Abstract
Objectives
To evaluate demographics, magnetic resonance imaging (MRI) measures, and Vascular risk among mild cognitive impairment (MCI) subtypes.
Design
Cross-sectional study.
Setting
Both clinics and the community.
Participants
A total of 153 subjects with MCI, 218 cognitively normal older individuals (controls), and 68 patients with Alzheimer disease.
Main Outcome Measures
Classification of subjects with MCI according to current subtype diagnostic convention based on neuropsychological performance, estimates of vascular risk based on medical history, research MRI unless there was a specific contraindication, and apolipoprotein E genotype.
Results
Of the 153 subjects with MCI, 65 were diagnosed with amnestic single-domain, 46 with amnestic multiple-domain, 27 with nonamnestic single-domain, and 15 with nonamnestic multiple-domain MCI. Analyses of control, MCI, and Alzheimer disease cases revealed significant differences in brain and hippocampal volumes between each group. Post hoc analyses of MRI measures among the MCI subtypes found that patients with amnestic single-domain MCI had significantly less brain atrophy and that hippocampal volume differed significantly from controls for the 2 amnestic forms of MCI. Apolipoprotein E genotype prevalence was significantly greater in the amnestic and nonamnestic subtypes of MCI. Conversely, the nonamnestic subtypes were more likely to have increased vascular risk and to be African American.
Conclusions
Amnestic forms of MCI appear to have demographic, genetic, and MRI findings suggestive of Alzheimer disease pathology, whereas the nonamnestic forms of MCI have findings suggestive of vascular disease. Importantly, however, all subjects with MCI showed evidence of brain injury, and the biological differences among subtypes are relatively subtle beyond the memory vs nonmemory groupings.
Keywords: Mild Cognitive Impairment, Subtypes, Magnetic Resonanace Imaging, white matter hyperintensities, cerebrovascular disease, Apolipoprotein E genotype, pathophysiology
Introduction
While concerns regarding the ever increasing prevalence of dementia are well publicized,1,2 attention has only recently focused on the transition state between normal cognitive aging and dementia, described as mild cognitive impairment (MCI).3,4 Like dementia, MCI is now recognized as an important public health problem, potentially effecting between 12% and 18% of individuals older than 65 years,5 and is associated with increased morbidity and mortality 6–8 as well as risk of Alzheimer disease (AD). The concept of MCI,9–12 however, has evolved.3,4 It is now recognized as a heterogeneous syndrome indicating risk of a variety of dementing illnesses.10,11 Various MCI clinical subtypes have been identified including amnestic single-domain (aMCI-S), amnestic multiple-domain (aMCI-M), nonamnestic single-domain (naMCI-S), and nonamnestic multiple-domain (naMCI-M) MCI, with potential relevance to differing underlying etiologies.11 For example, individuals with amnesia (ie, aMCI-S and aMCI-M) are thought to represent early AD pathology,13 and an average of 3% per year develop AD.14 The nonamnestic MCI subtypes, however, are more strongly associated with cerebrovascular 11,15–17 or other diseases.6,9 Recent Pittsburgh Compound B (PiB) imaging also supports the notion of etiological heterogeneity among MCI subtypes, as approximately one-third of subjects with MCI have significant PiB retention.18,19 This is particularly true for nonamnestic subjects,20 who also develop dementia more slowly than PiB-positive patients with MCI.21,22
These data support the notion of both etiological and prognostic relevance to the various clinical subtypes of MCI. Given potential etiologic differences, specific subtypes of MCI might also be amenable to different medications. Recognizing the high prevalence of both stroke and AD among individuals older than 65 years,23 correct early diagnosis and prognosis of these various subtypes is likely to be important for effective treatments. Neuroimaging techniques can provide differential diagnosis, help predict the probability of developing dementia, and measure the progression of neurodegenerative diseases.24 Despite this, few magnetic resonance imaging (MRI) studies have examined the differences in brain MRI among MCI subtypes and, to our knowledge, none have combined genetic and vascular risk factors with MRI. In this study, we compared the quantitative MRI measures, cerebrovascular risk factors, ethnicity, and apolipoprotein genotypes of the 4 MCI subtypes.
