Abstract
Objective. In clinical trials of RA patients on traditional DMARDs, the addition of TNF-α antagonists increased infections compared with addition of placebo. Our objective was to compare serious infections following initiation of different RA regimens. Prior comparative studies of DMARD initiation have yielded conflicting results.
Methods. We estimated hospitalization rates for infections following initiation of TNF-α antagonists, other DMARDs and oral glucocorticoids in Tennessee Medicaid-enrolled RA patients (1995–2005). Exposure time was measured using pharmacy information and infections were identified using validated definitions. Initiation of RA regimens was compared using Cox regression models with MTX as the reference. Sensitivity analyses excluded glucocorticoid users, applied a first exposure carried forward approach, restricted observations to 2002–05 and first episodes of use and explored effects of unmeasured confounders.
Results. We identified 28 906 new episodes of medication use, including TNF-α antagonists (8%), MTX alone (15%) and glucocorticoids alone (57%). Compared with MTX initiation, TNF-α antagonist initiation did not significantly increase the risk of hospitalizations for pneumonia [adjusted hazard ratio (aHR) 1.61; 95% CI 0.85, 3.03] or any infection (aHR 1.31; 95% CI 0.78, 2.19). Initiation of LEF, SSZ or HCQ did not increase serious infections, compared with MTX. Both initiation and concurrent glucocorticoid use were associated with a dose-dependent increase in serious infections. Sensitivity analyses showed consistent results.
Conclusions. Compared with initiation of MTX alone, initiation of TNF-α antagonists was not associated with a large increase in the risk of serious infections. Glucocorticoid use was associated with a dose-dependent increase in the risk of these infections.
Keywords: Rheumatoid arthritis, Biologic therapies, Epidemiology, Infections
Introduction
Although the introduction of TNF-α antagonists revolutionized the treatment of RA, concerns about the safety of these medications remain. Serious infections have been reported among users of TNF-α antagonists. Pooled data from nine randomized clinical trials of either adalimumab or infliximab found that the odds of serious infections were two times higher among RA patients randomized to TNF-α antagonists than among those randomized to placebo or MTX alone [1].
The use of TNF-α antagonists has been associated with relatively rare systemic opportunistic infections and tuberculosis [2–4]. However, the association of these medications with more common serious infections, such as pneumonia, remains debatable [5–10].
Safety information from clinical trials is limited because few trials had sufficient power to assess safety outcomes conclusively. Moreover, the selected populations participating in the trials warrant caution in extrapolation of safety results. Although placebo-controlled trials are widely used to study medications efficacy, placebo is not the most clinically applicable comparator when making decisions about treatment for RA. Nevertheless, few trials have provided safety data comparing initiation of TNF-α antagonists with initiation of other DMARDs [11–13].
Several randomized controlled trials of TNF-α antagonists added either placebo or TNF-α antagonists to ongoing MTX regimens [14–16]. Thus, initiators of TNF-α antagonists were compared with placebo initiators among prevalent users of MTX. Patients enrolled in these trials had disease insufficiently controlled with their current MTX regimen. In administrative databases without detailed clinical information, patients whose disease is poorly controlled are best identified by the initiation of a new therapeutic regimen. Moreover, the study of initiators (i.e. ‘new users’) is of interest because it avoids known selection bias in observational studies [17]. Hence, similar to the design of three other clinical trials [10–12], we compared the initiation of TNF-α antagonists with the initiation of MTX or other DMARDs on the risk of serious infections among RA patients enrolled in TennCare, the managed-care Medicaid programme in Tennessee.
Methods
TennCare provides health-care insurance to those who are Medicaid eligible and those who are uninsured or lack other access to health care. We assembled a retrospective cohort of RA patients, who were identified with one or more RA-coded (ICD9-CM: 714.**, except Juvenile Rheumatoid Arthritis [714.3*]) health-care encounters and a prescription filled for a DMARD. RA was also defined by two or more RA-coded encounters (⩾30 days apart) and an oral glucocorticoid prescription filled [18].
The cohort was restricted to RA patients with new episodes of DMARD use, which should reduce bias related to the inclusion of prevalent users in the study of medication effects [17]. A new episode of DMARD use started when an RA patient filled a prescription for a DMARD or glucocorticoid (t0) from 1995 to 2005, and had no prescription filled for the medication of interest during the 180 days preceding the fill date (baseline). As of t0, cohort members were aged ⩾18 years, had at least 180 days of continuous enrolment in TennCare and had filled one or more prescriptions for any medication during baseline (to assure active use of pharmacy benefits and active medical surveillance).
