Abstract
Background
Little is known about the effects of hypokalemia on outcomes in patients with chronic heart failure (HF) and chronic kidney disease (CKD).
Methods and Results
Of the 7788 chronic HF patients in the Digitalis Investigation Group trial, 2793 had CKD, defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. Of these, 527 had hypokalemia (serum potassium <4 mEq/L) and 2266 had normokalemia (4–4.9 mEq/L). Propensity scores for hypokalemia were used to assemble a balanced cohort of 522 pairs of patients with hypokalemia and normokalemia. All-cause mortality occurred in 48% and 36% of patients with hypokalemia and normokalemia respectively during 57 months of follow-up (matched hazard ratio {HR} when hypokalemia was compared with normokalemia, 1.56, 95% confidence interval {CI}, 1.25–1.95; P<0.0001). Matched HR’s (95% CI’s) for cardiovascular and HF mortalities, and all-cause, cardiovascular and HF hospitalizations were 1.65 (1.29–2.11; P<0.0001), 1.82 (1.28–2.57; P<0.0001), 1.16 (1.00–1.35; P=0.036), 1.27 (1.08–1.50; P=0.004) and 1.29 (1.05–1.58; P=0.014) respectively. Among 453 pairs of balanced patients with HF and CKD, all-cause mortality occurred in 47% and 38% of patients with mild hypokalemia (3.5–3.9 mEq/L) and normokalemia respectively (matched HR, 1.31, 95% CI, 1.03–1.66; P=0.027). Among 169 pairs of balanced patients with eGFR <45 ml/min/1.73 m2, all-cause mortality occurred in 57% and 47% of patients with hypokalemia (<4 mEq/L) and normokalemia respectively (matched HR, 1.53, 95% CI, 1.07–2.19; P=0.020).
Conclusions
In patients with HF and CKD, hypokalemia is common and associated with increased mortality and hospitalization.
Hypokalemia is common in heart failure (HF) and is associated with poor outcomes.1, 2 Chronic kidney disease (CKD) is also common in HF and is also associated with poor outcomes.3 However, little is known about the prevalence and effect of hypokalemia in chronic HF patients with CKD. While hyperkalemia is considered to be a more common potassium-related problem in CKD,4 hypokalemia may be potentially under-recognized in these patients. Therefore, the purpose of this study was to examine the effect of hypokalemia on outcomes in propensity-matched cohorts of chronic HF patients with CKD.
METHODS
Source of Data
The Digoxin Investigation Group (DIG) trial was a randomized clinical trial of digoxin in HF conducted in 302 centers in the United States and Canada between 1991 and 1993.5 We obtained a public-use copy of the DIG data from the National Heart Lung and Blood Institute. The DIG data was particularly suitable for the current analysis as it included a large sample of chronic HF patients with CKD and did not include any intervention that may have affected potassium homeostasis.
Study Patients
Of the 7788 ambulatory chronic systolic and diastolic HF patients in normal sinus rhythm enrolled in the DIG trial, 6800 had a left ventricular ejection fraction ≤45%. Over 90% of DIG participants were receiving angiotensin-converting enzyme (ACE) inhibitors and nearly 80% were receiving non-potassium-sparing diuretics. At the time of the DIG trial, beta-blockers were not approved for use in HF. Patients with a serum creatinine >2.5mg/dL were excluded. Of the 7788 patients, 6857 (88%) had data on baseline serum potassium. After excluding 579 patients with potassium ≥5 mEq/L, a cohort of 6278 patients were available for these analyses.6
Chronic Kidney Disease
Of the 6278 patients, 2793 (44%) had CKD, defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 body surface area.4, 7 To determine if the effect of hypokalemia in HF patients with CKD can be replicated in those with more advanced CKD, we assembled a separate cohort of 961 HF patients with more advanced CKD (Stage ≥3B, defined as eGFR <45 ml/min/1.73 m2).8
Hypokalemia
Although hypokalemia has traditionally been defined as serum potassium <3.5 mEq/L, in patients with HF, potassium levels <4 mEq/L are considered low and levels between 4 and 5 mEq/L are considered optimal.1, 6, 9 In HF patients, potassium levels of <4 and ≥5 mEq/L have been shown to be associated with poor outcomes when compared with 4–5 mEq/L.1, 6 Therefore, we defined hypokalemia as potassium <4 mEq/L and normokalemia as 4–4.9 mEq/L. Of the 2793 patients with HF and CKD (eGFR <60 ml/min/1.73 m2), 527 (19%) had hypokalemia.
Because hypokalemia was mild (3.5–3.9 mEq/L) in 87% of the 527 patients with hypokalemia, we separately examined the effect of mild hypokalemia and more severe hypokalemia (both versus normokalemia). Finally, to determine the effect of hypokalemia in HF patients with more advanced CKD (eGFR <45 ml/min/1.73 m2), we assembled a cohort of 961 HF patients with CKD Stage ≥3B (eGFR <45 ml/min/1.73 m2). Of these, 178 (19%) had hypokalemia and only 26 (3%) patients had more severe hypokalemia (potassium <3.5 mEq/L).
