Abstract
Objectives
Acute febrile illnesses consistent with malaria are the most common presentation at health clinics in sub-Saharan Africa, accounting for 30–50% of outpatient visits. The symptoms of acute human immunodeficiency virus (HIV) infection can mimic acute malaria. We investigated whether acute HIV infections could be identified among adults with suspected malaria at rural health centers in Uganda.
Design
Cross-sectional study of 1,000 consecutive patients referred for malaria blood smears at each of 7 government health centers, of which 2893 (41%) were age 13 years or older and tested for HIV.
Methods
HIV EIA antibody testing was performed on dried blood spots and confirmed by Western blot (WB). EIA non-reactive and EIA reactive, WB-unconfirmed samples were pooled (10/pool) and tested for HIV RNA by nucleic acid amplification testing. We defined acute HIV infection as HIV-1 RNA positive with a negative or indeterminate HIV-1 Western blot pattern and early HIV infection as HIV-1 RNA positive with a positive Western blot pattern, but with a BED corrected optical density (ODn) of <0.8.
Results
Of 2893 patients evaluated, 324 (11%) had test results indicating HIV infection. Overall, 30 patients (1.0%) had acute HIV infection, 56 (1.8%) had early HIV infection, and 238 (8%) had established HIV infection. Acute HIV infections were more prevalent at sites with higher HIV prevalence and lower malaria endemicity.
Conclusions
At multiple sites in Uganda, 1–3% of adults with suspected malaria had acute or early HIV infection. These findings highlight a major opportunity for expanding recognition of acute and early HIV infection in Africa.
Keywords: HIV Serodiagnosis, Malaria, Africa, Acute HIV Infection
INTRODUCTION
Acute HIV infection is characterized by very high levels of circulating HIV in blood and genital secretions [1]. Studies have shown that the risk of transmitting HIV to others during acute HIV infection is several fold higher than the risk during subsequent established HIV infection [1–5]. Although most patients with acute HIV infection will develop a ‘flu-like’ acute retroviral syndrome, the majority will not be recognized as being acutely HIV-infected either because they do not seek medical care or because, when they do access care, their symptoms do not trigger an evaluation for HIV infection [6–8]. It has been estimated that up to 50% of all new HIV infections may be due to transmission from individuals with acute HIV infection [2]. Given the impact of acute HIV infection on the spread of the disease and the infrequency with which it is identified, recognizing acute HIV infection is a critical missed opportunity for public health intervention to prevent HIV transmission.
Standard HIV antibody tests commonly fail to identify acute HIV infection, and the laboratory testing needed to detect acute HIV is complex and costly. Typically, acute HIV infection is identified by a combination of tests, including one to identify HIV RNA, DNA, or antigens, and a second to confirm that a robust antibody response has not yet developed [9–12]. This system requires an intact cold-chain for sample storage, which is not feasible in many developing world settings. Clinical identification of acute HIV infection is also challenging given that many of the presenting signs and symptoms such as fever, rash, arthralgias and malaise are non-specific and may be wrongly attributed to other diagnoses, including malaria [13, 14].
In many parts of the world most affected by HIV, malaria is also endemic. In sub-Saharan Africa, where the majority of the world’s HIV infections occur, more than 300 million cases of malaria are diagnosed each year [15, 16]. Moreover, in sub-Saharan Africa acute febrile syndromes are routinely diagnosed as malaria (often without laboratory confirmation) and account for 30–50% of all outpatient visits [17–19]. Given the significant overlap of symptoms between acute HIV and malaria, patients presenting to health centers in sub-Saharan Africa with acute HIV infection may initially be suspected of malaria. Thus, it may be possible to identify acute HIV infection in patients seeking outpatient care for febrile illnesses thought to be malaria. In this study we evaluated the prevalence of acute, early and established HIV infections among patients suspected to have malaria in government health clinics in rural Uganda.
