Abstract
Bcl-2 is the founding member of a family of proteins that influence apoptosis. Loss of bcl-2 results in renal hypoplasia/cystic dysplasia at birth. Here we examined whether reexpression of bcl-2 throughout the ureteric bud and its derived epithelia would restore a normal renal phenotype in bcl-2 −/− mice. Reexpression of bcl-2 in the ureteric bud/collecting duct of bcl-2 −/− mice increased nephron numbers, diminished glomerular hypertrophy and increased nephrogenic zone size. Unlike bcl-2 −/− mice which have gross renal cyst formation, few renal cysts were present in mice reexpressing bcl-2. We have previously shown increased apoptosis and proliferation, as well as aberrant protein tyrosine phosphatase 1B expression, accompanied cystic changes in bcl-2 −/− mice. These changes were not observed when bcl-2 was reexpressed in the ureteric bud/collecting duct system. Thus, expression of bcl-2 in the ureteric bud/collecting duct resulted in increased nephron numbers partially rescuing renal hypoplasia/cystic dysplasia in bcl-2 −/− mice.
INTRODUCTION
Apoptosis plays an integral role during development. Aberrant regulation of apoptosis can result in a variety of disease states including lymphoma and renal hypoplasia. Bcl-2 was discovered over 20 years ago and is the founding member of a family of proteins that influences apoptosis. It was first identified at the interchromosomal breakpoint of t(14;18) in follicular B cell lymphoma (Tsujimoto and Corce, 1986). Bcl-2 is widely expressed during development and becomes restricted upon maturation in many tissues. In the embryonic kidney, bcl-2 is highly expressed in the ureteric bud (Novak and Korsmeyer, 1994) and epithelial condensates of the metanephric blastema but not in uninduced mesenchyme (Chandler et al., 1994). During differentiation of the renal vesicle, the epithelium of the nascent glomerulus is initially strongly positive but upon maturation and vascularization, staining becomes limited to the parietal layer of the Bowman’s capsule.
Loss of bcl-2 has a profound effect on nephrogenesis. Mice deficient in bcl-2 develop renal hypoplasia/cystic dysplasia. These mice had fulminant apoptosis of the metanephric blastema at embryonic day 12 (E12) and decreased branching of the ureteric bud. At birth, the kidneys are hypoplastic with a diminished nephrogenic zone. Gross cyst formation in these kidneys was observed at postnatal day 20 (P20) at multiple sites along the nephron (Sorenson et al., 1996). Cystic kidneys from these mice displayed increased apoptosis and proliferation. The increased proliferation was due in part to an inablility to up-regulate phosphatases, such as protein tyrosine phosphatase (PTP 1B), that can dephosphorylate proteins involved in proliferative processes (Sorenson and Sheibani, 2002a; Sorenson and Sheibani, 2002b). Reduced renal mass was accompanied by decreased nephron numbers and compensatory hypertrophy of glomeruli. Thus, controlled regulation of apoptosis is important during normal kidney development.
Here we extended our previous studies, employing the Hox b7 promoter to drive specific expression of bcl-2 throughout the ureteric bud during kidney development and in its derived epithelia (collecting duct), in the absence of bcl-2 in the rest of the kidney. We observed increased renal mass, nephron numbers and fewer renal cysts in bcl-2 −/− mice with Hox b7 promoter driven bcl-2 expression. Restoration of bcl-2 expression in the ureteric bud and its derivatives increased nephrogenic zone, increased renal mass and averted glomerular hypertrophy. Thus, increasing nephron numbers to near normal levels restored a more normal renal phenotype.
RESULTS
Generation of Transgenic Mice that Express Bcl-2 in the Ureteric Bud/Collecting Duct
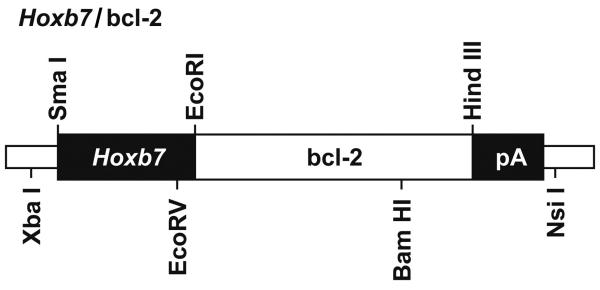
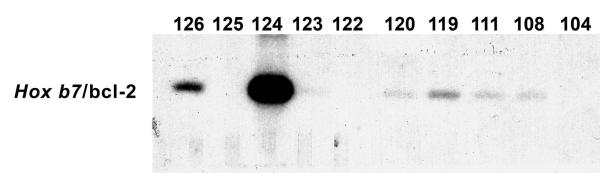
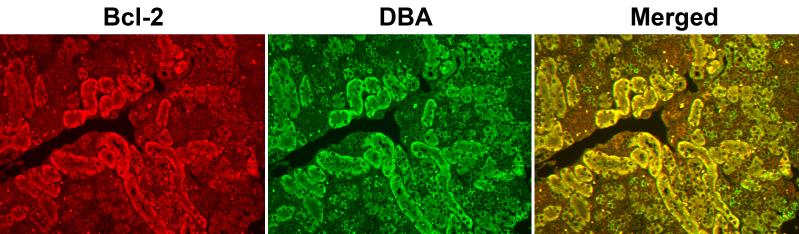