Study Outcomes
The primary outcome of our study was all-cause mortality. Secondary outcomes were cardiovascular and HF mortality, and all-cause, cardiovascular and HF hospitalizations. Vital status data were complete for 99% of patients during 57 months of follow-up.10
Assembly of Balanced Study Cohorts
Because of the imbalances in baseline patient characteristics between patients with normokalemia and hypokalemia (Table 1 and Figure 1), we used propensity score matching to assemble a cohort in which these two groups would be balanced on all measured baseline characteristics. 11–16 We began by estimating propensity scores for hypokalemia for each patient using a non-parsimonious multivariable logistic regression model.2, 16–22 A patient’s propensity for hypokalemia is his/her probability of having hypokalemia given his/her measured baseline characteristics. In the model, hypokalemia was the dependent variable and 32 measured baseline patient characteristics (Figure 1) and two significant clinically important interaction terms (Creatinine by diuretic use and creatinine by angiotensin-converting enzyme inhibitor use) were included as covariates.
Table 1.
Baseline characteristics of chronic heart failure patients with chronic kidney disease, by potassium levels, before and after propensity score matching
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Potassium 4–4.9 mEq/L (n = 2266) | Potassium <4 mEq/L (n = 527) | P value | Potassium 4–4.9 mEq/L (n = 522) | Potassium <4 mEq/L (n = 522) | P value | |
| Age, years | 68 ± 9 | 68 ± 10 | 0.405 | 68 ± 10 | 68 ± 10 | 0.718 |
| Female | 680 (30%) | 207 (39%) | <0.001 | 200 (38%) | 204 (39%) | 0.830 |
| Non-white | 180 (8%) | 52 (10%) | 0.150 | 54 (10%) | 51 (10%) | 0.830 |
| Body mass index, kg/m2 | 27 ± 5 | 27 ± 5 | 0.507 | 27 ± 5 | 27 ± 5 | 0.729 |
| Duration of heart failure, months | 30 ± 37 | 29 ± 37 | 0.684 | 28 ± 36 | 29 ± 37 | 0.379 |
| Ischaemic heart disease | 1674 (74%) | 353 (67%) | 0.001 | 340 (65%) | 350 (67%) | 0.513 |
| Prior myocardial infarction | 1508 (67%) | 318 (60%) | 0.007 | 300 (58%) | 315 (60%) | 0.361 |
| Hypertension | 1113 (49%) | 294 (56%) | 0.006 | 298 (57%) | 289 (55%) | 0.596 |
| Diabetes mellitus | 675 (30%) | 146 (28%) | 0.344 | 145 (28%) | 145 (28%) | 1.000 |
| Medications | ||||||
| Pre-trial digoxin use | 942 (42%) | 225 (43%) | 0.638 | 221 (50%) | 222 (50%) | 1.000 |
| Trial use of digoxin | 1133 (50%) | 254 (48%) | 0.456 | 238 (46%) | 252 (48%) | 0.409 |
| Angiotensin-converting enzyme inhibitors | 2096 (93%) | 475 (90%) | 0.071 | 484 (93%) | 475 (91%) | 0.362 |
| Diuretics | 1888 (83%) | 461 (88%) | 0.019 | 465 (89%) | 456 (87%) | 0.439 |
| Potassium-sparing diuretics | 189 (8%) | 50 (10%) | 0.397 | 53 (10%) | 50 (10%) | 0.838 |
| Potassium supplement | 721 (32%) | 247 (47%) | <0.001 | 233 (45%) | 242 (46%) | 0.504 |
| Symptoms and signs of heart failure | ||||||
| Dyspnea on exertion | 1751 (77%) | 403 (77%) | 0.693 | 395 (78%) | 398 (76%) | 0.882 |
| Jugular venous distension | 299 (13%) | 94 (18%) | 0.006 | 87 (17%) | 90 (17%) | 0.858 |
| Third heart sound | 549 (24%) | 155 (29%) | 0.014 | 173 (33%) | 152 (29%) | 0.182 |
| Pulmonary râles | 384 (17%) | 106 (20%) | 0.085 | 103 (20%) | 103 (20%) | 1.000 |
| Lower extremity edema | 507 (22%) | 148 (28%) | 0.005 | 146 (28%) | 144 (28%) | 0.942 |
| New York Heart Association class III–IV | 822 (36%) | 202 (38%) | 0.378 | 196 (38%) | 197 (38%) | 0.949 |
| Heart rate, beats per minute | 78 ± 13 | 80 ± 13 | 0.004 | 79 ± 13 | 80 ± 13 | 0.471 |
| Systolic blood pressure, mm Hg | 129 ± 21 | 129 ± 23 | 0.569 | 129 ± 23 | 129 ± 23 | 0.933 |
| Diastolic blood pressure, mm Hg | 74 ± 11 | 75 ± 12 | 0.206 | 75 ± 11 | 75 ± 12 | 0.715 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 327 (14%) | 98 (19%) | 0.016 | 99 (19%) | 95 (18%) | 0.793 |
| Cardiothoracic ratio >0.5 | 1400 (62%) | 374 (71%) | <0.001 | 358 (69%) | 369 (71%) | 0.472 |