Since some medical conditions could reduce follow-up and/or increase the risk of infections regardless of medication exposure, we excluded patients with solid organ transplantation, HIV/AIDS, cancer and serious kidney, liver or respiratory diseases, identified at baseline. We also identified and excluded patients who had two or more health-care encounters (⩾30 days apart) coded for JRA, SLE, Crohn's disease or ulcerative colitis during baseline (although some of these patients would receive DMARDs, their risk of infections could be different from RA patients).
New episodes of use began on t0 and continued through the earliest of the following dates: death, loss of enrolment, study outcome or 180th day of follow-up. We restricted the follow-up to 180 days because previous research suggested an increased risk of infections during the first months of use of TNF-α antagonists [5, 8, 10]. Moreover, a previous study in this population indicated short persistence on initial DMARD regimens [19], making the long-term classification of exposure person-time problematic. This strategy allowed the isolation of follow-up time after initiation of DMARD use and the assessment of study outcomes and medication adherence. Patients who left the cohort could subsequently re-enter and contribute new episodes of medication use if they fulfilled selection criteria.
Exposures
Study DMARDs included TNF-α antagonists (etanercept, infliximab and adalimumab), LEF, SSZ, HCQ and MTX. New episodes of DMARD use were identified applying a hierarchical algorithm to maximize the identification of newer and less frequently used study DMARDs in TennCare. This hierarchy was: TNF-α antagonists, LEF, SSZ, HCQ and MTX [20]. We also identified new episodes of use of oral glucocorticoids. This last group was stratified according to the estimated average daily dose of prednisone equivalents that the patient was initiating [⩽7.5 (low), 7.5–30 (medium) and >30 mg (high)] [21, 22]. For each new episode of use, the 180 person-days following initiation were included as part of a defined follow-up time. Once identified, these person-days could not be included as part of new use of another medication group.
To calculate person-time exposed to a study medication, we aggregated the person-time from t0 through the earliest of: end of the episode, death, loss of enrolment, occurrence of a study outcome, switch to another DMARD regimen or the discontinuation of the current regimen (defined as 14 days without medication). This approach reduced the potential misclassification introduced by concurrent use of other non-study DMARDs and allowed a short gap in which outcomes identified after drug supply exhaustion could be related to the most recent exposure. The TNF-α antagonist group allowed the concurrent use (continuation or addition) of MTX; all other exposure episodes ended with the addition of another DMARD [6, 8, 20]. Concurrent use of glucocorticoids was allowed among initiators of DMARDs, but initiation of DMARDs ended a glucocorticoid episode.
Outcomes
Study outcomes were serious infections that required hospitalization [5, 6, 23]. These infections were identified using computerized definitions based on principal discharge diagnoses [6, 24, 25]; and pneumonia, the most common serious infection in our cohort, was assessed separately; whereas, due to small number of events, all serious infections were aggregated into a composite outcome. Based on medical-chart reviews, computerized definitions for serious infections showed high positive predictive value in identifying study outcomes among TennCare RA patients [24]. The identification of systemic opportunistic infections is challenging in administrative databases and those rare infections were not included in this study.
Potential confounders
To control for potential confounders, we measured covariates during baseline including demographics: age, gender, race, residence (urban, suburban and rural), nursing home/community dwelling and calendar year; generic markers of comorbidity: number of hospitalizations, outpatient and emergency-room visits, enrolment in TennCare based on disability, number of different medication classes filled; surrogate markers of disease severity: extra-articular manifestations of disease, number of IA and orthopaedic procedures, number of laboratory tests ordered for inflammatory markers and days of drug supply for other DMARDs, oral glucocorticoids, NSAIDs and narcotics [5, 6, 18, 20, 26]; and risk factors for infections: previous hospitalization due to infection, chronic obstructive pulmonary disease (COPD), diabetes and previous use of antibiotics [6]. Among DMARD initiators, the average daily dose of oral glucocorticoids at t0 was categorized as described above.
Statistical analysis
Since MTX is considered to be the cornerstone of RA treatment and was the most prevalent DMARD used [20], initiation of MTX served as the reference for all comparisons [27, 28]. Cox proportional hazard regression models assessed the association between medication exposure and outcomes. Person-time of continuous exposure (including <14-day gaps) represented time at risk. Since patients could contribute one or more episodes of new use (with an updated set of covariates), we accounted for this clustering of observations using patient's study numbers to define clusters and accounted for this additional intra-group correlation using the Huber–White ‘sandwich’ variance estimator and calculated robust s.e. for all estimates [29]. The proportional hazard assumption was verified using generalized linear regressions of Schoenfeld residuals on functions of time [30].
