Abstract
The arcuate fasciculus (AF) is a white matter pathway traditionally considered to connect left Broca’s area with posterior language zones. We utilized diffusion tensor imaging (DTI) in eight healthy subjects (5M) to track pathways in the horizontal mid-portion of the AF (hAF) to subregions of Broca’s area - pars triangularis (PTr) and pars opercularis (POp); and to ventral premotor cortex (vPMC) in the right and left hemispheres (RH, LH). These pathways have previously been studied in the LH, but not in the RH. Only 1/8 subjects showed fiber tracts between PTr and hAF in the RH (also, only 1/8 in the LH). In contrast to PTr, 5/8 subjects showed fiber tracts between POp and hAF in the RH (8/8 in the LH). Fiber tracts for vPMC were similar to those of POp, where 7/8 subjects showed fiber tracts between vPMC and hAF in the RH (8/8 in the LH). Our designated hAF could have included some of the superior longitudinal fasciculus (SLF) III, because it is difficult to separate the two fiber bundles. The SLF III has been previously reported to connect supramarginal gyrus with POp and vPMC in the LH. Thus, although the present DTI study showed almost no pathways between PTr and hAF in the RH (and in the LH), robust pathways were observed between POp and/or vPMC with hAF in the RH (and in LH). These results replicate previous studies for the LH, but are new, for the RH. They could contribute to better understanding of recovery in aphasia.
Keywords: Aphasia, Arcuate Fasciculus, Broca’s area, Pars Triangularis, Pars Opercularis, Diffusion Tensor Imaging, Ventral Premotor Cortex
INTRODUCTION
The anatomy and function of the left (L) posterior inferior frontal gyrus has been studied since the time of Broca (Broca 1861). Broca’s area is critically involved in language, including syntactic, semantic and phonological processing (Price, 2000; Bookheimer, 2002; Grodzinsky and Amunts 2006 for review; Saur et al., 2008). In some functional imaging studies involving healthy subjects, the L pars triangularis (PTr) portion of Broca’s area has been observed to activate in semantic processing, whereas the L pars opercularis (POp), relatively more in phonological processing (Buckner et al., 1995; Price et al., 1996; Fiez 1997; Poldrack et al., 1999; Gold and Buckner 2002; Devlin et al., 2003; Nixon et al., 2004). Lesion in L Broca’s area continues to be associated with speech disturbance (Mohr et al., 1978; Alexander et al., 1990). Lesion deep to Broca’s area including periventricular white matter is associated with longer-lasting nonfluent aphasia (Naeser et al., 1989).
The contribution of right (R) Broca’s homologue to language includes prosody, discourse and processing of syntactic violations (Blumstein et al., 1977; Ross, 1981; Yeni-Komshian and Lafontaine 1983; Bradvik et al., 1991; Buckner et al., 1995; Nichelli et al., 1995; Winner et al., 1998; Meyer et al., 2000; Finger et al., 2003; Nishitani et al., 2005 for review). A role for the R POp and R ventral premotor cortex (vPMC) in promoting recovery of speech in nonfluent aphasia has been suggested since 1877 when Barlow reported his detailed anatomical study (Barlow, 1877). A 10 year-old boy lost speech for only 10 days following a first stroke restricted to L POp and L vPMC. One month later, however, a second stroke occurred, located in the same, right hemisphere (RH) homologous areas (R POp and R vPMC). Following the second stroke, he lost all speech again, and there was “loss of voluntary motor power over the muscles concerned in articulation and the first part of deglutition.” The boy died two months later, without any recovery of speech. See Fig. 1. More recently, a PET study by Blank et al., (2003) has supported a role for the R POp in aphasia recovery in patients with L frontal lesion. They observed that the R POp “contributed to processes involved in the assembly of the sound structure of speech” in these patients.
Figure 1.
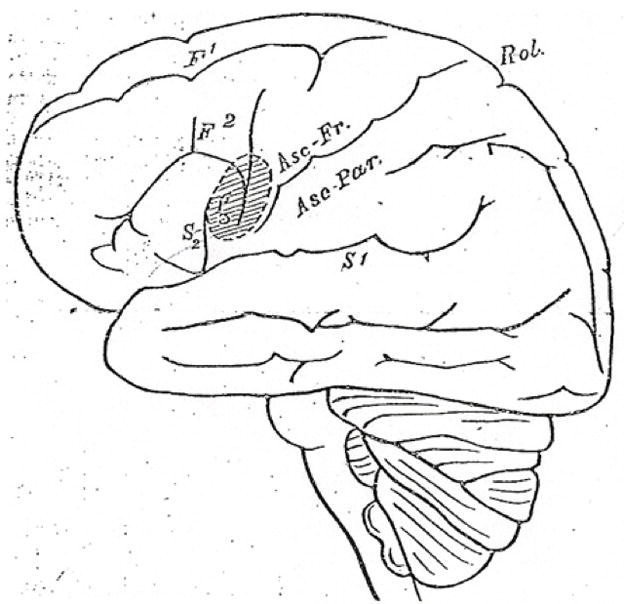
Aphasia case reported by Barlow (1877) showing detailed drawing of lesion in POp and vPMC following initial L hemisphere stroke. The second stroke (a few months later) occurred in identical areas in the RH; there was no recovery of speech. The L and R PTr remained intact, suggesting possibly different roles for POp and PTr in aphasia recovery. Permission to reprint would need to obtained from The British Medical Journal, for July 28, 1877, page 104.
In addition, repetitive transcranial magnetic stimulation (rTMS) studies with nonfluent aphasia patients have observed that suppression of R POp can impair naming in some patients (Naeser et al., 2002 and submitted; Naeser et al., 2005b). Surprisingly, however, suppression of R PTr for 20 minutes a day (10 days) improves naming in these same patients (lasting 2 – 8 months post-rTMS treatments) (Naeser et al., 2005a,b; Martin et al., 2009; Naeser et al., 2010; Hamilton et al., 2010). Thus, the two parts of R Broca’s homologue (PTr and POp) may play different roles in aphasia recovery that remain to be clarified.
The relevance of the L arcuate fasciculus (AF) in aphasia syndromes has been summarized by Geschwind (1965), and recently reviewed in historical context by Catani & Mesulam (2008). Dejerine (1895) considered the arcuate fasciculus (AF) to be part of a larger pathway, the superior longitudinal fasciculus (SLF). The AF/SLF is traditionally believed to be a system of fibers connecting frontal lobe with the parietal and temporal lobes. The horizontal portion of the AF (hAF) runs parallel to SLF II and SLF III (Petrides and Pandya, 1984; Makris et al., 2005; Glasser and Rilling, 2008).