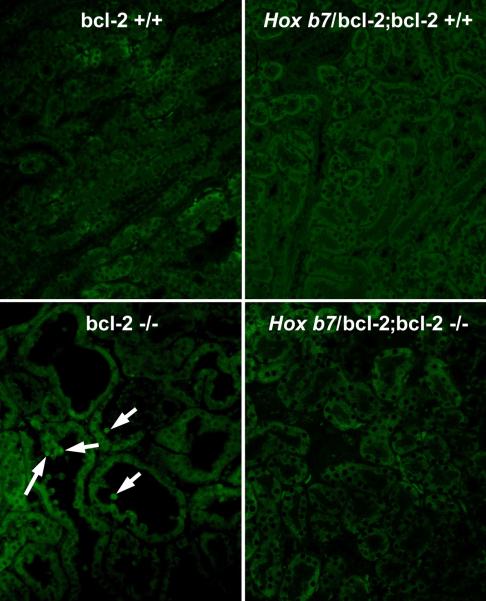
We previously characterized postnatal renal maturation in bcl-2 deficient mice (Sorenson et al., 1996; Sorenson and Sheibani, 1999; Sorenson and Sheibani, 2002a; Sorenson and Sheibani, 2002b). Here we set out to determine whether reexpression of bcl-2 in the ureteric bud/collecting duct would alleviate any of the renal hypoplasia observed in the absence of bcl-2. A transgene was constructed using the Hox b7 promoter to drive expression of bcl-2 (Hox b7/bcl-2) throughout the ureteric bud during kidney development and in its derived epithelia (collecting duct). A schematic representation of this transgene is shown in Figure 1A. The Hox b7 promoter has been successfully used to drive numerous transgenes and its expression is observed by E11.5 when nephrogenesis begins (Srinivas et al., 1999a; Srinivas et al., 1999b). Figure 1B demonstrates several founders with varying copy numbers of the transgene detected by Southern blot analysis. Founders 124 and 126 had the highest copy numbers. F1 generations were obtained from several founders, but unfortunately founder 126 never produced offspring. The other low copy number founders produced limited numbers of positive offspring at best. Therefore, founder 124’s progeny were chosen to breed to bcl-2 +/− mice. This facilitated the generation of mice that express bcl-2 in the ureteric bud/collecting duct, but are bcl-2 deficient in the rest of the kidney (Hox b7/bcl-2;bcl-2 −/−). These mice were maintained by crossing Hox b7/bcl-2; bcl-2 +/− mice and genotyping for bcl-2 and the Hox b7/bcl-2 transgene. Figure 1C demonstrates that kidney lysates from Hox b7/bcl-2;bcl-2 −/− mice had significant bcl-2 protein expression compared to bcl-2 −/− mice. We next co-stained kidney sections from P0 Hox b7/bcl-2;bcl-2 −/− mice with anti-bcl-2 and Dolichos biflorus agglutinin to examine the distribution of Hox b7 driven bcl-2 expression (Figure 1D). In the absence of bcl-2 in the rest of the kidney, we observed Hox b7 driven expression of bcl-2 in the collecting duct.
Figure 1. Hox b7 transgene expression of bcl-2.
Panel A is a schematic representation of the bcl-2 transgene. Panel B is Southern blot analysis of tail DNA that was employed to identify the founders. This blot demonstrates transgene expression in founders 108, 111, 119, 120, 124 and 126. In Panel C, Western blot analysis was done on kidney protein lysate (20 μg) from P25 bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice. The membrane was incubated with antibodies to bcl-2. Panel D is a kidney section from a P0 Hox b7/bcl-2;bcl-2 −/− mouse co-stained with anti-bcl-2 and Dolichos biflorus agglutinin (DBA). The staining is shown individually and with a merged image in the far panel. P0 bcl-2 −/− kidney sections stained with anti-bcl-2 demonstrated no detectable immunostaining (data not shown).
Increased Renal Mass and Less Cysts in Hox b7/bcl-2;bcl-2 −/− Mice
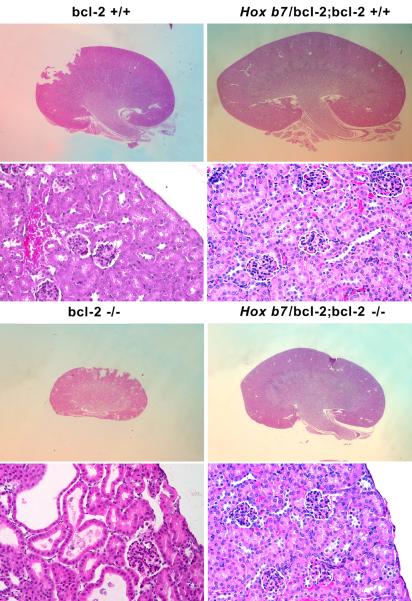
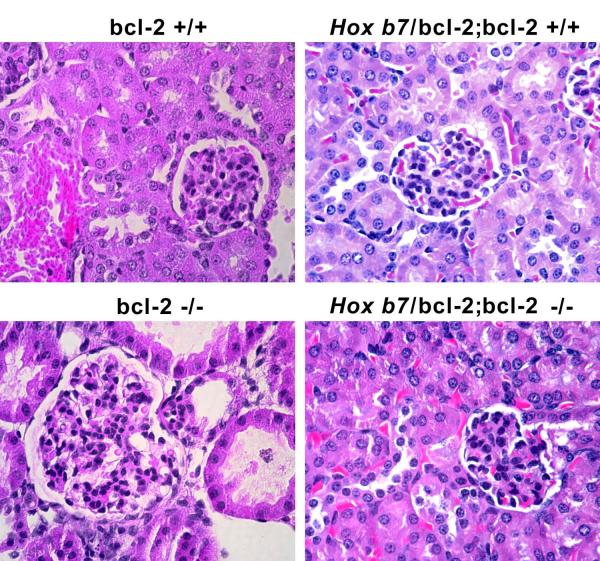
Previous work from this laboratory demonstrated renal hypoplasia and gross renal cyst formation by P21 in bcl-2 −/− mice (Sorenson et al., 1996). We first examined whether reexpression of bcl-2 in the ureteric bud and its epithelial derivatives would impact renal morphology and/or renal mass in the absence of bcl-2 in the rest of the kidney. In Figure 2A, hematoxylin and eosin (H&E) stained mid-sagittal kidney sections from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice are shown at low (x40) and high (x200) magnification. Kidneys from bcl-2 +/+ and Hox b7/bcl-2;bcl-2 +/+ mice have similar morphology. Kidneys from bcl-2 −/− mice are hypoplastic with apparent renal cysts. In contrast, mid-sagittal kidney sections from Hox b7/bcl-2;bcl-2 −/− mice showed little if any cyst formation and appeared less hypoplastic compared to kidneys from bcl-2 −/− mice.