| Serum creatinine, mg/dL | 1.53 ± 0.34 | 1.53 ± 0.40 | 0.703 | 1.52 ± 0.35 | 1.52 ± 0.39 | 0.963 |
| Estimated GFR, ml/min/1.73 m2 | 47 ± 9 | 47 ± 10 | 0.527 | 47 ± 9 | 47 ± 10 | 0.701 |
| Ejection fraction, % | 32 ± 13 | 32 ± 14 | 0.969 | 33 ± 14 | 32 ± 14 | 0.995 |
Values are presented as n (%) or mean ± standard deviation. GFR=glomerular filtration rate
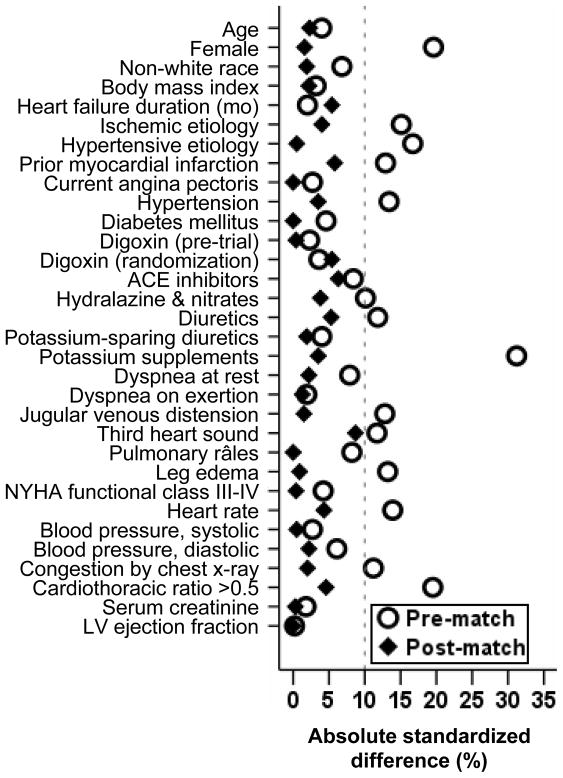
Figure 1.
Love plot displaying pre- and post-match absolute standardized differences for baseline covariates between patients with normokalemia (4–4.9 mEq/L) and hypokalemia (<4 mEq/L)
The efficacy of the propensity score model was assessed by estimating absolute standardized differences for each covariate between the groups.13, 16, 23 Standardized differences directly quantify biases in the means (or proportions) of covariates across the groups, and are expressed as percentages of the pooled standard deviations,11, 13, 24, 25 which are presented as a Love plot.16–22 An absolute standardized difference of 0% on a covariate indicates no residual bias for that covariate and values <10% suggests inconsequential residual bias.16–22 Using a 1 to 1 greedy matching protocol, described elsewhere in detail, we matched 522 (99% of 527) patients with hypokalemia with 522 patients with normokalemia, who had similar propensity scores.16–22
We repeated the above process to assemble three additional cohorts of patients as follows: (1) Using 2724 HF and CKD (GFR <60 ml/min/1.73 m2) patients with normokalemia (n=2266) and mild hypokalemia (potassium 3.5–3.9 mEq/L; n=458), we assembled a matched cohort of 453 pairs of patients; (2) Using 2335 HF and CKD (GFR <60 ml/min/1.73 m2) patients with normokalemia (n=2266) and more severe hypokalemia (potassium <3.5 mEq/L; n=69), we assembled a matched cohort of 65 pairs of patients; and (3) Using 961 patients with HF and CKD Stage ≥3B (GFR <45 ml/min/1.73 m2) with normokalemia (n=783) and hypokalemia (potassium <4 mEq/L; n=178), we assembled a matched cohort of 169 pairs of patients.
Statistical Analysis
For descriptive analyses, we used Pearson Chi square and Wilcoxon rank-sum tests for the pre-match data, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate. Kaplan-Meier plots and matched Cox regression analysis were used to estimate associations of hypokalemia with various outcomes. Matched Cox regression models are essentially stratified Cox regression models, in which the matching variable is the unit for stratification. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. We conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate conclusions based on significant associations between hypokalemia and outcomes among matched patients.27 To determine the homogeneity of the associations of hypokalemia with all-cause mortality among patients with HF and CKD, we examined the association in various subgroups of matched patients. We then formally tested for first-order interactions using Cox proportional hazards models, entering interaction terms for the subgroup (e.g. sex by hypokalemia for the sex subgroup). All statistical tests were evaluated using two-tailed 95% confidence levels and a p-value <0.05 considered significant. Data analyses were performed using SPSS for Windows version 15.26
RESULTS
Patient Characteristics
The mean (±SD) age of the 1044 matched patients was 68 (±10) years, 404 (39%) were women and 105 (10%) were non-whites. Before matching, patients with mild hypokalemia were more likely to be women, have a history of hypertension and cardiomegaly, and receive diuretics and potassium supplements. These and other pre-match imbalances were balanced after matching (Table 1 and Figure 1). Post-match absolute standardized differences for all observed covariates were below 10% suggesting substantial improvement in covariate balance between the groups (Figure 1).3, 16, 25 Pre- and post-match absolute standardized differences for propensity scores were 48.3% and 0.04% respectively.