The MRI technology of diffusion tensor imaging (DTI) is based on examining the directional dependence of water diffusion throughout the brain (Moseley et al., 1990; Beaulieu et al., 2002) and permits visualization of white matter fiber tracts in vivo. Several DTI studies have confirmed white matter pathways between L Broca’s area and posterior language zones (via the AF/SLF), including superior or middle temporal gyrus and the supramarginal gyrus (Catani et al., 2005; Parker et al., 2005; Powell et al., 2006; Frey et al., 2008; Saur et al 2008). Saur et al., (2008) observed separate (dorsal and ventral) white matter pathways from POp and PTr to posterior language zones in the LH. The major pathway from ventrolateral premotor cortices (including POp) followed a more dorsal route via the AF/SLF to supramarginal gyrus (SMG) and superior temporal lobe; a pathway believed to be important for sensory-motor mapping of sound to articulation. Hickok and Poeppel (2004; 2007) have proposed the dorsal stream to be LH dominant, and critical for auditory-motor integration. The major pathway from ventrolateral prefrontal cortex (including PTr) followed a ventral route via extreme capsule, to middle temporal lobe; this pathway is believed to be important for linguistic processing of sound to meaning. Other DTI studies have observed pathways between L ventral premotor cortex (vPMC) and the supramarginal gyrus (Croxson et al., 2005; Makris et al., 2005; Rushworth et al., 2006).
As reviewed above, pathways between the AF and parts of Broca’s area, and the vPMC, have previously been examined in the LH, but not in the RH. The primary purpose of the present study was to utilize DTI to examine pathways between the horizontal mid-portion of the AF (hAF) and parts of Broca’s area (PTr, POp), and vPMC in the RH in healthy control subjects. These pathways were also examined in the LH. This study was undertaken as a first step, and reference point for future clinical studies (Mori et al., 2002b). In aphasia patients, knowledge of pathways between the AF/SLF and inferior frontal areas in the RH could contribute to better understanding of recovery in aphasia.
MATERIALS AND METHODS
Participants
Eight healthy subjects participated in the study (ages 55–71, 5 M). All subjects were native speakers of English, right-handed, with no history of neurological or psychiatric disease. Demographics are presented in Supplemental Table 1. Each subject provided signed, informed consent for this research as approved by each institution.
A 3-Tesla whole body scanner (Intera, Philips; Boston University Center for Biomedical Imaging) was used to acquire structural and diffusion-weighted imaging. A 3D SPGR series of structural images was acquired for each subject.
Structural Image Acquisition
For half of the subjects (N1–N4), the following parameters were used to acquire the structural images: TR=9.9 ms; TE=4.6 ms; matrix size 256 × 256; FOV 256; slice thickness=1 mm. For half of the subjects (N5–N8), who were scanned at a later date, the parameters were: TR=6.85 ms, TE=3.2 ms, matrix size 256 × 256, FOV 256, slice thickness=1.20 mm. All the participants’ images were acquired in the sagittal view and re-sliced into the axial plane.
Diffusion Weighted Image Acquisition
For subjects N1–N4, the acquisition parameters for diffusion-weighted images (DWI) were as follows: TR=10686 ms; TE=84 ms; matrix size 128 × 128, FOV 256, fat suppression, number of slices=44, slice thickness=3.00 mm, b=1000 s/mm2, directions, gradient strength and SENSE (sensitivity encoding) reduction factor=2.00. For subjects N5–N8, the acquisition parameters for DWI were as follows: TR=10686 ms, TE=91 ms, matrix size 128 × 128, FOV 230, fat suppression, number of slices=73, slice thickness=2.00 mm, b=1000 s/mm2, directions, gradient strength and SENSE (sensitivity encoding) reduction factor=2.00. Although the scans were acquired at different times, the slice thickness for all subjects fell within typical DTI parameters (1–5mm) (Mori & van Zijl, 2002).
For each subject, three DWI data sets were acquired in 15 directions. These were corrected for motion, co-registered and averaged within and between acquisitions using Philips averaging software (Philips Medical Systems). Structural images were co-registered to DTI images in SPM99 (http://www.fil.ion.ucl.ac.uk/spm/) using mutual information.
Fiber Tracking Parameters
Diffusion tensors, fractional anisotropy (FA), and fiber tracts were calculated using Diffusion Toolkit software (Wang et al., 2007) that uses a deterministic tractography algorithm based on the FACT tractography algorithm (Mori and van Zijl 2002). Fibers were generated across the entire brain with the following parameters: Mask 1: b0 field map, lower threshold of 120 and upper threshold of 3000, angle threshold 40, Invert Y; Mask 2: FA map, lower threshold 150 and upper threshold 900. The masks and thresholds were utilized to remove the background and “non-trackable” areas in the brain.
First, the B0 field map was used to segment grey and white matter, then the FA map was overlaid to constrain data within the white matter. A threshold of 0.15 was set in Diffusion Toolkit (Wang et al., 2007) so that tracts were terminated if the FA dropped below this threshold (Mori and van Zijl 2002).
Fiber Tracking Analyses
Tractography data were obtained using TrackVis, an interactive program for fiber tracking reconstruction, display and analysis (Wang et al., 2007). This software permitted the use of hand-drawn selected regions of interest (ROIs). The white matter pathways were studied between one ROI (beginning seedpoint) and a second ROI (target seedpoint).
The beginning seedpoint was always the horizontal, mid-portion of the AF (hAF), defined in more detail later. Tractography was conducted sequentially and separately between a pair of seedpoints - e.g., always the hAF, and then one of four, separate cortical ROIs. The cortical ROIs included the anterior pars triangularis (A-PTr, defined later), posterior pars triangularis (P-PTr), POp, and vPMC.
Seedpoint Placements for Broca’s Area and Subregions within Broca’s Area
Placement for each cortical ROI was initially estimated using cortical reconstruction and volumetric segmentation from the Freesurfer image analysis suite (Desikan et al., 2006). The automated cortical parcellation feature in FreeSurfer was used as an initial tool for the identification of POp and PTr on sagittal and axial slices. The ROIs for POp and PTr were then manually identified on a series of structural MRI sagittal and axial slices using TrackVis (Wang et al., 2007). Each target cortical ROI was drawn to the depth of the deepest sulcus for that ROI, in order to include the targeted white matter; these ROIs did not extend into the insula.
