Abstract
Articular cartilage extracellular matrix and cell function change with age and are considered to be the most important factors in the development and progression of osteoarthritis. The multifaceted nature of joint disease indicates that the contribution of cell death can be an important factor at early and late stages of osteoarthritis. Therefore, the pharmacologic inhibition of cell death is likely to be clinically valuable at any stage of the disease. In this article, we will discuss the close association between diverse changes in cartilage aging, how altered conditions influence chondrocyte death, and the implications of preventing cell loss to retard osteoarthritis progression and preserve tissue homeostasis.
Keywords: aging, apoptosis, autophagy, cartilage, cell death, chondrocyte, necrosis, osteoarthritis
Aging and the development of cartilage degeneration involve many factors, which either alone or in combination may precipitate the onset of osteoarthritis (OA). Much evidence indicates that a single factor may induce a number of sequential responses and structural changes, which either affects the cartilage extracellular matrix (ECM) or cell function, or which makes the tissue more vulnerable to compressive loads or injury. These changes eventually lead to a disruption of tissue homeostasis and reduced capacity for regeneration, which manifest as OA and eventual tissue destruction. Cell-based or ECM-based factors identified to play a major role in the onset and progression of OA include cell senescence, accumulation of glycation end products, oxidative damage, reduced growth factor responsiveness, altered mitochondrial function and apoptosis [1–4].
Aging has been associated with progressively reduced cellularity in articular cartilage [5,6], probably a consequence of cell death over time. Cell death in the form of apoptosis has been linked with OA, yet the strength of this causal link has yet to be determined. The difficulty in establishing causality is partly owing to the fact that primary OA presumably develops over many years, which is contrary to some reports showing high numbers of dying cells in diseased tissue. Nevertheless, a number of proapoptotic stimuli have been associated with chondrocyte apoptosis and have been linked to OA development [7,8]. The major mechanisms of chondrocyte apoptosis include the involvement of Fas, TNF, TNF-related apoptosis-inducing ligand (TRAIL)-R1, TRAIL-R2 and nitric oxide (NO) exposure [4].
Cartilage homeostasis is mediated by resident chondrocytes, and loss of cells due to death leads to the characteristic features of OA tissue, including loss of cartilage ECM and abnormal tissue remodeling; the latter most likely an attempt of the remaining cells to repair degenerating tissue [9,10]. Parallel changes in companion structures that make up the joint are also evident, such as the subchondral bone and inflammation of the synovium [11–15]. In this article, we outline significant associations between aging, cell death, and the initiation and progression of OA. A number of mechanisms have been proposed, each alone may be generally involved or may present as a secondary consequence of the diseased state over time (Figures 1 & 2).
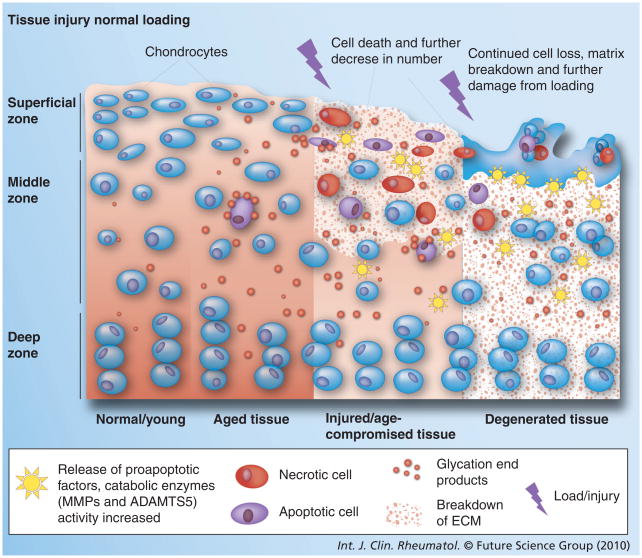
Figure 1. Cellular and extracellular matrix changes associated with age that lead to chondrocyte death and osteoarthritis development in articular cartilage.
Cell density reduces with age and ECM properties are altered with age, resulting in reduced load-bearing capacity. All of these changes increase the tissue’s vulnerability to loading/injury cell death (necrotic and apoptotic). Continued loading on compromised tissue leads to further cell death and matrix degradation.
ECM: Extracellular matrix; MMP: Matrix metalloproteinase.
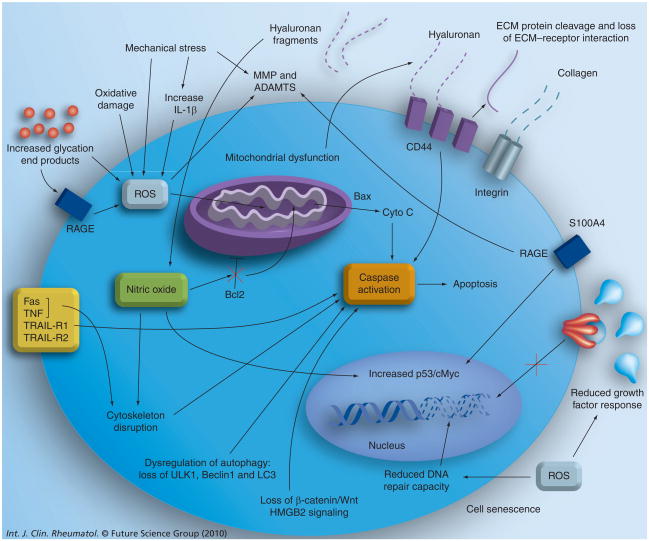
Figure 2. Cellular and extracellular matrix changes associated with age that lead to chondrocyte death and osteoarthritis development in articular cartilage.
Summary of cell-based (intrinsic and extrinsic) factors known to change with age and alter cell viability (see text for details).
ECM: Extracellular matrix; MMP: Matrix metalloproteinase; RAGE: Receptor for advanced glycation end products; ROS: Reactive oxygen species.
Types of cell death
Various types of cell death have been described, primarily including apoptosis (type I), autophagy-associated cell death (type II) and necrosis (type III). Specific biochemical events, such as caspase activation, cytochrome c release and internucleosomal DNA fragmentation, have been used to identify cell death associated with apoptosis or morphological features such as double membrane vacuole formation in autophagy. Necrosis is classically distinguished by the absence of these events. Without phagocytosis, defining true necrosis can be difficult since apoptotic cells eventually become secondary necrotic cells sharing the morphological features of primary necrosis [16]. While the definitions of each type of cell death are distinct, in actuality a spectrum of modes of death exist with a variety of morphologic and molecular manifestations [17]. The latest review by the Nomenclature Committee on Cell Death (NCCD) outlines and defines these phenomena [18]. Apoptosis is classified as a form of programmed cell death (PCD) that is either physiologic (e.g., part of natural developmental processes) or pathologic. In most circumstances, necrosis represents cell death as a consequence of a pathological incident. In principle, necrosis represents the final stage of any form of cell death, including oncosis and apoptosis [3]. Criteria used to distinguish the distinct modalities of cell death are presented in Box 1.
Box 1. Criteria for classifying the major types of cell death.
Morphological features
-
Apoptosis (type I):
Rounding of cells
Plasma membrane blebbing
Nuclear fragmentation
Chromatin condensation
Reduction in cellular and nuclear volume
Apoptosis body formation
Mitochondrial swelling (rare)
-
Autophagy-associated cell death (type II):
Accumulation of autophagic vacuoles (double membrane)
Lack of chromatin condensation
Late-stage mitochondrial swelling
-
Necrosis (type III):
Plasma membrane rupture
Mitochondrial and cytoplasmic swelling (oncosis)
No vesicle formation
Moderate chromatin condensation
Biochemical features & molecular pathways
-
Apoptosis (type I):
Activation of Bcl-2 proteins
Mitochondrial transmembrane permeabilization
Cytochrome c release
Caspase activation
PARP cleavage
DNA fragmentation
ATP dependent
Death-associated proteins
Reactive oxygen species overgeneration
-
Autophagy-associated cell death (type II):
Cathepsin B activity (lysosomal)
Death-associated proteins
PI3K and mTOR
LC3-1 to LC3-II conversion
Beclin-1 dissociation from Bcl-2/XL
Dependency on atg gene products
Degradation of p62Lck
-
Necrosis (type III):
PARP activation
Loss of ion homeostatsis
Drop in ATP levels
Death-associated proteins
Activation of calpains and cathepsins
HMGB1 release
PARP: Poly(ADP-ribose) polymerase.
