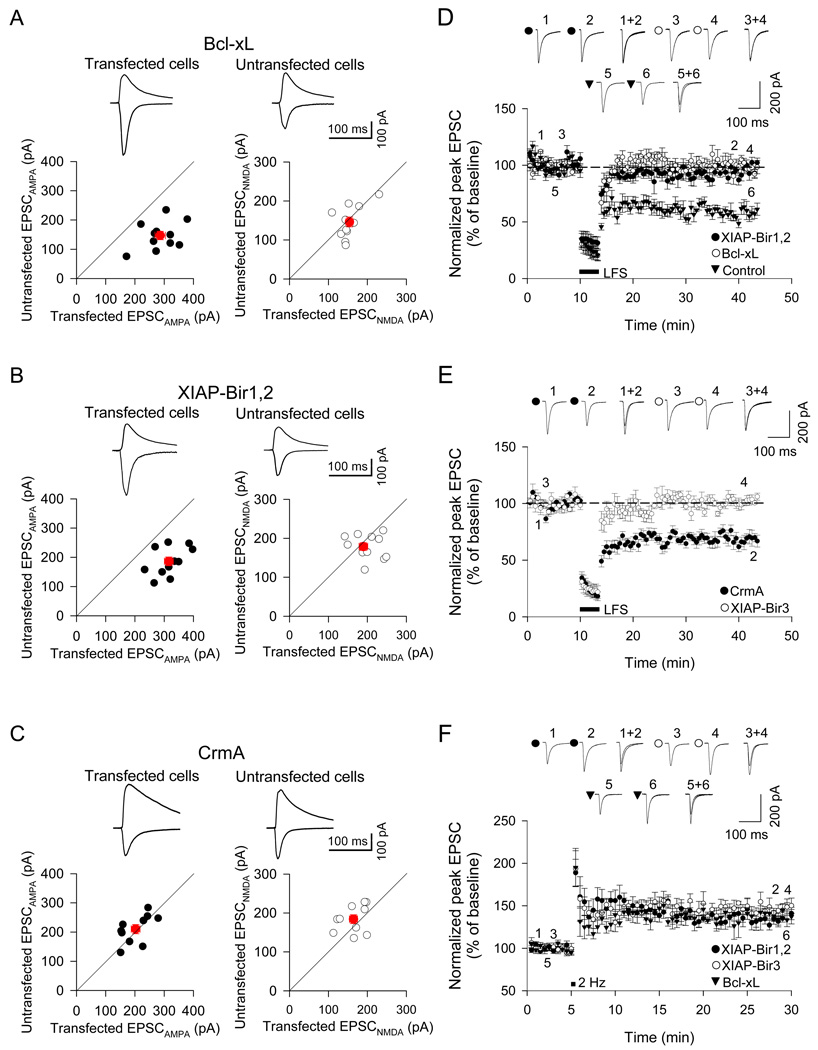
CA1 neurons were biolistically transfected with plasmids expressing Bcl-xL or XIAP (plus GFP as transfection marker) and recorded in whole-cell patch-clamp mode. LTD or LTP of Schaffer collateral-CA1 synapses was induced by pairing protocols (see Experimental Procedures). We first analyzed the effect on basal synaptic transmission by comparing EPSCs evoked in pairs of transfected and neighboring untransfected cells in the same slice using the same stimulus position and intensity. Overexpression of Bcl-xL caused an increase in amplitude of AMPA receptor-mediated EPSCs (Bcl-xL-transfected cells: 286 ± 18 pA, n=11; untransfected cells: 147 ± 14 pA, n=11, p<0.01; Figure 2A left). However, Bcl-xL had no effect on the NMDA receptor-mediated component of EPSC compared to untransfected cells in the same slices (transfected cells: 154 ± 9 pA; untransfected: 146 ± 13 pA, p>0.05; Figure 2A right). Overexpression of XIAP-Bir1,2 also caused an increase in basal AMPA-EPSCs (XIAP-Bir1,2: 316 ± 15 pA, n=11; untransfected: 186 ± 15 pA, n=11, p<0.01; Figure 2B), without affecting NMDA-EPSCs (XIAP-Bir1,2: 188 ± 11 pA, n=11; untransfected: 179 ± 10 pA, n=11; Figure 2B). In contrast to Bcl-xL and XIAP-Bir1,2, CrmA did not change either AMPA-EPSC (CrmA: 202 ±15 pA, n=10; untransfected: 210 ±16 pA, n=10, p>0.05; Figure 2C) or NMDA-EPSC amplitudes (CrmA: 164 ± 10 pA; untransfected: 174 ±11 pA, p>0.05; Figure 2C).
Figure 2.
Overexpression of Bcl-xL and XIAP constructs blocks LTD in CA1 neurons of organotypic hippocampal slice cultures. CA1 neurons were biolistically transfected with Bcl-xL, XIAP-Bir1,2, XIAP-Bir3 or CrmA (with GFP cotransfected as a visual marker) and recorded in whole-cell patch-clamp mode. (A–C) Pairwise analysis of the effects of Bcl-xL (11 pairs of transfected and neighboring untransfected cells), XIAP-Bir1,2 (11 pairs) or CrmA transfection (10 pairs) on basal AMPA-EPSC amplitude (left graph, recorded at a holding potential of −70mV) and NMDA-EPSC amplitude (right graph, recorded at a holding potential of +40mV) versus nearby untransfected cells (pairs of transfected and neighboring untransfected cells are individually plotted; black circles). Red symbol and error bars indicate mean ± SEM. Bcl-xL and XIAP caused an increased basal EPSCAMPA (rightward shift on graph) but had no effect on the EPSCNMDA, in comparison with neighboring untransfected cells. (D) Induction of LTD was blocked in CA1 cells overexpressing XIAP-Bir1,2 (p>0.05, n=7), and BclxL (p>0.05, n=8). LTD was intact in non-transfected CA1 neurons from organotypic hippocampal culture (p<0.01, n=6). (E) LTD induction was blocked by overexpression of XIAP-Bir3 (n=8) but not CrmA (n=8). (F) LTP was unaffected by overexpression of Bcl-xL (n=7), XIAP-Bir1,2 (n=7) or XIAP-Bir3 proteins (n=7). Graphs show mean ± SEM.