Five sulcal landmarks were visually identified on each subject’s structural MRI scan in order to further define boundaries and placements for the PTr and POp target seedpoints (Amunts et al., 2004; Keller et al., 2007). See Fig. 2. These included the following: 1) the inferior frontal sulcus defined the superior border for POp and PTr; 2) the Sylvian fissure, the inferior border for POp and PTr; 3) the inferior precentral sulcus, the posterior border for POp; 4) the vertical, anterior ascending ramus (AR), the anterior border of POp and posterior border of PTr; and 5) the horizontal ramus (HR), the anterior border of PTr. Additionally, the PTr was further divided into anterior (A-PTr) and posterior (P-PTr) using the triangular sulcus as the dividing marker (Keller et al., 2007) (Fig. 2).
Figure 2.

(A) Sulcal landmarks used to define boundaries and location of the four cortical ROIs. The ascending vertical ramus was used to delimit PTr from POp in Broca’s area. (B) Location of the four cortical ROIs in the LH and RH are superimposed on one of several sample sagittal slices used for ROI tracings. LH sample ROIs are for subject N4; RH sample ROIs are for subject N1. Colors: red, anterior PTr; yellow, posterior PTr; light blue, POp; and dark blue, ventral premotor cortex. Abbreviations: PTr, pars triangularis; POp, pars opercularis; ROI, region of interest; LH, left hemisphere; RH, right hemisphere.
Without cytoarchitectonics, the issue of which anatomical landmark (sulcus) to use as a dividing marker between PTr and POp is not straightforward, especially if a diagonal sulcus (DS) is present. When present, the DS is located caudal to the vertical ascending ramus (AR) within the inferior frontal gyrus. Amunts et al., (2004) observed in their cytoarchitectonic studies, that a DS was present, however, only in every second hemisphere examined. They wrote that the DS can either mark the border between Brodmann area (BA) 45 and 44, or it can be inside BA 44. Thus, without cytoarchitectonics, it was not possible in the present study to know whether the DS, if present, was a border between PTr and POp, or if it was within the POp. Across our 8 subjects, 6 subjects (75%) had a DS in the LH; 4 subjects (50%) in the RH; and only 2 of those subjects had a DS in both. Therefore, for consistency across all subjects and both hemispheres, the AR was arbitrarily chosen to define the border between the PTr and POp in the LH and in the RH (Fig. 2).
Seedpoint Placement for Ventral Premotor Cortex
The sulcal boundaries defining the vPMC were the following: 1) the Sylvian fissure was defined as the inferior border; 2) the inferior frontal sulcus was extended caudally to define the superior border; 3) the inferior precentral sulcus, the anterior border; 4) the inferior central sulcus, the posterior border (Fig. 2).
Seedpoint Placement for the horizontal, mid-portion of Arcuate Fasciculus (hAF)
The placement for this seedpoint was in the mid-portion of the horizontal aspect of the AF (hAF). A methodology similar to that of Makris et al., (2005), and Glasser and Rilling (2008) was used. The size of the entire hAF seedpoint was about 100 voxels in each hemisphere. It was drawn across 5–7 coronal slices on the DTI FA color map in TrackVis (Wang et al., 2007) (Fig. 3A). This region was superior to the insula, extreme capsule, claustrum, external capsule, and internal capsule (Fig. 3B). This region borders the SLF II and III (Makris et al., 2005; Glasser and Rilling 2008). Thus, the hAF seedpoint could have included some of the SLF II and III fibers, because it is difficult to separate the AF from these SLF fibers.
Figure 3.
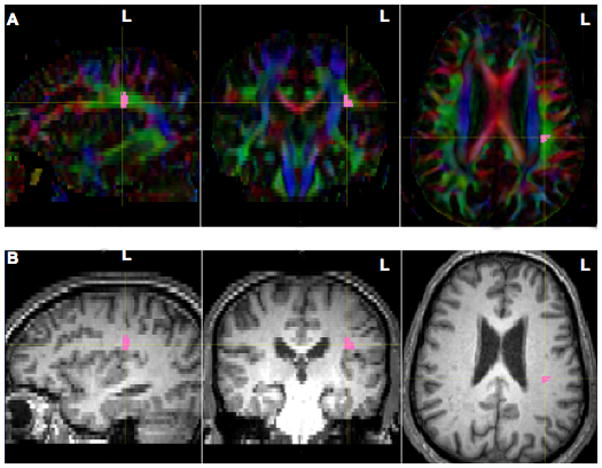
Illustration of method used for locating the hAF seedpoint (pink) in sagittal, coronal and axial views in the LH (subject N4). The hAF seedpoint was drawn on 5–7 coronal slices in the horizontal mid-portion of the AF fibers oriented in the anterior-posterior direction (green). The AF seedpoint was located superior to the insula, extreme capsule, claustrum, external capsule, and internal capsule. A similar hAF seedpoint was drawn in the RH (not shown). (A) Fractional Anisotropy Color Maps; (B) Structural MRI Images. Abbreviations: hAF, horizontal mid-portion of the arcuate fasciculus; LH, left hemisphere; RH, right hemisphere.
Initial Statistical Analyses
Laterality Index for Each Cortical ROI and the Arcuate Fasciculus
Because the study included a small number of subjects, a laterality index (LI) was first computed for relative L and R size of each ROI, in order to establish that the distribution of the relative L and R sizes for the seedpoints was not skewed in an atypical (rightward) direction. Either a leftward asymmetry or symmetry has been previously reported for the majority of subjects in studies that have examined relative size of total Broca’s area, the PTr, the POp (Keller et al., 2009 for review); and the AF (Buchel et al., 2004; Nucifora et al., 2005; Parker et al., 2005; Hagmann et al., 2006; Powell et al., 2006; Vernooij et al., 2007; Upadhyay et al., 2008; Catani et al., 2007).
In the present study, the LI was computed using sample size (mm3) for each L and R seedpoint, where LI = L − R/(L + R) × 100. A positive LI value was considered to reflect leftward asymmetry; a negative LI value, rightward asymmetry. The LI for each seedpoint ROI (for each subject) is provided in Supplemental Table 1, and plotted in graph form in Supplemental Fig. 1.
Chi square results indicated that the probability of the relative size of the ROIs and volume of AF fibers being lateralized leftward or rightward, was not random (x2=15.55, df=8, p=0.05), with a higher probability of being lateralized to the left. Since the relative sizes of the cortical ROIs, and volume of AF fibers in the LH and RH were not skewed in an atypical (rightward), direction this small population sample was considered to be not atypical of that found in previous studies, and further comparisons were performed.