Methods
PARTICIPANTS
Recruitment
All participants were evaluated at the University of California, Davis, Alzheimer Disease Center. Approximately 59% of participants were recruited through protocols designed to enroll ethnic and racial minorities in research. These individuals were recruited through various outreach methods such as soliciting in a community hospital lobby, a community survey, health fairs, and word of mouth. The remaining 40% were recruited either when seeking an evaluation at the disease center or as volunteers. Thus, while this is a sample of convenience, it represents a concerted effort to be broadly inclusive. Inclusion criteria were limited to age greater than 60 years. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder (history of schizophrenia, bipolar disorder, or recurrent major depression), and substance abuse or dependence in the last 5 years. All participants signed informed consent forms, and all subject involvement was overseen by institutional review boards at the University of California, Davis, the Veterans Administration Northern California Health Care System, and San Joaquin General Hospital in Stockton, California.
Clinical Evaluation
All participants received a multidisciplinary clinical evaluation through the University of California, Davis, Alzheimer Disease Center. These evaluations included detailed medical history and physical and neurological examination. A physician fluent in Spanish examined subjects who spoke only Spanish. A family member or other informant in close contact with the participant was interviewed to obtain information about the participant’s level of independent functioning. Routine dementia laboratory tests and MRI were obtained for all participants with cognitive impairment.
Clinical neuropsychological evaluation used the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery 25–27 (Mini-Mental State Examination, List Learning, Animal Fluency, Constructional Praxis, 15-item Boston Naming Test for Spanish speakers, and 60-item version for English speakers) supplemented by the Wechsler Adult Intelligence Scale, Revised Digit Symbol,28 and the Trail Making Test.
Diagnosis of cognitive syndrome (control, MCI, or dementia) and for individuals with dementia, underlying etiology, was made according to standardized criteria. Dementia was diagnosed using Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) (DSM-III-R)29 criteria for dementia, modified to exclude the requirement of memory impairment. Alzheimer disease was diagnosed using National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria.30 Vascular dementia was diagnosed using the California Alzheimer’s Disease Diagnostic and Treatment Centers diagnostic criteria for ischemic vascular dementia.31 Mild cognitive impairment was diagnosed according to standard clinical criteria, modified to include the 4 subtypes.32 Memory impairment was identified based on results of the list-learning task, whereas nonmemory impairments were defined by performance on animal fluency, Boston Naming, digit symbol, or trail-making tasks. Normal cognitive function was diagnosed if performance was in the reference range for all tests. All subject diagnoses were blinded to research neuropsychological testing or quantitative brain image analysis. Individuals diagnosed as having clinically probable vascular dementia (n=5), frontotemporal dementia (n=3), or dementias in which the etiology was uncertain (n=6) were excluded from analysis. Patients clinically diagnosed with dementia with Lewy bodies (n=3) were included in the study, as these individuals often have coincident AD pathology.
MRI Acquisition
Research brain imaging was obtained at the University of California, Davis, MRI research center on a 1.5-T GE Signa Horizon LX Echospeed system (Sound Imaging, San Diego, California) or the Veterans Administration at Martinez on a 1.5-T Marconi system (Frontier Medical Imaging LLC, Phoenix, Arizona) using an axial T2-weighted double echo image, a coronal 3-dimensional spoiled gradient recalled echo (Inversion recovery prepped Spoiled GRASS) acquisition and an axial high-resolution fluid-attenuated inversion recovery image. Image quantification was performed by a rater who was blind to age, sex, race, educational achievement, ethnicity, and diagnostic status.