Even though they showed a higher basal EPSC, neurons overexpressing Bcl-xL or XIAP-Bir1,2 were unable to undergo LTD (Bcl-xL: 97 ± 4%, n=8; XIAP-Bir1,2: 97± 5%, n=8; Figure 2D). Similarly, LTD was abolished in CA1 neurons transfected with XIAP-Bir3 (101 ± 3%, n=8; Figure 2E). In cells transfected with CrmA, however, LTD was unaffected (66 ± 6%, n=8; Figure 2E). The induction of LTP was normal in cells transfected with Bcl-xL, XIAP-Bir1,2 or XIAP-Bir3 (138 ± 5%, 146 ± 5% and 143 ± 8% of baseline, respectively; n=7 for each condition; Figure 2F). Control untransfected neurons, as expected, showed robust LTD (64 ± 4%, n=6; Figure 2D) as well as LTP (147 ± 9 %, n=6). The lack of effect on LTP implies that Bcl-xL, XIAP-Bir3 and XIAP-Bir-1,2 overexpression are not non-specifically inhibiting synaptic function and plasticity. In line with the pharmacological data, the overexpression experiments support the idea that activity of caspases -9 and -3/7 are crucial for LTD. Moreover, the blockade of LTD by Bcl-xL and XIAP-Bir3 suggests that these caspases are activated by the mitochondrial pathway.
Loss of LTD in caspase-3 KO mice
The pharmacological approach has inherent problems with specificity, and there are also caveats with overexpression of proteins such as XIAP, Bcl-xL for several days. For further corroboration, we studied synaptic plasticity in caspase-3 knockout mice. In the C57BL/6 background, the homozygous caspase-3 knockout mice are viable, reach adulthood and display minimal brain histopathology (Leonard et al., 2002).
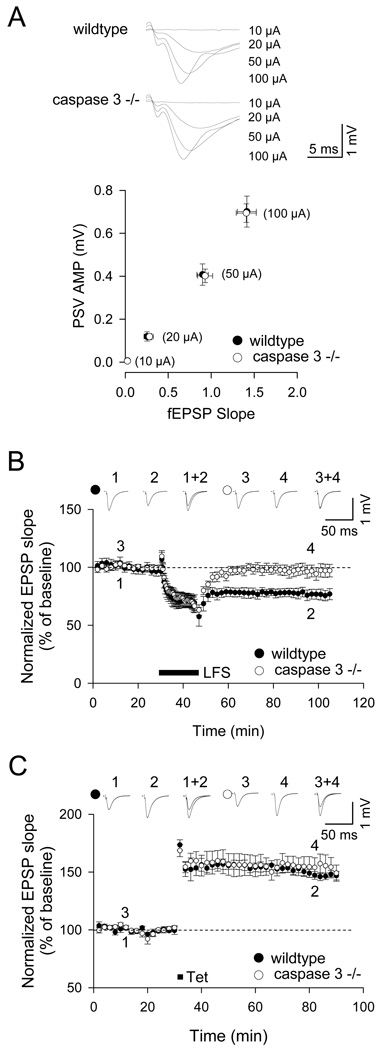
LTD and LTP were measured in acute hippocampal slices taken from caspase-3 knockout (−/−) mice or wildtype controls (2–4 weeks age). There was no difference between wildtype and knockout animals with respect to input/output relationship upon stimulation of Schaffer collateral-CA1 synapses (Figure 3A: p > 0.05, n = 16), indicating that loss of caspase-3 did not affect the strength of basal synaptic transmission.
Figure 3.
LTD is abolished in hippocampal slices from caspase-3 knockout (−/−) mice. (A) Input-output relationship in caspase-3 −/− and wildtype mice (2–4 weeks age). fEPSP slope was plotted against amplitude of presynaptic volley at four different stimulus intensities (10 µA, 20 µA, 50 µA and 100 µA). Graph indicates mean ± SEM (p>0.05, n=16). (B) Low frequency stimulation (LFS, 1 Hz, 15 min) failed to induce LTD in caspase-3 −/− slices (p<0.01, n=6). (C) Magnitude of LTP induced by tetanic stimulation (Tet) was not different between caspase-3 −/− and wildtype slices (p>0.05, n=6). See also Figure S2.
Although it robustly induced synaptic depression in wildtype hippocampal slices, LFS (1 Hz, 900 pulses) failed to induce LTD in caspase-3 knockout slices (caspase-3 −/−: 97 ± 6% of baseline; wildtype: 77 ± 5%, n = 6 slices from 6 animals, p < 0.01; Figure 3B). The plasticity defect in caspase-3 deficient animals was specific for LTD, as induction of LTP was remarkably normal. Two trains of tetanus (100 Hz, 100 pulses) resulted in LTP that was similar in magnitude in caspase-3 knockout and wildtype hippocampus (caspase-3 −/−: 154 ± 8% of baseline; wildtype: 149 ± 3%, n = 6 slices from 6 animals, p > 0.05; Figure 3C).
The electrophysiological findings in knockout mice indicate a crucial role for caspase-3 in LTD, consistent with the results from pharmacological and overexpression experiments. The expression of AMPA receptors (GluR1 and GluR2) and NMDA receptors (NR1, NR2A and NR2B) in caspase-3 −/− hippocampi was similar to wildtype by immunoblotting (Figure S2).
DEVD-FMK and LEHD-FMK prevent AMPA receptor internalization
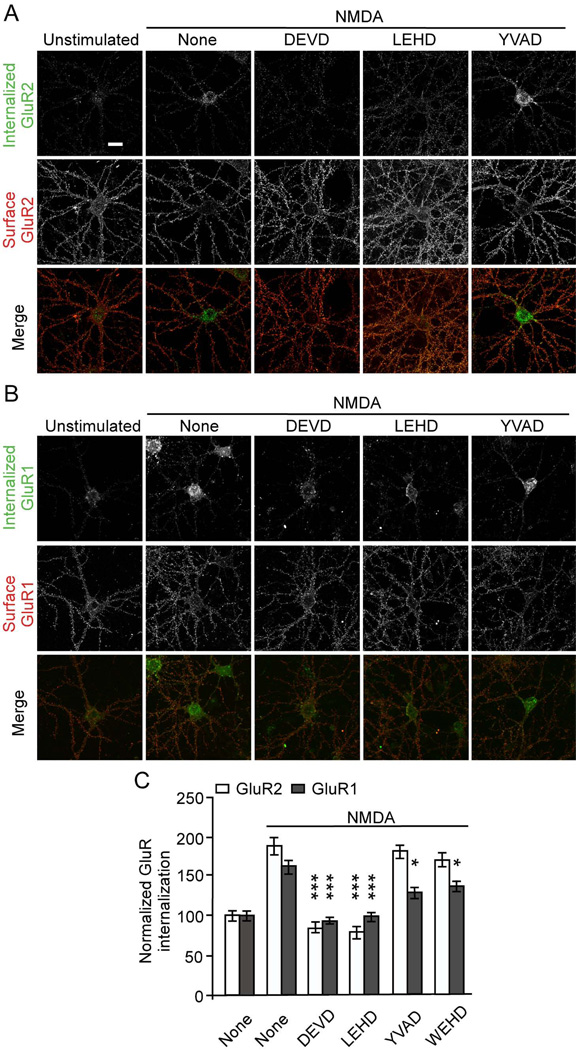
The block of LTD by DEVD-FMK and LEHD-FMK suggests that caspases may regulate the inducible internalization of AMPA receptors. To test this hypothesis, we measured AMPA receptor endocytosis in dissociated hippocampal neurons using an “antibody feeding” internalization assay (Lee et al., 2002). Treatment of hippocampal neurons (DIV18) with NMDA (50 µM for 10 min) stimulated internalization of endogenous AMPA receptors (Figure 4). The internalization of GluR2 induced by NMDA was abolished by treatment with DEVD- and LEHD-, but not by YVAD- or WEHD-FMK (Figure 4A, C). DEVD and LEHD also blocked NMDA-induced internalization of AMPA receptor GluR1 subunit (Figure 4B, C). The basal internalization of GluR2 (i.e. that occurring in unstimulated neurons) was unchanged by caspase inhibitors (Figure S3). These data indicate that DEVD- and LEHD-sensitive caspases are specifically required for the inducible internalization of AMPA receptors.