In cartilage tissue, the classical morphological features described in other tissue systems, for example cell blebbing, are often absent. Roach et al. coined the term ‘chondroptosis’ to indicate a specific form of chondrocyte apoptosis [19]. This type of PCD involves altered protein synthesis as evidenced by increased endoplasmic reticulum (ER) and Golgi apparatus, distinct from typical receptor-mediated or mitochondrial pathways. The ER membranes segment the cytoplasm to produce autophagic vacuoles in the cytoplasm where organelles are digested and finally disposed into the lacunae. This divergent cell death process appears to be consistent with the avascular nature of cartilage, where chondrocytes are isolated within their lacunae and cannot rely on the phagocytotic removal typical in other tissues [20].
Structure-forming or developmental PCD can also be the consequence of autophagy: a type of cell death that is mechanistically distinct from apoptosis and is dependent on the lysosomal machinery of the cell. Autophagy has been investigated in yeast and some of the involved genes are found in higher vertebrates, including humans. Chondrocytes express autophagic proteins [21]. A recent study indicates that autophagy has a protective role for the maintenance of the homeostatic state in normal cartilage, but aging leads to reduced autophagic protein levels and increased apoptosis (see section on autophagy) [22].
An alternative process, termed ‘oncosis’, has been proposed as another distinct form of cell death that is principally regulated by changes in adhesion to ECM (see section on regulators of cell death: matrix components). This process displays some features associated with necrosis, such as increased membrane permeability or cell and organelle swelling, but is not associated with internucleosomal DNA fragmentation [23,24]. However, since oncosis involves distinct cellular processes, studies suggest that it is a form of PCD [25,26]. Some evidence suggests that failure of ionic pumps and ATP depletion may be among the causes of oncosis [25,27]. Cell death resembling oncosis has been observed in atherosclerotic lesions [28] and in ischemic heart disease [29] and may also occur in bone and cartilage [30].
Regulators of chondrocyte death
Matrix components
The cartilage ECM is a dynamic network of proteins secreted by chondrocytes, which serves as a structural support and as a reservoir for cytokines and growth factors to regulate cell behavior by modulating their proliferation and differentiation, thus providing cues that are critical for cell survival [31–33]. Changes in the structure of the chondrocyte environment during the aging process can alter the physical forces experienced by the cell, as well as the biochemical signals that regulate cell response [34]. As degeneration continues, the loss of matrix leads to the propagation of cell death and tissue degeneration (Figure 1). There are two major influences of ECM: adhesion changes and signaling through receptors. Either influence directly initiates apoptotic pathways (e.g., Fas and TNF-α receptor) or indirectly alters the cytoskeleton, which leads to induction of apoptosis (Figure 2) [35–38].
The Greek word anoikis, meaning ‘homelessness’, was used to describe apoptotic cell death as a result of lost, reduced or inappropriate cell adhesion in endothelial cells (review in [34]). Initiation and execution of this apoptotic process are mediated through various pathways that eventually converge to activate caspases. The signals may be intrinsic, usually mitochondrial based, or extrinsic through cell surface death receptors (Figure 2). Extrinsic pathways are initiated by extracellular death ligands, such as Fas ligand (FasL/CD95L) or TNF-α, through their respective cell surface death receptors, Fas and TNF-α receptor [39,40].
Among the different cartilage ECM components, collagen type II is critical in maintaining chondrocyte viability and preventing apoptosis, as demonstrated in transgenic mice lacking this protein [41]. Integrin receptors bind many ECM proteins, including laminin, fibronectin and collagen types II and IV [32,42], and appear to be an important interface between the ECM and mediators of cell survival. Antibodies against the integrin α5-subunit (CD49e) induce death in human chondrocytes [43], and RGD peptides reduce cell viability in cultured chicken chondrocytes [44]. RGD peptides induced apoptosis in cultured chondrocytes and in cartilage explants, probably through direct activation of caspase-3 [45]. Type X collagen deposition and chondrocyte survival in chicken sterna were dependent on CD49b and CD49c integrin subunits [35]. These studies indicate a direct involvement of integrin–ligand interactions in chondrocyte death.
While intact ECM proteins modulate cell survival, ECM protein fragments can elicit different effects. For example, the 29-kDa fragment of fibronectin induces inflammatory responses [46], including an increase in catabolic proteases such as matrix metalloproteinase (MMP)-13 [47], although it does not directly induce cell death in cultured human chondrocytes. A 24-mer synthetic peptide of type II collagen (residues 195–218; CB12-II) lacking any RGD sequence has been demonstrated to induce apoptosis in bovine cartilage explants. This type of cell death may be related to chondrocyte hypertrophic events [48,49]. Blocking CD44 and hyaluronan interaction decreases chondrocyte survival [50] and fragments of hyaluronan may augment the production of NO in a CD44-dependent manner [51]. Hyaluronan oligosaccharides can induce MMP-13 production and cause further matrix breakdown [52]. Conversely, hyaluronan fragments of 500–730 kDa interact with CD44 and CD54 to inhibit Fas ligand-induced apoptosis [53].
Alteration in the ECM properties leads to tissues less able to bear normal load or withstand low-impact injuries, which leads to a chain reaction of events that damage and further drive disease progression. Changes in cartilage ECM due to aging include altered aggrecan sizes [54,55] and increased fibril crosslinking of collagen type II. The latter process increases the stiffness of cartilage [56,57], and increased stiffness of the matrix has been attributed to an accumulation of glycation end products (nonenzymatic protein modifications) over time [58,59]. The accumulation of glycation end products results in the activation of receptor for advanced glycation end products (RAGE) receptors [60] and induces reactive oxygen species (ROS) and catabolic pathways (Figure 2) [61]. ROS can be produced by chondrocytes or by the synovial lining [62], which impairs chondrocyte response to growth factors, such as IGF-1, and inhibits mitochondrial function [63–65] and DNA repair capacity [66].
Small calcium-binding S100 proteins have been implicated in various inflammatory conditions, including arthritis. S100A4 may play an important role in cartilage degradation by mediating ECM destruction and indirectly altering chondrocyte viability [67]. The interaction of S100A4 with RAGE increases MMP-13 production in cartilage (Figure 2) [67], and is also known to upregulate MMP-13 and other MMPs in rheumatoid arthritis-derived synovial fibroblasts [68]. S100A4 was reported to bind p53 tumor suppressor and to regulate its function [69], possibly promoting apoptosis (see section on p53 and c-myc).
Mechanical stress & injury
As previously outlined, changes in ECM not only alter cellular response, but also modify the mechanical properties of the tissue, leaving the cell more vulnerable to normal loading. Mechanical injury has been demonstrated to induce cell death, and cartilage matrix degradation in response to mechanical injury has been reported in bovine [70–74] and human cartilage [74–77]. Loss of glycosaminoglycan may indirectly predispose cells to necrotic cell death following mechanical injury, which later precipitates PCD (Figure 1) [78].
Mechanical stimuli releases proteoglycans from cartilage explants [74,79,80] and induces the production of inflammatory or catabolic peptides, such as MMPs [81], NO [82], ADAMTS-5 [83] and IL-1β (Figure 2) [79]. A single episode of mechanical injury in human cartilage explants resulted in a time-dependent increase in apoptotic cells. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was partially inhibited by incubating the impacted explants with the pan-caspase inhibitor z-VAD-fmk [74]. Apoptosis has also been demonstrated to be induced by repetitive trauma in vitro [84]. In vivo cartilage degeneration, generated by anterior cruciate ligament transaction in rabbit knees, was significantly reduced with caspase inhibition, supporting a potential therapeutic role [85]. Other studies also demonstrate the potential therapeutic role of caspase inhibitors [74,77], BMP7 [86] and P188 surfactant [76] for chondroprotection following impact trauma or tissue injury as a result of surgical procedure [87].
It has been hypothesized that mechanical injury releases ROS. This hypothesis has been supported by reports of antioxidants (N-acetylcysteine and a superoxide dismutase [SOD] mimetic) increasing cell viability after mechanical injury [88,89].