IMAGE ANALYSIS
Brain and White Matter Hyperintensity Volumes
Analysis of brain and white matter hyperintensity (WMH) volumes was based on a fluid-attenuated inversion recovery sequence designed to enhance WMH segmentation.33 Brain and WMH segmentation was performed in a 2-step process according to previously described methods.34,35 In brief, nonbrain elements were manually removed from the image by operator guided tracing of the dura matter in the cranial vault including the middle cranial fossa, but excluding the posterior fossa and cerebellum. The resulting measure of the cranial vault was defined as the total cranial volume to correct for differences in head size among the subjects. Image intensity nonuniformities36 were then removed from the image, and the resulting corrected image was modeled as a mixture of 2 gaussian probability functions with the segmentation threshold determined at the minimum probability between these 2 distributions,34 followed by a single gaussian distribution fitted to the image data using an a priori threshold of 3.5 SD in pixel intensity above the mean to identify WMH. Intrarater and interrater reliability of these methods are high and have been published previously.37
Hippocampal Volumes
The boundaries of the hippocampus were manually traced according to previously described methods38 that emphasize analysis of the anterior two-thirds of the hippocampus. Intrarater reliability for both the right and left hippocampus using this method is good, with intraclass correlation coefficients of 0.98 for the right hippocampus and 0.96 for the left hippocampus. MRI Infarctions The presence or absence of cerebral infarction on MRI was determined according to previously published protocols.35,37 The presence of MRI infarction was determined from the size, location, and imaging characteristics of the lesion. Only lesions 3 mm or larger qualified for consideration as cerebral infarcts. Other necessary imaging characteristics included (1) cerebrospinal fluid density on a T1-weighted or fluid-attenuated inversion recovery image and (2) distinct separation from the circle of Willis vessels if the stroke was in the basal ganglia area. Previously reported κ values for agreement between the 3 raters were generally good, ranging from 0.73 to 0.90.37
Cerebrovascular Risk Factors
The presence or absence of 5 cerebrovascular risk factors (ie, stroke, transient ischemic attack, diabetes, hypertension, and coronary artery disease) was systematically assessed using subject and informant medical histories as well as review of pertinent medical records to create a summed composite score, reported as a percentage.
STATISTICAL ANALYSES
Data were analyzed in JMP, version 5.1.2 (SAS Institute, Cary, North Carolina). We used multiple linear analyses to detect the total hippocampus volume, WMH, and brain volume differences among groups. Contingency analysis and correspondence analysis were used to compare the differences of ethnicity and apolipoprotein E4 (APOE-4) among subtypes. Multiple linear or logistic analyses were also used to test the association of cerebrovascular risks with MCI subtypes.
Results
SUBJECT CHARACTERISTICS
Subject characteristics are summarized in the Table 1. A total of 153 subjects with MCI (mean [SD] age, 75.29[7.20] years) were included in the study. In the MCI group, there were 65 subjects with aMCI-S, 46 with aMCI-M, 15 with naMCI-S, and 27 with naMCI-M. For the purposes of this study, these individuals were also compared with 218 cognitively normal individuals (controls) (mean [SD] age, 73.48[7.11] years) and 68 patients with AD (mean [SD] age, 77.80[6.64] years). Three older patients with aMCI-S, 1 with aMCI-M, 3 with naMCI-S, 3 with AD, and 4 without dementia did not receive MRI, while 10 subjects (5 cognitively normal and 5 with AD) were missing hippocampal volume data. Of the subjects, 27% self-identified as Hispanic, 26% as African American, and 43% as white. Although the ethnic distribution of cognitively normal individuals was quite balanced (ie, 33% in each group), white individuals were significantly more likely to have cognitive impairment (χ2=15.7; P=.003).
Table 1.