METHODS
Study sites and patient population
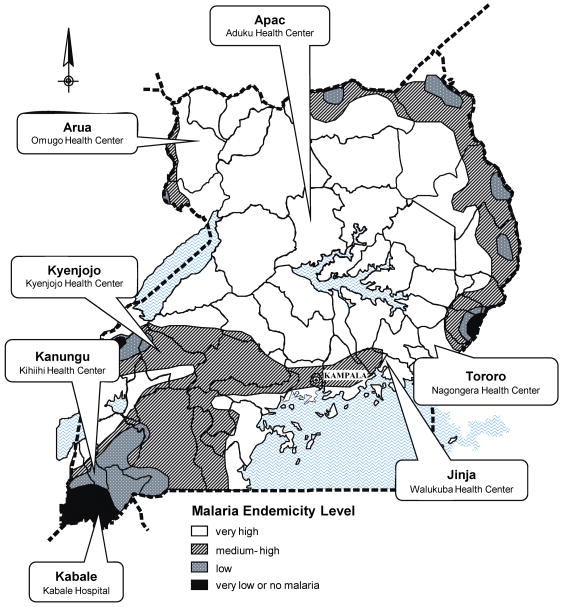
This study was conducted at seven government health clinics representing the diversity of malaria transmission intensity and HIV prevalence in Uganda (Figure 1). Six of seven sites were level IV health clinics mainly serving the local community, where outpatients were primarily evaluated by non-physician health workers and at least one microscopist was available. One site (Kabale) was a regional referral hospital staffed by physicians. Services at these government sponsored clinics were provided free of charge.
Figure 1.
Map of malaria endemicity levels according to the Uganda Malaria Control Program with sites where samples were collected.
We used a cross-sectional design to prospectively screen 1000 consecutive patients (of any age) at each site who were referred by providers to on-site laboratories for malaria blood smears over 1–2 months in 2006/2007. Study staff did not impact upon patient referral, diagnosis, or management. We collected data on patient age and gender at the time of presentation, and finger-prick blood was used for malaria blood smears and blotted onto filter paper.
Microscopy
Thick blood smears were stained with 10% Giemsa for 10 minutes. Smears were considered positive for malaria if any asexual parasites were detected and negative if examination of 100 high power fields did not reveal asexual parasites. Initial readings were done by on-site microscopists. Final microscopy results were derived after re-reading of all smears by an expert microscopist and resolution of any discrepancies by an additional expert microscopist.
Identification and staging of acute, early and established HIV infection
We used well-accepted methodology to identify and stage acute and early HIV infections [20], based on the following assays which were adapted for use on dried blood: HIV-1 RNA nucleic acid amplification; HIV antibody immunoassay; HIV antibody Western blot; and the Calypte HIV-1 BED Incidence EIA [21]. Using this methodology, acute HIV infection was defined as the period of roughly three weeks where patients with acute HIV infection exhibit detectable HIV-1 RNA but negative or indeterminate results on antibody immunoassay and Western blot [20, 22]. For those patients who had already developed HIV-specific antibody responses as measured by positive results on antibody immunoassay, qualitative antibody response was used to differentiate early from established HIV infection. Immature antibody responses to HIV, including absence of the p31 band on Western blot [20] and a low ratio of HIV specific IgG to total IgG, as measured by a Calypte HIV-1 BED Incidence EIA ratio less than 0.4 [23, 24, pers comm., B. Parekh] have been associated with having infection of less than four months’ duration, and BED ratios less than 0.8 can indicate infection less than 12 months’ duration. These immature antibody responses were classified as early HIV infection.
Thus, based on this methodology, we defined acute HIV infection as HIV-1 RNA positive with a negative or indeterminate HIV-1 Western blot pattern. We defined early HIV infection as HIV-1 RNA positive with a positive HIV-1 Western blot pattern, but with a BED corrected optical density (ODn) of <0.8 or absence of the p31 band on Western blot. We defined established HIV as HIV-1 DNA or RNA positive with a BED ODn of ≥0.8 and positive Western blot.
HIV antibody testing
Dried blood spots were collected on Whatman 3M filter paper, stored individually at room temperature and transported to our molecular research laboratory at the University of California San Francisco for HIV testing. Screening for HIV infection was done using the Murex HIV 1.2.0 Enzyme Immunoassay (EIA) (Abbott Laboratories, Abbott Park, Illinois, USA), a 3rd generation (IgM-sensitive) EIA, according to the manufacturer’s instructions. Briefly, dried blood spots were eluted using 100μL PBS with 0.05% Tween 20 in 1.5 mL tubes at 4°C for at least three hours. Samples with a corrected optical density at 450nm of <0.2 were considered HIV negative.
HIV-1 DNA and RNA detection
Confirmatory testing of antibody positive specimens for HIV-1 DNA was performed using the Roche Amplicor HIV-1 DNA test (Roche Systems, Basel, Switzerland). HIV DNA was extracted from samples using chelex resin, [25] and testing was performed according to the manufacturer’s instructions as previously described [26].