Hypokalemia and Mortality in Patients with HF and CKD
All-cause mortality occurred in 48% and 36% of patients with hypokalemia and normokalemia respectively (matched hazard ratio {HR} when hypokalemia was compared with normokalemia, 1.56, 95% confidence interval {CI}, 1.25–1.95; P<0.0001; Table 2 and Figure 2). Associations of hypokalemia with cardiovascular and HF mortalities among matched patients are displayed in Table 2.
Table 2.
Serum potassium <4 mEq/L and outcomes in patients with chronic HF and CKD
| Events (%); rate per 10,000 person-years | Absolute rate difference* (per 10,000 person-years) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Outcomes | Serum potassium 4–4.9 mEq/L (N=522) | Serum potassium <4 mEq/L (N=522) | |||
| Mortality | |||||
| All-cause | 187 (36%); 1276 | 249 (48%); 1864 | + 587 | 1.56 (1.25–1.95) | <0.001 |
| Cardiovascular | 145 (28%); 990 | 204 (39%); 1527 | + 537 | 1.65 (1.29–2.11) | <0.001 |
| Progressive HF | 71 (14%); 485 | 111 (21%); 831 | + 346 | 1.82 (1.28–2.57) | <0.001 |
| Hospitalization | |||||
| All-cause | 358 (69%); 4403 | 376 (72%); 5288 | + 885 | 1.16 (1.00–1.35) | 0.036 |
| Cardiovascular | 275 (53%); 2753 | 309 (59%); 3657 | + 904 | 1.27 (1.08–1.50) | 0.004 |
| Worsening HF | 177 (34%); 1451 | 203 (39%); 1931 | + 481 | 1.29 (1.05–1.58) | 0.014 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the serum potassium 4–4.9 mEq/L group from the event rates in the serum potassium <4 mEq/L group
Figure 2.
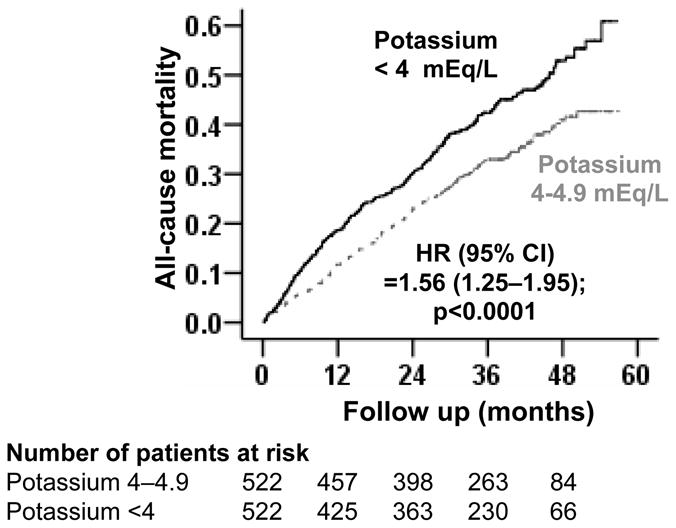
Kaplan-Meier plots for all-cause mortality by serum potassium levels
Hypokalemia and Hospitalization in Patients with HF and CKD
Cardiovascular hospitalization occurred in 59% and 53% of patients with hypokalemia and normokalemia respectively (matched HR, 1.27, 95% CI, 1.08–1.50; P=0.004; Table 2). Associations of hypokalemia with all-cause and HF hospitalizations among matched patients are displayed in Table 2.
Mild Hypokalemia and Outcomes in Patients with HF and CKD
All-cause mortality occurred in 47% and 38% of patients with mild hypokalemia and normokalemia respectively (matched HR, 1.31, 95% CI, 1.03–1.66; P=0.027; Table 3). Associations of mild hypokalemia with other outcomes are displayed in Table 3.
Table 3.