Size of ROI Cortical Seedpoints
Additionally, to ensure that any potential white matter tractography volume differences would not be a direct result of differences in size of the ROI cortical seedpoints, statistical comparisons were performed for relative size of each cortical ROI. There were no significant differences in size of the ROI cortical seedpoints, with the exception of the following two: 1) The POp seedpoint was significantly larger (p<0.05) than the ipsilateral A-PTr and P-PTr in each hemisphere (Supplemetal Table 1 and Supplemental Fig. 2C); 2) The POp seedpoint was also significantly larger (p<0.05) than the ipsilateral vPMC in each hemisphere (see Supplemental Table 1 for the means and SDs).
Volume of hAF
In order to establish the volume (mm3) for the AF in each hemisphere, the TrackVis program was run for the hAF seedpoint alone, without any separate target, cortical ROI seedpoint. A paired t-test (2-tailed) was used to compare the volume of the AF fibers in the LH versus the RH. There was a significantly greater volume of AF fibers in the LH (mean=6747.0 mm3, SD=2588.83) versus the RH (mean=5842.7 mm3, SD=2353.99); (t=2.5, df=7, p=0.04).
RESULTS
hAF Pathways with Separate Cortical ROIs
hAF Fiber Tracts with Anterior-PTr and Posterior-PTr
Only one subject (N3) showed fiber tracts between the hAF and the entire PTr as a seedpoint (A-PTr plus the P-PTr combined) in either hemisphere. With PTr separated into anterior and posterior, no fiber tracts were observed between A-PTr and the hAF in the RH or LH, for all eight subjects (Table 1). Only one of the eight subjects showed fiber tracts between P-PTr and hAF in the RH and LH (subject N3).
Table 1.
Volume of hAF fibers to each cortical ROI: Subregions within Broca’s area, and the ventral premotor cortex in the left hemisphere and the right hemisphere, for each subject.
| Left hAF Fibers (mm3) to each ROI | Right hAF Fibers (mm3) to each ROI | |||||||
|---|---|---|---|---|---|---|---|---|
| A-PTr | P-PTr | POp | vPMC | A-PTr | P-PTr | POp | vPMC | |
| N1, 68 Yr., M | 0 | 0 | 2707.0 | 63.2 | 0 | 0 | 1742.3 | 1679.1 |
| N2, 57 Yr., M | 0 | 0 | 1489.6 | 3635.3 | 0 | 0 | 1239.3 | 1275.8 |
| N3, 58 Yr., M | 0 | 668.3 | 1540.6 | 1222.3 | 0 | 733.9 | 1598.9 | 2245.3 |
| N4, 55 Yr., M | 0 | 0 | 3042.4 | 2240.5 | 0 | 0 | 600.2 | 527.3 |
| N5, 58 Yr., F | 0 | 0 | 1023.8 | 1134.0 | 0 | 0 | 706.3 | 907.2 |
| N6, 69 Yr., F | 0 | 0 | 2643.8 | 5287.7 | 0 | 0 | 0.0 | 1309.0 |
| N7, 71 Yr., F | 0 | 0 | 693.4 | 1483.9 | 0 | 0 | 0.0 | 0.0 |
| N8, 62 Yr., M | 0 | 0 | 1859.8 | 3065.0 | 0 | 0 | 0.0 | 1205.3 |
| Mean | 0 | 83.5 | 1875.0 | 2266.5 | 0 | 91.7 | 735.9 | 1143.6 |
| SD | 0 | 236.3 | 847.5 | 1668.2 | 0 | 259.5 | 722.6 | 685.8 |
Abbreviations: hAF, horizontal mid-portion of arcuate fasciculus; A-PTr, anterior pars triangularis; P-PTr, posterior pars triangularis; POp, pars opercularis; vPMC, ventral premotor cortex.
hAF Fiber Tracts with Pars Opercularis
In contrast to PTr, 5/8 subjects showed pathways between R POp and R hAF, and all eight subjects showed pathways between L POp and L hAF (Table 1 and Fig. 4).
Figure 4.
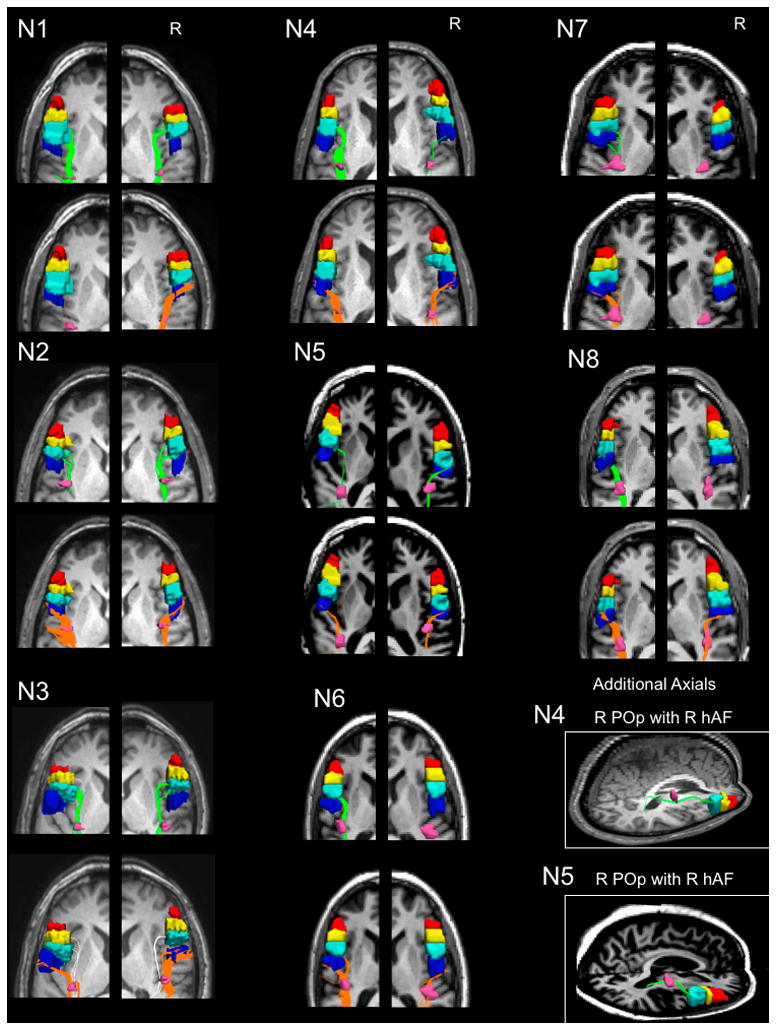
Fiber tracts between the horizontal mid-portion of the AF (hAF) seedpoint (pink) and the separate cortical ROI seedpoints in the LH and RH for each subject. The cortical seedpoints are anterior PTr (red); posterior PTr (yellow); POp (light blue); and vPMC (dark blue). The fiber tracts between hAF and POp are shown in green; and those between hAF and POp, orange. Fiber tracts between hAF and posterior PTr (present only in N3) are shown in white (shown on the same slice as those for vPMC, to conserve space; tractography was performed separately between hAF and each cortical ROI seedpoint). There were no fiber tracts between hAF and A-PTr in either hemisphere for any subjects. See Table 1. Additional axial views are provided for subjects N4 and N5, showing fiber tracts between the hAF and R POp, where view of R POp was obscured by presence of R vPMC on other axial views above, for these two subjects. Abbreviations: hAF, horizontal, mid-portion arcuate fasciculus; POp, pars opercularis; PTr, pars triangularis; vPMC, ventral premotor cortex.