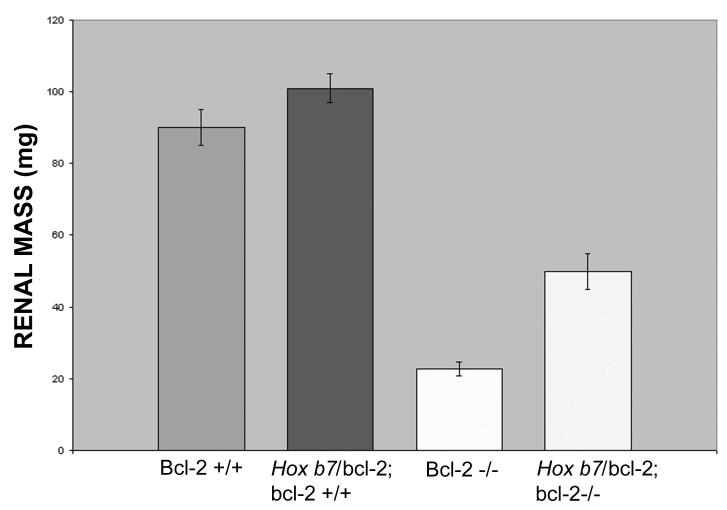
Figure 2. Hox b7 driven bcl-2 expression decreased renal hypoplasia and cyst formation in bcl-2 −/− mice.
In Panel A, mid-sagittal kidney sections from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice were hematoxylin and eosin stained. Sections are representative of > 6 litters. The upper panel shows a lower (10X) magnification and the lower panel a higher (200X) magnification. Panel B is the summarization of renal mass from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice. Please note that expression of bcl-2 in ureteric bud and its derivatives increased renal mass to nearly half of that of normal mice (P<0.01). * Denotes that the differences are statistically significant. Values are means ± SD. Data are representative of a minimum of 30 mice.
Next, renal mass was examined to determine whether increased renal mass was observed in the Hox b7/bcl-2;bcl-2 −/− mice. We observe approximately a two-fold increase in renal mass in the Hox b7/bcl-2;bcl-2 −/− mice compared to the bcl-2 −/− mice (Figure 2B). These data are consistent with the mid-sagittal sections shown in Figure 2A.
Glomerular Hypertrophy in Bcl-2 −/− Mice
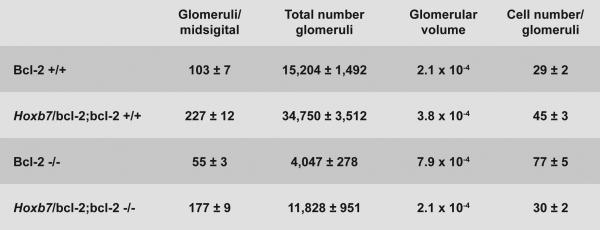
In the absence of bcl-2, we previously observed glomerular hypertrophy (Sorenson et al., 1996). Here we examined whether glomerular hypertrophy, glomerular volume and nephron numbers were affected in Hox b7/bcl-2;bcl-2 −/− mice compared to the bcl-2 −/− mice. Glomeruli from P25 bcl-2 −/− mice are significantly larger compared to bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+ and Hox b7/bcl-2;bcl-2 −/− mice (Figure 3A). This was associated with increased glomerular volume as well as increased numbers of cells within the glomerulus (Figure 3B). In contrast, glomeruli from Hox b7/bcl-2;bcl-2 −/− mice were normal in size and volume. To determine whether the lack of glomerular hypertrophy in Hox b7/bcl-2;bcl-2 −/− mice could be due, in part, to increased numbers of nephrons, total numbers of glomeruli on mid-sagittal sections and total glomerular numbers were determined. Glomerular counts increased nearly three fold in Hox b7/bcl-2;bcl-2 −/− mice verus bcl-2 −/− mice (Figure 3B). Interestingly, Hox b7/bcl-2;bcl-2 +/+ mice had twice as many glomeruli as their wild-type counterpart.
Figure 3. Glomerular hypertrophy in the absence of bcl-2.
Panel A is representative photomicrographs of renal cortex from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice were hematoxylin and eosin stained. Please note glomerular hypertrophy in bcl-2 −/− mice. In Panel B, glomeruli/midsigittal section, glomerular volume and cell numbers/glomeruli were determined (N> 30 mice). Please note that Hoxb7 driven expression of bcl-2 significantly increased total glomerular numbers (bcl-2 +/+ 15,204 ±1,492 vs Hox b7/bcl-2;bcl-2 +/+ 34,750±3,512;P<0.03 and bcl-2 −/− 4,047± 278 vs Hox b7/bcl-2;bcl-2 −/− 11,828±951;P<0.03; n=5). In Panel C, mid-sagittal kidney sections from P 25 bcl-2 +/+ and Hox b7/bcl-2;bcl-2 +/+ mice were stained with Lotus tetragonobolus agglutinin to identify proximal tubules.
Proximal convoluted tubules undergo a 5-fold increase in length during the first month of life in the rodent (Spitzer and Brandis, 1974). To begin to understand how a two-fold increased glomerular numbers in Hox b7/bcl-2;bcl-2 +/+ mice did not result in a similar increase in renal mass we stained mid-sagittal sections from P25 kidneys with Lotus tetragonobolus agglutinin to identify proximal tubules (Figure 3C). Figure 3C demonstrates that proximal tubules were identified deeper into the cortex in kidneys from P25 bcl-2 +/+ mice compared to Hox b7/bcl-2;bcl-2 +/+ mice (1.1 ± 0.059 mM bcl-2 +/+ vs 0.8 ± 0.045 mM Hox b7/bcl-2;bcl-2 +/+; P <0.0001).
Decreased Proliferation and Apoptosis in the Hox b7;bcl-2/bcl-2 −/− Mice
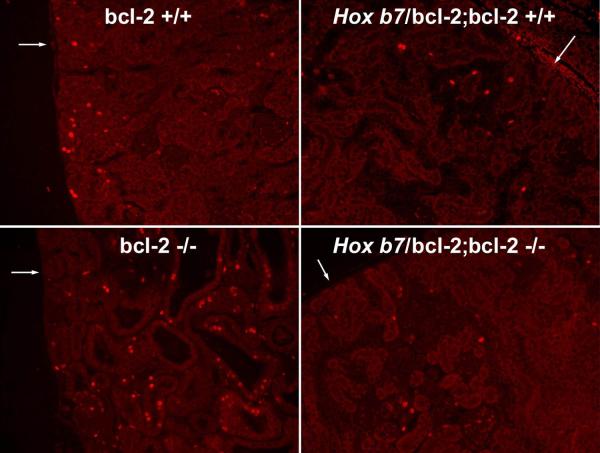
Proliferation and apoptosis are typically down-regulated in conjunction with renal maturation in the kidney. Our previous data demonstrated that proliferation and apoptosis were not down-regulated in the kidney in the absence of bcl-2 at P21 (Sorenson and Sheibani, 2002b). Here we show that bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, and Hox b7/bcl-2;bcl-2 −/− mice had similarly low rates of proliferation in the renal cortex (1% ± 0.03% Hox b7/bcl-2;bcl-2 +/+ vs. 1% ± 0.03% Hox b7/bcl-2;bcl-2 −/−; Figure 4A). In contrast, cystic kidneys from bcl-2 −/− mice had a higher rate of proliferation compared to Hox b7/bcl-2;bcl-2 −/− mice, as shown by Ki-67 staining (14% ± 1.2% bcl-2 −/− vs. 1% ± 0.03% Hox b7/bcl-2;bcl-2 −/−; P< 0.001; Figure 4A).