Serum potassium 3.5–3.9 mEq/L and outcomes in patients with chronic HF and CKD
| Events (%); rate per 10,000 person-years | Absolute rate difference* (per 10,000 person-years) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Outcomes | Serum potassium 4–4.9 mEq/L (N=453) | Serum potassium 3.5–3.9 mEq/L (N=453) | |||
| Mortality | |||||
| All-cause | 173 (38%); 1401 | 212 (47%); 1819 | + 418 | 1.31 (1.03–1.66) | 0.027 |
| Cardiovascular | 135 (30%); 1094 | 176 (39%); 1511 | + 417 | 1.34 (1.03–1.74) | 0.030 |
| Progressive HF | 60 (13%); 486 | 92 (20%); 790 | + 304 | 1.58 (1.06–2.34) | 0.025 |
| Hospitalization | |||||
| All-cause | 306 (68%); 4454 | 320 (71%); 5031 | + 577 | 1.17 (0.96–1.44) | 0.121 |
| Cardiovascular | 227 (50%); 2633 | 264 (58%); 3515 | + 882 | 1.31 (1.05–1.64) | 0.016 |
| Worsening HF | 145 (32%); 1408 | 171 (38%); 1851 | + 443 | 1.29 (0.99–1.67) | 0.057 |
Absolute differences in rates of events per 10,000 person-years of follow-up were calculated by subtracting the event rates in the serum potassium 4 to 4.9 mEq/L group from the event rates in the serum potassium 3.5 to 3.9 mEq/L group.
More Severe Hypokalemia and Outcomes in Patients with HF and CKD
All-cause mortality occurred in 55% and 38% of patients with more severe hypokalemia and normokalemia respectively (matched HR, 2.07, 95% CI, 1.12–3.83; P=0.021; Table 4). Associations of more severe hypokalemia with other outcomes in patients with HF and CKD are displayed in Table 4. Among the 527 patients with hypokalemia, all-cause mortality occurred in 55% and 47% of those with more severe and mild hypokalemia respectively (propensity-score adjusted HR for more severe hypokalemia, 1.36; 95% CI, 0.94–1.95; P=0.102).
Table 4.
Serum potassium <3.5 mEq/L and outcomes in patients with chronic HF and CKD
| Events (%); rate per 10,000 person-years | Absolute rate difference* (per 10,000 person-years) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Outcomes | Serum potassium 4–4.9 mEq/L (N=65) | Serum potassium <3.5 mEq/L (N=65) | |||
| Mortality | |||||
| All-cause | 25 (38%); 1276 | 36 (55%); 2236 | + 961 | 2.07 (1.12–3.83) | 0.021 |
| Cardiovascular | 19 (29%); 969 | 27 (42%); 1677 | + 708 | 2.09 (1.02–4.29) | 0.044 |
| Progressive HF | 9 (14%); 459 | 20 (31%); 1242 | + 783 | 2.83 (1.12–7.19) | 0.028 |
| Hospitalization | |||||
| All-cause | 52 (80%); 5714 | 55 (85%); 8209 | + 2495 | 1.18 (0.71–1.95) | 0.523 |
| Cardiovascular | 44 (68%); 3894 | 44 (68%); 5116 | + 1222 | 1.29 (0.76–2.20) | 0.347 |
| Worsening HF | 30 (46%); 1974 | 33 (51%); 2821 | + 847 | 1.24 (0.65–2.34) | 0.517 |
Absolute differences in rates of events per 10,000 person-years of follow-up were calculated by subtracting the event rates in the serum potassium 4 to 4.9 mEq/L group from the event rates in the serum potassium <3.5 mEq/L group.
Hypokalemia and Outcomes in Patients with HF and More Advanced CKD
All-cause mortality occurred in 57% and 47% of patients with hypokalemia and normokalemia respectively (matched HR, 1.53, 95% CI, 1.07–2.19; P=0.020; Table 5). Associations of hypokalemia with other outcomes in these patients are displayed in Table 5.
Table 5.
Serum potassium <4 mEq/L and outcomes in patients with chronic HF and stage ≥3B CKD (eGFR <45 ml/min/1.73 m2)
| Events (%); rate per 10,000 person-years | Absolute rate difference* (per 10,000 person-years) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Outcomes | Serum potassium 4–4.9 mEq/L (N=169) | Serum potassium <4 mEq/L (N=169) | |||
| Mortality | |||||
| All-cause | 80 (47%); 1822 | 97 (57%); 2487 | + 665 | 1.53 (1.07–2.19) | 0.020 |
| Cardiovascular | 63 (37%); 1435 | 79 (47%); 2026 | + 591 | 1.49 (1.00–2.21) | 0.049 |
| Progressive HF | 25 (15%); 569 | 53 (31%); 1359 | + 790 | 2.47 (1.41–4.34) | 0.002 |
| Hospitalization | |||||
| All-cause | 119 (70%); 5085 | 135 (80%); 7258 | + 2173 | 1.54 (1.11–2.14) | 0.010 |
| Cardiovascular | 93 (55%); 3218 | 109 (64%); 4781 | + 1563 | 1.33 (0.94–1.90) | 0.110 |
| Worsening HF | 68 (40%); 1960 | 71 (42%); 2383 | + 423 | 1.26 (0.83–1.93) | 0.282 |
Absolute differences in rates of events per 10,000 person-years of follow-up were calculated by subtracting the event rates in the serum potassium 4 to 4.9 mEq/L group from the event rates in the serum potassium <4 mEq/L group.