Figure 4.
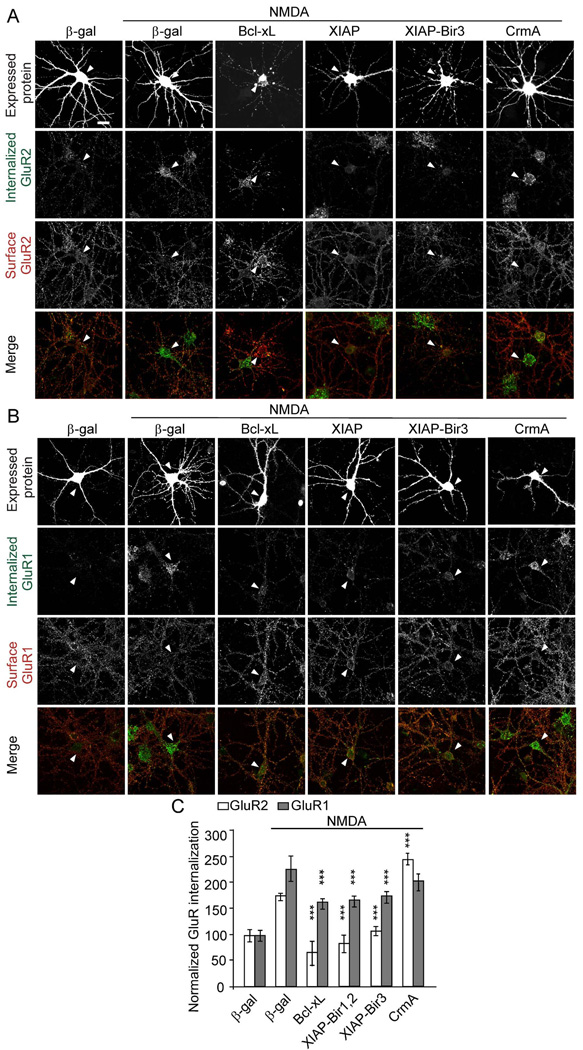
Caspase inhibitors DEVD- and LEHD-FMK block NMDA-induced AMPA receptor internalization. Antibody-feeding internalization assay for endogenous GluR2 (A) and GluR1 (B) in hippocampal neurons stimulated with NMDA (50 µM for 10 min). Neurons were pretreated with various caspase inhibitors (5 µM) as indicated. A and B show double-label immunostaining for internalized GluR (green, first row) and surface-remaining GluR (red, second row), and merge (in color, third row). Individual channels are shown in grayscale. Quantitation in C shows internalization index (integrated fluorescence intensity of internalized GluR / integrated fluorescence intensity of internalized GluR plus surface GluR) normalized to unstimulated untreated cells (Lee et al., 2002). For all quantitations, n=15 neurons for each group. *p<0.05, ***p<0.001, compared to NMDA treated cells transfected with µ-gal. The graph shows mean ± SEM. Scale bar, 20 µm. See also Figure S3.
Bcl-xL, XIAP and caspase-3 knockout block AMPA receptor internalization
To obtain additional non-pharmacological evidence to support the importance of caspases in AMPA receptor internalization, we overexpressed in neurons XIAP, Bcl-xL or CrmA. Transfection of XIAP-Bir1,2, XIAP-Bir3 or Bcl-xL in hippocampal neurons prevented NMDA-induced internalization of GluR2 (Figure 5A, C). NMDA-stimulated GluR1 internalization was also inhibited by XIAP-Bir1,2, XIAP-Bir3 and Bcl-xL, though not as completely as for GluR2 (Figure 5B, C). In contrast, overexpression of CrmA did not significantly reduce NMDA-induced internalization of GluR2 or GluR1 (Figure 5A–C). Basal internalization of GluR2 (Figure S4A) and surface expression of GluR2 (Figure S4B, C) were unaffected by any of these constructs. Finally, we found that NMDA-induced GluR2 internalization was completely abolished in hippocampal neurons from caspase-3 knockout mice (Figure S4D, E). Together, the molecular genetic and pharmacological data argue that the activity of caspases-9 and -3/7 is critical for inducible AMPA receptor internalization.
Figure 5.
Anti-apoptotic proteins inhibit AMPA receptor internalization. Cultured hippocampal neurons (DIV14) were transfected with Bcl-xL, XIAP-Bir1,2, XIAP-Bir3 or CrmA, or control β-gal. At 2–4 days after transfection, neurons were treated with NMDA as indicated, and GluR2 (A) and GluR1 internalization (B) was measured by the antibody-feeding internalization assay, as in Figure 4. A and B show immunostaining for the transfected protein (upper row), internalized GluR (second row), surface-remaining GluR (third row), and merge of internalized and surface GluR (bottom row). All individual channels of this triple labeling experiment are shown in grayscale. Arrowheads mark the cell body of transfected cells. Note that the cell body of a Bcl-xL transfected neuron in (A) lies adjacent to an untransfected neuron. Histograms show internalization index for GluR normalized to unstimulated control-transfected neurons (C). n=15–30 neurons for each group. ***p<0.001 (compared to NMDA stimulated β-gal control). The graph shows mean ± SEM. Scale bar, 20 µm. See also Figure S4.
Caspase-7 is expressed at low levels in the brain (Van de Craen et al., 1997), and we could not detect caspase-7 in rat brain or cultured neurons by immunoblotting (Figure S4F). Although we cannot exclude a role for caspase-7, we suppose that caspase-3 and -9 are probably the main caspases involved in LTD and AMPA receptor internalization.