Figure 4. Decreased proliferation and apoptosis in the Hox b7;bcl-2/bcl-2 −/− mice.
Panel A are fluorescence photomicrographs of histological section of kidney cortex from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice stained with anti-Ki-67 to visualize proliferating cells. The arrow points to the renal capsule. In Panel B, a TUNEL assay was employed to identify apoptotic cells. Arrows point to apoptotic cells in sections from bcl-2 −/− mice. Please note that the level of proliferation and apoptosis is higher in kidney sections from bcl-2 −/− mice. These results are representative of those obtained from 5 mice.
We next examined whether apoptosis was affected in the Hox b7/bcl-2;bcl-2 −/− mice. Sections from P25 bcl-2 +/+, bcl-2−/−, Hox b7/bcl-2;bcl-2 +/+ and Hox b7/bcl-2;bcl-2 −/− mice were subjected to a TUNEL assay to identify apoptotic cells. Kidney sections from bcl-2 −/− mice demonstrated apoptotic cells being sloughed into the cyst interiors (Figure 4B). In sections from P25 bcl-2 +/+, Hox b7/bcl-2;bcl-2 +/+ and Hox b7/bcl-2;bcl-2 −/− mice, we detected few if any apoptotic cells. The levels of apoptosis observed in the bcl-2 +/+ and bcl-2 −/− mice are consistent with our previous studies (Sorenson et al., 1996). Thus, the decrease in apoptosis and proliferation typically associated with renal maturation was observed in Hox b7/bcl-2;bcl-2 −/− mice.
Restoration of PTP 1B Distribution in Hox b7/bcl-2;bcl-2 −/− Mice
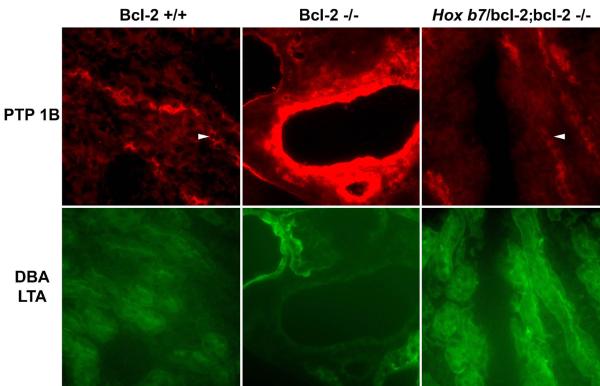
Previous work from this laboratory suggested that proper activation and recruitment of PTP 1B to focal adhesion complexes may play an important role during renal maturation. Cystic kidneys from bcl-2 −/− mice displayed loss of PTP 1B expression on the cell periphery and increased staining for PTP 1B in cystic distal tubules (Sorenson and Sheibani, 2002a). In the lower panel of Figure 5A, nephron segments were stained with Lotus tetragonobolus agglutinin and Dolichos biflorus agglutinin to identify proximal tubules and collecting duct, respectively. Kidneys from Hox b7/bcl-2;bcl-2 −/− mice exhibited cell periphery PTP 1B staining in distal tubules similar to that observed in kidneys from bcl-2 +/+ (Figure 5A) and Hox b7/bcl-2;bcl-2 +/+ mice (data not shown). In contrast, kidneys from bcl-2 −/− mice demonstrated intense PTP 1B staining in cystic distal tubules (Figure 5A), confirming our previous data (Sorenson and Sheibani, 2002a).
Figure 5. Restoration of PTP 1B distribution in Hox b7;bcl-2/bcl-2 −/− mice.
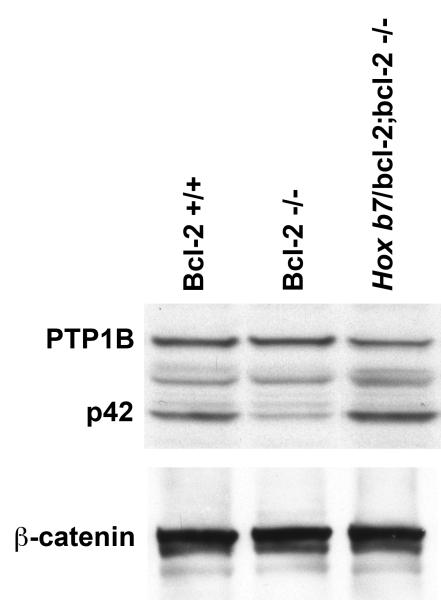
In Panel A, fluorescence photomicrographs of kidney cortex from P25 bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice stained with anti-PTP 1B and co-stained with Lotus tetragonobus agglutinin (to identify proximal tubules) and Dolichos biflorus agglutinin (to identify collecting ducts) in the lower panel. The arrow head points to staining on the cell periphery as observed in normal kidneys. Please note the intense PTP1B staining of distal tubules in kidney cortex from bcl-2 −/− mice. In Panel B, Western blot analysis was done on kidney protein lysate (20 μg) from P25 bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice. The membrane was incubated with antibodies to PTP 1B and reprobed with antibodies to β-catenin to control loading. Blots are representative of protein lysates obtained from 4 individual mice.