Because of limited number of outcomes by exposure group, we summarized the distribution of covariates using propensity scores. A multinomial logistic regression model was fitted to estimate the probability of initiating use of each study medication regimen using MTX as the reference [31]. The visual inspection of the distribution of predicted probabilities across exposure groups indicated appropriate overlap. Calendar year and the average daily dose of glucocorticoids among DMARD initiators were not included in the propensity score model, but were added to the final outcome models to assess their effects independently.
Specific measurements of RA disease severity were not available in our data. Although our strategies accounted for constructs that correlated with RA disease severity, residual confounding could persist. Hence, we explored the potential effect of an unmeasured confounder, using an array-based sensitivity analysis [32, 33]. All analyses were done in Stata 10.1, and this study was approved by the Vanderbilt University IRB and by the Bureau of TennCare.
Results
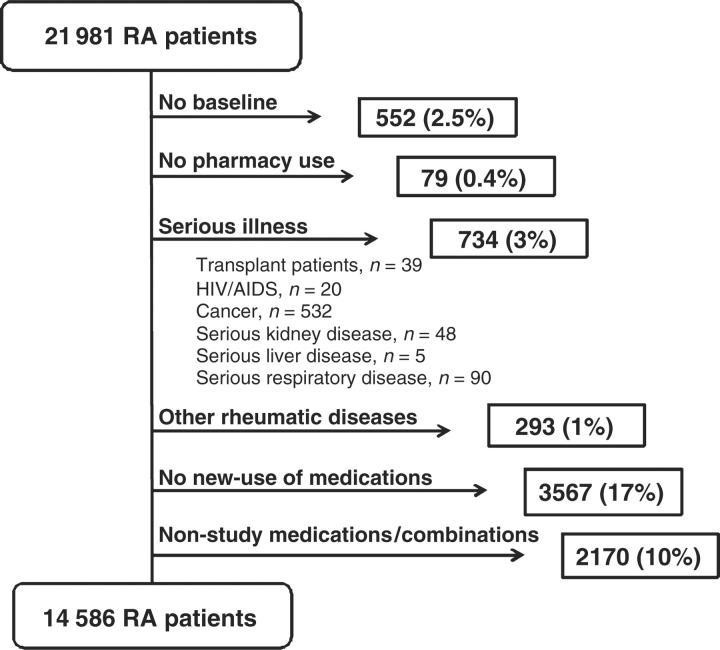
There were 21 981 TennCare enrollees who met our RA definition. After application of selection criteria, our cohort encompassed 14 586 (66%) RA patients (Fig. 1). These RA patients contributed 28 906 new episodes of medication use, including TNF-α antagonists with or without MTX (8%), LEF (4%), SSZ (4%), HCQ (12%), MTX (15%) and glucocorticoids (57%).
Fig. 1.
Selection criteria and cohort assembly. RA TennCare cohort, 1995–2005.
Initiators of TNF-α antagonists had more orthopaedic procedures, inflammatory markers assessed and joint aspirations performed during baseline than other groups. Moreover, the baseline use of DMARDs, NSAIDs and narcotics was consistently higher among initiators of TNF-α antagonists, suggesting higher disease severity, compared with other groups. Initiators of TNF-α antagonists were also more likely to be enrolled in a TennCare disability category and to be urban residents than initiators of other regimens (Table 1).
Table 1.