Nitric oxide
Nitric oxide is present in normal and young cartilage, yet its production is elevated in aged tissue [90,91]. NO mediates apoptosis through a mitochondria-dependent mechanism [92–94] and contributes to the breakdown of the ECM by enhancing the expression of proinflammatory cytokines [95–97]. High concentrations of the NO donor sodium nitroprusside (SNP) can induce apoptosis-like cell death in cultured human chondrocytes [98]. Incubation of human articular chondrocytes with SNP can induce events characteristic of apoptosis, including increased caspase-3 and caspase-7 gene expression and downregulating Bcl-2 (an antiapoptotic molecule) mRNA levels (Figure 2) [93]. SNP induces apoptosis of human chondrocytes through sequential events, involving cytoskeletal remodeling (disruption and reduced polymerization of F-actin and microtubule cytoskeleton), MEKK1/JNK activation, Bax translocation, mitochondrial dysfunction (decreased complex I NADH dehydrogenase activity and cytochrome c release) and sequential caspase activation (caspase-9, -3 and -6), leading to DNA fragmentation [94].
IL-1β, an inducer of inducible NO synthase expression and production of NO in chondrocytes, did not induce chondrocyte apoptosis [98,99]. Nevertheless, combining IL-1β with dimethyl sulfoxide resulted in hypoploidy and DNA fragmentation. Use of a specific inhibitor of inducible NO synthase reduced apoptosis induced by IL-1β, indicating that cell death is dependent on endogenous NO generation. In addition, the proapoptotic effect of NO could be blocked by ROS [99]. The balance between intracellular NO and ROS has been proposed to determine whether chondrocytes die through apoptosis or necrosis, with a low concentration of ROS promoting apoptosis in the presence of NO and a high concentration of ROS promoting necrosis.
Interestingly, del Carlo and Loeser found that incubation with NO alone does not induce apoptotic cell death in chondrocytes [100]. They also demonstrated that both NO and ROS are required to induce apoptosis, suggesting that NO alone may have beneficial effects in chondrocytes. Other studies on the effect of NO on chondrocyte apoptosis have focused on the role of apoptosis in terminal differentiation, again illustrating an alternative, nonpathological role for NO in development. However, most reports indicate that NO is primarily a catabolic factor in OA that can induce cell death and further contribute to disease progression.
Death receptors
Members of the TNF-receptor family are transmembrane receptors that activate well-characterized apoptosis pathways. Fas (CD95) is expressed on the cell surface of cultured chondrocytes and is detected in cartilage from normal and OA donors [101]. Fas activation by agonistic antibody leads to apoptotic cell death in cultured chondrocytes, linking this receptor to functional apoptosis signaling components. In tissue, however, antibody to Fas has not been shown to induce cell death [102], which has been attributed to the barrier created by matrix proteins that prevents antibody interaction with the chondrocytes. In addition, chondrocytes in ECM may not respond to Fas stimulation, since they may be protected from Fas-dependent apoptosis through survival signals generated by the interaction of cell membrane receptors, such as integrins, with their respective ECM ligands [3]. However, in degraded tissues the Fas/FasL system may be more effective in inducing cell death. To date, TNF-α-mediated chondrocyte death has not been conclusively established. TNF-α stimulation (48 h) of monolayer chondrocytes led to a small increase in the number of TUNEL- or in situ nick-end labeling (ISNEL)-positive cells [103]. In another report, DNA fragmentation in response to TNF-α was detected with a sensitive ELISA-based technique when the chondrocytes were simultaneously stimulated with proteasome inhibitors [102]. Therefore, TNF-α alone may have no effect on apoptosis. However, TNF-α in combination with actinomycin-D or Ro 31–8220 induces an increase in caspase-1 and -8 mRNA and protein levels [104,105].
The sensitivity of T cells to TNF-α-induced apoptosis appears to be age dependent [106]. In aged lymphocytes, apoptosis is associated with increased expression of TRADD, FADD and Bax [107,108], and decreased expression of Bcl-2, TRAF2 and TNFRII. To date, such age-related changes have not been established in articular cartilage.
Mitochondria
The influence or involvement of mitochondria in apoptosis and cell necrosis has been extensively investigated (for reviews see [109–112]). Altered mitochondrial function has been associated with apoptosis, aging and a number of pathological conditions, including OA [4,113–116].
In addition to the mitochondrial involvement in NO-induced apoptosis [94], oxidative stress and mitochondrial dysregulation play an important role in OA development and progression [117,118]. Oxidation changes occur during the aging process, which illustrates a possible relationship between aging, chronic inflammation and cartilage degradation in OA [119].
Reactive oxygen species activity is balanced by enzymatic and nonenzymatic antioxidants, which act by inhibiting oxidative enzymes or scavenging free radicals [113]. Decreased mitochondrial SOD2 within OA chondrocytes affects chondrocyte intracellular metabolism [120]. Degenerating regions of OA cartilage [121] possessed lower anti-oxidative capacity and the resulting oxidative stress induced replicative senescence and telomere genomic instability. This result indicates that inadequate control of ROS is an essential factor in OA pathophysiology.
Mitochondria may be involved in epiphysial chondrocyte death during bone development. Mitochondria in the avian growth plate show a maturation-dependent reduction of oxidative phosphorylation [122] and changes in Bcl-2 protein levels [123]. Loss of mitochondrial function may be linked to NO production induced by phosphate ions [124]. A causal relationship between phosphate ions, NO production and mitochondrial dysfunction in avian growth plate chondrocytes has recently been established [125], although no evidence is available to directly link this relationship to OA.
p53 & c-myc
A majority of studies suggest that p53 is involved in increased cell death in aging cartilage. Experiments using mice with hyperactive p53 tumor suppressor gene show a senescence phenotype and signs of premature aging [126], although cartilage from p53-knockout mice shows no significant effect on cell death [127]. In degenerated lesions of arthritic cartilage, ISNEL, denoting apoptotic cells, correlated with the expression of p53 and c-myc [128]. Hydrostatic pressure induced apoptosis in cultured human chondrocytes, which was associated with increased p53 expression [129]. In aged rabbits, the expression of p53 was increased and was associated with decreased viable cell density [130]. NO was shown to cause cell death and induce p53 via p38 MAPK and NF-κB, indicating that p53 plays a role in chondrocyte survival in the presence of NO (Figure 2) [131].
Levels of c-myc increased in fully differentiated hypertrophic chondrocytes [132] and in hypertrophic chondrocytes in rat growth plates, indicating a role for c-myc in terminal chondrocyte differentiation [133]. Subcellular localization also changed with intranuclear concentration of c-myc, which decreased with the maturing of chondrocytes. Another study demonstrated that the s-myc protein was expressed in rat embryo cells committed to undergo differentiation into hypertrophic chondrocytes [134]. In rabbit growth plates, c-myc staining frequently colocalized with cells showing DNA strand breaks. In a canine model of OA, high levels of c-myc were found in areas of cartilage erosion [135]. Furthermore, c-myc expression also colocalized with apoptotic cells in human arthritic cartilage [128]. Similar to p53, apoptosis induced by hydrostatic loading was linked to c-myc [129]. These findings suggest that in addition to p53, c-myc may also regulate developmental and OA-related chondrocyte death.
Wnt/β-catenin signaling
Two recent studies reveal a close relationship between aging, Wnt/β-catenin signaling and apoptosis [136]. In these studies, Col2a1– inhibitor of β-catenin and T-cell factor (ICAT)-transgenic mice inhibited β-catenin signaling in chondrocytes and significantly increased cleaved PARP, caspase-3 and TUNEL-positive cells (Figure 2) expression. Conversely, Bcl-2 and Bcl-XL were decreased and caspase-9 and caspase-3/7 activity were increased, suggesting that increased cell apoptosis may contribute significantly to the articular cartilage destruction observed in Col2a1–ICAT-transgenic mice.
In another study, both Wnt signaling and chromatin protein high mobility group box protein 2 (HMGB2) expression decreased with aging in mouse joints, and conditional deletion of β-catenin in cultured mouse chondrocytes induced apoptosis (Figure 2) [137]. The loss of HMGB2–Wnt signaling interaction represents a new mechanism in aging-related cartilage pathology.