Because 3/8 subjects showed no fibers between R POp and R hAF (Table 1, N6, N7, N8), a paired t-test (2-tailed) was completed with only the five subjects who showed pathways POp pathways with hAF in each hemisphere. There was no significant difference between the volume of hAF fibers to L POp (mean=1961 mm3, SD=866) versus R POp (mean=1177 mm3, SD=514); (t=1.75, df=4, p=0.15) when only data for these five cases were compared.
hAF Fiber Tracts with ventral Premotor Cortex
Seven of eight subjects showed fiber tracts between R vPMC and R hAF; and all eight subjects, between L vPMC and L hAF (Table 1 and Fig. 4). Two of the three subjects who did not have pathways between R hAF and R POp, did have fiber tracts between R hAF and R vPMC (subjects N6, N8) (Table 1 and Fig. 4).
A paired t-test (2-tailed) was used to compare the volume of fibers between vPMC and hAF in the LH and RH, for all eight subjects. There was no significant difference in volume of L hAF fibers to L vPMC, (mean=2266.5 mm3, SD=1668.16), versus R hAF fibers to R vPMC (mean=1143.6 mm3, SD=685.85); (t=1.73, df=7, p=0.13).
The observed presence of hAF fiber tracts with specific cortical ROIs was not dependent on sample size of the cortical ROIs. For example, in the RH, the seedpoint size for the vPMC was significantly smaller (p <.05) than that for the POp (see Supplemental Table 1 for means and SDs). However, in the RH, 7/8 subjects showed pathways between vPMC and hAF, but only 5/8 subjects showed pathways between POp and hAF. Similarly, in the LH, the seedpoint size for the vPMC was significantly smaller (p <.05) than that for the POp (see Supplemental Table 1). In the LH, all 8 subjects showed pathways between vPMC and hAF, as well as between POp and hAF.
DISCUSSION
Our DTI study observed fiber tracts between hAF and POp and/or vPMC in both the RH and in the LH, but almost no fiber tracts between the hAF and the PTr in either hemisphere. In the RH, these findings are new. In the LH they support and replicate previous studies. For example, in the DTI studies of Frey et al., (2008) and Saur et al., (2008) pathways were observed between the L posterior Broca’s area (not L anterior Broca’s area) and the SMG via the AF (and likely SLF III). Frey et al., 2008 observed pathways between the L anterior Broca’s area with the superior temporal gyrus to be via the extreme capsule, not via the AF. Thus, major pathways from premotor cortices in the LH have been observed to follow a more dorsal route via the AF/SLF III to SMG (Crosxon et al., 2005; Frey et al., 2008; Saur et al., 2008); whereas major pathways from ventrolateral prefrontal cortex (including PTr) follow a more ventral route via extreme capsule, to part of the superior temporal gyrus, or middle temporal gyrus (Frey et al., 2008; Saur et al., 2008).
The dorsal route in the LH, as recently summarized by Frey et al., (2008), is mainly restricted to sensory-motor mapping of sound to articulation and higher-order articulatory control of speech, where the POp is connected directly with premotor area 6 (involved with orofacial musculature) (Petrides, 2006; Petrides et al., 2005). The ventral route in the LH likely performs linguistic processing of sound to meaning, requiring temporo-frontal interaction and top-down regulation of linguistic processing such as that involved in verbal retrieval (Petrides, 2006), and lexical/semantic aspects of language processing (Devlin et al., 2003; Gold and Buckner, 2002; Nixon et al., 2004; Poldrack et al., 1999; Price et al., 1996; Saur et al., 2008). A dissociation between the roles of L PTr versus L POp in semantic versus phonological tasks has been supported by TMS application to these two areas in normals, with differential/opposite effects observed (Gough et al., 2005).
The two parts of Broca’s area differ in cytoarchitectonics. The PTr and the POp in both LH and RH, are often considered to correspond in a general manner with the cytoarchitectonic BA 45 and 44, respectively (Amunts et al., 1999; 2004). The primary distinguishing cytoarchitectonic feature between these two areas is located in cortical layer IV, which is granular in BA 45 and dysgranular in BA 44. The ventral premotor cortex (vPMC), located immediately posterior to BA 44, is agranular in layer IV (Amunts et al., 1999; 2004; 2006; Keller et al., 2009 for review). These differences in cytoarchitectonics may also support differences in connectivity and function for these two areas. In a detailed anatomical and fMRI study with verbal fluency, Amunts et al., (2004) described L BA 45 to be “involved in semantic aspects of language processing, while area 44 is probably involved in high-level aspects of programming speech production per se.”
Our DTI results in the RH showed the presence of pathways between the hAF and the POp and/or vPMC, but not between the hAF and the PTr. Thus, there may be a dorsal route in the RH which is parallel to that in the LH. This dorsal route would include R hAF fiber tracts with R POp and vPMC which are likely connecting with R SMG (although this posterior pathway with SMG was not examined as part of our DTI study). Along these lines, a possible parallel role of the R POp might be related to articulation, and the R vPMC, to the movement of orofacial musculature (Petrides et al 2005; Petrides, 2006). The relevance of a RH dorsal route in normal speech/language remains to be clarified; it may have relevance for speech/language recovery in aphasia, as discussed below.
A possible role for the R POp and R vPMC in promoting recovery of speech in nonfluent aphasia has been suggested since 1877, when Barlow reported his detailed anatomical study. The absence of any speech recovery following the second stroke, which destroyed the R POp and R vPMC (after initial destruction in the L POp and L vPMC when recovery did occur) suggests that following the first stroke, a supportive, parallel system for orofacial musculature and articulation had possibly been present in the R POp and R vPMC. Fig. 1.