Characteristics of RA patients initiating new episodes of medication use, RA TennCare cohort, 1995–2005
| TNF-α antagonists | LEF | SSZ | HCQ | MTX | GC (low dose) | GC (medium dose) | GC (high dose) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| New episodes of use, n | 2192 | 1097 | 1283 | 3398 | 4355 | 2058 | 11 117 | 3406 | |
| Age, median (IQR), years | 54 (45–62) | 56 (48–64) | 52 (42–61) | 52 (43–62) | 55 (45–64) | 57 (47–67) | 55 (45–65) | 55 (45–64) | <0.001 |
| Female | 74.86 | 77.03 | 68.04 | 81.11 | 76.21 | 77.75 | 76.38 | 75.60 | <0.001 |
| Race | <0.001 | ||||||||
| White | 83.90 | 84.32 | 84.88 | 79.64 | 80.60 | 80.47 | 84.72 | 84.44 | |
| Black | 12.73 | 13.49 | 12.70 | 18.01 | 16.67 | 17.15 | 13.28 | 13.30 | |
| Other | 3.38 | 2.19 | 2.42 | 2.35 | 2.73 | 2.38 | 2.01 | 2.26 | |
| Residence | <0.001 | ||||||||
| Rural | 47.40 | 50.41 | 54.25 | 52.77 | 51.76 | 59.52 | 59.64 | 55.23 | |
| Sub-urban | 27.14 | 25.80 | 23.54 | 24.51 | 26.80 | 19.97 | 22.39 | 24.05 | |
| Urban | 25.46 | 23.79 | 22.21 | 22.72 | 21.45 | 20.51 | 17.97 | 20.73 | |
| Disability | 69.75 | 67.37 | 61.26 | 58.92 | 60.16 | 62.15 | 64.08 | 65.27 | <0.001 |
| Nursing home resident | 0.64 | 0.82 | 0.62 | 1.12 | 1.65 | 3.50 | 1.91 | 1.88 | <0.001 |
| Markers of disease activity | |||||||||
| RA visit in baseline | 87.18 | 88.61 | 71.78 | 63.39 | 70.47 | 41.11 | 36.20 | 33.35 | <0.001 |
| Extra articular disease | 1.23 | 3.01 | 1.56 | 2.24 | 1.19 | 0.92 | 0.88 | 1.06 | <0.001 |
| Orthopaedic surgeries | 26.55 | 25.34 | 23.30 | 20.42 | 20.94 | 20.31 | 18.71 | 18.32 | <0.001 |
| Inflammatory markers | 42.11 | 34.28 | 33.20 | 37.26 | 32.61 | 17.15 | 14.99 | 13.83 | <0.001 |
| Joint aspirations | 21.44 | 20.42 | 19.02 | 15.92 | 16.74 | 15.74 | 13.73 | 13.12 | <0.001 |
| Concurrent GC use on t0 | <0.001 | ||||||||
| ⩽7.5 mg/day | 70.26 | 67.91 | 74.67 | 77.02 | 70.31 | – | – | – | |
| 7.5–30 mg/day | 25.36 | 28.08 | 21.67 | 19.39 | 24.52 | – | – | – | |
| >30 mg/day | 4.38 | 4.01 | 3.66 | 3.59 | 5.17 | – | – | – | |
| No. of inj. GC | <0.001 | ||||||||
| 0 | 77.83 | 83.68 | 83.40 | 81.61 | 80.53 | 78.43 | 81.51 | 82.41 | |
| 1 | 8.67 | 6.93 | 8.42 | 8.59 | 8.01 | 6.46 | 8.30 | 7.57 | |
| ⩾2 | 13.50 | 9.39 | 8.18 | 9.80 | 11.46 | 15.11 | 10.19 | 10.01 | |
| No. of days on DMARDs, median (IQR) | 160 (56–180) | 101 (0–180) | 0 (0–97) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | <0.001 |
| No. of days on NSAIDs, median (IQR) | 59 (0–162) | 52 (0–155) | 37 (0–128) | 30 (0–118) | 39 (0–120) | 30 (0–136) | 30 (0–125) | 30 (0–125) | <0.001 |
| No. of days on narcotics, median (IQR) | 78 (7–180) | 44 (0–142) | 15 (0–99) | 19 (0–105) | 22 (0–97) | 30 (0–157) | 30 (0–141) | 37 (0–153) | <0.001 |
| Other risk factors for infections | |||||||||
| Diabetes | 12.86 | 11.21 | 10.37 | 10.92 | 12.33 | 11.37 | 10.07 | 10.80 | <0.001 |
| Infection | 5.20 | 5.56 | 3.27 | 4.91 | 5.30 | 6.22 | 6.29 | 5.96 | <0.001 |
| No. of days on antibiotics, median (IQR) | 25 (5–104) | 30 (5–107) | 16 (0–56) | 10 (0–30) | 10 (0–38) | 16 (0–51) | 17 (1–47) | 16 (2–45) | <0.001 |