Autophagy
Autophagy is an important cellular process involved in recycling of long-lived proteins and organelles and is upregulated in response to ischemia/reperfusion and pressure overload in the heart [138,139]. Therefore, autophagy may be similarly altered in mechanoresponsive tissues such as cartilage. The role of autophagy in cartilage biology has only recently received attention. Observations by Caramés et al. indicate that aged and OA articular cartilage are associated with reduced expression or loss of ULK1, Beclin1 and light chain 3, which was accompanied by an increase in apoptosis (Figure 2) [22]. Hypoxia-inducible factor (HIF) activity influences the expression of Beclin 1 (a major factor in autophagy) and regulates HIF’s interaction with caspase-8 and members of the Bcl-2 family of proteins [21]. The induction of autophagy appears to delay chondrocyte death until completion of the maturation process; however, prolonged autophagy may play a role in PCD.
Evidence of crosstalk has been reported between autophagy and apoptosis [140,141]. The autophagy protein, Atg5, induces mitochondria-based apoptosis, while Bcl-2 overexpression protected against Atg5-mediated mitochondrial dysfunction. Beclin 1, an essential autophagy protein, is regulated by the Bcl-2 proteins in normal conditions. Bcl-2 and Bcl-XL suppress autophagy by associating with Beclin 1 [142]. Reduced Beclin 1 heterozygous mice (Beclin 1+/−) have reduced autophagy and apoptosis and heart infarct size after ischemia/reperfusion injury [143], suggesting that Beclin 1 might activate apoptosis. Continued research into the relationship between age, apoptosis and autophagy may reveal alternative means to preserve cartilage viability.
Growth factor responsiveness
TGF-β and IGF-I are major growth factors regulating chondrocyte survival, proliferation, differentiation and matrix synthesis. IGF-I is essential in maintaining chondrocyte viability [144]. Studies demonstrate that as mice and men age, chondrocyte responses to growth factors are reduced (Figure 2) [6,145,146]. van der Kraan and van den Berg [147] propose that after the age of 40 years, chondrocytes lose the ability to maintain a normal phenotype or resist terminal differentiation. Disruption in normal TGF-β signaling (absence of ALK5 and or Smad2/3) appears to be the major underlying cause, as illustrated by knockout mice and human family studies [148–150]. While the proliferative response to TGF-β1, FGF2 and PDGFbb was not reduced in human articular chondrocytes, the cartilage-forming capacity following expansion with growth factors was lower in older individuals [6]. These age-related changes in growth factor response then shift cartilage tissue homeostasis toward tissue destruction and eventual cell death. Such age-related changes are thought to be significant factors in the increased susceptibility to injury and degeneration and to the reduced repair response with aging.
Apoptosis inhibitors
Mitochondria-associated proteins play key roles in activating apoptosis. The Bcl-2 family regulates the release of proteins (such as cytochrome c) from the space between the inner and outer mitochondrial membrane that, once in the cytosol, activate caspase proteases. Bcl-2 is an antiapoptotic protein and is expressed typically in the mid-zone of normal cartilage. Overexpression of Bcl-2 protects against apoptosis [151] and Bcl-2 expression appears to be regulated by IL-1β and NO (Figure 2) [99,152]. The overall expression of Bcl-2 is reduced in OA, although there is relatively greater expression in chondrocytes near arthritic defects [153,154]. Bcl-2 is also implicated in cell death associated with collagen type II deficiency [41]. In mice, Bcl-2 expression decreased with age, indicating a decline in antiapoptotic activity [155].
Cellular control of apoptosis is complex and several intracellular inhibitors of apoptotic signaling cascades have been characterized. Inhibitor of apoptosis (IAP) proteins inhibit caspases or block the pathways that activate them. The IAP proteins primarily function as ubiquitin E3 ligases and possess protein–protein interaction domains (reviewed in [156,157]). XIAP is a potent inhibitor of the catalytic self-activation of caspase-3. Fas activation in cultured chondrocytes often leads to incomplete caspase-3 processing [102]. This suggests potent apoptotic inhibitory mechanisms at or above the level of caspase-3 activation. The mediators of these mechanisms are still being elucidated. However, evidence implicates that low expression of caspase-8 and expression of FLICE inhibitory protein (FLIP) leads to suppression of the Fas signal [102,158].
Cytokines have also been implicated to prevent apoptosis. In human chondrocytes, TNF-α increases NF-κB activity, which alone does not induce apoptosis [102,104,105]. NF-κB has been shown to block TNF-α-induced apoptosis [159]. However, at least partial inhibition of NF-κB activation protects chondrocytes against Fas- and NO-induced death [99,152]. IL-1β can also block Fas-induced apoptosis, which is thought to be dependent on tyrosine phosphorylation [99]. IL-4 downregulated cyclic tensile stress-induced inducible NO synthase mRNA expression and NO production by chondrocytes and reduced NO induced apoptosis [160]. Intra-articular injection of IL-4 into rat joints appeared to exert chondroprotective properties against mechanical stress-induced cartilage destruction, probably by inhibiting NO production by chondrocytes [161]. Cilostazol, a selective phosphodiesterase type III inhibitor, inhibited NO-induced apoptosis by preventing the upregulation of phosphorylated p53 and p38, reducing heme oxygenase 1 and caspase-3, -7 and -8 activation [162].
Cell death, aging & OA
Osteoarthritis is generally thought to be a slowly progressive disease. In humans, as well as in animal models, it is linked with chondrocyte death, which is assumed to be largely apoptotic in nature. Experiments with articular cartilage of C57BL/6 mice and Wistar rats demonstrated a significant age-dependent increase of the percentage of apoptotic cells (TUNEL-positive) for all joint surfaces in both species [163]. Electron microscopy of human OA cartilage reveals cytoplasmic and nuclear features consistent with apoptosis. In very early OA, when the superficial zone is still intact, empty lacunae, lysosome-like structures, matrix vesicle-like structures, fragmented chondrocytes and nuclear condensation are observed [7]. To illustrate the spectrum of cell death, Kuhn et al. showed evidence of chondrocytes with the ultrastructural features of apoptosis or necrosis in OA cartilage [3]. Chondrocyte death correlates strongly with age and severity of OA. Several reports have associated a significantly greater number of TUNEL- or ISNEL-positive cells in OA cartilage relative to normal [7,8,128,164]. The number of potentially apoptotic cells also correlated significantly with the OA grade [8]. Flow cytometric analysis of chondrocytes isolated from osteoarthritic tissue demonstrated increased rates of apoptosis (by TUNEL) when compared with cells from normal cartilage. The matrix surrounding TUNEL-positive cells contained lower proteoglycan concentrations [8]. Increased numbers of empty lacunae in cartilage were associated with higher arthritic grade when compared with age-matched normal cartilage [165,166]. TUNEL has been frequently used to quantify apoptosis and can sometimes label necrotic cells. This false-positive staining can lead to overestimation of the number of apoptotic cells (thus, a combination of techniques is recommended) [77,167]. However, the significant difference in TUNEL-positive cells between normal and OA cartilage indicates that increased cell death (regardless of mechanism) is an integral feature of OA pathology. These dead cells tend to persist in their lacunae owing to lack of vascularity or phagocytotic removal. Eventually, disintegration of apoptotic chondrocytes leads to formation of membrane-enclosed structures resembling matrix vesicles [7,164,168]. These structures may be responsible for the matrix mineralization often associated with OA.
Animal models of OA (such as the cartilage degeneration induced by anterior cruciate ligament transection) have linked the histologic severity of cartilage lesions with chondrocyte death, matrix loss, production of NO, increased intracellular caspase-3 activity and an increased frequency of TUNEL-positive cells [8,85,165]. More research is necessary to elucidate the precise role of conventional apoptosis pathways and the potential role of caspases other than caspase-3 in OA-linked chondrocyte death. In mice activation of caspase-12, which is located in the ER, led to apoptosis-like cell death [169]. Nonclassical pathways of PCD may also be useful candidates for future investigations.
Novel mechanisms of age-induced reduction in cellularity are being discovered. The nonhistone chromatin protein HMGB2 is a transcriptional regulator, which is specifically expressed in the superficial zone of human articular cartilage, and which gradually reduces with aging [170]. Genetic deficiency of HMGB2 in mouse chondrocytes increases susceptibility to apoptosis induction in vitro. In vivo, a dramatic reduction in cellularity is followed by an accelerated and more severe form of OA. HMGB2 in human articular cartilage is therefore an important chondrocyte survival factor and directly links aging with OA.