Transient activation of caspase-3 in neurons by NMDA receptor stimulation
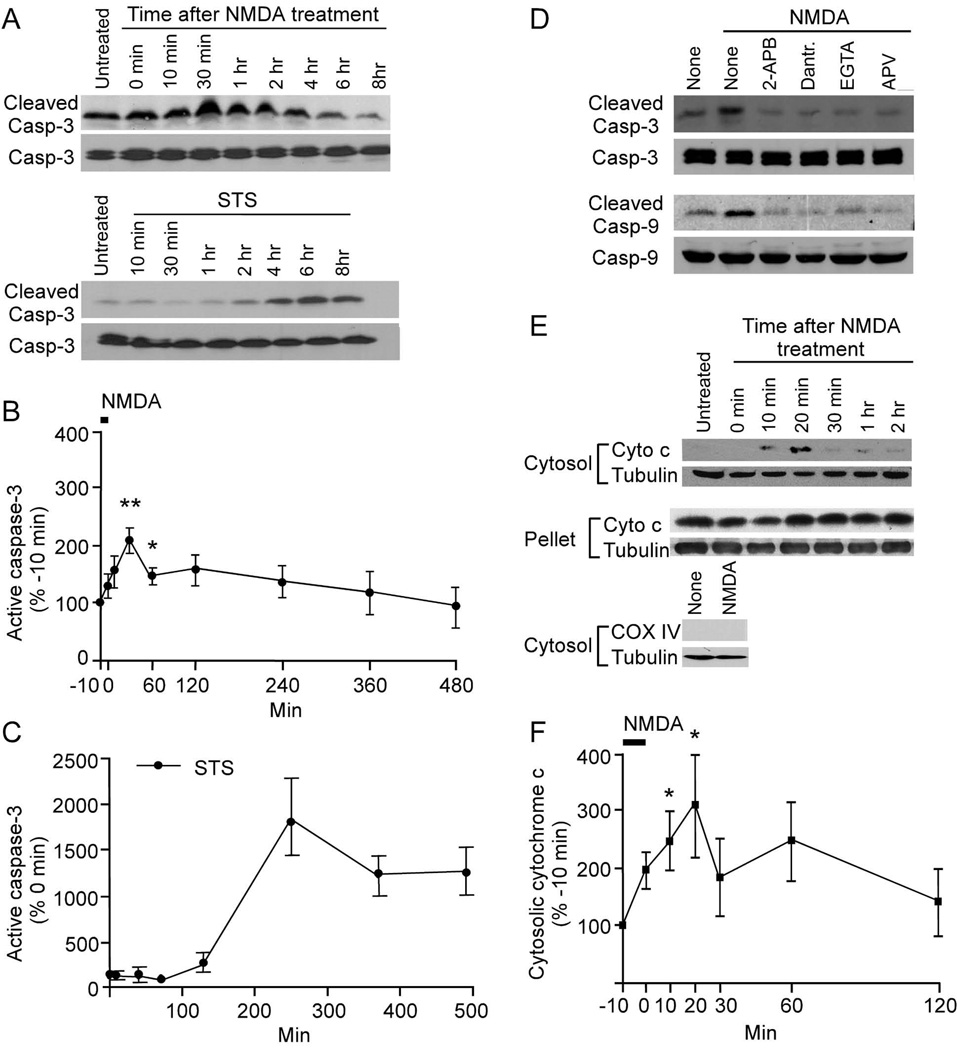
If caspase-3 is involved in LTD and AMPA receptor internalization, then caspase-3 should be activated by stimuli that induce LTD or AMPA receptor endocytosis. To test this possibility, we measured active caspase-3 by immunoblotting for the cleaved form of caspase-3 (active caspase-3) (Figure 6). Prolonged exposure to staurosporine (STS), an inducer of neuronal apoptosis, caused a delayed but sustained accumulation of active caspase-3 in cortical neuron cultures (Figure 6A, C). At the peak, active caspase-3 was ~17-fold higher than in untreated neurons, and remained >10-fold elevated for 8 hr. The NMDA treatment that induced AMPA receptor internalization also caused caspase-3 activation, but to a much lower level than STS and with more rapid and transient kinetics. Caspase-3 activation increased by 33 ± 21 % immediately (at 0 min) after a 10 min treatment with NMDA, although not reaching statistical significance. Active caspase-3 continued to rise, peaking at ~30 min after NMDA (213 ± 23% of baseline, n=4, p<0.01), before gradually returning to pre-treatment levels (Figure 6A, B). The maximal increase of active caspase-3 after NMDA stimulation was only ~2–3-fold (cf 10–20 fold for STS) and it occurred much more quickly (Figure 6B). Caspase-9, which lies upstream of caspase-3 in the mitochondrial apoptotic pathway, was also activated by NMDA stimulation (measured 30 min after NMDA stimulation; Figure 6D). The activation of caspase-3 and -9 by NMDA was blocked by antagonist of NMDA receptors (APV), chelation of extracellular calcium (EGTA) and inhibitors of intracellular calcium release (2-APB (IP3 receptor inhibitor) and dantrolene (ryanodine receptor inhibitor)) (Figure 6D).
Figure 6.
Transient activation of caspase-3 in neurons by NMDA. (A, D, E) Immunoblots showing cleaved (active) caspase-3 and caspase-9, and cytosolic cytochrome c in cortical neuronal cultures (DIV18) at various times after treatment with NMDA (50 µM for 10 min) or staurosporine (STS, 1 µM throughout the experimental period). In (D), neurons were preincubated as indicated with 2-APB (30 µM), dantrolene (100 µM), DL-APV (100 µM) or EGTA (5 mM) for 15 min before NMDA treatment, and were immunoblotted 30 min after NMDA stimulation for active (cleaved) and total caspase-3,-9; and cytochrome c in the cytosolic and pellet fractions. (B, C, F) Quantitation (mean ± SEM) of immunoblot band intensities normalized to values before treatment (NMDA was applied from −10 to 0 min; STS treatment began at 0 min); n=3 for each time point. See also Figure S5.
We also measured activation of caspase-3 by an “in situ” binding assay, in which cultured hippocampal neurons were labelled with a biotin-conjugated DEVD probe that has selective affinity for activated caspase-3/7 (Huesmann and Clayton, 2006; Thornberry et al., 1997). The bound biotin-DEVD was then visualized with Oregon Green-conjugated NeutrAvidin. In the absence of stimulation, the level of biotin-DEVD labelling was low in the cell bodies of cultured hippocampal neurons (DIV18), and almost undetectable in dendrites (Figure S5A, top row). The biotin-DEVD signal was enhanced by NMDA stimulation and was distributed throughout the neuron including dendrites (Figure S5A). The DEVD staining in dendrites was often punctate or patchy in appearance (Figure S5A, insets). We quantified the level of biotin-DEVD labelling specifically in neuronal dendrites (integrated intensity of biotin-DEVD labelling per µm dendrite). NMDA caused a rapid and transient increase in active caspase-3; active caspase-3 levels rose ~44% immediately following 10 min NMDA treatment and reached a maximum of ~2.7-fold at 30 min after NMDA stimulation (Figure S5B). By 8 hr after NMDA treatment the level of biotin-DEVD staining was not significantly different from unstimulated neurons (Figure S5B).