| Smoking-related disease | 7.62 | 7.38 | 6.00 | 5.50 | 6.22 | 7.34 | 7.48 | 9.45 | <0.001 |
| COPD | 9.67 | 11.85 | 9.98 | 10.18 | 10.75 | 14.63 | 16.48 | 19.64 | <0.001 |
| Health-care utilization | |||||||||
| Baseline hospitalizations | <0.001 | ||||||||
| 0 | 81.25 | 79.31 | 82.00 | 80.40 | 78.92 | 76.87 | 76.84 | 75.69 | |
| 1 | 13.37 | 14.13 | 13.17 | 13.39 | 14.26 | 14.87 | 15.52 | 16.24 | |
| ⩾2 | 5.38 | 6.56 | 4.83 | 6.21 | 6.82 | 8.26 | 7.65 | 8.07 | |
| Baseline ED visits | <0.001 | ||||||||
| 0 | 69.75 | 68.55 | 68.28 | 63.48 | 65.26 | 61.71 | 57.61 | 53.02 | |
| 1 | 19.16 | 18.69 | 18.39 | 20.48 | 18.81 | 20.60 | 22.38 | 24.16 | |
| 2 | 5.38 | 6.47 | 5.77 | 7.89 | 7.51 | 7.29 | 9.21 | 10.13 | |
| ⩾3 | 5.70 | 6.29 | 7.56 | 8.15 | 8.43 | 10.40 | 10.80 | 12.68 | |
| No. of different drugs, median (IQR) | 14 (10–20) | 13 (9–18) | 11 (7–16) | 12 (7–17) | 11 (7–17) | 13 (8–19) | 13 (8–19) | 14 (9–20) | <0.001 |
| No. of outpatient visits, median (IQR) | 6 (4–9) | 6 (3–9) | 5 (3–8) | 5 (3–8) | 5 (3–8) | 5 (2–8) | 5 (2–8) | 5 (2–8) | <0.001 |
Values indicate percentages unless otherwise specified. GC: glucocorticoid (low dose: <7.5 mg; medium dose: 7.5–30 mg; high dose: >30 mg of prednisone equivalents per day); ED: emergency department; IQR: interquartile range.
Initiators of LEF, MTX or glucocorticoids were older than other groups. Initiators of MTX or glucocorticoids were also more likely to be nursing-home residents, and had more hospitalizations and emergency department visits, suggesting more frailty than other groups. Patients initiating MTX were the most likely of all groups to be using high doses of glucocorticoids at t0. Initiators of glucocorticoid regimens were more likely to have a history of smoking-related diseases, COPD, a previous infection hospitalization and to be residents of rural areas. Initiators of glucocorticoids had less extra-articular disease, orthopaedic surgeries, assessments of inflammatory markers or joint aspirations than other groups. By definition, glucocorticoid initiators were non-users of these drugs during baseline (Table 1).
Pneumonia hospitalizations
There were 3842 person-years of follow-up and 192 pneumonia hospitalizations, yielding five pneumonia hospitalizations per 100 person-years. Compared with initiators of MTX, the risk of pneumonia hospitalizations was not significantly increased among initiators of TNF-α antagonists [adjusted hazard ratio (aHR) 1.61; 95% CI 0.85, 3.03]. Similarly, the risk of pneumonia was not increased among initiators of LEF, SSZ or HCQ compared with MTX. However, the risk of pneumonia hospitalizations was consistently increased with initiation of glucocorticoids (aHR 2.30, 2.36 and 4.33 for low, medium and high doses, respectively) (Table 2). In addition, baseline use of medium and high doses of glucocorticoids were associated with increased pneumonia risk compared with low doses or no use (aHR 1.92; 95% CI 1.25, 2.95 and aHR 3.56; 95% CI 1.85, 6.85 for medium and high doses, respectively).
Table 2.