Qualitative HIV RNA testing was performed by Target-Capture Transcript Mediated Amplification (TC-TMA) using the APTIMA HIV-1 Assay (Procleix dHIV blood screening assay Gen-Probe Incorporated, San Diego, USA); rated limit of detection >100 copies/mL for fresh or frozen blood/plasma samples. The Gen-Probe Aptima NAT assay was validated for screening pooled dried blood spots by replicate testing of 9 HIV negative dried blood spots with 1 known (or spiked) HIV positive dried blood spot. Assay sensitivity on dried blood spots was assessed by spiking panels with HIV-1 from tissue culture supernatant of cells infected with HIV-1 subtype B [27] which was quantitated using a validated, TMA-based HIV-1 assay calibrated against the Virology Quality Assurance Laboratory standard (Virology Quality Assurance Laboratory, Rush-Presbyterian St. Luke’s Medical Center, Chicago, IL). No decrement in analytical sensitivity was observed when samples were pooled 9:1 compared to non-pooled samples [27]. From these studies, the threshold for detection of HIV viral load by pooled dried blood spot screening was inferred to be approximately 10,000 copies/mL.
All EIA negative, EIA positive but Western blot negative or indeterminate and EIA positive but DNA negative samples were pooled for RNA detection by TMA. 10 individual 6 mm dried blood spot punches were eluted simultaneously with 0.8 mL Dilution-Elution Buffer, the manufacturer-provided proprietary lysis buffer solution. Tubes were eluted at 95 °C for 20 minutes, and 0.6 mL eluate was transferred to a clean tube, frozen, and shipped to Blood Systems Laboratory for HIV TC-TMA. For reactive pools, additional 6 mm punches from each dried blood spot were repeat tested individually using 0.6 mL buffer for initial elution. Samples testing positive in both pooled and individual testing were classified as HIV RNA positive.
Western blot confirmation
All samples testing HIV positive by DNA or RNA nucleic acid amplification testing were subjected to Western blot confirmation using an assay validated and FDA approved for dried blood spots (Genetic Systems HIV-1 Western Blot, Bio-Rad Laboratories, Redmond, WA, USA). Samples were classified as Western blot positive if two of the following three HIV-1 specific bands were detectable: p24, gp 120/160, and/or gp41. Samples were classified as Western blot negative if no bands were visible. Samples were classified as having an indeterminate Western blot pattern if one HIV-1 specific band was visible. Samples were classified as p31-negative if the p31 HIV-1 specific band was absent or <1+ intensity, but all other HIV-1 specific bands were present.
HIV BED testing
Early HIV infection was identified by testing HIV EIA-reactive samples with the Calypte HIV-1 BED Incidence EIA (Calypte Biomedical, Portland, OR, USA), a de-tuned EIA utilizing the HIV-specific IgG to total sample IgG ratio to detect early HIV infection, according to the manufacturer’s instructions. Samples were classified as early HIV infection if the BED corrected optical density on triplicate testing was <0.8.
Ethical approval
Human subjects research approval was obtained from the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research. All results were de-linked from patient identifiers and anonymized.
Data management and statistical analysis
Data were double-entered into EpiInfo version 6.4 (Centers for Disease Control and Prevention, Atlanta, GA, USA) and statistical analyses were performed using STATA version 8 (Stata, College Station, TX, USA). Categorical variables were compared using the chi-square test. Independent predictors of acute HIV infection were identified using multivariate logistic regression models.