Expression of PTP 1B is developmentally regulated during kidney development and renal maturation (Sorenson and Sheibani, 2002a). The calpain-cleaved 42-kDa form (p42) is more catalytically active and increases with renal maturation. Figure 5B demonstrates that kidneys from bcl-2 +/+ and Hox b7/bcl-2;bcl-2 −/− mice have similar expression of PTP 1B (p50) and p42 PTP 1B. In contrast, kidneys from bcl-2 −/− mice have decreased expression of p42 PTP 1B as shown previously (Sorenson and Sheibani, 2002a). Thus, reexpression of bcl-2 in the ureteric bud and its epithelial derivatives, in the absence of bcl-2 in the rest of the kidney, results in a more normal renal expression and distribution of PTP1B.
Enhanced Pax 2 Expression in Kidneys from P0 Hox b7/bcl-2;bcl-2 −/− Mice
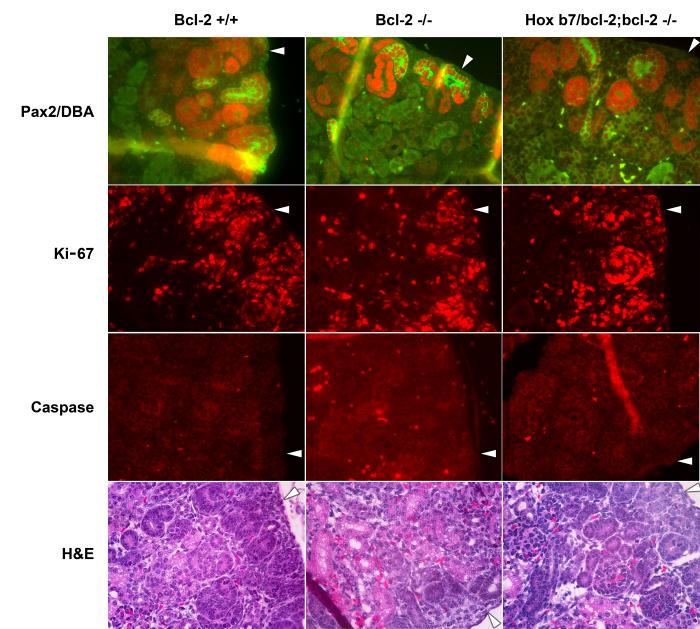
The rodent is born with immature kidneys in which nephrons continue to develop from the nephrogenic zone, located at the periphery of the kidney. This period of time is termed renal maturation (Koseki et al., 1992). Here we have examined Pax-2 expression, proliferation and apoptosis in kidneys from newborn mice. Pax-2 is expressed in induced mesenchyme and is essential for epithelial conversion. In newborn kidneys, Pax-2 staining is mainly confined to the nephrogenic zone where we also observed the highest level of proliferation (arrow heads). Consistent with the reduced nephrogenic zone in kidneys from bcl-2 −/− mice, we also observed less Pax-2 and Ki-67 staining compared to bcl-2 +/+ mice (Figure 6). The Hox b7/bcl-2;bcl-2 −/− mice demonstrated partial restoration of the nephrogenic zone as demonstrated by Pax 2 and Ki-67 staining (Figure 6). To determine apoptotic cells, the sections were stained with anti-cleaved caspase 3 antibody. Sections from P0 bcl-2 +/+ and Hox b7/bcl-2;bcl-2 −/− mice demonstrated similar levels of apoptotic cells. In contrast, kidney sections from P0 bcl-2 −/− mice demonstrated increased amounts of apoptotic cells. Hematoxylin and eosin (H&E) staining of the P0 kidneys are shown in the lower panels. Consistent with the increased nephron numbers in Hox b7/bcl-2;bcl-2 −/− mice, the nephrogenic zone depth was also increased (bcl-2 +/+ 0.14 mm ± 0.01 vs. bcl-2 −/− 0.058 mm ± 0.005 vs. Hox b7/bcl-2;bcl-2 −/− 0.1 mm ± 0.01 mm).
Figure 6. Increased nephrogenic zone in Hox b7/bcl-2;bcl-2 −/− mice.
Fluorescence photomicrographs of kidney cortex from P0 bcl-2 +/+, bcl-2 −/− and Hox b7/bcl-2;bcl-2 −/− mice stained with anti-Pax-2 and Dolichos biflorus agglutinin (to identify collecting ducts), anti-Ki-67, anti-cleaved caspase 3 or hematoxylin and eosin. The arrow head points to periphery of the kidney. Stained sections are representative of staining performed on 5 individual mice.
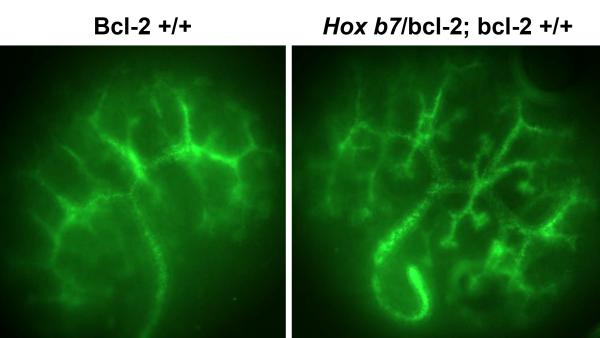
Hox b7/bcl-2;bcl-2 +/+ Embryos Demonstrated Increased Ureteric Bud Branch Tips and Branch Points
The increased number of nephrons observed in mice expressing the Hox b7/bcl-2 transgene, suggested that ureteric bud branching may be enhanced in the presence of the bcl-2 transgene. In Figure 7, metanephroi from E12 bcl-2 +/+ and Hox b7/bcl-2;bcl-2 +/+ embryos were wholemount stained with Dolichos biflorus agglutinin to visualize the ureteric bud. We observed increased ureteric branching in metanephroi from Hox b7/bcl-2;bcl-2 +/+ embryos. Specifically, we observed increased branch points (20±2 branch points/bcl-2 metanephroi vs. 30±2 branch points/Hox b7/bcl-2;bcl-2 +/+ metanephroi) and increased numbers of branch tips (21±2 branch tips/bcl-2 +/+ metanephroi vs. 30±3 branch tips/Hox b7/bcl-2;bcl-2 +/+ metanephroi). Thus, increased ureteric bud branching may contribute to the increase nephron number observed in the presence of Hox b7/bcl-2 transgene.
Figure 7. Increased ureteric bud branch tips and branch points in Hox b7/bcl-2;bcl-2 +/+ embryos.