Findings from Sensitivity Analyses
For all-cause mortality, in the absence of a hidden bias, a sign-score test for matched data with censoring provided strong evidence (P <0.0001) that patients with normokalemia clearly outlived those with hypokalemia. A hidden covariate that is a near-perfect predictor of total mortality would need to increase the odds of hypokalemia by 25.2% to explain away this association. Hypokalemia was also associated with reduction in cardiovascular mortality (sign-score test P <0.0001), all-cause hospitalization (sign-score test P =0.004) and cardiovascular hospitalization (sign-score test P=0.003), and a hidden covariate would need to increase the odds of hypokalemia by 28.9%, 8.9% and 11.1% respectively to explain away these associations.
Findings from Subgroups Analyses
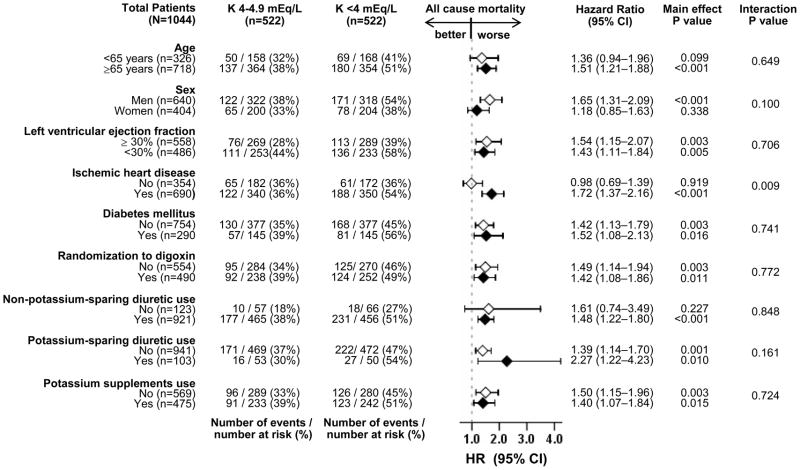
The effect of hypokalemia on all-cause mortality was significant only in patients with IHD but not in those without (p for interaction, 0.009; Figure 3). The effect of hypokalemia on cardiovascular hospitalization was significant only among matched patients with IHD (HR, 1.35, 95% CI, 1.11–1.64; P=0.003), but not in those without (HR, 1.13, 95% CI, 0.84–1.51; P=0.420; p for interaction, 0.321; data not shown). HR’s (95% CIs) for HF hospitalization for matched patients with and without IHD were 1.46, 95% CI, 1.14–1.87; P=0.003) and 1.00 (95% CI, 0.70–1.42; P=0.978; p for interaction, 0.073; data not shown).
Figure 3.
Association of hypokalemia (serum potassium <4 mEq/L) with all-cause mortality in subgroups of patients with chronic heart failure with chronic kidney disease
DISCUSSION
The findings of the current study suggest that in ambulatory patients with chronic HF and CKD receiving ACE inhibitors and non-potassium-sparing diuretics, hypokalemia (<4 mEq/L) was common and was associated with increased mortality and hospitalizations. Further, we demonstrate that hypokalemia was mild (3.5–3.9 mEq/L) in most patients, and that even mild hypokalemia was associated with poor outcomes. Additionally, hypokalemia also increased risk of death in those with more advanced CKD (eGFR <45 ml/min/1.73 m2). To the best of our knowledge this is the first report of an association between hypokalemia and poor outcomes in propensity-matched cohorts of HF patients with CKD. The findings are important as both CKD and hypokalemia are highly prevalent in HF. While the presence of CKD increases the risk of hyperkalemia and associated complications, these findings demonstrate that underestimating the presence and the risk of hypokalemia in HF patients with CKD is also a concern.
There are several potential explanations for the associations between hypokalemia and poor outcomes in patients with chronic HF and CKD: confounding by imbalances in measured baseline characteristics, confounding by unmeasured baseline characteristics, and/or an intrinsic effect of low serum potassium. Bivariate associations between hypokalemia and poor outcomes may potentially be explained by residual bias. However, all measured baseline characteristics were well-balanced among our propensity-matched patients with normokalemia and hypokalemia. Therefore, hypokalemia-associated poor outcomes observed in our study may not be explained by imbalances in any of the measured baseline characteristics.
Confounding by an unmeasured baseline characteristic may also potentially explain the poor outcomes associated with hypokalemia. For example, we had no data on diuretic doses, which may be a potential confounder, as sicker HF patients were more likely to receive larger doses of diuretics and develop more severe hypokalemia. Diuretic use is associated with poor outcomes, which has been shown to be dose dependent.16, 28, 29 Although the prevalence of diuretic use was similar, it is possible that those with hypokalemia were using higher doses of diuretics. However, this is unlikely to explain away the observed associations as the findings from our sensitivity analysis suggest that these associations were robust and rather insensitive to the potential confounding effect of an unmeasured covariate. Further, the potential effect of an unmeasured confounder can also be indirectly assessed by examining balance on variables that might be strongly correlated with that unmeasured confounder.23 For example, NYHA class and symptoms and signs of fluid volume overload would be strongly correlated with the diuretic doses. However, in our study, these markers of higher diuretic doses were balanced after matching, suggesting that any confounding effect by diuretic dose would likely be minimal. Finally, the observation that the associations between hypokalemia and poor outcomes were observed at various degrees of hypokalemia and at various stages of CKD also highlights the robustness of those associations.