RESULTS
Patient characteristics
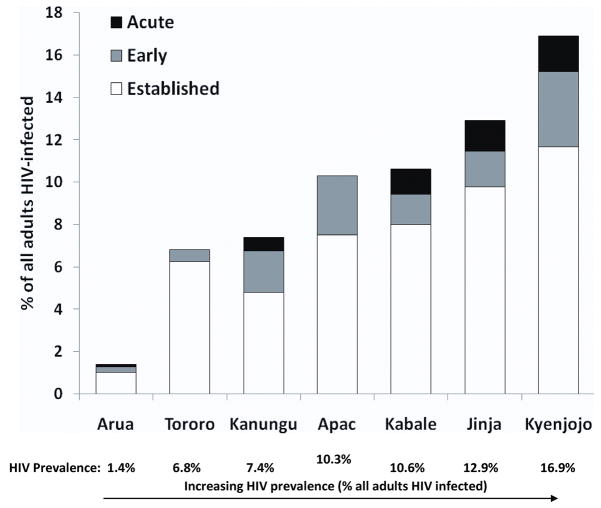
We evaluated 1000 consecutive patients of all ages who presented to each of seven rural clinics (Figure 1) and were referred for malaria blood smears. Of these 7000 patients, 2893 (41%) were adults aged 13 years or older; only these adults were included in this study (Table 1). A majority of adult patients were female, with the proportions ranging from 65% to 75% at different sites, consistent with known disproportionate representation of females seeking health care in rural Uganda. Median age of those >13 years ranged from 27–32 years. Among the 2893 adults studied, 494 (17%) had blood smears positive for malaria parasites, with the proportion of positive smears varying greatly by site (3.4% to 30%). A total of 324 adults (11.2%) were HIV infected. HIV prevalence in adults varied markedly between sites, from 1.4% to 16.9% (Figure 2).
Table 1.
Patient characteristics of adults ≥13years according sample collection site and included in this study (from an initial n=1000 consecutive samples of all ages collected per site).
| Site | |||||||
|---|---|---|---|---|---|---|---|
| Apac (n=82) | Tororo (n=339) | Arua (n=574) | Kyenjojo (n=508) | Kanungu (n=299) | Jinja (n=443) | Kabale (n=643) | |
| Date samples collected | May 2006 | Jan–Feb 2007 | Nov 2006 | Dec 2006–Jan 2007 | Nov 2006 | Jan 2006 | Sept–Oct 2006 |
| Median age in years (IQR†) | 27.4 (20–30) | 32.7 (21–41) | 30.6 (20–38) | 30.5 (20–38) | 28.5 (18–35) | 28.4 (19–35) | 30.4 (20–37) |
| Female sex (%) | 62 (71%) | 255 (75%) | 406 (71%) | 357 (70%) | 214 (72%) | 309 (70%) | 415 (65%) |
| Malaria smear-positive (%) | 18 (20.7%) | 22 (6.5%) | 158 (27.5%) | 153 (30.1%) | 51 (17.1%) | 70 (20.8%) | 22 (3.4%) |
| HIV-1 infected (%) | 11 (12.6%) | 25 (7.4%) | 11 (1.9%) | 100 (19.7%) | 34 (11.4%) | 62 (14.0%) | 81 (12.6%) |
IQR= Inter-quartile range
Figure 2.
Prevalence acute, early and established HIV infection by site.
Prevalence of acute and early HIV infection
Among 324 adults determined to be HIV positive, 238 were classified as having established HIV infection (Appendix 1). The remaining 86 HIV positive adults (3.0% of all patients, 26.5% of all HIV positive adults) met criteria for acute or early HIV infection: 30 patients (9.3% of HIV infections; 1.0% of all patients) had RNA and serologic patterns defining acute HIV infection and 56 patients had patterns defining early HIV infection (17.3% of HIV infections; 1.9% of all patients) [Table 2].
Table 2.
Prevalence of acute, early and established HIV infection by risk category. Classification criteria for each stage of infection are enumerated in Appendix 1.
| Stage of HIV infection | Estimated duration (months) | n | % | % in Malaria Smear+ | % in Malaria Smear- |
|---|---|---|---|---|---|
| Acute | <1 | 30 | 1.0 | 0.4 | 1.2 |
| Early | <4–12 | 56 | 1.9 | 1.6 | 2.0 |
| Established | >4–12 | 238 | 8.2 | 10.1 | 7.8 |
| Acute or Early | <12 | 86 | 3.0 | 2.0 | 3.2 |
| All HIV+ | All | 324 | 11.2 | 12.1 | 11.0 |
Predictors of acute and early HIV infection
The prevalence of acute and early HIV infection varied by site, ranging from 0.5% to 6.0% of all adult patients (Figure 2). Site level predictors of acute HIV infection (among all subjects) included high HIV prevalence (>10%) (OR=4.5, p=.006) and low malaria endemicity (OR=2.8, p=.015). Potential individual predictors of acute HIV infection (among all subjects) included a negative malaria smear (OR=0.36, p=0.159), male sex (OR=2.1, p=0.049), and age <20 (OR=3.2, p=0.055) however these individual level predictors were all of borderline significance. Predictors of a combined outcome of acute or early HIV infection (among all subjects) were high site HIV prevalence (OR=3.0, p<.001), low malaria endemicity (OR=0.36, p<.001), and subject age < 20 (OR=3.6, p=0.001). HIV prevalence was not related to malaria transmission intensity (data not shown). The ratio of malaria cases to acute HIV cases was 14:1 overall, but varied between 158:1 in Arua, the site with the highest malaria transmission intensity and 2.4:1 in Kabale, the site with the lowest malaria transmission intensity.