Embryonic kidneys from E12 bcl-2 +/+ and Hox b/bcl-2;bcl-2 +/+ embryos were wholemount stained with Dolchios biflorus agglutinin to visualize the ureteric bud. Quantitation of ureteric bud branch points demonstrated 20±2 branch points/bcl-2 metanephroi vs. 30±2 branch points/Hox b7/bcl-2;bcl-2 +/+ metanephroi (P<0.03) and 21±2 ureteric bud branch tips/bcl-2 +/+ metanephroi vs. 30±3 branch tips/Hox b7/bcl-2;bcl-2 +/+ metanephroi (P< 0.03). Values are means ± SD. Data are representative of 4 animals.
DISCUSSION
Bcl-2 plays an important role during kidney development. Although kidney development does occur in the absence of bcl-2, it is significantly affected by the increased apoptosis that occurs early during embryonic kidney development. As a result postnatal kidneys are hypoplastic and become cystic. Here we reexpressed bcl-2 in the ureteric bud and its epithelial derivatives, in the absence of bcl-2 in the rest of the kidney, and observed the following results: 1) Increased renal mass and less renal cysts; 2) Lack of glomerular hypertrophy; 3) Increased numbers of nephrons; 4) Increased size of nephrogenic zone; and 5) Similar levels of apoptosis and proliferation as wild-type controls. Thus, reexpression of bcl-2 results in increased renal mass and restores a more normal renal morphology.
Increased levels of apoptosis and proliferation are observed in renal cystic diseases as well as aberrant regulation of signaling pathways, such as tyrosine phosphatases, involved in these processes (Grantham, 1990; Welling and Grantham, 1991; Wilson and Sherwood, 1991; Avner, 1993; Calvet, 1993; Sorenson, 1998; Grantham, 2001; Sorenson and Sheibani, 2002b; Sorenson and Sheibani, 2002a). Typically, postnatal renal maturation is concomitant with down-regulation of proliferation and apoptosis. Here reexpression of bcl-2 increased renal mass and restored a more normal, less cystic morphology. These changes were concomitant with proliferation and apoptosis at levels similar to kidneys from wild type mice.
Reduced nephron number at birth is thought to predispose individuals toward pathological conditions such as hypertension and susceptibility to injury later in life (Luyckx, 2005). Bcl-2 −/− mice display reduced nephron numbers and glomerular hypertrophy. The glomerular hypertrophy was due, at least in part, to increased cell numbers (Figure 3). This is consistent with reported hyperplasia observed following early loss of renal mass. Interestingly, Hox b7 promoter driven bcl-2 expression increased nephron number in the presence and absence of bcl-2 in the rest of the kidney. Hox b7/bcl-2;bcl-2 −/− mice demonstrated increased renal mass to 50% of that of wild type mice and glomeruli of normal size and volume. In contrast, oligosyndactyl (Os/+) mice, which are born with a 50% decrease in renal mass and nephron number undergo glomerular hypertrophy (Sorenson, 1996). These data suggest that glomeruli hypertrophy correlates with nephron number, perhaps relieving glomerular overload described in the absence of bcl-2 (Gassler, 1998).
Renal coloboma syndrome is due to heterozygous mutations in PAX2 genes resulting in renal hypoplasia. These mutations are associated with increased apoptosis and reduced ureteric bud branching as a result of reduced PAX2 dosage during development (Porteous, 2000). As a result, this syndrome is characterized by reduced numbers of glomeruli that undergo hypertrophy. Goodyer’s group proposed a model in which the rate of ureteric bud arborization is influenced by the balance of factors that influence apoptosis (Dziarmaga, 2006). This model is further supported by their work demonstrating PAX2 promotor driven expression of bax disturbs ureteric bud branching (Dziarmaga, 2003). Targeted expression of bcl-2 to the ureteric bud of Pax21Neu mutant mice suppresses the aberrant apoptosis restoring ureteric bud branching and nephron numbers (Dziarmaga, 2006). The work presented here further supports this model.
Suboptimal nephron endowment at birth may be a predisposing factor for hypertension later in life. During kidney development, bcl-2 not only acts as a survival factor, but may also impact cell adhesive mechanisms and by extension branching morphogenesis. Previous work in this laboratory demonstrated decreased ureteric bud branching in the absence of bcl-2 including a decreased number of branch tips (Sheibani, 2007). Postnatally, this resulted in renal hypoplasia, decreased nephron numbers and glomerular hypertrophy (Sorenson et al., 1996). Here Hox b7 driven expression of bcl-2 led to an increase in nephron numbers in the presence or absence of bcl-2 in the rest of the kidney. Once the nephron number was increased to normal levels, glomerular hypertrophy was averted with a modest increase in renal mass. Thus, bcl-2 expression appears to positively influence nephron number.
EXPERIMENTAL PROCEDURES
Generation and Screening of Transgenic Mice
We have inserted full-length murine bcl-2 cDNA downstream of the Hoxb7 promoter (Kress et al., 1990; Vogels et al., 1993; Srinivas et al., 1999a; Srinivas et al., 1999b) and upstream of a bovine growth hormone (BGH) poly A signal sequence as shown in Figure 1A. Briefly, the full-length cDNA for murine bcl-2 as a 0.7 Kbp EcoRI/SmaI fragment was ligated with a 1.3 Kbp SmaI/EcoRI fragment encoding Hoxb7 promoter and pGEM7Zf vector (Promega, Madison, WI) digested with SmaI. This vector was then digested with Xba I/Hind III to remove the insert (Hoxb/bcl-2) and ligated with a Hind III/Pvu II fragment encoding BGH polyA signal sequence (370 bp) and pGEM7zf vector digested with Xba I/Sma I. This generated pGEM7Hoxb7-Bcl-2 with a poly A signal. The integrity of the vector was confirmed by restriction enzyme digestion and DNA sequencing. The transgene was excised by XbaI/NsiI digestion (Figure 1A), isolated by agarose gel electrophoresis and purified for microinjection by extraction using QIAEX gel extraction kit (Qiagen, Valencia, CA). Transgenic mice were generated at the University of Wisconsin Biotechnology Center’s Transgenic Facility. Each founder mouse was bred to a bcl-2 +/− mouse.