Future perspective
The aging process is inevitable, yet our understanding of the consequences of this phenomenon is incomplete. The breakdown of cartilage tissue structure and aging leads to the development and progression of OA. OA and other rheumatic diseases are among the most common of all health conditions and are the number one cause of disability in the USA, affecting an estimated 27 million Americans [171]. At present, the most common treatment of advanced OA is joint replacement, which is estimated to reach 2 million knees and hips per year by 2015 in the USA alone [172]. The impact of arthritic conditions is also expected to grow as the population increases and ages in the coming decades. Current North American (American College of Rheumatology [ACR]) and European (European League Against Rheumatism [EULAR]) recommendations for the treatment of OA include only symptom-modifying therapies [173,174]. Unlike rheumatoid arthritis, presently there are no intervention therapies available for altering OA.
The multifaceted nature of joint disease indicates that the contribution of cell death can be an important factor at all stages of the disease. Matrix homeostasis relies on a balance between net anabolic and catabolic activities, which are directly influenced by the number of available chondrocytes. The weight of existing evidence offers chondrocyte death as an excellent target for therapeutic intervention in OA. To achieve prophylactic and therapeutic success, further research into chondrocyte death, cartilage degeneration and arthritic progression is required.
Within the next 5–10 years, it is envisaged that researchers and clinicians will develop more distinct pharmacological and/or cell-based methods to slow or reverse age-related tissue degeneration. A better resolution of the mechanistic links between aging changes in ECM, receptor and signaling pathways that instigate initial matrix degradation that leads to cell death, and OA progression will ensue. Therapies that preserve cell viability modulate and control cellular response in vivo will probably be the main focus of research over the next decade, which should lead to clinical application soon thereafter. The main areas that may evolve to clinical application include: inhibition of apoptosis; pharmacological approaches to retard overtly catabolic and ROS activities that lead to accelerated ECM degradation and cell death; and utilization of cartilage progenitor cells.
Inhibition of apoptosis
This could occur either through caspase inhibitors or other chondropreserving factors such as BMP7 [86]. The clinical efficacy of caspase inhibition in other diseases currently under investigation includes acute and chronic neurodegenerative diseases, myocardial infarction and liver apoptosis [175–177]. Application of these inhibitors should firstly translate into treatment of acute post-traumatic joint injuries. More precise identification of the intrinsic and extrinsic mechanisms leading to chondrocyte death will be established to provide novel targets for therapeutic interventions.
Pharmacological approaches to retard overtly catabolic & ROS activities that lead to accelerated ECM degradation & cell death
Such treatments may include use of antioxidants or enhancement of SOD to combat excess ROS [178,179]. Further development of aggrecanase (specifically ADAMTS-4 and ADAMTS-5) [180–182] and matrix metalloproteinase inhibitors [183] will be better refined and employed to slow tissue degradation. Means to inhibit or reduce excessive matrix extracellular sulfatase activity, observed in OA cartilage [184], are currently being developed and will offer yet another means to control loss of tissue homeostasis and OA progression.
Utilization of cartilage progenitor cells
Over the last 20 years, cell-based methods to repair cartilage defects and diseased tissues using chondrocytes [185] and mesenchymal stem cells have been studied [186]. In fact, human chondrocytes have been used for almost 20 years with unclear clinical outcomes [187]. Nevertheless, over the past decade, mounting evidence shows that progenitor cell populations reside in articular cartilage [188–196], which are principally located in the articular cartilage superficial zone [189,190,196]. Loss of these progenitors during aging and resulting from injury may be an important factor that leads to loss of tissue homeostasis. These cells may be a better cell source to repair injured or degenerated tissue; thus, preserving this subpopulation may be critical. Furthermore, therapies that utilize these cells in situ would represent a more elegant means to restore joint function.
Executive summary.
Cell death is a normal consequence of aging
Cellularity in articular cartilage progressively reduces with age.
Reduced cellularity is compounded by cell senescence, accumulation of glycation end products, oxidative damage and reduced growth factor responsiveness.
Chondrocyte death has many faces
Three primary modes of chondrocyte death are apoptosis, autophagy-associated cell death and necrosis.
These modes of cell death often have overlapping rather than distinct modes of morphologic features.
Cell–matrix interactions are critical to survival
Collagen type II supports cell survival via integrin binding.
Matrix protein fragments have inflammatory effects on cartilage matrix.
Aging- or osteoarthritis (OA)-related matrix changes can alter the mechanical environment of the chondrocyte making it more susceptible to injury and death.
Stresses associated with cell death
Mechanical injury induces cell death: a major component of which is apoptotic in nature.
Mitochondrial dysfunction is associated with aging, OA and cell death.
Exogenous nitric oxide induces cell death, while the balance between endogenous nitric oxide and reactive oxygen species determines potential for cell death.
p53 and c-myc are implicated in cell death and OA.
OA is intimately associated with increased cell death
Cell density is inversely correlated with the grade of OA.
The matrix surrounding apoptotic cells has lower proteoglycan concentration.
Apoptotic bodies resemble matrix vesicles found in OA tissues and can be sites of nucleation for calcific deposits.
Animal models have linked OA with increased caspase activity, nitric oxide, matrix loss and cell death.
Prevention of cell death holds promise as a treatment of post-traumatic OA
Caspase inhibitors and antixoxidants can prevent cell death induced by mechanical injury.
Inhibition of caspases in vivo has significantly reduced the grade of OA.
Acknowledgments
The authors would like to thank Judy Blake for manuscript formatting and copyediting.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This research was supported by the NIH (P01 AG007996) and by Donald and Darlene Shiley. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328(3):700–708. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12(1):1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8(2):333–345. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 5.Temple MM, Bae WC, Chen MQ, et al. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15(9):1042–1052. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12(6):476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998;41(7):1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15(5):364–372. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 10.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Layton MW, Goldstein SA, Goulet RW, Feldkamp LA, Kubinski DJ, Bole GG. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988;31(11):1400–1405. doi: 10.1002/art.1780311109. [DOI] [PubMed] [Google Scholar]
- 12.Revell PA, Mayston V, Lalor P, Mapp P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47(4):300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–79. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 14.Goldring SR. The role of bone in osteoarthritis pathogenesis. Rheum Dis Clin North Am. 2008;34(3):561–571. doi: 10.1016/j.rdc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;213:34–40. [PubMed] [Google Scholar]
- 16▪.Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44(3):205–221. doi: 10.1016/j.ymeth.2007.12.001. Excellent overview of techniques to distinguish apoptosis and necrotic cell death. [DOI] [PubMed] [Google Scholar]
- 17.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2(8):589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 18▪.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. Establishes current definitions of many types of cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9(3):265–277. doi: 10.1023/b:appt.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 20.Searle J, Lawson TA, Abbott PJ, Harmon B, Kerr JF. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- 21.Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3(3):207–214. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 22▪▪.Caramés B, Taniguchi N, Otsuki S, Blanco F, Lotz M. Autophagy is a protective mechanism in normal cartilage and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305. First comprehensive study to show a clear connection between aging and autophagy in osteoarthritis (OA) in human articular cartilage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings BS, McHowat J, Schnellmann RG. Phospholipase A2s in cell injury and death. J Pharmacol Exp Ther. 2000;294(3):793–799. [PubMed] [Google Scholar]
- 24.Zhang C, Xu Y, Gu J, Schlossman SF. A cell surface receptor defined by a mAb mediates a unique type of cell death similar to oncosis. Proc Natl Acad Sci USA. 1998;95(11):6290–6295. doi: 10.1073/pnas.95.11.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps PC, Smith MW, Trump BF. Cytosolic ionized calcium and bleb formation after acute cell injury of cultured rabbit renal tubule cells. Lab Invest. 1989;60(5):630–642. [PubMed] [Google Scholar]