HRs for pneumonia and any infection requiring hospitalization, RA TennCare cohort (1995–2005)
| Number of episodes | Time, median (IQR), days* | Events | Crude HR (95% CI) | Age, gender- adjusted HR (95% CI) | PS-adjusted HRa (95% CI) | |
|---|---|---|---|---|---|---|
| Pneumonia | ||||||
| MTX | 4355 | 60 (41–133) | 32 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| TNF-α antagonists | 2192 | 68 (27–168) | 19 | 1.17 (0.67, 2.05) | 1.28 (0.73, 2.24) | 1.61 (0.85, 3.03) |
| LEF | 1097 | 43 (20–114) | 11 | 1.64 (0.79, 3.39) | 1.64 (0.79, 3.39) | 1.65 (0.77, 3.54) |
| SSZ | 1283 | 43 (28–51) | 3 | 0.47 (0.14, 1.53) | 0.51 (0.16, 1.68) | 0.60 (0.19, 1.97) |
| HCQ | 3398 | 43 (43–120) | 27 | 1.11 (0.67, 1.85) | 1.20 (0.72, 2) | 1.24 (0.73, 2.08) |
| Glucocorticoids (low dose)b | 2058 | 34 (23–43) | 17 | 2.00 (1.1, 3.64) | 1.71 (0.94, 3.1) | 2.30 (1.2, 4.41) |
| Glucocorticoids (medium dose) | 11 117 | 19 (19–23) | 53 | 2.08 (1.31, 3.3) | 1.92 (1.22, 3.01) | 2.36 (1.44, 3.87) |
| Glucocorticoids (high dose) | 3406 | 19 (18–25) | 30 | 3.91 (2.31, 6.62) | 3.67 (2.19, 6.15) | 4.33 (2.49, 7.54) |
| Any infection | ||||||
| MTX | 4355 | 60 (41–133) | 55 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| TNF-α antagonists | 2192 | 67 (27–167) | 29 | 1.04 (0.66, 1.64) | 1.12 (0.71, 1.77) | 1.31 (0.78, 2.19) |
| LEF | 1097 | 43 (20–114) | 16 | 1.39 (0.78, 2.48) | 1.39 (0.78, 2.48) | 1.48 (0.81, 2.69) |
| SSZ | 1283 | 43 (28–51) | 9 | 0.82 (0.4, 1.66) | 0.89 (0.44, 1.8) | 1.03 (0.51, 2.1) |
| HCQ | 3398 | 43 (43–119) | 44 | 1.06 (0.71, 1.56) | 1.13 (0.76, 1.67) | 1.20 (0.81, 1.79) |
| Glucocorticoids (low dose)b | 2058 | 34 (23–43) | 21 | 1.46 (0.88, 2.43) | 1.28 (0.77, 2.13) | 1.62 (0.94, 2.78) |
| Glucocorticoids (medium dose) | 11 117 | 19 (19–23) | 90 | 2.12 (1.47, 3.05) | 1.98 (1.38, 2.83) | 2.39 (1.63, 3.51) |
| Glucocorticoids (high dose) | 3406 | 19 (18–25) | 43 | 3.37 (2.19, 5.19) | 3.21 (2.1, 4.9) | 3.72 (2.37, 5.84) |
aPS-adjusted HRs accounted for all study covariates. bLow dose: <7.5 mg; medium dose: 7.5–30 mg; high dose: >30 mg of prednisone equivalents per day. PS: propensity score; IQR: interquartile range.
Serious infections hospitalizations
For the serious infections composite outcome, there were 3831 person-years of follow-up and 307 hospitalizations yielding eight serious infection-related hospitalizations per 100 person-years. Serious infections included 192 (63%) cases of pneumonia, 27 (9%) sepsis/septicaemia, 44 (14%) pyelonephritis, 37 (13%) cellulitis, 2 (1%) septic arthritis, 1 (0.3%) endocarditis, 1 (0.3%) meningitis and 3 (1%) osteomyelitis.
Hospitalizations due to serious infections were not significantly increased among initiators of TNF-α antagonists (aHR 1.31; 95% CI 0.78, 2.19), compared with initiation of MTX. Initiation of LEF, SSZ and HCQ did not increase serious infections, compared with MTX. Initiation of oral glucocorticoids was consistently associated with an increased risk of serious infections (aHR 1.62, 2.39 and 3.72 for low, medium and high doses, respectively). Furthermore, baseline use of medium and high doses of glucocorticoids were associated with increased serious infections risk compared with low doses or no use (aHR 1.78; 95% CI 1.26, 2.52 and aHR 3.72; 95% CI 2.26, 6.13, respectively).
Sensitivity analyses
Our findings were robust to a number of planned sensitivity analyses. After exclusion of patients initiating glucocorticoids with prescriptions of <30 days supply (14 565 episodes), the association of glucocorticoid initiation and the risk of serious infections remained (e.g. aHR 1.59; 95% CI 0.82, 3.10; aHR 2.23; 95% CI 1.31, 3.80; and aHR 1.69; 95% CI 0.51, 5.57 for low, medium and high doses, respectively). Furthermore, the daily dose of glucocorticoid used at the time of DMARD initiation was consistently associated with the study outcomes in all analyses and after exclusion of all glucocorticoid exposure groups from the analyses. Restricting analyses to episodes that started in 2002–05 (14 672 episodes excluded) showed similar patterns. Restricting the analyses to the first episode per patient (14 320 episodes excluded) and analyses using propensity scores quintiles also yielded similar results. Finally, analyses of drug initiation without regard to subsequent regimen changes (first exposure carried forward) yielded similar conclusions although most HRs were closer to the null (Table 3).