Subject demographics
| NL | AD | aMCI-S | aMCI-M | naMCI-S | naMCI-M | |
|---|---|---|---|---|---|---|
| No (F/M) | 218 (149/69) | 68 (45/23) | 65 (30/35) | 46 (25/21) | 27 (18/9) | 15 (8/7) |
| Age (yr) | 73.5 ± 7.1 | 77.8 ± 6.6 | 74.7 ± 6.5 | 77.2 ± 7.0 | 73.0 ± 8.6 | 76.0 ± 7.1 |
| Education (yr) | 12.5 ± 4.5 | 11.1 ± 4.7 | 13.4 ± 5.4 | 11.5 ± 4.9 | 12.2 ± 5.1 | 12.5 ± 5.2 |
| Minority (%) | 64.2 | 47.1 | 39.1 | 52.2 | 70.4 | 60.0 |
| Brain Volume* | 0.79 ± 0.04 | 0.75 ± 0.05 | 0.78 ± 0.04 | 0.76 ± 0.04 | 0.78 ± 0.06 | 0.76 ± 0.04 |
| WMH** | −5.55 ± 0.92 | −4.98 ± 0.94 | −5.43± 1.1 | −5.13 ± 1.0 | −5.21 ± 0.99 | −5.18 ± 0.71 |
| Hippocampal Volume | 0.31± 0.04 | 0.26± 0.06 | 0.28 ± 0.05 | 0.280 ± 0.05 | 0.29 ± 0.05 | 0.30 ± 0.04 |
| MR Infarct (%) | 24 | 25 | 17 | 40 | 24 | 53 |
| Vascular Risk Score | 0.24 ± 0.20 | 0.25 ± 0.22 | 0.23 ± 0.18 | 0.28 ± 0.26 | 0.28 ± 0.20 | 0.50 ± 0.23 |
| APOE4 No (%)-*** | 30 | 64 | 54 | 43 | 30 | 18 |
All brain volume measuresare reported as the percentage of intracranial volume.
WMH volumes were log transformed to normalize variance.
ApoE4 was considered present if one or both alleles were E4.
DIFFERENCES BY SYNDROME
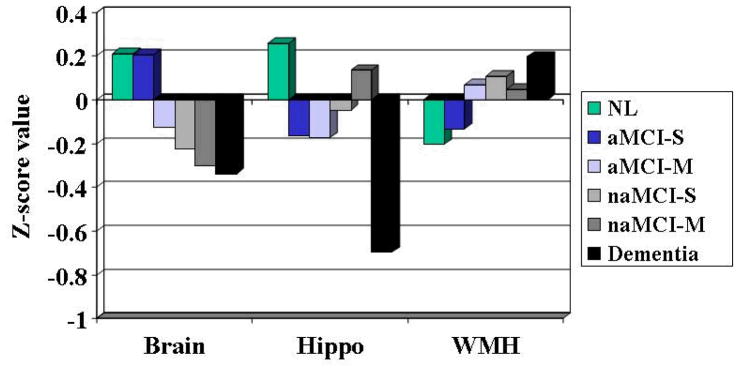
Initial multiple regression analyses across groups according to cognitive syndrome (AD, controls, and MCI), adjusting for age, sex, and ethnicity found significant differences in brain volume (F=13.0; P =.001), hippocampal volume (F=22.7; P = 001), and WMH(F=6.2; P = .002). The Figure shows the differences between mean values for the 3 MRI measures across groups adjusted for age, sex, and ethnicity. Post hoc analyses found that brain and hippocampal volumes differed significantly between all 3 groups, whereas WMH volumes for both MCI and AD differed significantly from controls, but not each other. No significant group differences were found for vascular risk factors or prevalent magnetic resonance infarcts. Presence of APOE-4 varied significantly between the groups (30% for controls, 43% for MCI, and 64% for AD; χ2=18.5; P=.001).
FIGURE.
Least square means of 3 MRI measures among groups
QUANTITATIVE MRI DIFFERENCES
Post hoc analysis of mean differences in brain volume for all 6 groups is summarized in Table 2. The aMCI-S group was not statistically significantly different from controls, but differed significantly from the aMCI-M (P=.02), naMCI-M (P=.02), naMCI-S (P=.02), and AD groups (P=.001). Post hoc analyses of the hippocampus showed that the control group differed significantly from the aMCI-S (P_.001) and aMCI-M groups (P=.004), but not the naMCI-M (P=.61) or naMCI-S (P=.13) group. Conversely, hippocampal volumes of all MCI subtypes differed significantly from those of patients with AD(aMCI-S P=.008; aMCI-M P=.01; naMCI-S P=.009; and naMCI-M P=.003). Finally, we found a trend toward WMH differences between controls and aMCI-M (P=.06), but not any other significant differences among the MCI subtypes.
Table 2.