Notably, the increase in biotin-DEVD staining induced by NMDA (measured at 30 min after NMDA stimulation) was blocked in neurons transfected with Bcl-xL or XIAP-Bir3 (Figure S5C, D; NB the “beaded” pattern of staining in Bcl-xL and XIAP transfected neurons reflects the subcellular distribution of the transfected protein, not a toxic effect on the transfected cells). This result implies that the in situ biotin-DEVD signal really reflects activation of caspase-3, and it supports the idea that caspase-3 activation by NMDA is mediated by the mitochondrial pathway. We noted that active caspase-3, as imaged by DEVD-biotin labelling, partially overlapped with Bassoon immunostaining, implying that some active caspase-3 is present at or close to synapses (Figure S5E).
The transient time course and low magnitude of caspase-3 activation induced by NMDA suggest that caspase-3 activation in NMDA-treated neurons may not be involved in cell death. To directly assess neuronal death, we stained cultured hippocampal neurons with propidium iodide (PI) 12 hr after NMDA or staurosporine treatment. 77.3% of neurons treated with staurosporine were positive for PI staining but there was no increase in PI staining following NMDA treatment (% PI-stained cells: untreated, 7.6%; NMDA, 6.3%). Thus the transient caspase-3 activation induced by 10 min NMDA stimulation is not associated with eventual neuronal death, which supports a non-apoptotic role of caspase-3 in AMPA receptor internalization and synaptic plasticity.
Cytochrome c release after NMDA stimulation
The inhibition of LTD by Bcl-xL suggests that during LTD the caspase-9-caspase-3 cascade is activated by the mitochondrial pathway. Bcl-xL inhibits mitochondrial release of proapoptotic molecules such as cytochrome c. To test whether cytochrome c is released from mitochondria following chemical LTD, we measured cytochrome c in the cytosolic (mitochondria-free) fraction prepared from cortical neurons by ultracentrifugation. Cytochrome c levels in cytosol were almost undetectable by immunoblotting in unstimulated cultures, but were significantly increased in cultures treated with NMDA (Figure 6E). The level of cytosolic cytochrome c peaked at ~10–20 min after NMDA stimulation (Figure 6E, F). COX IV (cytochrome c oxidase subunit IV) was not found in the cytosol of NMDA-treated neurons (Figure 6E), indicating lack of contamination with mitochondria. These data show that NMDA stimulates rapid and transient cytochrome c release from mitochondria.
Identification of a caspase-3 resistant Akt1 mutant
What is the molecular mechanism by which caspase-3 acts in LTD? We sought to identify downstream substrates of caspase-3 that are involved in synaptic plasticity and that would disrupt LTD when they are rendered resistant to caspase 3-mediated cleavage. Akt1 is a well-characterized prosurvival protein kinase and a substrate of caspase-3 during apoptosis (Bachelder et al., 1999; Widmann et al., 1998). Akt is known to phosphorylate Ser-9 of glycogen synthase kinase-3β (GSK3β) and to inhibit the activity of GSK3β, whose function is critical for LTD (Peineau et al., 2007). Thus, proteolysis and inactivation of Akt1 by caspase-3 represents a possible mechanism for caspase-3 involvement in LTD.
In order to find a mutant Akt1 that is resistant to caspase-3 cleavage but still functional as a kinase, we characterized a series of Akt1 mutants that had substitutions in one or more of four putative caspase cleavage sites: Asp-108, Asp-119, Asp-434 and Asp-462 (Rokudai et al., 2000; Xu et al., 2002) (Figure S6A). These mutant Akt1 proteins expressed at similar levels to wild-type Akt1 in HeLa cells (Figure S6B), and they also showed comparable levels of phosphorylation at Thr-308 and Ser-473, which is correlated with activation of Akt1 kinase (Figure S6B). In contrast, phosphorylation at Thr-308 and Ser-473 is abolished in a kinase-defective Akt1 deletion mutant, Akt1-Mut(1–462) (Figure S6B) (Xu et al., 2002).
The Akt1 mutants were characterized for their degree of resistance to caspase cleavage in HeLa cells treated with STS to induce apoptosis. By immunoblotting, wild-type Akt1 showed reduced protein levels (~50%) at 4 hours after staurosporine addition, consistent with proteolysis by caspase (Figure S6C, D). Of the Akt1 mutants, only the triple mutant D108A/D119A/D462N and the quadruple mutant (D108A/D119A/D434/D462N) showed significantly less drop in levels than wildtype following STS treatment (Figure S6C, D).