Genomic DNA was prepared from tail biopsies and the transgenic mice were identified by PCR screening for bcl-2 as previously described [5]. PCR screening for the Hox b7 bcl-2 transgene utilized the Hox b7 (5′-GGGGGTCCTTTGGTGTAAATC-3′) and bcl-2 (5′-GCCATATAGTTCCACAAA-3′) primers. The amplified fragments were electrophoresed and visualized by ethidium bromide staining. For Southern blot analysis, genomic DNA (10 μg) was digested with BamHI/EcoRV, and electrophoresed on a 0.8% agarose gel, transferred to Zeta-probe membrane and hybridized with digoxigenin random-prime-labeled probe as described by the supplier (Roche Applied Science, Indianapolis, IN). The probe was specific to a 0.7 Kbp fragment of the Hox b7/ bcl-2 transgene (Figure 1A), but not endogenous mouse bcl-2. These transgenic mice were produced on the same background as our bcl-2 −/− mice (B6/129Sv) so that direct comparisons and crosses could be performed. The F1 generation was then bred to a bcl-2 +/− mouse. This resulted in bcl-2 +/+ mice that expressed the Hox b7/bcl-2 transgene (Hox b7/bcl-2;bcl-2 +/+) and bcl-2 −/− mice that expressed the Hox b7/bcl-2 transgene (Hox b7/bcl-2 ;bcl-2 −/−).
Western Blot Analysis
Total protein lysates from postnatal kidneys were prepared in a modified RIPA buffer containing 142.5 mM KCl, 5 mM MgCl2, 10 mM Hepes pH 7.4, 1% NP40 and Complete protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). The protein concentration was determined using a Bio-rad DC protein assay (Bio-rad, Hercules, CA). 20 μg of the sample was Western blotted for bcl-2 (1:1000; R&D Systems, Minneapolis, MN), PTP 1B (1:100; Millipore, Billerica, MA) or β-catenin (1:4000; Sigma, St. Louis, MO).
Processing of Kidneys for Histological Studies and Immunochemistry
Following surgical removal from mice, the kidneys were fixed overnight in formalin and processed for paraffin sectioning or placed in optimum cutting temperature (OCT) compound (VWR Scientific, St. Louis MO) and rapidly frozen. Sections of 7 μm each were placed on slides. Some sections were stained with Hematoxylin and Eosin (H&E). Paraffin sections were deparaffinized with xylene and rehydrated. Antigen unmasking was performed using antigen-unmasking solution (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Frozen sections were fixed in cold acetone. The sections were then washed in phosphate buffered saline (PBS) and incubated for 15 min in PBS blocking buffer (PBS containing 1% bovine serum albumin, 0.3% Triton X-100 and 0.2% skim milk powder). The sections were incubated overnight with anti-Ki-67 (1:100; TEC-3; DAKO Glostrup, Denmark), Pax-2 (:100; Santa Cruz, Santa Cruz, CA), anti-bcl-2 (1:250; R&D Systems, Minneapolis, MN) or PTP 1B (1:100; Millipore). In some cases, the nephron segments were double stained with Lotus tetragonobolus agglutinin (Vector Laboratories), to identify proximal tubules and Dolichos biflorus agglutinin (Vector Laboratories), to identify collecting ducts. For these experiments, fluorescein-labeled lectins (1:40) were incubated overnight in the presence of primary antibody. The sections were then incubated with indocarbocyanine (CY3)-labeled secondary antibody (Jackson ImmunoResearch, West Grove, PA). The slides were then photographed. Proliferation rates were assessed by determining the number of Ki-67 positive cells in a field as a percentage of the total number of cells in a field. The depth of the nephrogenic zone was determined by measuring in 6 areas around the periphery of a P0 kidney. Nephrogenic zones from 4 mice of each genotype were measured and a mean obtained.
Total numbers of glomeruli
Whole mouse kidneys were embedded in OCT and sectioned at 20 μM, collecting every tenth and eleventh sections. The sections were stained with either anti-vimentin (Sigma, St. Louis MO ;1:100) or H&E. The total number of glomeruli were estimated as previously described by Bertram and colleagues (Bertram et al., 1992; Bertram, 2001; Samuel et al., 2005) using the formula Nglom,kid =10 × Ps/Pf × 1/2fa × Q−. Q− are those glomeruli that are present in the first microscope but not present in the look-up section. 10 is the reciprocal of the section sampling fraction. Ps is the total number of points overlying all tenth kidney sections and Pf is the number of points overlying the complete kidney sections. 1/2 fa is the fraction of the total section area used to count glomeruli.
UB visualization in embryonic kidneys
Embryos were removed from timed pregnant mice at embryonic day 12 and genotyped. Embryonic kidneys were surgically dissected from the embryos. To localize the ureteric bud, wholemount staining of embryonic kidneys with FITC-labeled Dolichos biflorus (DBA) (Vector, Burlingame,CA) was performed (Sorenson, 2004). The embryonic kidneys were fixed overnight in 2% paraformaldehyde and blocked in 50 mM NH4Cl. The embryonic kidneys were incubated for 30 minutes with 0.075% saponin and then 0.075% saponin with 0.2% gelatin. The embryonic kidneys were next incubated with the saponin-gelatin solution containing DBA (1:80) for 1 hour at room temperature, washed in PBS and coverslipped. Images were obtained using a Zeiss microscope (AxioPhot, Carl Zeiss, Chester, VA) equipped with a digital camera (Axiovision, Carl Zeiss). For quantification, the numbers of ureteric bud branch points and ureteric bud branch tips were determined.
ACKNOWLEDGEMENTS
This research was funded by National Institutes of Health DK067120. The authors wish to thank Dr. Nader Sheibani for insightful discussion and critical review of this manuscript. The authors wish to thank Drs. John Bertram and Luise Cullen-McEwen for their valuable assistance calculating total glomeruli numbers. The authors also wish to thank Elizabeth Scheef and Cathy Grutzmacher for assistance with total glomerular counts and Robert Gordon for assistance with graphics.