Comparing mean values among groups (p-value)
| aMCI-S | aMCI-M | naMCI-S | naMCI-M | NL | AD | ||
|---|---|---|---|---|---|---|---|
| Brain volume | aMCI-S | -- | 0.02 | 0.02 | 0.02 | <0.0001 | |
| aMCI-M | -- | 0.006 | |||||
| naMCI-S | -- | 0.008 | |||||
| naMCI-M | -- | 0.01 | |||||
| NL | -- | <0.0001 | |||||
| AD | -- | ||||||
| Hippocampus volume | aMCI-S | -- | 0.0009 | 0.008 | |||
| aMCI-M | -- | 0.004 | 0.01 | ||||
| aMCI-S | -- | 0.13 | 0.009 | ||||
| aMCI-M | -- | 0.61 | 0.003 | ||||
| NL | -- | <0.0001 | |||||
| AD | -- | ||||||
| WMH | aMCI-S | -- | 0.04 | ||||
| aMCI-M | -- | 0.06 | |||||
| naMCI-S | -- | ||||||
| naMCI-M | -- | ||||||
| NL | -- | 0.001 | |||||
| AD | -- | ||||||
aMCI-S=amnestic single domain MCI
aMCI-M=amnestic multiple domain MCI
naMCI-S=non-amnestic single domain MCI
naMCI-M=non-amnestic multiple domain MCI
NL=normal elderly
AD= dementia with Alzheimer’s disease
WMH=white matter hyperintensities
MAGNETIC RESONANCE INFARCTIONS
Owing to the low frequency of cortical infarction in the sample, magnetic resonance infarcts were considered present or absent independent of size or location. The prevalence of magnetic resonance infarcts is shown in Table 1 and varied significantly by subtype (χ2=12.6; P=.006). Although the numbers are small and the true estimates likely unstable, individuals with multiple-domain cognitive impairment had greater prevalent MRI infarcts than controls, whereas aMCI-S had the lowest prevalence (multiple vs single-domain MCI, P=.001).
CEREBROVASCULAR RISK FACTORS
Significant differences in prevalent cerebrovascular risk factors after adjusting for age, sex, and ethnicity (P=.001) were found. Given previous reports of increased vascular risk for nonmemory subtypes.16 we performed post hoc analysis of memory vs nonmemory MCI. There was a significant difference (P=.03), with nonmemory MCI subtypes having significantly more concomitant vascular risk factors (36% vs 25%).
APOLIPOPROTEIN E4
There was no significant difference in APOE-4 genotype when comparing all 4 MCI subtypes (χ2=6.80; P=.08). Because previous reports suggesting that individuals with amnestic MCI are at high risk of AD,9,11,13 we also examined differences in APOE-4 genotype prevalence between the memory and nonmemory groups. Individuals with amnestic MCI had nearly twice the prevalence of APOE-4 (48.96%) compared with the nonmemory MCI group (26%; χ2=5.18; P=.02).
ETHNICITY
Given previous reports of increased vascular disease among African American and Hispanic individuals with MCI17 and the relationship between vascular disease and nonmemory MCI, we examined the ethnic composition (white, African American, and Hispanic) among the 4 MCI subtypes. Although there was a trend toward differences in distribution of ethnicity across the 4 MCI subtypes, this did not reach statistical significance (χ2=11.69; P=.07). There were, however, significant differences between amnestic and nonamnestic subtypes (χ2=8.10; P=.02), with African American individuals being more likely to have naMCI and white individuals more likely to have aMCI. This may reflect the fact that African American individuals had the highest prevalence of vascular risk factors (29% vs 26% and 23% for Hispanic and white persons, respectively), although these differences in prevalent vascular risk factors among the groups were not significant. This finding was not explained by differences in APOE-4 prevalence by ethnicity (χ2=5.6; P=.05) in the MCI population.
Comment
Petersen9 recently noted the potential “heuristic value” of clinical subtyping of subjects with MCI. Our data support the notion that MCI subtyping may be biologically meaningful, particularly at the level of distinguishing memory from nonmemory MCI. Our findings show that persons with amnestic forms of MCI have significantly smaller hippocampi, are significantly more likely to carry at least 1 APOE-4 allele, and have a lower prevalence of vascular risk factors and WMH. Conversely, nonamnestic individuals with MCI are more likely to have prevalent cerebrovascular disease risk factors.