In addition, the PET study by Blank et al., (2003), observed that after lesion of the L POp, the R POp “contributed to processes involved in the assembly of the sound structure of speech.” Results from our rTMS studies in nonfluent aphasia patients also suggest a possible role for the R POp in recovery of speech following L frontal lesion, where suppression of R POp with 1 Hz rTMS was observed to impair naming in some aphasia cases (Naeser et al., 2002 and submitted; Naeser et al., 2005b). Suppression of R POp with 1 Hz rTMS would likely interrupt connections between R POp and R vPMC, as well as connections via the “dorsal route” to R SMG and directly interrupting control of articulation and orofacial musculature, as well as indirectly interrupting connections with other parts of the bilateral neural network for naming (Price et al., 2001; Gold and Buckner, 2002; Damasio et al., 2004).
Conversely, we have observed that suppression of the R PTr with rTMS improves naming in nonfluent aphasia. Although the mechanism for this beneficial effect is unknown, we would posit the following possibility: In cases with nonfluent aphasia where lesion is present in L inferior frontal cortex, and/or subcortical white matter deep to it (adjacent to ventricle), possible hyperactivity of neurons in R PTr (among other RH areas) may be present, due to interhemispheric disinhibition from the damaged L frontal lobe. This R PTr hyperactivity could excessively suppress the R POp, via their shared U-fibers, which could possibly hinder recovery from aphasia.
The POp (BA 44) and PMC (BA 6) in the human brain are thought to be analogous to the monkey’s F5 region, a locus of mirror neurons (Rizzolatti and Craighero, 2004). The mirror neuron system is bilateral, and these neurons fire during both production and perception of similar actions (Wilson et al., 2004; Iacoboni, 2008 for review). They are important in child language acquisition (Rizzolatti and Craighero, 2004). The potential contribution of parts of the mirror neuron system to recovery in aphasia is unknown; however, some of the results post-rTMS in our aphasia patients may provide some insight. For example, one category of naming where we have consistently observed improvement is that of naming “tools/implements” (Naeser et al., 2005a; 2005b; Martin et al., 2009; Naeser et al., 2010; Hamilton et al., 2010). Although hypothetical at this time, this may be related in part, to the mirror neuron system. The POp (BA 44, an area with mirror neurons) “mediates observation-execution matching for the goals of arm/hand actions” (Kemmerer and Gonzalez-Castillo, 2010, for review); and the neural network for “tool use” is widely distributed, including temporal, parietal and frontal regions in the LH (Kemmerer et al., 2008). Thus, following rTMS suppression of R PTr, modulation of R POp (and vPMC) may have permitted access to this uniquely, widespread neural network for naming tools/implements. These possibilities require further study.
The results from the present DTI study suggest that in the RH the fiber tracts between the hAF and POp and vPMC may be parallel, and similar in structure to those in the LH. The relevance of these similarities for speech/language in normals, and for aphasia recovery in stroke patients remains to be further explored.
Limitations
Some limitations may have restricted our findings.
Even though DTI has been prevalently used to analyze white matter connectivity, it has inherent limitations. The deterministic tractography algorithm used here is unable to track through areas of crossing fibers when a competing pathway is significantly stronger, or when subject motion reduces the quality of the dataset. Multi-fiber orientations within a voxel are not captured robustly by deterministic tracking, thus limiting tracking performance to areas of high anisotropy and low uncertainty. Weak pathways would be more reliably found with the probabilistic algorithm (Behrens et al., 2007). However, even with this limitation in methodology, we were able to replicate previously published findings for the LH, where the hAF was observed to have fiber tracts with POp and vPMC, but not with PTr, in most subjects.
The AF is located parallel to the inferior part of the SLF II and SLF III. The SLF III has been found to connect BA 40 (SMG) with BA 44 (POp) and ventral BA 6 (vPMC) (Crosxon et al., 2005; Makris et al., 2005; Rushworth et al., 2006). We observed hAF pathways with POp and vPMC in each hemisphere; our designated hAF could have included some of the SLF III.
The present study only examined fiber tracts between the horizontal mid-portion of the AF to parts of Broca’s area, and the vPMC, not pathways caudal to the mid-portion of the AF towards parieto-temporal areas. Nor did we examine the extreme capsule connections. Although our results support the notion of a dorsal route with fiber tracts between the hAF, and the POp and vPMC (but not PTr) in each hemisphere, we cannot comment directly on a possible ventral route for the PTr with the extreme capsule. A more detailed mapping of complete white matter pathway connections between parts of R Broca’s homologue and the vPMC in the RH, with specific posterior zones (inferior parietal and/or superior and middle temporal gyrus areas) was beyond the scope of this study, and would be appropriate for a future study.
The relatively older ages of the healthy subjects in this study may have affected the data, and reduced the potential pathways observed from the hAF to the cortical target ROIs. If younger subjects had been studied, it is possible that additional white matter tracts could have been observed. These ages were chosen, however, so that future comparisons could be performed with aphasic stroke patients of a comparable age.
The number of subjects examined in this DTI study was small, with only eight healthy controls. However, the major observations were consistent across the majority of subjects - e.g., there was a dorsal route pathway between the hAF with the POp and vPMC (but not with the PTr), in both the RH and the LH. Also, our initial LI analyses showed that relative sizes for the L and R ROIs, and the relative volume of the AF fibers, were not skewed in the atypical (rightward) direction for this small population sample.
Conclusions
We observed the hAF fiber tracts were primarily with the POp and the vPMC (not with the PTr) in each hemisphere; these pathways likely followed the dorsal route pathway to SMG, as has been previously described in the LH, but not studied here. These results suggest different network connections, and different language roles for the two parts of Broca’s area, POp and PTr. In aphasia recovery, the POp and the vPMC may support a common role (primarily orofacial musculature and articulation) which is different from that of the PTr. Further examination of these pathways in both aphasia patients and controls with a probabilistic algorithm, and their connections with the bilateral neural networks for speech/language and naming is recommended.
Supplementary Material
Distribution for laterality index (LI) calculated with L and R sizes for each cortical ROI seedpoint, and the horizontal mid-portion of the arcuate fasciculus (hAF), across all subjects (n=8). Distribution shows no atypical (rightward) directional bias, across the 8 subjects.