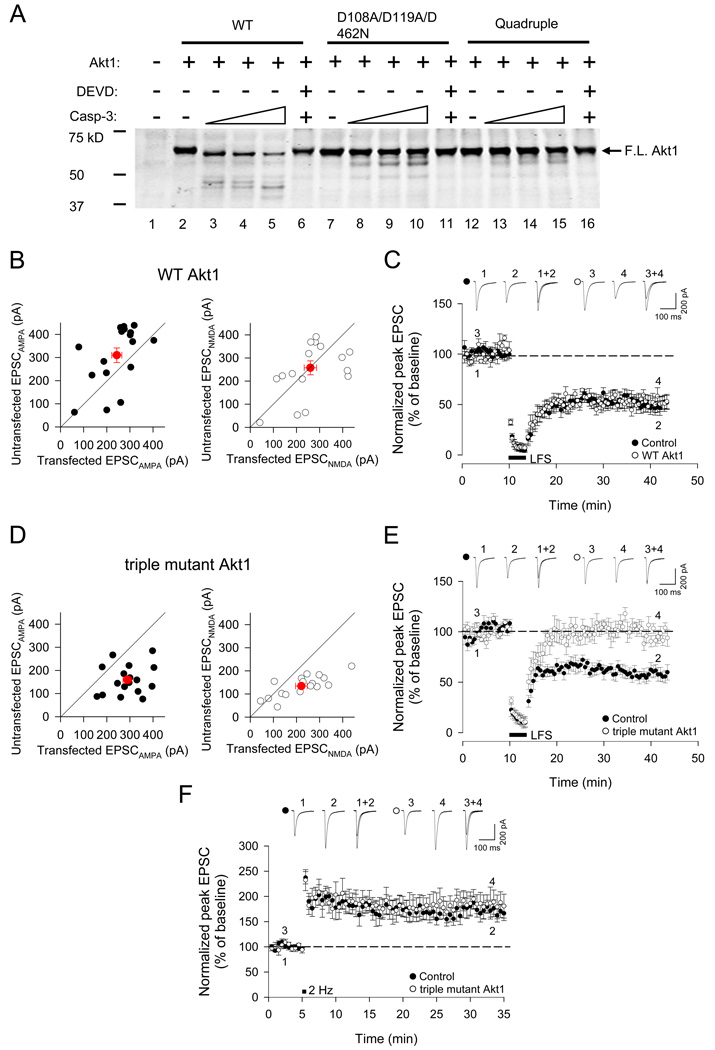
To confirm caspase-3 resistance directly, the mutant Akt1 constructs were transcribed and translated in vitro and tested for proteolysis by purified active recombinant caspase-3 in vitro (Figure 7A). When incubated with caspase-3, wild-type Akt1 was completely cleaved into shorter polypeptides (including a large fragment just slightly shorter than full-length Akt1); the loss of full-length Akt1 protein was blocked by DEVD-FMK (Figure 7A). In contrast, the triple mutant Akt1-D108A/D119A/D462N and the quadruple mutant remained largely unaltered by caspase-3 (Figure 7A). The other mutant Akt1 proteins were all efficiently cleaved into shorter polypeptides by caspase-3 (Figure S6E). Thus Asp-108, Asp-119, and Asp-462 in Akt1appear to be the predominant sites for cleavage by caspase-3.
Figure 7.
Overexpression of caspase-3-resistant triple mutant of Akt1 (D108A/D119A/D462N) blocks LTD in CA1 neurons of organotypic hippocampal slice cultures. (A) Cleavage of wildtype or mutant Akt1 proteins by caspase-3 in vitro. In vitro translated WT Akt1, D108A/D119A/D462N triple mutant or Quadruple mutant were incubated with increasing amounts of recombinant active caspase-3 (10, 20, and 40 ng) at 37°C for 18 h. The caspase-3 inhibitor DEVD-fmk was either omitted or included in the reactions containing 40 ng caspase-3. Position of full-length Akt1 is indicated. (B) Pairwise analysis of wildtype Akt1 (Akt1) transfected cells and nearby untransfected cells on basal AMPA receptor-mediated EPSCs (left panel, EPSCAMPA, recorded at a holding potential of −70mV) and NMDA receptor-mediated EPSCs (right panel, EPSCNMDA, recorded at +40mV). Red symbols show mean ± SEM. (C) Overexpression of WT Akt1 had no effect on LTD compared to neighboring untransfected cells (p<0.01, n=6). (D) Pairwise analysis of Akt1triple mutant (D108A/D119A/D462N)-transfected cells and nearby untransfected cells on basal AMPA receptor-mediated EPSCs and NMDA receptor-mediated EPSCs. (E) Induction of LTD was blocked in Akt1 triple mutant transfected cells (p>0.05, n=6) but LTD was intact in neighboring untransfected cells. (F) LTP was unaffected by overexpression of Akt1 triple mutant. Graphs show mean ± SEM. See also Figure S6.
Finally, we tested in vitro the ability of cleavage-resistant Akt1 mutants to phosphorylate Ser-9 of GSK3β, a natural substrate. Akt1 triple mutant D108A/D119A/D462N was as good as WT Akt1 in phosphorylation of GSK3β Ser-9, but the quadruple mutant was inactive (Figure S6F). Based on these data, we identified the triple mutant D108A/D119A/D462N as an Akt1 mutant that is relatively resistant to caspase-3-mediated proteolysis but retains normal kinase activity.
Caspase-3 resistant Akt1 blocks LTD
If Akt1 is a critical substrate of caspase-3 in LTD, then overexpression of the caspase-3-resistant Akt1 mutant should block LTD induction. Overexpression of WT Akt1 in CA1 cells of hippocampal slice cultures caused a slight reduction in AMPA receptor-mediated EPSCs (WT Akt1: 234 ± 22 pA, n=17; untransfected neighboring cells: 310 ± 32 pA, n=17, p<0.05; Figure 7B) and no effect on NMDA receptor-mediated EPCSs (WT Akt1: 260 ± 27 pA, n=17; untransfected: 257 ± 30 pA, n=17, p>0.05; Figure 7A). Induction of LTD, however, was unaffected by overexpression of WT Akt1 (transfected: 51 ± 8%, n=6; untransfected: 53 ± 7%, n=6, p>0.05; Figure 7C). In cells transfected with the caspase-3-resistant triple mutant of Akt1 (D108A/D119A/D462N), both AMPA and NMDA receptor-mediated EPSCs were significantly increased (triple mutant Akt1 EPSCAMPA: 298 ± 18 pA, n=17; untransfected: 160 ± 15 pA, n=17, p<0.001; triple mutant Akt1 EPSCNMDA: 221 ± 25 pA, n=17; untransfected: 134 ± 11 pA, n=17, p<0.001; Figure 7D). More importantly, LTD was abolished in cells tranfected with the triple mutant Akt1, while untransfected cells in the same slice (measured simultaneously in dual recording conditions) showed normal LTD (triple mutant Akt1: 98 ± 7%; untransfected: 58 ± 7%, n=6, p<0.001; Figure 7E). There was no difference between transfected and untransfected cells with respect to LTP (triple mutant Akt1: 179 ± 24%; untransfected: 176 ± 17%, n=6, Figure 7F). These data imply that proteolysis of Akt1 by caspase-3 is necessary for the induction of LTD.
Is Akt1 is degraded in hippocampal neurons during chem-LTD? At 10–20 min post-NMDA stimulation, GluR1 was dephosphorylated on Ser-845, as expected; however, there was no measurable loss of total Akt1 protein in NMDA-stimulated neuron cultures by immunoblotting (Figure S6G, H). A plausible explanation for the non-detection of Akt1 degradation is that only a small fraction of Akt1 is cleaved by caspase-3 locally in dendrites during chem-LTD.
Discussion
A role for caspases in NMDA receptor-dependent LTD
Caspase-3 and caspase-9 are thought to function in neuronal apoptosis during development, brain trauma and various neurodegenerative diseases (Chan and Mattson, 1999; Yuan and Yankner, 2000). These caspases, as well as apoptosis regulatory factors such as BAD, BAX, Bcl-xL and Bcl-2, are expressed in the mature nervous system, well after the major stage of cell death in rodent brain development, which occurs during embryogenesis and the first few postnatal weeks. Although these pro-apoptotic molecules could be expressed in the brain merely to “prepare” neurons for programmed cell death in response to trauma or disease, it seems plausible that the molecular pathways used in apoptosis are also exploited in neurons for purposes other than cell death. This study implicates the regulators and executors of apoptosis in the molecular mechanisms of synaptic plasticity.