DISCUSSION
In this cross-sectional study conducted at rural government health clinics in Uganda, acute HIV infection was found to be highly prevalent, with 1.0 % of symptomatic patients initially suspected of malaria found to have HIV RNA and serology consistent with acute HIV infection. These patients presented to clinicians with signs, symptoms and clinical history consistent with malaria, triggering a laboratory evaluation for that infection, but not consideration for acute HIV infection. Indeed, at the three study sites with the highest HIV prevalence, 4–6% of adults with suspected malaria met criteria for acute HIV infection. This range of acute HIV infection prevalence among patients not typically suspected of this diagnosis was surprisingly high, highlighting the need to increase recognition of acute HIV infection in Africa.
Our findings suggest that it may be possible to identify large numbers of Africans with acute and early HIV infection by focusing on the evaluation of patients presenting to clinics with acute febrile illnesses. This approach would have significant implications for HIV prevention, since acute HIV is highly infectious [2, 4, 10]. A third or more of all sex partners may become infected following contact with individuals with acute HIV infection [3, 5], and many of these infections may be preventable. Steward and colleagues [28] found that patients with acute HIV infection reduced their HIV transmission risk behavior by 98% in the 8 weeks following acute HIV diagnosis, when compared with the 8 weeks prior to diagnosis. Previous calls for expanded acute HIV prevention [29] have been criticized on the basis that identifying acute infection is too difficult, amounting to searching for “a needle in a haystack” [30]. Our results suggest, to the contrary, that there may be significant potential for acute HIV identification and intervention in Ugandan general medical settings. The potential for frequent identification of acute HIV infection further suggests an opportunity to find HIV-sero-discordant couples where transmission to the uninfected partner has yet to occur, a situation where behavioral interventions have been shown to be effective in reducing HIV transmission [31].
The results of this study are subject to several limitations. Identifying acute and early HIV infection through cross-sectional analysis can lead to misclassification. For instance, our methods may have underestimated the true prevalence of acute HIV infections in our population, due to the use of dried blood spots and the pooling techniques, which may not detect some acutely infected patients with low level viremia. However, we employed multiple, highly sensitive NAT and serologic tests in our research algorithm to distinguish acute from non-acute infections. Identification of antibody-positive HIV infections as early infections can also be problematic: the BED assay used in the study has been shown to occasionally misclassify patients with late-stage HIV infection as having early HIV, and the test’s performance characteristics vary depending on HIV subtype [33, 34]. For this study, we applied a more stringent BED ODn cut-off than is commonly used for surveillance purposes in order to increase the specificity of the assay for early HIV infection cases. Improved methods may be necessary in order to demonstrate the true proportion of antibody-positive early HIV infections in our testing population. Ideally in a cohort setting, follow-up serological testing would be used to confirm evolving HIV infection.
An additional practical concern in identifying acute HIV infections in sub-Saharan Africa is the complexity and resource intensive nature of the serologic testing employed in this study. In this manuscript, we describe a new method of acute HIV detection using RNA in pooled dried blood spots. The use of dried blood spots collected on filter paper is highly feasible in the developing world setting. However, the nucleic acid amplification testing performed for this study required a central laboratory with specialized equipment and skills and may not be applicable to real-time detection of acute HIV cases. However, we expect that the new methods described here will be useful in further research studies and in confirming clinical cases in areas lacking a cold chain for sample storage and transport, including much of the developing world. Of note is the recent development of simple rapid diagnostic tests for HIV antigen detection [35] and portable laboratory devices for nucleic acid amplification [36] that may bridge the diagnostic technology gap, bringing acute HIV testing to the point-of-care.
The findings of this study may not be generalizable to all medical clinics, in Uganda or elsewhere. The study was limited to patients who had blood drawn for malaria testing and provided no HIV testing information on febrile patients not referred for malaria testing. However, the patients included in this study are typical of those evaluated for malaria at community health centers, and this limitation is mitigated by the diversity of the clinic sites, large number of subjects studied, and large number of acute and early HIV infections identified. Given the conservative case-finding definitions employed in this study, these results may be considered a lower bound of what is feasible in terms of acute HIV case-finding.