Graphs of volumes (mm3) for size of Broca’s area and its subregions in the LH and RH. Bars indicate standard error. (A) Total Broca’s area (PTr plus POp). There was no significant difference in size between total Broca’s area in the LH versus RH (p>0.05). (B) PTr and POp. There was no significant difference in size of PTr or POp in the LH versus RH (p>0.05). There was also no significant difference in size between PTr and POp within each hemisphere (p>0.05). (C) POp and subregions within PTr. There was no significant difference in the size of each part or subregion of Broca’s area in the LH, compared to each respective part or subregion in the RH (p>0.05). There were significant differences, however, in size of these within each hemisphere (p<0.05), where the POp was significantly larger than the ipsilateral subregions within Broca’a area (A-PTr, P-PTr). These significant differences within each hemisphere are marked with a star (p<0.05). Abbreviations: A-PTr, anterior pars triangularis; P-PTr, posterior pars triangularis; POp, pars opercularis. Results for the statistical analyses are provided below:
Supplemental Statistical Analyses for Seedpoint Sizes, LH vs. RH, and Ipsilateral Comparisons
Total Broca’s Area, Pars Triangularis plus Pars Opercularis
The volume (mm3) of total Broca’s area (grey matter and subjacent white matter), including both PTr and POp in the LH was compared to its RH homologue using a paired samples t-test (2-tailed). There was no significant difference in size between total Broca’s area in the LH (mean=7051.2 mm3, SD=1758.49) and RH (mean=6670.1 mm3, SD=1758.51); (t=0.394, df=7, p=0.705) (Supplemental Figure 2A).
Pars Triangularis and Pars Opercularis Parts of Broca’s Area, Separately
A two-way repeated measures analysis of variance (ANOVA) was conducted to compare the size of the PTr and POp for each hemisphere. The two factors were hemispheres (L, R) and parts of Broca’s area (PTr, POp). There was no significant difference in size of PTr or POp between LH and RH (F=0.15, df=7, p=0.71). or between PTr and POp within each hemisphere (F=0.51, df=7, p=0.50) (Supplemental Figure 2B).
Subregions within Broca’s area, Anterior-PTr, Posterior-PTr), and the Pars Opercularis
A two-way repeated measures ANOVA was performed. The two factors were hemisphere (L, R) and subregions within Broca’s area (A-PTr, P-PTr and POp). There was no significant difference in the size of each subregion in the LH compared to each respective subregion in the RH (F=0.15, df=7 p=0.71).
There was a significant difference in size for the ipsilateral subregions within each hemisphere (F=48.3, df=14, p=0.00). Tukey B test was used to make post hoc comparisons between conditions. In the LH and RH, the size of POp was significantly larger (p<0.05) than the P-PTr, as well as A-PTr. In the LH, the size of P-PTr was also significantly larger (p<0.05) than A-PTr. These differences were anticipated because the PTr had been divided into two smaller subregions (A-PTr and P-PTr) (Supplemental Figure 2C).
Ventral Premotor Cortex
A paired t-test (2-tailed) was used to compare the size of L vPMC versus R vPMC. There was no significant difference in size between L vPMC (mean=2047.4 mm3, SD=605.20) and R vPMC (mean=1967.5 mm3, SD=647.59); (t=0.61, df=7, p=0.56).
The vPMC and the POp are adjacent, therefore, a two-way repeated measures ANOVA was conducted to compare the size of vPMC and POp in each hemisphere. The two factors were hemisphere (L, R) and ROI (vPMC, POp). There was no significant difference in size of vPMC or POp between LH and RH (F=0.68, df=7, p=0.438). There was, however, a significant difference in size between vPMC and POp within each hemisphere (F=18.2, df=7, p=0.004). Tukey B test was performed for post hoc comparisons between conditions. POp was significantly larger (p<0.05) than vPMC, in each hemisphere.
Acknowledgments
FUNDING
This work was supported by a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); and NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders (NIDCD), Bethesda, MD, (to M.A.N.); and a K24 NIH award (RRO18875, to A.P.-L) and the Harvard-Thorndike General Clinical Research Center (NCRR MO1 RR01032); and a P30 DC05207 NIDCD grant to the Harold Goodglass BU Aphasia Research Center.s
The authors would like to thank Jacquie Kurland Ph.D., formerly at Boston University Aphasia Research Center, currently at University of Massachusetts Amherst, for her contribution in DTI data acquisition for half of the subjects. The authors would also like to thank Andrew Bogdan, Ph.D., Andrew Ellison B.S., Dae-Shik Kim, Ph.D., Itamar Ronan, Ph.D., and Jaymin Upadhyay, Ph.D., for assistance with DTI scan acquisition and analysis at the Boston University Center for Biomedical Imaging. The authors also thank Ethan Treglia, MS, CCC-SLP for assistance with graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MP, Naeser MA, Palumbo CL. Broca’s area aphasias: Aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. A multimodal analysis of structure and function in Broca’s region. In: Grodzinsky Y, Amunts K, editors. Broca’s Region. Oxford University Press; New York: 2006. pp. 31–46. [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Barlow T. A case of double hemiplegia, with cerebral symmetrical lesions. Brit Med J. 1877 July;28:103–104. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54:310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Cooper WE, Zurif EG, Caramazza A. The perception and production of voice-onset time in aphasia. Neuropsychologia. 1977;15:371–383. doi: 10.1016/0028-3932(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bradvik B, Dravins C, Holtas S, Rosen I, Ryding E, Ingvar DH. Disturbances of speech prosody following right hemisphere infarcts. Acta Neurol Scand. 1991;84:114–126. doi: 10.1111/j.1600-0404.1991.tb04919.x. [DOI] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, ramollissement chronique et destruction partielle du lobe anterieur gauche. Bull Soc Antropol. 1861;2:235–238. [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des Centres Nerveux. Paris: Rueff et Cie; 1895. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Finger S, Buckner RL, Buckingham H. Does the right hemisphere take over after damage to Broca’s area? the Barlow case of 1877 and its history. Brain Lang. 2003;85:385–395. doi: 10.1016/s0093-934x(03)00060-9. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. 585–644. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathways. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y, Amunts K. Broca’s Region. New York: Oxford University Press; 2006. [Google Scholar]