How does caspase-3 mediate its effects on synaptic depression? We provide evidence that Akt1 (a known target of caspase-3 during apoptosis (Bachelder et al., 1999; Widmann et al., 1998)) is a critical substrate of caspase-3 during LTD. In addition to Akt1, there are likely to be multiple other caspase-3 substrates relevant to synaptic function. Several synaptic proteins, including GRASP-1 (a GRIP-associated Ras-guanine nucleotide exchange factor), actin, and AMPA receptor subunit GluR1, are reported to be cleaved by caspase-3 (Lu et al., 2002; Mashima et al., 1997; Ye et al., 2002). Caspases may also cleave signaling molecules (e.g. MEKK1, calcium calmodulin-dependent protein kinase IV) to modify synaptic strength. That LTD involves degradation of specific proteins by caspases is an appealing concept, because synapse weakening is associated with spine shrinkage and synapse elimination and presumably requires dismantling of existing postsynaptic structures.
Non-apoptotic function of caspases in synapses
Several lines of evidence argue that caspase-3 and -9 have specific functional roles in synapses that are unrelated to cell death. Caspase-3 and -9 are critical for LTD and AMPA receptor internalization, but not for LTP. If caspase activation inevitably signals impending cell death, or if caspase inhibitors are non-specifically toxic to neurons, one would expect less selective effects on synaptic function. In fact, inhibitors of caspase-3 or -9 should protect against cell death, rather than cause harm. In addition, short-term NMDA receptor stimulation that is sufficient to induce AMPA receptor redistribution and synaptic depression causes caspase-3 activation that is transient and mild, compared with a conventional agent (staurosporine) that induces massive apoptosis. The activation of caspase-3 induced by short-duration glutamate receptor stimulation was not associated with increased cell death, implying that caspase-3 activation does not inevitably lead to cell demise.
Mattson and colleagues have also reported that caspases can be reversibly activated, and that apoptotic insults such as trophic factor withdrawal, amyloid beta peptide or staurosporine can suppress AMPA receptor-mediated responses via a caspase-dependent mechanism (Glazner et al., 2000; Lu et al., 2002). Bcl-xL overexpression has been reported to enhance synaptic transmission and synapse number (Li et al., 2008).
It is notable that the MAP kinases p38 and JNK, which are well-known players in neuronal apoptosis (Kawasaki et al., 1997; Yang et al., 1997), have been implicated in synaptic depression (Zhu et al., 2002; Zhu et al., 2005). Recently, Peineu et al showed that activity of the protein kinase GSK-3β is increased during, and critical for, NMDA receptor-dependent LTD (Peineau et al., 2007). GSK3 is also known to promote cell death, at least in part via the intrinsic apoptosis pathway (Beurel and Jope, 2006). Interestingly, the prosurvival PI3 kinase-Akt pathway suppresses GSK3 activity, thereby antagonizing LTD (Peineau et al., 2007). This idea is in keeping with our finding that Akt1 needs to be curtailed by caspase-3 proteolysis to allow for LTD. PP2B/calcineurin -- an established player in LTD —is known to promote apoptosis by dephosphorylating BAD (a proapoptotic Bcl-2 family protein), which then activates the mitochondrial pathway (Wang et al., 1999). Thus overall, the signaling mechanisms implicated in LTD are closely linked to those that regulate cell death and survival.
Mitochondria involvement in LTD
Mitochondria are well-suited to play a key role in synaptic plasticity. Mitochondria are present in dendrites and occasionally even in individual spines, and their distribution, morphology and motility are regulated by synaptic activity (Li et al., 2004). Mitochondria in dendrites take up Ca2+ after synaptic stimulation (Pivovarova et al., 2002), and Ca2+ uptake promotes the release of proapoptotic factors from mitochondria (Pacher and Hajnoczky, 2001; Szalai et al., 1999). So mitochondria can act as sensors of elevated postsynaptic Ca2+, which is required for induction of LTD.
Most dendritic mitochondria span lengths that encompass multiple synapses. If mitochondrial release of proapoptotic factors is involved in induction of LTD, one would predict that LTD is not absolutely synapse-specific. Indeed, LTD tends to “spread” more than LTP (Nishiyama et al., 2000), and heterosynaptic LTD (LTD occurring at unstimulated synapses) has been described in a variety of neurons (Barry et al., 1996; Chevaleyre and Castillo, 2003; Christie et al., 1995; Scanziani et al., 1996).
Once the apoptotic mechanism is activated, what decides the balance between synaptic depression versus cell death? We hypothesize that LTD-inducing stimulation only moderately activates the mitochondrial apoptotic pathway locally in dendrites, thereby inducing LTD without ensuing cell death (Gilman and Mattson, 2002; Lu et al., 2002). The involvement of common mechanisms in both programmed cell death and synapse depression/elimination is particularly intriguing because both these processes are believed to result from competition for limiting trophic factors.
Experimental Procedures
Electrophysiology of acute hippocampal slices
Standard whole-cell or field recordings were made from hipocampal slices from Wistar rats (3–4 weeks old) (details in Supplemental Experimental Procedures). Briefly, field recordings were made from stratum pyramidale in area CA1. Stimulating electrodes were placed in the Schaffer collateral-commissural pathway. To induce LTP, tetanic stimuli (100 pulses at 100 Hz, 4 trains) were delivered. To induce LTD, low frequency stimulation (LFS; 1 Hz) was delivered for 200 sec at −40 mV holding potential (whole-cell recording) or 900 sec (field recordings) to Schaffer collateral-CA1 synapses. EPSC amplitude, series resistance and input resistance were monitored and analyzed on-line and off-line using the LTP program (Anderson, 2001). Only cells with series resistance < 25 MΩ with a change in series resistance < 10% from the start were included.
For caspase-3 knockout experiments, mouse hippocampal slices (350 µm) were prepared from 2~4-week-old mice. 100 Hz stimulation (100 pulses, 2 trains, 30 sec interval) was delivered to induce LTP and 1 Hz LFS stimulation (900 pulses, 15 min) was delivered to induce LTD.
Data were collected from one slice per animal. Data pooled across experiments are expressed as the mean ± SEM and effects of conditioning stimulation were measured between 30–35 min after induction of LTD and LTP. Statistical significance was calculated by Student’s two-tailed t-test.