The notion that the associations between hypokalemia and poor outcomes may be intrinsic in nature is biologically plausible. Hypokalemia is known to enhance membrane excitability, increase cardiac automaticity, delay ventricular repolarization and predispose patients to reentrant arrhythmias.30–33 Hypokalemia-associated deaths have often been attributed to cardiac arrhythmias and sudden cardiac death. We have previously demonstrated that in HF patients with and without CKD, hypokalemia was associated with increased risk of death without an increase in hospitalization suggesting sudden death may have precluded hospitalization in those patients.1, 2 However, in the current analysis, we observed that hypokalemia was associated with both increased death and hospitalization, suggesting that the effect of hypokalemia in HF patients with CKD may be both sudden and non-sudden in nature. The progressive deleterious effects of hypokalemia in HF patients with CKD may also be mediated by aldosterone, which has been shown to cause myocardial fibrosis, diastolic dysfunction and disease progression in HF.33–36 Although the effect of hypokalemia in the setting of acute myocardial infarction is well known,37–39 little is known about the effect of hypokalemia in patients with chronic IHD. Although the prevalence of hypokalemia was lower in patients with IHD (Table 1, pre-match), the effects of hypokalemia were worse in those with IHD (Figure 3), suggesting that infarcted/ischemic myocardium may provide a more suitable substrate for the adverse effects of hypokalemia.
An interesting observation of our study is that the prevalence of hypokalemia in patients with HF and CKD was high (19%) and similar to that in HF patients in general.1, 2 Among the 3739 patients without CKD and with valid serum potassium (excluded from the current analysis), only 18% had potassium <4 mEq/L (data not shown). This is important as hyperkalemia is often considered a more common problem of potassium homeostasis in patients with CKD. However, findings from our study suggest that hypokalemia is common in patients with HF and CKD receiving ACE inhibitors and that even a mild reduction in serum potassium level (3.5–3.9 mEq/L) was associated with poor outcomes. These findings are important because patients with HF and CKD often require larger doses of diuretics increasing their risk of hypokalemia. Yet, hypokalemia in these patients is less likely to be treated for fear of causing hyperkalemia. Therefore, taken together with our prior reports and expert opinions, it may be suggested that serum potassium should be routinely monitored in HF patients with CKD and carefully maintained between 4 and 5 mEq/L.1, 2, 6, 9, 40
There are a few limitations of our study. The MDRD formula may underestimate GFR in patients with GFR >60 ml/min/1.73 m2.41 However, all patients in our analysis had eGFR <60 ml/min/1.73 m2. Further, we were able to replicate our key findings in more advanced CKD patients. As previously mentioned, diuretic dose was not available. B-type natriuretic peptide (BNP) levels were also not available and could have provided further data on HF severity. Findings of our study are based on predominantly white men in normal sinus rhythm. Data on beta-blocker use was not collected in the DIG trial as these drugs were not approved for use in HF at that time. The transfer of potassium from plasma into cells is facilitated by stimulation of beta-2 receptors.42–44 Therefore, the prevalence of hypokalemia may be somewhat lower in patients receiving carvedilol and metoprolol extended-release, the two most commonly used beta-blockers in HF.45 However, the effect of hypokalemia on outcomes is unlikely to be substantially different from that observed in our study. Future studies may examine the effect of hypokalemia in contemporary HF patients with CKD.
In conclusion, in ambulatory patients with chronic HF and CKD, hypokalemia (<4 mEq/L) is common and associated with increased mortality and hospitalization. Further, hypokalemia in these patients is mostly mild (3.5–3.9 mEq/L) but even the mild hypokalemia is associated with poor outcomes. Serum potassium should be routinely monitored in HF patients with CKD, and should be carefully maintained between 4 and 5 mEq/L.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the NIH through grants (R01-HL085561 and R01-HL097047) from the NHLBI and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama; Dr. Sanders is supported by NIH grants R01 DK046199 and P30 DK079337
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interests
No authors have any conflicts of interest in relation to this manuscript.