- 26.Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25(1):82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57(10):1835–1840. [PubMed] [Google Scholar]
- 28.Crisby M, Kallin B, Thyberg J, et al. Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis. 1997;130(1–2):17–27. doi: 10.1016/s0021-9150(96)06037-6. [DOI] [PubMed] [Google Scholar]
- 29.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98(14):1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 30.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 31.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 32.Shakibaei M, Csaki C, Mobasheri A. Diverse roles of integrin receptors in articular cartilage. Adv Anat Embryol Cell Biol. 2008;197:1–60. doi: 10.1007/978-3-540-78771-6. [DOI] [PubMed] [Google Scholar]
- 33▪.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. Demonstrates that the extent of cell death is associated with the degree of cartilage matrix damage and varied with each cartilage zone. [DOI] [PubMed] [Google Scholar]
- 34.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76(11):1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch MS, Lunsford LE, Trinkaus-Randall V, Svoboda KK. Chondrocyte survival and differentiation in situ are integrin mediated. Dev Dyn. 1997;210(3):249–263. doi: 10.1002/(SICI)1097-0177(199711)210:3<249::AID-AJA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Hwang SG, Kim IC, Chun JS. Actin cytoskeletal architecture regulates nitric oxide-induced apoptosis, dedifferentiation, and cyclooxygenase-2 expression in articular chondrocytes via mitogen-activated protein kinase and protein kinase C pathways. J Biol Chem. 2003;278(43):42448–42456. doi: 10.1074/jbc.M304887200. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Lian G, Lenkinski R, et al. Filamin B mutations cause chondrocyte defects in skeletal development. Hum Mol Genet. 2007;16(14):1661–1675. doi: 10.1093/hmg/ddm114. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Woods A, Agoston H, Ulici V, Glogauer M, Beier F. Genetic ablation of Rac1 in cartilage results in chondrodysplasia. Dev Biol. 2007;306(2):612–623. doi: 10.1016/j.ydbio.2007.03.520. [DOI] [PubMed] [Google Scholar]
- 39.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 40.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296(5573):1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 41.Yang C, Li SW, Helminen HJ, Khillan JS, Bao Y, Prockop DJ. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp Cell Res. 1997;235(2):370–373. doi: 10.1006/excr.1997.3692. [DOI] [PubMed] [Google Scholar]
- 42.Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37(1–2):109–116. [PubMed] [Google Scholar]
- 43.Pulai JI, Del Carlo M, Jr, Loeser RF. The α5β1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46(6):1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 44.Perlot RL, Jr, Shapiro IM, Mansfield K, Adams CS. Matrix regulation of skeletal cell apoptosis II: role of Arg–Gly–Asp-containing peptides. J Bone Miner Res. 2002;17(1):66–76. doi: 10.1359/jbmr.2002.17.1.66. [DOI] [PubMed] [Google Scholar]
- 45▪.Matsuki K, Sasho T, Nakagawa K, et al. RGD peptide-induced cell death of chondrocytes and synovial cells. J Orthop Sci. 2008;13(6):524–532. doi: 10.1007/s00776-008-1281-z. Authors demonstrate that RGD peptides specifically activate caspase-3 without initiating caspase-8 or -9 activation. [DOI] [PubMed] [Google Scholar]
- 46.Homandberg GA, Hui F, Wen C, Kuettner KE, Williams JM. Hyaluronic acid suppresses fibronectin fragment mediated cartilage chondrolysis: I. In vitro. Osteoarthritis Cartilage. 1997;5(5):309–319. doi: 10.1016/s1063-4584(97)80035-0. [DOI] [PubMed] [Google Scholar]
- 47.Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60(9):1118–1124. doi: 10.1093/gerona/60.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26(4):247–258. doi: 10.1016/j.matbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Chetkina EV, Pul AR. Type II collagen fragment capacity to induce collagen cleavage and hypertrophy of articular chondrocyte. Vestn Ross Akad Med Nauk. 2008;(5):15–21. [PubMed] [Google Scholar]
- 50.Brun P, Panfilo S, Daga Gordini D, Cortivo R, Abatangelo G. The effect of hyaluronan on CD44-mediated survival of normal and hydroxyl radical-damaged chondrocytes. Osteoarthritis Cartilage. 2003;11(3):208–216. doi: 10.1016/s1063-4584(02)00352-7. [DOI] [PubMed] [Google Scholar]
- 51.Iacob S, Knudson CB. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int J Biochem Cell Biol. 2006;38(1):123–133. doi: 10.1016/j.biocel.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFκB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281(26):17952–17960. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisignoli G, Grassi F, Zini N, et al. Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum. 2001;44(8):1800–1807. doi: 10.1002/1529-0131(200108)44:8<1800::AID-ART317>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Bihari-Varga M, Farkas T, Biro T. Changes in the cartilage proteoglycans in relation to age and osteoarthrosis. Acta Biol Hung. 1984;35(2–4):325–331. [PubMed] [Google Scholar]
- 55.Lark MW, Bayne EK, Flanagan J, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100(1):93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerin A, Patwari P, Kuettner K, Cole A, Grodzinsky A. Molecular basis of osteoarthritis: biomechanical aspects. Cell Mol Life Sci. 2002;59(1):27–35. doi: 10.1007/s00018-002-8402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4(3):301–305. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 59.DeGroot J, Verzijl N, Wenting-van Wijk MJ, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50(4):1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 60.Loeser RF, Yammani RR, Carlson CS, et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52(8):2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeGroot J, Verzijl N, Jacobs KM, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthritis Cartilage. 2001;9(8):720–726. doi: 10.1053/joca.2001.0469. [DOI] [PubMed] [Google Scholar]
- 62.Zhai G, Aviv A, Hunter DJ, et al. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann Rheum Dis. 2006;65(11):1444–1448. doi: 10.1136/ard.2006.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43(7):1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 64.Studer R, Jaffurs D, Stefanovic-Racic M, Robbins PD, Evans CH. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7(4):377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 65.Studer RK, Levicoff E, Georgescu H, Miller L, Jaffurs D, Evans CH. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRβ tyrosine phosphorylation. Am J Physiol Cell Physiol. 2000;279(4):C961–969. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- 66.Grishko VI, Ho R, Wilson GL, Pearsall AW., 4th Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartilage. 2009;17(1):107–113. doi: 10.1016/j.joca.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪.Yammani RR, Long D, Loeser RF. Interleukin-7 stimulates secretion of S100A4 by activating the JAK/STAT signaling pathway in human articular chondrocytes. Arthritis Rheum. 2009;60(3):792–800. doi: 10.1002/art.24295. Elucidates the pathways involved in mediating S100A4 and matrix metalloproteinase13 production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Senolt L, Grigorian M, Lukanidin E, et al. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann Rheum Dis. 2006;65(12):1645–1648. doi: 10.1136/ard.2005.047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grigorian M, Andresen S, Tulchinsky E, et al. Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: functional consequences of their interaction. J Biol Chem. 2001;276(25):22699–22708. doi: 10.1074/jbc.M010231200. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto S, Nishiyama T, Hayashi S, et al. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthritis Rheum. 2009;60(8):2340–2349. doi: 10.1002/art.24706. [DOI] [PubMed] [Google Scholar]
- 71.Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 72.Natoli RM, Athanasiou KA. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J Biomech Eng. 2008;130(4):041012. doi: 10.1115/1.2939368. [DOI] [PubMed] [Google Scholar]
- 73.Stevens AL, Wishnok JS, Chai DH, Grodzinsky AJ, Tannenbaum SR. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis-liquid chromatography tandem mass spectrometry analysis of bovine cartilage tissue response to mechanical compression injury and the inflammatory cytokines tumor necrosis factor α and interleukin-1β. Arthritis Rheum. 2008;58(2):489–500. doi: 10.1002/art.23120. [DOI] [PubMed] [Google Scholar]
- 74.D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 75.Hembree WC, Ward BD, Furman BD, et al. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J Bone Joint Surg Br. 2007;89(10):1388–1395. doi: 10.1302/0301-620X.89B10.18907. [DOI] [PubMed] [Google Scholar]
- 76.Pascual-Garrido C, Hakimiyan AA, Rappoport L, Oegema TR, Wimmer MA, Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17(9):1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dang AC, Kim HT. Chondrocyte apoptosis after simulated intraarticular fracture: a comparison of histologic detection methods. Clin Orthop Relat Res. 2009;467(7):1877–1884. doi: 10.1007/s11999-009-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Otsuki S, Brinson DC, Creighton L, et al. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58(4):1076–1085. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 79.Patwari P, Cook MN, DiMicco MA, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48(5):1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 80.Sui Y, Lee JH, Dimicco MA, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60(10):2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 81.Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat. 2005;187(5–6):473–485. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Green DM, Noble PC, Ahuero JS, Birdsall HH. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006;54(5):1509–1517. doi: 10.1002/art.21812. [DOI] [PubMed] [Google Scholar]