Hippocampal slice culture
Hippocampal slice cultures were prepared from 6~8-day-old Wistar rats and transfected at DIV3-4; whole cell patch recordings of CA1 neurons were performed 3–4 days after transfection (details of methods in Supplemental Experimental Procedures). To induce LTD, 1 Hz stimulation (200 stimuli) at −40 mV was delivered. LTP was induced by pairing 2 Hz stimulation with depolarization of the postsynaptic cell to 0 mV for 100 s. AMPAR-mediated EPSC amplitude (EPSCAMPA) was measured as the peak EPSC amplitude at a holding potential of −70 mV, and NMDAR-mediated EPSC amplitude (EPSCNMDA) was measured at +40 mV at 50–70 ms after the peak of EPSCAMPA.
Caspase-3 knockout mice, DNA constructs and reagents
Caspase-3 knockout mice were purchased from the Jackson Laboratory (Kuida et al., 1996; Leonard et al., 2002). The following constructs were obtained as gifts: XIAP-Bir3 (Colin S. Duckett, University of Michigan (Wilkinson et al., 2004)), Bcl-xL (Azad Bonni, Harvard Medical School). Generation of Akt1 constructs, and sources of commercial chemicals and antibodies are described in Supplemental Experimental Procedures.
Neuronal culture and transfection
Hippocampal neuron cultures were prepared from embryonic day (E) 18–19 rat embryos (Sala et al., 2001), and grown in Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 0.5 mM glutamine and 12.5 µM glutamate. Neurons were transfected using Lipofectamine™ 2000 (Invitrogen).
GluR internalization assay and surface staining
Internalization assays were performed as described (Lee et al., 2002). Briefly, neurons were incubated with antibodies against the N-terminus of GluR2 (mouse monoclonal, Chemicon) or the N-terminus of GluR1 (rabbit polyclonal, Calbiochem) for 10 min at 37°C, then stimulated with NMDA (50 µM) or left unstimulated for 10 min. Neurons were fixed with 4% formaldehyde and 4% sucrose immediately after the stimulation, and surface-remaining antibody-labeled GluR was saturated by incubation with Cy5-conjugated secondary antibody (Jackson ImmunoResearch). Neurons were permeabilized with methanol (−20°C), and internalized antibody-labeled GluR was stained with Alexa 488-conjugated secondary antibody (Invitrogen). To measure steady-state GluR levels on the cell surface, neurons were incubated with antibodies against the N-terminus of GluR2 or GluR1 for 10 min at 37°C, then fixed with 4% formaldehyde and 4% sucrose and stained with Alexa 488-conjugated secondary antibody.
Image acquisition and image analysis
Images were acquired using a Zeiss LSM510 confocal microscope with a 63× (NA 1.4) objective. Confocal images were collapsed to make 2D projections. MetaMorph software was used to measure total integrated intensity of internalized receptors and surface-remaining receptors in the same region which includes the cell body and dendrites within ~50 µm of the cell body. Threshold was set on the basis of cell-free regions and kept constant for all conditions in each experiment. Statistical analysis was performed using Student's t-test. All image acquisition and image analysis were done blind to the treatment.
Supplementary Material
Acknowledgements
We thank Colin S. Duckett, Azad Bonni and Jochen H. M. Prehn for plasmid constructs. M.S. was Investigator of the Howard Hughes Medical Institute. Z. L. was a recipient of NIH fellowship (F32-NS046126) and is supported by NIMH Division of Intramural Research Programs. This work is supported by BBSRC (K.C.) Alzheimer’s Research Trust UK (K.C.), and the Intramural Program of the NIH, NIMH (Z.L., J.J. and S.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson WWaC, GL The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR, Mercurio AM. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MF, Vickery RM, Bolsover SR, Bindman LJ. Intracellular studies of heterosynaptic long-term depression (LTD) in CA1 of hippocampal slices. Hippocampus. 1996;6:3–8. doi: 10.1002/(SICI)1098-1063(1996)6:1<3::AID-HIPO2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Progress in neurobiology. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Chan SL, Griffin WS, Mattson MP. Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer's disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Evidence for common expression mechanisms underlying heterosynaptic and associative long-term depression in the dentate gyrus. J Neurophysiol. 1995;74:1244–1247. doi: 10.1152/jn.1995.74.3.1244. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Denny JB, Polan-Curtain J, Ghuman A, Wayner MJ, Armstrong DL. Calpain inhibitors block long-term potentiation. Brain Res. 1990;534:317–320. doi: 10.1016/0006-8993(90)90148-5. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Embo J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med. 2002;2:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesmann GR, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron. 2006;52:1061–1072. doi: 10.1016/j.neuron.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Leonard JR, Klocke BJ, D'Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. Journal of neuropathology and experimental neurology. 2002;61:673–677. doi: 10.1093/jnen/61.8.673. [DOI] [PubMed] [Google Scholar]
- Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lu C, Fu W, Salvesen GS, Mattson MP. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1:69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mashima T, Naito M, Noguchi K, Miller DK, Nicholson DW, Tsuruo T. Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene. 1997;14:1007–1012. doi: 10.1038/sj.onc.1200919. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. Embo J. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Pozzo-Miller LD, Hongpaisan J, Andrews SB. Correlated calcium uptake and release by mitochondria and endoplasmic reticulum of CA3 hippocampal dendrites after afferent synaptic stimulation. J Neurosci. 2002;22:10653–10661. doi: 10.1523/JNEUROSCI.22-24-10653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Rokudai S, Fujita N, Hashimoto Y, Tsuruo T. Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. J Cell Physiol. 2000;182:290–296. doi: 10.1002/(SICI)1097-4652(200002)182:2<290::AID-JCP18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. Embo J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Nicoll RA, Malenka RC. Heterosynaptic long-term depression in the hippocampus. J Physiol Paris. 1996;90:165–166. doi: 10.1016/s0928-4257(97)81416-7. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW. NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem. 2000;275:33777–33781. doi: 10.1074/jbc.M006226200. [DOI] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Van de Craen M, Vandenabeele P, Declercq W, Van den Brande I, Van Loo G, Molemans F, Schotte P, Van Criekinge W, Beyaert R, Fiers W. Characterization of seven murine caspase family members. EBS Lett. 1997;403:61–69. doi: 10.1016/s0014-5793(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004;24:7003–7014. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu D, Songyang Z. The role of Asp-462 in regulating Akt activity. J Biol Chem. 2002;277:35561–35566. doi: 10.1074/jbc.M203805200. [DOI] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Ye B, Sugo N, Hurn PD, Huganir RL. Physiological and pathological caspase cleavage of the neuronal RasGEF GRASP-1 as detected using a cleavage site-specific antibody. Neuroscience. 2002;114:217–227. doi: 10.1016/s0306-4522(02)00142-2. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yuan J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.