Conclusion
Our results show how important synergies could be achieved by coordinating malaria and HIV control strategies in sub-Saharan Africa. For both diseases, case-finding and treatment are key strategies in reducing transmission. Thus, medical settings providing routine primary care, and typically focusing on diagnosis and management of malaria, may be ideal sites for expanded point-of-care HIV testing and counseling in sub-Saharan Africa. With the arrival of reliable diagnostics capable of identifying acute HIV infections at the point-of-care, screening of populations suspected to have malaria could identify significant numbers of acutely HIV infected persons. These individuals could then be provided pharmacologic and/or behavioral interventions to reduce HIV transmission. Institution of such a strategy in areas that are endemic for malaria could represent a major opportunity for global HIV prevention.
Acknowledgments
Sources of Support: Doris Duke Charitable Foundation, NIH grant R01-MH068686
We thank study participants and families, staffs of the district health facilities, Wilson Kambale, Yoel Lubell, Joaniter Nankabirwa, and Christina Angle for assistance in the field, Heidi Hopkins for making sample collection possible, Chris Dokomajilar, Bruce Lilley and Hong Kim for laboratory assistance, and the staffs of Bio-Rad, Gen-Probe, BSRI and Blood Systems Laboratories for their assistance with HIV testing assays. This work was funded in part by the National Institutes of Health/NIMH R01 MH068686. We thank the Doris Duke Charitable Foundation for their support for LMB as a Clinical Research Fellow, GD with a Clinical Scientist Development Award, and PJR as a Distinguished Clinical Scientist.
Footnotes
Conflicts of Interest: C. Nugent is employed by Gen-Probe Incorporated and C. Bentsen is employed by Bio-Rad.
References
- 1.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS (London, England) 2007 Aug 20;21(13):1723–30. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. The Journal of infectious diseases. 2007 Apr 1;195(7):951–9. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 3.Brooks JT, Robbins KE, Youngpairoj AS, et al. Molecular analysis of HIV strains from a cluster of worker infections in the adult film industry, Los Angeles 2004. AIDS (London, England) 2006 Apr 4;20(6):923–8. doi: 10.1097/01.aids.0000218558.82402.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. The Journal of infectious diseases. 2008 Sep 1;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 5.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. The Journal of infectious diseases. 2005 May 1;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 6.Lindback S, Thorstensson R, Karlsson AC, et al. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS (London, England) 2000 Oct 20;14(15):2333–9. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996 Aug 15;125(4):257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007 Mar;21(1):19–48. vii. doi: 10.1016/j.idc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. The New England journal of medicine. 2005 May 5;352(18):1873–83. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 10.Pilcher CD, Price MA, Hoffman IF, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS (London, England) 2004 Feb 20;18(3):517–24. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 11.Stekler J, Maenza J, Stevens CE, et al. Screening for acute HIV infection: lessons learned. Clin Infect Dis. 2007 Feb 1;44(3):459–61. doi: 10.1086/510747. [DOI] [PubMed] [Google Scholar]
- 12.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS (London, England) 2005 Aug 12;19(12):1323–5. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 13.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS (London, England) 2002 May 24;16(8):1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kuo AM, Haukoos JS, Witt MD, Babaie ML, Lewis RJ. Recognition of undiagnosed HIV infection: an evaluation of missed opportunities in a predominantly urban minority population. AIDS patient care and STDs. 2005 Apr;19(4):239–46. doi: 10.1089/apc.2005.19.239. [DOI] [PubMed] [Google Scholar]
- 15.(UNAIDS) JUNPoHA. Report on the global AIDS epidemic 2008. Geneva, Switzerland: 2008. [Google Scholar]
- 16.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006 Dec 8;314(5805):1603–6. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 17.Font F, Alonso Gonzalez M, Nathan R, et al. Diagnostic accuracy and case management of clinical malaria in the primary health services of a rural area in south-eastern Tanzania. Trop Med Int Health. 2001 Jun;6(6):423–8. doi: 10.1046/j.1365-3156.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.(PMI) President’s Malaria Initiative. Rapid Assessment Report. Uganda: 2005. pp. 1–25. [Google Scholar]
- 19.(PMI) President’s Malaria Initiative. Uganda Malaria Operational Plan. 2007 http://www.fightingmalaria.gov/countries/uganda_mop-fy07.pdf.