- Hagmann P, Cammoun L, Martuzzi R, Maeder P, Clarke S, Thiran JP, Meuli R. Hand preference and sex shape the architecture of language networks. Hum Brain Mapp. 2006;27:828–835. doi: 10.1002/hbm.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, Martin P, Coslett HB. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113:45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. The role of premotor cortex in speech perception: evidence from fMRI and rTMS. J Physiol Paris. 2008;102:31–34. doi: 10.1016/j.jphysparis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N. Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. J Anat. 2007;211:534–555. doi: 10.1111/j.1469-7580.2007.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008;107:16–43. doi: 10.1016/j.bandl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez-Castillo J. The Two-Level Theory of verb meaning: An approach to integrating the semantics of action with the mirror neuron system. Brain Lang. 2010;112:54–76. doi: 10.1016/j.bandl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009;111:20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY. Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Brain Res Cogn Brain Res. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: Pathologic and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin P, Fregni F, Theoret H, Kobayashi M, Nicholas M, Baker E, Maria-Tormos J, Steven M, Pascual-Leone A. Modulation of cortical areas with repetitive transcranial magnetic stimulation to improve naming in nonfluent aphasia [Abstract#133]. NeuroImage; Proceedings of the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. Submitted. [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005a;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005b;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112 ( Pt 1):1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, Ho M, Nicholas M, Alonso M, Pascual-Leone A. Improved Language in a Chronic Nonfluent Aphasia Patient After Treatment With CPAP and TMS. Cogn Behav Neurol. 2010;23:29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. Neuroreport. 1995;6:2309–2313. doi: 10.1097/00001756-199511270-00010. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Schurmann M, Amunts K, Hari R. Broca’s region: from action to language. Physiology (Bethesda) 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16:791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Com Neurol. 1984;228 doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Petrides M. Broca’s area in the human and the non-human primate brain. In: Grodzinsky Y, Amunts K, editors. Broca’s Region. Oxford University Press; New York: 2006. pp. 31–46. [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. NeuroImage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119 ( Pt 3):919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13:419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Ross ED. The aprosodias. Functional-anatomic organization of the affective components of language in the right hemisphere. Arch Neurol. 1981;38:561–569. doi: 10.1001/archneur.1981.00510090055006. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proc Natl Acad Sci. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I. Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. NeuroImage. 2008;39:1–9. doi: 10.1016/j.neuroimage.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage. 2007;35:1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Weeden VJ. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography (Abstract #3720). Poster presented at the Joint Annual Meeting of the International Society for Magnetic Resonance Medicine and European Society for Magnetic Resonance in Medicine and Biology; May 19–25. Berlin, Germany. ; May 19–25, 2007. http://trackvis.org/ [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Winner E, Brownell H, Happe F, Blum A, Pincus D. Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Lang. 1998;62:89–106. doi: 10.1006/brln.1997.1889. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian GH, Lafontaine L. Discrimination and identification of voicing and place contrasts in aphasic patients. Can J Psychol. 1983;37:107–131. doi: 10.1037/h0080694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution for laterality index (LI) calculated with L and R sizes for each cortical ROI seedpoint, and the horizontal mid-portion of the arcuate fasciculus (hAF), across all subjects (n=8). Distribution shows no atypical (rightward) directional bias, across the 8 subjects.
Graphs of volumes (mm3) for size of Broca’s area and its subregions in the LH and RH. Bars indicate standard error. (A) Total Broca’s area (PTr plus POp). There was no significant difference in size between total Broca’s area in the LH versus RH (p>0.05). (B) PTr and POp. There was no significant difference in size of PTr or POp in the LH versus RH (p>0.05). There was also no significant difference in size between PTr and POp within each hemisphere (p>0.05). (C) POp and subregions within PTr. There was no significant difference in the size of each part or subregion of Broca’s area in the LH, compared to each respective part or subregion in the RH (p>0.05). There were significant differences, however, in size of these within each hemisphere (p<0.05), where the POp was significantly larger than the ipsilateral subregions within Broca’a area (A-PTr, P-PTr). These significant differences within each hemisphere are marked with a star (p<0.05). Abbreviations: A-PTr, anterior pars triangularis; P-PTr, posterior pars triangularis; POp, pars opercularis. Results for the statistical analyses are provided below:
Supplemental Statistical Analyses for Seedpoint Sizes, LH vs. RH, and Ipsilateral Comparisons
Total Broca’s Area, Pars Triangularis plus Pars Opercularis
The volume (mm3) of total Broca’s area (grey matter and subjacent white matter), including both PTr and POp in the LH was compared to its RH homologue using a paired samples t-test (2-tailed). There was no significant difference in size between total Broca’s area in the LH (mean=7051.2 mm3, SD=1758.49) and RH (mean=6670.1 mm3, SD=1758.51); (t=0.394, df=7, p=0.705) (Supplemental Figure 2A).
Pars Triangularis and Pars Opercularis Parts of Broca’s Area, Separately
A two-way repeated measures analysis of variance (ANOVA) was conducted to compare the size of the PTr and POp for each hemisphere. The two factors were hemispheres (L, R) and parts of Broca’s area (PTr, POp). There was no significant difference in size of PTr or POp between LH and RH (F=0.15, df=7, p=0.71). or between PTr and POp within each hemisphere (F=0.51, df=7, p=0.50) (Supplemental Figure 2B).
Subregions within Broca’s area, Anterior-PTr, Posterior-PTr), and the Pars Opercularis
A two-way repeated measures ANOVA was performed. The two factors were hemisphere (L, R) and subregions within Broca’s area (A-PTr, P-PTr and POp). There was no significant difference in the size of each subregion in the LH compared to each respective subregion in the RH (F=0.15, df=7 p=0.71).
There was a significant difference in size for the ipsilateral subregions within each hemisphere (F=48.3, df=14, p=0.00). Tukey B test was used to make post hoc comparisons between conditions. In the LH and RH, the size of POp was significantly larger (p<0.05) than the P-PTr, as well as A-PTr. In the LH, the size of P-PTr was also significantly larger (p<0.05) than A-PTr. These differences were anticipated because the PTr had been divided into two smaller subregions (A-PTr and P-PTr) (Supplemental Figure 2C).
Ventral Premotor Cortex
A paired t-test (2-tailed) was used to compare the size of L vPMC versus R vPMC. There was no significant difference in size between L vPMC (mean=2047.4 mm3, SD=605.20) and R vPMC (mean=1967.5 mm3, SD=647.59); (t=0.61, df=7, p=0.56).
The vPMC and the POp are adjacent, therefore, a two-way repeated measures ANOVA was conducted to compare the size of vPMC and POp in each hemisphere. The two factors were hemisphere (L, R) and ROI (vPMC, POp). There was no significant difference in size of vPMC or POp between LH and RH (F=0.68, df=7, p=0.438). There was, however, a significant difference in size between vPMC and POp within each hemisphere (F=18.2, df=7, p=0.004). Tukey B test was performed for post hoc comparisons between conditions. POp was significantly larger (p<0.05) than vPMC, in each hemisphere.