References
- 1.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper AB, Campbell RC, Anker SD, Bakris G, Wahle C, Love TE, Hamm LL, Mujib M, Ahmed A. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int J Cardiol. 2009;137:1–8. doi: 10.1016/j.ijcard.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 5.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed MI, Ekundayo OJ, Mujib M, Campbell RC, Sanders PW, Pitt B, Perry GJ, Bakris G, Aban I, Love TE, Aronow WS, Ahmed A. Mild hyperkalemia and outcomes in chronic heart failure: A propensity matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Archibald G, Bartlett W, Brown A, Christie B, Elliott A, Griffith K, Pound S, Rappaport I, Robertson D, Semple Y, Slane P, Whitworth C, Williams B. UK Consensus Conference on Early Chronic Kidney Disease--6 and 7 February 2007. Nephrol Dial Transplant. 2007;22:2455–2457. doi: 10.1093/ndt/gfm268. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched. Sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33–38. [Google Scholar]
- 12.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 13.Austin PC. Report Card on Propensity-Score Matching in the Cardiology Literature From 2004 to 2006: A Systematic Review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. doi: 10.1161/CIRCOUTCOMES.108.790634. [DOI] [PubMed] [Google Scholar]
- 14.Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–583. doi: 10.1016/j.nut.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: A propensity-matched study. Int J Cardiol. 2009;136:270–277. doi: 10.1016/j.ijcard.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–253. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekundayo OJ, Adamopoulos C, Ahmed MI, Pitt B, Young JB, Fleg JL, Love TE, Sui X, Perry GJ, Siscovick DS, Bakris G, Ahmed A. Oral potassium supplement use and outcomes in chronic heart failure: A propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2008.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekundayo OJ, Dell’Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd-Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie C, Ekundayo OJ, Muchimba M, Campbell RC, Frank SJ, Liu B, Aban IB, Ahmed A. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: A propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui X, Gheorghiade M, Zannad F, Young JB, Ahmed A. A propensity matched study of the association of education and outcomes in chronic heart failure. Int J Cardiol. 2008;129:93–99. doi: 10.1016/j.ijcard.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297:314–316. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 26.SPSS for Windows, Rel. 15 [computer program]. Version. Chicago, IL: SPSS Inc., Chicago, IL; 2009. [Google Scholar]
- 27.Rosenbaum PR. Sensitivity to Hidden Bias. In: Rosenbaum PR, editor. Observational Studies. 2. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 28.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 29.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 30.Fitzovich DE, Hamaguchi M, Tull WB, Young DB. Chronic hypokalemia and the left ventricular responses to epinephrine and preload. J Am Coll Cardiol. 1991;18:1105–1111. doi: 10.1016/0735-1097(91)90774-4. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro JI, Banerjee A, Reiss OK, Elkins N. Acute and chronic hypokalemia sensitize the isolated heart to hypoxic injury. Am J Physiol. 1998;274:H1598–1604. doi: 10.1152/ajpheart.1998.274.5.H1598. [DOI] [PubMed] [Google Scholar]
- 32.Yano K, Hirata M, Matsumoto Y, Hano O, Mori M, Ahmed R, Mitsuoka T, Hashiba K. Effects of chronic hypokalemia on ventricular vulnerability during acute myocardial ischemia in the dog. Jpn Heart J. 1989;30:205–217. doi: 10.1536/ihj.30.205. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava TN, Young DB. Impairment of cardiac function by moderate potassium depletion. J Card Fail. 1995;1:195–200. doi: 10.1016/1071-9164(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 34.Young DB, Lin H, McCabe RD. Potassium’s cardiovascular protective mechanisms. Am J Physiol. 1995;268:R825–837. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 35.Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 36.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 37.Kafka H, Langevin L, Armstrong PW. Serum magnesium and potassium in acute myocardial infarction. Influence on ventricular arrhythmias. Arch Intern Med. 1987;147:465–469. [PubMed] [Google Scholar]
- 38.Cooper WD, Kuan P, Reuben SR, VandenBurg MJ. Cardiac arrhythmias following acute myocardial infarction: associations with the serum potassium level and prior diuretic therapy. Eur Heart J. 1984;5:464–469. doi: 10.1093/oxfordjournals.eurheartj.a061692. [DOI] [PubMed] [Google Scholar]
- 39.Nordrehaug JE, von der Lippe G. Serum potassium concentrations are inversely related to ventricular, but not to atrial, arrhythmias in acute myocardial infarction. Eur Heart J. 1986;7:204–209. doi: 10.1093/oxfordjournals.eurheartj.a062052. [DOI] [PubMed] [Google Scholar]
- 40.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 41.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 42.Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414–1419. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]
- 43.Lim M, Linton RA, Wolff CB, Band DM. Propranolol, exercise, and arterial plasma potassium. Lancet. 1981;2:591. doi: 10.1016/s0140-6736(81)90987-9. [DOI] [PubMed] [Google Scholar]
- 44.Rosa RM, Silva P, Young JB, Landsberg L, Brown RS, Rowe JW, Epstein FH. Adrenergic modulation of extrarenal potassium disposal. N Engl J Med. 1980;302:431–434. doi: 10.1056/NEJM198002213020803. [DOI] [PubMed] [Google Scholar]
- 45.Zebrack JS, Munger M, Macgregor J, Lombardi WL, Stoddard GP, Gilbert EM. Beta-receptor selectivity of carvedilol and metoprolol succinate in patients with heart failure (SELECT trial): a randomized dose-ranging trial. Pharmacotherapy. 2009;29:883–890. doi: 10.1592/phco.29.8.883. [DOI] [PubMed] [Google Scholar]