- 20.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS (London, England) 2003 Sep 5;17(13):1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Parekh BS, McDougal JS. Application of laboratory methods for estimation of HIV-1 incidence. The Indian journal of medical research. 2005 Apr;121(4):510–8. [PubMed] [Google Scholar]
- 22.Owen SM, Yang C, Spira T, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. Journal of clinical microbiology. 2008 May;46(5):1588–95. doi: 10.1128/JCM.02196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbs T, Kennedy S, Pau CP, McDougal JS, Parekh BS. Performance characteristics of the immunoglobulin G-capture BED-enzyme immunoassay, an assay to detect recent human immunodeficiency virus type 1 seroconversion. Journal of clinical microbiology. 2004 Jun;42(6):2623–8. doi: 10.1128/JCM.42.6.2623-2628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargrove JW, Humphrey JH, Mutasa K, et al. Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS (London, England) 2008 Feb 19;22(4):511–8. doi: 10.1097/QAD.0b013e3282f2a960. [DOI] [PubMed] [Google Scholar]
- 25.Fischer A, Lejczak C, Lambert C, et al. Simple DNA extraction method for dried blood spots and comparison of two PCR assays for diagnosis of vertical human immunodeficiency virus type 1 transmission in Rwanda. Journal of clinical microbiology. 2004 Jan;42(1):16–20. doi: 10.1128/JCM.42.1.16-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasasira AF, Dorsey G, Kamya MR, et al. False-Positive Results of Enzyme Immunoassays for Human Immunodeficiency Virus in Patients with Uncomplicated Malaria. Journal of clinical microbiology. 2006;44(8):3021–4. doi: 10.1128/JCM.02207-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugent CT, Dockter J, Giachetti C, et al. Detection of HIV-1 in Alternative Specimen Types Using the APTIMAR HIV-1 RNA Qualitative Assay. Journal of Virological Methods. 2009;159:10–4. doi: 10.1016/j.jviromet.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steward W, Remien R, Truong T, et al. A Move Toward Serosorting Following Acute HIV Diagnosis: Part 1 of 4 on Findings from the NIMH Multi-Site Acute HIV Infection Study. National HIV Prevention Conference; Atlanta, GA. 2007. [Google Scholar]
- 29.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. The Journal of infectious diseases. 2005 May 1;191(9):1391–3. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 30.Pinkerton SD, Holtgrave DR, Galletly CL. Infections prevented by increasing HIV serostatus awareness in the United States, 2001 to 2004. Journal of acquired immune deficiency syndromes (1999) 2008 Mar 1;47(3):354–7. doi: 10.1097/QAI.0b013e318160d57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegbreit J, Bertozzi S, DeMaria LM, Padian NS. Effectiveness of HIV prevention strategies in resource-poor countries: tailoring the intervention to the context. AIDS (London, England) 2006 Jun 12;20(9):1217–35. doi: 10.1097/01.aids.0000232229.96134.56. [DOI] [PubMed] [Google Scholar]
- 32.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. The Journal of infectious diseases. 2006 Jan 1;193(1):9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 33.Karita E, Price M, Hunter E, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS (London, England) 2007 Feb 19;21(4):403–8. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 34.Sakarovitch C, Rouet F, Murphy G, et al. Do tests devised to detect recent HIV-1 infection provide reliable estimates of incidence in Africa? Journal of acquired immune deficiency syndromes (1999) 2007 May 1;45(1):115–22. doi: 10.1097/QAI.0b013e318050d277. [DOI] [PubMed] [Google Scholar]
- 35.Inverness Medical UK [Internet] Bedford, United Kingdom: Inverness Medical; 2008. Determine: HIV-1/2 Ag/Ab Combo, Detecting Acute HIV Infection; 2008. [cited 2009 Aug 28]; [about 2 screens]. Available from: http://www.invmeduk.com/pdf/030309111951-Determine%20HIV%20Combo%20BROCHURE.pdf. [Google Scholar]
- 36.Pilcher CD, Christopoulos KA, Golden M. The Public Health Rationale for Rapid Nucleic Acid or p24 Antigen Tests for HIV. J Infect Dis. 2009 doi: 10.1086/650393. in press. [DOI] [PubMed] [Google Scholar]
- 37.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]