- 83.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8):2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 84.Clements KM, Bee ZC, Crossingham GV, Adams MA, Sharif M. How severe must repetitive loading be to kill chondrocytes in articular cartilage? Osteoarthritis Cartilage. 2001;9(5):499–507. doi: 10.1053/joca.2000.0417. [DOI] [PubMed] [Google Scholar]
- 85.D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 86.Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27(5):602–611. doi: 10.1002/jor.20787. [DOI] [PubMed] [Google Scholar]
- 87.Costouros JG, Kim HT. Preventing chondrocyte programmed cell death caused by iatrogenic injury. Knee. 2007;14(2):107–111. doi: 10.1016/j.knee.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123–130. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- 90.Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99(6):1231–1237. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Min BH, Kim HJ, Lim H, Park CS, Park SR. Effects of ageing and arthritic disease on nitric oxide production by human articular chondrocytes. Exp Mol Med. 2001;33(4):299–302. doi: 10.1038/emm.2001.48. [DOI] [PubMed] [Google Scholar]
- 92▪.Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101(6):1520–1531. doi: 10.1002/jcb.21268. Demonstrates interactions between nitric oxide, cytoskeleton and mitochondria proteins that lead to caspase activation and apoptosis. [DOI] [PubMed] [Google Scholar]
- 93.Maneiro E, Lopez-Armada MJ, de Andres MC, et al. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005;64(3):388–395. doi: 10.1136/ard.2004.022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cherng YG, Chang HC, Lin YL, Kuo ML, Chiu WT, Chen RM. Apoptotic insults to human chondrocytes induced by sodium nitroprusside are involved in sequential events, including cytoskeletal remodeling, phosphorylation of mitogen-activated protein kinase kinase kinase-1/c-Jun N-terminal kinase, and Bax-mitochondria-mediated caspase activation. J Orthop Res. 2008;26(7):1018–1026. doi: 10.1002/jor.20578. [DOI] [PubMed] [Google Scholar]
- 95.Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. discussion 65. [DOI] [PubMed] [Google Scholar]
- 96.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 97.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10(Suppl 2):S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- 99.Kuhn K, Hashimoto S, Lotz M. IL-1 β protects human chondrocytes from CD95-induced apoptosis. J Immunol. 2000;164(4):2233–2239. doi: 10.4049/jimmunol.164.4.2233. [DOI] [PubMed] [Google Scholar]
- 100.Del Carlo M, Jr, Loeser RF. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46(2):394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 101.Hashimoto S, Setareh M, Ochs RL, Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997;40(10):1749–1755. doi: 10.1002/art.1780401004. [DOI] [PubMed] [Google Scholar]
- 102.Kuhn K, Lotz M. Regulation of CD95 (Fas/APO-1)-induced apoptosis in human chondrocytes. Arthritis Rheum. 2001;44(7):1644–1653. doi: 10.1002/1529-0131(200107)44:7<1644::AID-ART287>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 103.Fischer BA, Mundle S, Cole AA. Tumor necrosis factor-α induced DNA cleavage in human articular chondrocytes may involve multiple endonucleolytic activities during apoptosis. Microsc Res Tech. 2000;50(3):236–242. doi: 10.1002/1097-0029(20000801)50:3<236::AID-JEMT7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 104.Lopez-Armada MJ, Carames B, Lires-Dean M, et al. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14(7):660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Carames B, Lopez-Armada MJ, Cillero-Pastor B, et al. Differential effects of tumor necrosis factor-α and interleukin-1β on cell death in human articular chondrocytes. Osteoarthritis Cartilage. 2008;16(6):715–722. doi: 10.1016/j.joca.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Gupta S, Chiplunkar S, Kim C, Yel L, Gollapudi S. Effect of age on molecular signaling of TNF-α-induced apoptosis in human lymphocytes. Mech Ageing Dev. 2003;124(4):503–509. doi: 10.1016/s0047-6374(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 107.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases. J Immunol. 1999;162(4):2154–2161. [PubMed] [Google Scholar]
- 108.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160(4):1627–1637. [PubMed] [Google Scholar]
- 109.Skulachev VP, Anisimov VN, Antonenko YN, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta. 2009;1787(5):437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann NY Acad Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111▪.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8(2):119–130. doi: 10.2174/156652408783769571. Use of proteomics to identify important mitochondrial proteins differentially expressed in OA cartilage. [DOI] [PubMed] [Google Scholar]
- 112.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev. 2007;39(2–3):443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz-Romero C, Calamia V, Mateos J, et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2009;8(1):172–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terkeltaub R, Johnson K, Murphy A, Ghosh S. Invited review: the mitochondrion in osteoarthritis. Mitochondrion. 2002;1(4):301–319. doi: 10.1016/s1567-7249(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 115.Yasuhara R, Miyamoto Y, Akaike T, et al. Interleukin-1β induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389(Pt 2):315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 117▪.Johnson K, Svensson CI, Etten DV, et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. 2004;50(4):1216–1225. doi: 10.1002/art.20149. Reviews the changes in oxidation, aging and chronic inflammation that lead to cartilage degradation in OA. [DOI] [PubMed] [Google Scholar]
- 118.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 119.Henrotin Y, Blanco FJ, Aigner T, Kurz B. The significance of oxidative stress in articular cartilage ageing and degradation. Curr Rheumatol Rev. 2007;3(4):261–274. [Google Scholar]
- 120.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74(4):324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7(2):R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajpurohit R, Mansfield K, Ohyama K, Ewert D, Shapiro IM. Chondrocyte death is linked to development of a mitochondrial membrane permeability transition in the growth plate. J Cell Physiol. 1999;179(3):287–296. doi: 10.1002/(SICI)1097-4652(199906)179:3<287::AID-JCP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 123.Pucci B, Adams CS, Fertala J, et al. Development of the terminally differentiated state sensitizes epiphyseal chondrocytes to apoptosis through caspase-3 activation. J Cell Physiol. 2007;210(3):609–615. doi: 10.1002/jcp.20857. [DOI] [PubMed] [Google Scholar]
- 124▪▪.Mansfield K, Teixeira CC, Adams CS, Shapiro IM. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone. 2001;28(1):1–8. doi: 10.1016/s8756-3282(00)00409-9. Dysregulation of p53 tumor suppressor gene leads to senescence and premature aging. [DOI] [PubMed] [Google Scholar]
- 125.Teixeira CC, Mansfield K, Hertkorn C, Ischiropoulos H, Shapiro IM. Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am J Physiol Cell Physiol. 2001;281(3):C833–839. doi: 10.1152/ajpcell.2001.281.3.C833. [DOI] [PubMed] [Google Scholar]
- 126.Varela I, Cadinanos J, Pendas AM, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437(7058):564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- 127.Ohyama K, Chung CH, Chen E, et al. p53 influences mice skeletal development. J Craniofac Genet Dev Biol. 1997;17(4):161–171. [PubMed] [Google Scholar]
- 128.Yatsugi N, Tsukazaki T, Osaki M, Koji T, Yamashita S, Shindo H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J Orthop Sci. 2000;5(2):150–156. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 129.Islam N, Haqqi TM, Jepsen KJ, et al. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-α, inducible nitric oxide synthase, p53, c-myc, and bax-α, and suppression of Bcl-2. J Cell Biochem. 2002;87(3):266–278. doi: 10.1002/jcb.10317. [DOI] [PubMed] [Google Scholar]
- 130.Todd Allen R, Robertson CM, Harwood FL, et al. Characterization of mature vs aged rabbit articular cartilage: analysis of cell density, apoptosis-related gene expression and mechanisms controlling chondrocyte apoptosis. Osteoarthritis Cartilage. 2004;12(11):917–923. doi: 10.1016/j.joca.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 131.Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFκB-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277(36):33501–33508. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Toury R, Hauchecorne M, Balmain N. Expression and subcellular localization of the Myc superfamily proteins: c-Myc, Max, Mad1 and Mxi1 in the epiphyseal plate cartilage chondrocytes of growing rats. Cell Mol Biol (Noisy-le-grand) 1997;43(2):175–188. [PubMed] [Google Scholar]
- 133.Farquharson C, Hesketh JE, Loveridge N. The proto-oncogene c-myc is involved in cell differentiation as well as cell proliferation: studies on growth plate chondrocytes in situ. J Cell Physiol. 1992;152(1):135–144. doi: 10.1002/jcp.1041520118. [DOI] [PubMed] [Google Scholar]
- 134.Asai A, Miyagi Y, Sugiyama A, et al. The s-Myc protein having the ability to induce apoptosis is selectively expressed in rat embryo chondrocytes. Oncogene. 1994;9(8):2345–2352. [PubMed] [Google Scholar]