Table 3.
Sensitivity analyses exploring the HR of serious infections associated with initiation of TNF-α antagonists use, RA TennCare cohort, 1995–2005
| Sensitivity analysis | Serious infection, PS-adjusted HRa (95% CI) |
|---|---|
| Main analysis | 1.31 (0.78, 2.19) |
| Excluding GC initiators with <30 days supply | 1.20 (0.68, 2.13) |
| Excluding all GC initiators | 1.39 (0.79, 2.44) |
| Restricted to 2002–2005 | 1.33 (0.69, 2.56) |
| Restricted to first episode per subject | 1.23 (0.50, 3.03) |
| Adjustment using PS quintiles | 1.19 (0.71, 1.99) |
| First exposure carried forwardb | 1.15 (0.83, 1.60) |
aAll HRs considered MTX as reference. PS-adjusted HRs accounted for all study covariates. bBased on initiation of medication use only. GC: glucocorticoids; PS: propensity scores.
Since RA disease severity would increase the risk of infections [34] and TNF-α antagonist initiators had more surrogates of disease severity than other groups, our study HRs comparing initiation of TNF-α antagonists with initiation of MTX would overestimate the real HR [6]. Thus, the fully adjusted HR, accounting for RA disease severity, would be closer to the null (for a quantitative sensitivity analysis, see supplementary data available at Rheumatology Online).
Discussion
Our findings indicate that among RA patients enrolled in TennCare, initiation of TNF-α antagonists was not associated with a large increase in the risk of serious infections requiring hospitalization compared with initiation of MTX. However, compared with MTX, the initiation of glucocorticoid regimens increased the risk of serious infections.
Although most randomized clinical trials reported effects of TNF-α antagonists compared with placebo in patients who continue traditional DMARDs, few trials provided information on the risk of serious infections comparing initiation of TNF-α antagonists with initiation of MTX. Available data suggested that infliximab increased the risk of serious infections compared with initiation of MTX [11], whereas initiation of either adalimumab [12] or etanercept [13] did not. A pooled estimate of these three randomized trials comparing initiation of TNF-α antagonists with initiation of MTX yielded an overall risk ratio of 1.48 (95% CI 0.93, 2.35), encompassing the estimates reported in this study.
We considered some of the methodological challenges that could explain differences in results of observational studies in this area [35]. Previous research suggested a time-dependent risk of infections after initiation of TNF-α antagonists [5, 8, 10]. To assure comparability of exposure groups, we applied a new-user design and focused on the period immediately after treatment initiation [17]. We reduced exposure misclassification by using pharmacy data to classify each day of follow-up during the new episodes of medication use. To reduce outcome misclassification, we identified infections using algorithms that had previously shown high positive predictive values in our population [24]. Furthermore, although direct measurements of disease severity were not available, adjustment for measured covariates (including surrogates for disease severity) was performed and the potential role of unmeasured confounders was examined.
In our study, patients initiating TNF-α antagonists had an increased prevalence of surrogates for severe RA, suggesting channeling of patients with severe disease to these medications. However, TNF-α antagonists initiators were younger and had more baseline exposure to DMARDs than MTX initiators, suggesting that TNF-α antagonist initiators were less frail than MTX initiators. Adjustment for these latter factors resulted in increased HRs for TNF-α antagonists initiators. Although residual confounding could not be ruled out, our sensitivity analyses indicated that improving our imperfect adjustment for disease severity would reduce our HR within the confidence intervals of our estimate (see supplementary data available at Rheumatology Online) [6, 35].
Glucocorticoid use increased the risk of serious infections requiring hospitalizations consistently and in a dose-dependent manner, compared with MTX initiation. Although glucocorticoid use could also be a surrogate for severe RA, these associations persisted after adjustment for measured confounders and in a number of sensitivity analyses. Furthermore, these findings are consistent with results from randomized clinical trials and from previous observational studies [5–7, 36].
A retrospective cohort study of 609 RA patients reported 3.1 pneumonia hospitalizations per 100 person-years, but was not restricted to patients exposed to DMARDs [37]. Although our crude pneumonia hospitalization rate was 5/100 person-years, this likely reflects a sicker population of RA patients enrolled in a Medicaid plan and initiating DMARDs or glucocorticoids and observed during the initial months of medication use, when the risk for infections is considered to be the highest [5, 6, 8].