LITERATURE CITED
- Avner E. Epithelial polarity and differentiation in polycystic kidney disease. J. Cell Science. 1993;17:217–222. doi: 10.1242/jcs.1993.supplement_17.30. [DOI] [PubMed] [Google Scholar]
- Bertram JF. Counting in the kidney. Kidney Int. 2001;59:792–796. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res. 1992;270:37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- Calvet JP. Polycystic kidney disease: Primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int. 1993;43:101–108. doi: 10.1038/ki.1993.17. [DOI] [PubMed] [Google Scholar]
- Chandler D, El-Naggar AK, Brisbay S, Redline RW, McDonnell TJ. Apoptosis and expression of the bcl-2 proto-oncogene in the fetal and human adult kidney: Evidence for the contribution of bcl-2 expression to renal carcinogenesis. Human Pathology. 1994;25:789–796. doi: 10.1016/0046-8177(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Dziarmaga A, Clark P, Styner C, Julien JP, Torban E, Goodyer P, Eccles M. Ureteric bud apoptosis and renal hypoplasia in transgenic PAX2-Bax fetal mice mimics the renal-coloboma syndrome. J Am Soc Nephrol. 2003;14:2767–2774. doi: 10.1097/01.asn.0000094082.11026.ee. [DOI] [PubMed] [Google Scholar]
- Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17:1568–1575. doi: 10.1681/ASN.2005101074. [DOI] [PubMed] [Google Scholar]
- Gassler N, Elger M, Inoue D, Kriz W, Amling M. Oligonephronia, not exuberant apoptosis, accounts for the development of glomerulosclerosis in the bcl-2 knockout mouse. Nephrol Dial Transplant. 1998;13:2509–2518. doi: 10.1093/ndt/13.10.2509. [DOI] [PubMed] [Google Scholar]
- Grantham J. Polycystic kidney disease: from the bedside to the gene and back. 2001. pp. 1–10. [DOI] [PubMed]
- Grantham JJ. Polycystic kidney disease: neoplasia in disguise. Am. J. Kid. Dis. 1990;15:110–116. doi: 10.1016/s0272-6386(12)80507-5. [DOI] [PubMed] [Google Scholar]
- Koseki C, Herzlinger D, Al-Awqati Q. Apoptosis in metanephric development. J. Cell Biol. 1992;119:1327–1333. doi: 10.1083/jcb.119.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress C, Vogels R, De Graaff W, Bonnerot C, Meijlink F, Nicolas J, Deschamps J. Hox-2.3 upstream sequences mediate lacZ expression in intermediate mesoderm derivatives of transgenic mice. Development. 1990;109:775–786. doi: 10.1242/dev.109.4.775. [DOI] [PubMed] [Google Scholar]
- Luyckx VA, Brenner BM. Low birth weight, nephron number and kidney disease. Kidney Int. 2005;97:568–577. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- Novak DV, Korsmeyer SJ. Bcl-2 protein expression during murine development. Am. J. Pathology. 1994;145:61–73. [PMC free article] [PubMed] [Google Scholar]
- Porteous S, Torban E, Cho N-P, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, Sata T, Yun K, Favor J, Sicotte M, Goodyer P, Eccles M. Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys with Pax21Neu +/− mutant mice. Human Molec Genetics. 2000;9:1–11. doi: 10.1093/hmg/9.1.1. [DOI] [PubMed] [Google Scholar]
- Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Scheef EA, DiMaio TA, Wang Y, Kondo S, Sorenson CM. Bcl-2 expression modulates cell adhesion and migration promoting branching of ureteric bud cells. J Cell Physiol. 2007;210:616–625. doi: 10.1002/jcp.20858. [DOI] [PubMed] [Google Scholar]
- Sorenson CM. Life, death and kidneys: regulation of renal programmed cell death. Curr. Opin. Nephrol. Hypertens. 1998;7:5–12. doi: 10.1097/00041552-199801000-00002. [DOI] [PubMed] [Google Scholar]
- Sorenson CM. Interaction of bcl-2 with paxillin through its BH4 domain is important during ureteric bud branching. Journal of Biological Chemistry. 2004;279:11368–11374. doi: 10.1074/jbc.M310079200. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Padanilam B, Hammerman MR. Abnormal postpartum renal development and cystogenesis in the bcl-2 −/− mouse. Am. J. Physiol. 1996;271:F184–F193. doi: 10.1152/ajprenal.1996.271.1.F184. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Rogers SA, Hammerman MR. Abnormal renal development in the Os/+ mouse is intrinsic to the kidney. Am J Physiol. 1996;271:F234–F238. doi: 10.1152/ajprenal.1996.271.1.F234. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Sheibani N. Focal adhesion kinase, paxillin and bcl-2: Analysis of expression. phosphorylation and association during morphogenesis. Dev. Dynamics. 1999;215:371–382. doi: 10.1002/(SICI)1097-0177(199908)215:4<371::AID-AJA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Sheibani N. Altered regulation of SHP-2 and PTP1B tyrosine phosphatases in cystic kidneys from Bcl-2 −/− mice. Am. J. Physiol. 2002a;282:F442–F450. doi: 10.1152/ajprenal.00184.2001. [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Sheibani N. Sustained activation of MAPK/ERK’s signaling pathway in cystic kidneys from Bcl-2 −/− mice. American Journal of Physiology. 2002b;283:F1085–F1090. doi: 10.1152/ajprenal.00380.2001. [DOI] [PubMed] [Google Scholar]
- Spitzer A, Brandis M. Functional and morphologic maturation of the superficial nephrons. Relationship to total kidney function. J Clin Invest. 1974;53:279–287. doi: 10.1172/JCI107548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Goldberg MR, Watanabe T, D’Agati V, Al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Developmental Genetics. 1999a;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Wu Z, Chen C-M, D’Agati V, Costantini F. Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development. 1999b;126:1375–1386. doi: 10.1242/dev.126.7.1375. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Corce CM. Analysis of the structure, transcripts, and protein products of bcl, the gene involved in human follicular lymphoma. Proc Natl Acad Sci. 1986;83:5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Charite J, de Graaff W, Deschamps J. Proximal cis-acting elements cooperate to set Hoxb-7 (Hox-2.3) expression boundaries in transgenic mice. Development. 1993;118:71–82. doi: 10.1242/dev.118.1.71. [DOI] [PubMed] [Google Scholar]
- Welling LW, Grantham JJ. Cystic and developmental diseases of the kidney. The Kidney; Philedelphia PA: 1991. [Google Scholar]
- Wilson PD, Sherwood AC. Tubulocystic epithelium. Kidney Int. 1991;39:450–463. doi: 10.1038/ki.1991.56. [DOI] [PubMed] [Google Scholar]