- 135▪▪.Pelletier JP, Faure MP, DiBattista JA, Wilhelm S, Visco D, Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993;142(1):95–105. Demonstrates increased cell death via apoptosis due to age-related decreases in signaling involving β-catenin, Wnt and HMGB2. [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu M, Chen M, Zuscik M, et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58(7):2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taniguchi N, Carames B, Kawakami Y, Amendt BA, Komiya S, Lotz M. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via β-catenin pathway. Proc Natl Acad Sci USA. 2009;106(39):16817–16822. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44(4):654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14(4):536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 140.Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280(21):20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 141.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 142.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 143.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 144.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43(7):1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 145.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-β signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7(6):R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guerne PA, Blanco F, Kaelin A, Desgeorges A, Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38(7):960–968. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- 147.van der Kraan PM, van den Berg WB. Osteoarthritis in the context of ageing and evolution. Loss of chondrocyte differentiation block during ageing. Ageing Res Rev. 2008;7(2):106–113. doi: 10.1016/j.arr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 148.Dabovic B, Chen Y, Colarossi C, et al. Bone abnormalities in latent TGF-β binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-β bioavailability. J Cell Biol. 2002;156(2):227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li TF, Darowish M, Zuscik MJ, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serra R, Johnson M, Filvaroff EH, et al. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feng L, Precht P, Balakir R, Horton WE., Jr Evidence of a direct role for Bcl-2 in the regulation of articular chondrocyte apoptosis under the conditions of serum withdrawal and retinoic acid treatment. J Cell Biochem. 1998;71(2):302–309. [PubMed] [Google Scholar]
- 152.Notoya K, Jovanovic DV, Reboul P, Martel-Pelletier J, Mineau F, Pelletier JP. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000;165(6):3402–3410. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- 153.Erlacher L, Maier R, Ullrich R, et al. Differential expression of the protooncogene Bcl-2 in normal and osteoarthritic human articular cartilage. J Rheumatol. 1995;22(5):926–931. [PubMed] [Google Scholar]
- 154.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27(2):455–462. [PubMed] [Google Scholar]
- 155.Kinkel MD, Yagi R, McBurney D, Nugent A, Horton WE., Jr Age-related expression patterns of Bag-1 and Bcl-2 in growth plate and articular chondrocytes. Anat Rec A Discov Mol Cell Evol Biol. 2004;279(2):720–728. doi: 10.1002/ar.a.20063. [DOI] [PubMed] [Google Scholar]
- 156.Shi Y. A structural view of mitochondria-mediated apoptosis. Nat Struct Biol. 2001;8(5):394–401. doi: 10.1038/87548. [DOI] [PubMed] [Google Scholar]
- 157.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29(9):486–494. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 158.Masuko-Hongo K, Sakata M, Yuan GH, et al. Expression of Fas-associated death domain-like interleukin-1β-converting enzyme (FLICE) inhibitory protein (FLIP) in human articular chondrocytes: possible contribution to the resistance to Fas-mediated death of in vitro cultured human articular chondrocytes. Rheumatol Int. 2001;21(3):112–121. doi: 10.1007/s00296-001-0144-0. [DOI] [PubMed] [Google Scholar]
- 159.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 160▪.Doi H, Nishida K, Yorimitsu M, et al. Interleukin-4 downregulates the cyclic tensile stress-induced matrix metalloproteinases-13 and cathepsin B expression by rat normal chondrocytes. Acta Med Okayama. 2008;62(2):119–126. doi: 10.18926/AMO/30956. Demonstrates a chondroprotective effect of IL-4 intra-articular injections in a rat model following mechanical stress. [DOI] [PubMed] [Google Scholar]
- 161.Yorimitsu M, Nishida K, Shimizu A, et al. Intra-articular injection of interleukin-4 decreases nitric oxide production by chondrocytes and ameliorates subsequent destruction of cartilage in instability-induced osteoarthritis in rat knee joints. Osteoarthritis Cartilage. 2008;16(7):764–771. doi: 10.1016/j.joca.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 162.Lee SW, Song YS, Shin SH, et al. Cilostazol protects rat chondrocytes against nitric oxide-induced apoptosis in vitro and prevents cartilage destruction in a rat model of osteoarthritis. Arthritis Rheum. 2008;58(3):790–800. doi: 10.1002/art.23220. [DOI] [PubMed] [Google Scholar]
- 163.Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250(4):418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 164.Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol. 2000;27(4):1005–1019. [PubMed] [Google Scholar]
- 165.Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44(6):1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 166.Mitrovic D, Quintero M, Stankovic A, Ryckewaert A. Cell density of adult human femoral condylar articular cartilage. Joints with normal and fibrillated surfaces. Lab Invest. 1983;49(3):309–316. [PubMed] [Google Scholar]
- 167.Grogan SP, Aklin B, Frenz M, Brunner T, Schaffner T, Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198(1):5–13. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 168.Hashimoto S, Ochs RL, Rosen F, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci USA. 1998;95(6):3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 170.Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci USA. 2009;106(4):1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59(4):481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 173.Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee – the MOVE consensus. Rheumatology (Oxford) 2005;44(1):67–73. doi: 10.1093/rheumatology/keh399. [DOI] [PubMed] [Google Scholar]
- 174.Zhang W, Doherty M, Peat G, et al. EULAR evidence based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2009;69(3):483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 175.Daniel PT. Dissecting the pathways to death. Leukemia. 2000;14(12):2035–2044. doi: 10.1038/sj.leu.2401940. [DOI] [PubMed] [Google Scholar]
- 176.Baskin-Bey ES, Washburn K, Feng S, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7(1):218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 177.Kreuter M, Langer C, Kerkhoff C, et al. Stroke, myocardial infarction, acute and chronic inflammatory diseases: caspases and other apoptotic molecules as targets for drug development. Arch Immunol Ther Exp (Warsz) 2004;52(3):141–155. [PubMed] [Google Scholar]
- 178.Henrotin Y, Kurz B. Antioxidant to treat osteoarthritis: dream or reality? Curr Drug Targets. 2007;8(2):347–357. doi: 10.2174/138945007779940151. [DOI] [PubMed] [Google Scholar]
- 179.Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared with joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515–521. doi: 10.1016/j.joca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 180.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26(1):139–145. [PubMed] [Google Scholar]
- 181.Fosang AJ, Little CB. Drug insight: aggrecanases as therapeutic targets for osteoarthritis. Nat Clin Pract Rheumatol. 2008;4(8):420–427. doi: 10.1038/ncprheum0841. [DOI] [PubMed] [Google Scholar]
- 182.Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36(6):1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 183.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4(3):128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 184.Otsuki S, Taniguchi N, Grogan SP, D’Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10(3):R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 186.Vinatier C, Bouffi C, Merceron C, et al. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 2009;4(4):318–329. doi: 10.2174/157488809789649205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hettrich CM, Crawford D, Rodeo SA. Cartilage repair: third-generation cell-based technologies – basic science, surgical techniques, clinical outcomes. Sports Med Arthrosc. 2008;16(4):230–235. doi: 10.1097/JSA.0b013e31818cdc98. [DOI] [PubMed] [Google Scholar]
- 188.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 189.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 190.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hiraoka K, Grogan S, Olee T, Lotz M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology. 2006;43(3–4):447–454. [PubMed] [Google Scholar]
- 192.Karlsson C, Thornemo M, Henriksson HB, Lindahl A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat. 2009;215(3):355–363. doi: 10.1111/j.1469-7580.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koelling S, Kruegel J, Irmer M, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 194.Kruegel J, Miosge N, Koelling S. The role of progenitor cells in osteoarthritis. Curr Rheumatol Rev. 2008;4(3):210–213. [Google Scholar]
- 195.Otsuki S, Grogan SP, Miyaki S, Kinoshita M, Asahara H, Lotz MK. Tissue neogenesis and STRO-1 expression in immature and mature articular cartilage. J Orthop Res. 2010;28(1):96–102. doi: 10.1002/jor.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ustunel I, Ozenci AM, Sahin Z, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008;110(5):397–407. doi: 10.1016/j.acthis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 197.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157(3):455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 199.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4(8):592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]