Since several DMARD therapies require months to achieve a satisfactory response, we hypothesized that studying medication effects during a short, defined follow-up time after initiation would maximize the potential for complete persistence. However, both stopping and switching were common shortly after initiation of a new DMARD [19]. We reduced the potential effects of changes in exposure categories by studying new episodes of medication use and by truncating the exposure follow-up when an original study regimen was changed. A sensitivity analysis based on initiation of regimens ignoring subsequent regimen changes showed results consistent with our main findings.
Our study has several limitations. First, although pharmacy files provide excellent information on medications dispensed through TennCare and they are virtually free of information bias [38], the actual use of these medications is unknown. Even though use of medications filled outside the system could not be ruled out, we consider this unlikely because cohort members had full access to TennCare pharmacy benefits and because some medications, such as TNF-α antagonists, are expensive. Secondly, we relied on coded information to identify study outcomes. Misclassification makes it more difficult to demonstrate true associations [5, 24]. However, we minimized outcome misclassification by using computerized definitions that were previously validated in our population [24]. Thirdly, we had insufficient numbers to evaluate the role of specific TNF-α antagonists on serious infections. Furthermore, the relatively short, exposed person-time during episodes of medication use limited our power to detect small increases in the risk of serious infections. Indeed, our findings are also consistent with up to a 2-fold increased risk of serious infections hospitalization. Finally, TennCare enrollees may not be representative of the general population.
In conclusion, we found no increased risk of hospitalizations due to serious infections among initiators of TNF-α antagonists compared with initiators of MTX. Although we could not rule out small increases in risk or differences between TNF-α antagonists, our results were robust to a number of sensitivity analyses and we did not observe significant increases in the risk of infections among other DMARD regimens commonly used in our population. However, our study demonstrated a strong association between glucocorticoid use (especially at high doses) and the risk of serious infections requiring hospitalization among RA patients.

Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
Acknowledgement
We gratefully acknowledge the Tennessee Bureau of TennCare and the Department of Health, which provided the study data.
Funding: This work was supported by the Vanderbilt Multidisciplinary Clinical Research Center (NIH/NIAMS Grant P60 AR056116).
Disclosure statement: M.R.G. has received grant support from Pfizer. All other authors have declared no conflicts of interest.
References
- 1.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J Am Med Assoc. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 2.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit.2008.10.002. Advance Access published December 29, 2008, doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124–34. doi: 10.7326/0003-4819-148-2-200801150-00192. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–33. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 6.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–34. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 8.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896–904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–76. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 10.Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 11.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 12.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The premier study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 13.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 14.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343:1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 15.Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 16.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 18.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–8. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 19.Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF, Jr, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007;45:S66–76. doi: 10.1097/MLR.0b013e318041384c. [DOI] [PubMed] [Google Scholar]
- 20.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology. 2008;47:1061–4. doi: 10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buttgereit F, da Silva JA, Boers M, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61:718–22. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijlsma JW, Boers M, Saag KG, Furst DE. Glucocorticoids in the treatment of early and late RA. Ann Rheum Dis. 2003;62:1033–7. doi: 10.1136/ard.62.11.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doran MF, Crowson CS, Pond GR, O’Fallon M, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 24.Grijalva CG, Chung CP, Stein CM, et al. Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 2008;17:890–5. doi: 10.1002/pds.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Ting G, Schneeweiss S, Katz JN, et al. Performance of a rheumatoid arthritis records-based index of severity. J Rheumatol. 2005;32:1679–87. [PubMed] [Google Scholar]
- 27.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 28.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 30.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 31.Cadarette SM, Katz JN, Brookhart MA, Sturmer T, Stedman MR, Solomon DH. Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med. 2008;148:637–46. doi: 10.7326/0003-4819-148-9-200805060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 33.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 34.Caporali R, Caprioli M, Bobbio-Pallavicini F, Montecucco C. DMARDs and infections in rheumatoid arthritis. Autoimmun Rev. 2008;8:139–43. doi: 10.1016/j.autrev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Solomon DH, Lunt M, Schneeweiss S. The risk of infection associated with tumor necrosis factor alpha antagonists: making sense of epidemiologic evidence. Arthritis Rheum. 2008;58:919–28. doi: 10.1002/art.23396. [DOI] [PubMed] [Google Scholar]
- 36.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 37.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 38.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–49. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.