Limited prior neuroimaging studies support our observations. For example, Mariani et al16 found increased vascular risk factors, WMH, and history of stroke in persons with nonmemory MCI and multiple-domain MCI. A second study, however, found no differences in WMH among the subtypes,39 similar to our own findings. Pittsburgh Compound B studies20 also find lower frequency of PiB retention among subjects with nonmemory MCI, suggesting that persons with nonamnestic MCI are likely to have brain pathologies other than AD. Our findings of increased vascular risk and lower APOE-4 genotype prevalence support this possibility.
Hippocampal atrophy has also been an important predictor of progression from MCI to AD and may be a marker for early AD in patients with MCI.40–42 As noted, the memory subtypes of MCI had the greatest degree of hippocampal atrophy and were associated with increased prevalence of APOE-4 genotype, making it likely that these individuals will be similarly at risk for future progression to AD.43–45 Our results are also consistent with another study that found that aMCI-S and aMCI-M groups have gray matter loss in the medial and inferior temporal lobes, whereas nonamnestic persons have a different pattern.46 Global brain atrophy has also been measured in a number of studies of MCI. Given that increased rates of brain atrophy predict progression from MCI to dementia, 40,47,48 it is interesting to note that our aMCI-M group had significant atrophy compared with the aMCI-S group. This is consistent with at least 1 previous article49 and with presumably more advanced disease in the aMCI-M group.
Evaluation of ethnic differences in MCI subtype distribution was a unique aspect of our study. We found that African American individuals were more likely to have naMCI compared with white and Hispanic persons, while white persons were more likely to have aMCI. This difference is consistent with the fact that African American individuals in our study were more likely to have more vascular risk factors and with epidemiological studies that find increased prevalence of similar risk factors among African American persons.50 A number of studies have examined the prevalence of MCI in different ethnic groups, with mixed results.51,52 Although neither group subtyped MCI according to current convention,9 both groups found increased prevalence of vascular risk factors among African American persons, consistent with our findings. Because previous reports suggest that vascular disease is more prevalent among subjects with MCI,51,53 the increased prevalence of vascular disease repeatedly noted among African American individuals may also be associated with increased prevalence of cognitive impairment.54
This study is not without limitations. First, this cross-sectional study limits conclusions regarding prognostic significance of biological differences among the MCI subtypes. Second, subjects from ethnic minorities were significantly more likely to be recruited from the community. Some of the findings, therefore, may relate to recruitment bias rather than biology. One argument against this limitation is that both Hispanic and African American individuals were recruited primarily from the community, yet biological and clinical differences between these 2 groups were seen. In addition, previous study by our group has shown similar relationships between cognitive impairment and imaging variables across ethnic groups.38 Finally, despite the recruitment of more than 150 subjects with MCI, the number of non memory impaired subjects was still quite small, making comparison among the groups limited in some cases.
In conclusion, our results support the notion of biological heterogeneity, particularly between those with memory vs non memory predominant MCI. Such findings are likely to affect disease progression as well as potential treatment options. For example, recent data suggest that vascular disease may have a longer time course of cognitive impairment than AD.55 These findings, however, must be cautiously interpreted. While our findings do provide some support for differences in the underlying etiologies, such differences are relative. In fact, it is possible that multiple disease etiologies may co-occur in a given individual. For example, both AD and vascular disease are common to advancing age,23 may affect cognition in a similar manner,56 and are likely to combine to be the most common cause of dementia.57 Recognizing these limitations, however, treatment of cerebrovascular disease has been shown to influence dementia incidence in high-risk populations 58,59; therefore, clinical trials of non memory-predominant MCI subtypes that emphasize control of vascular risk factors may prove to be an avenue of further investigation. Conversely, clinical trials focused on prevention of AD should focus on memory-predominant MCI subtypes with limited evidence of cerebrovascular disease by imaging or cerebrovascular risk factors by history.
Acknowledgments
This research was supported by NIA grants P30 AG10129, R01 AG 10220 and R01 AG021028.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62(9):1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- 3.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2(1):15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22(4):312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 7.Guehne U, Luck T, Busse A, Angermeyer MC, Riedel-Heller SG. Mortality in individuals with mild cognitive impairment: results of the Leipzig Longitudinal Study of the Aged (LEILA75_) Neuroepidemiology. 2007;29(3–4):226–234. doi: 10.1159/000112479. [DOI] [PubMed] [Google Scholar]
- 8.Guehne U, Angermeyer MC, Riedel-Heller S. Is mortality increased in mildly cognitively impaired individuals? a systematic literature review. Dement Geriatr Cogn Disord. 2006;21(5–6):403–410. doi: 10.1159/000092846. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC. Challenges of epidemiological studies of mild cognitive impairment. Alzheimer Dis Assoc Disord. 2004;18(1):1–2. doi: 10.1097/00002093-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Bennett D. Mild cognitive impairment: is it Alzheimer’s disease or not? J Alzheimers Dis. 2005;7(3):241–245. doi: 10.3233/jad-2005-7307. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents earlystage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 15.Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis. 2007;12(1):23–35. doi: 10.3233/jad-2007-12104. [DOI] [PubMed] [Google Scholar]
- 16.Mariani E, Monastero R, Ercolani S, et al. ReGAl Study Group. Vascular risk factors in mild cognitive impairment subtypes: findings from the ReGAl project. Dement Geriatr Cogn Disord. 2007;24(6):448–456. doi: 10.1159/000110653. [DOI] [PubMed] [Google Scholar]
- 17.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 18.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 21.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68(19):1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 22.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment [published online ahead of print May 11, 2007] Neurobiol Aging. 2008;29(10):1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Edland S, Clark C, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD) part IV: rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43(12):2457–2465. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD) part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Welsh KA, Butters N, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s Disease (CERAD) part V: a normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42 (3 pt 1):473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC, Weintraub S, Chui HC, et al. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14(6):668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 35.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 36.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 37.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 38.DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, Whites, and Hispanics. Alzheimer Dis Assoc Disord. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bombois S, Debette S, Delbeuck X, et al. Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke. 2007;38(9):2595–2597. doi: 10.1161/STROKEAHA.107.486407. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farlow M, Lane R, Kudaravalli S, He Y. Differential qualitative responses to rivastigmine in APOE epsilon 4 carriers and noncarriers. Pharmacogenomics J. 2004;4(5):332–335. doi: 10.1038/sj.tpj.6500267. [DOI] [PubMed] [Google Scholar]
- 44.Grundman M, Sencakova D, Jack CR, Jr, et al. Alzheimer’s Disease Cooperative Study. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19(1–2):23–27. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- 45.DeCarli C, Frisoni GB, Clark CM, et al. Alzheimer’s Disease Cooperative Study Group. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64 (1):108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 46.Whitwell JL, Petersen RC, Negash S, et al. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64(8):1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease [letter] Lancet. 1999;353(9170):2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 48.Erten-Lyons D, Howieson D, Moore MM, et al. Brain volume loss in MCI predicts dementia. Neurology. 2006;66(2):233–235. doi: 10.1212/01.wnl.0000194213.50222.1a. [DOI] [PubMed] [Google Scholar]
- 49.Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62(9):1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 50.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32(8):1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 51.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch Neurol. 2003;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 52.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 53.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 54.Hendrie HC, Murrell J, Gao S, Unverzagt FW, Ogunniyi A, Hall KS. Alzheimer Dis Assoc Disord. 3 suppl 2. Vol. 20. 2006. International studies in dementia with particular emphasis on populations of African origin; pp. S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63(5):581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 56.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43(11):1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 58.Hansson L. Antihypertensive treatment and the prevention of dementia: further insights from the Syst-Eur trial. J Hypertens. 1999;17(3):307–308. doi: 10.1097/00004872-199917030-00001. [DOI] [PubMed] [Google Scholar]
- 59.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomized double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 98;352(9137):1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]