Abstract
Objectives. RA is a common, relapsing autoimmune disease primarily affecting the joints. Fibroblast-like synovial (FLS) cells are thought to be responsible for pannus formation and secretion of factors that recruit leucocytes to affected joints, thereby promoting bone and cartilage destruction. Fibrocytes are multipotent circulating stem cells that may have a role in RA pathogenesis, perhaps as the precursors of the FLS cells, or by regulating FLS cell function.
Methods. We utilized multidimensional phospho-specific flow cytometry to characterize the activation status of peripheral blood (PB) fibrocytes derived from human RA patients at different stages of disease and from mice with CIA.
Results. Human PB fibrocytes from RA patients exhibited phosporylation activation of the p44/42 and p38 MAP kinases (MAPKs), and STAT3 (signal transducer and activator of transcription) and STAT-5 early in disease, within the first year of diagnosis. Similarly, in murine CIA, an increase in the total number of PB phosphoSTAT5-positive fibrocytes was observed at early time points in disease. Notably, in the affected paws of mice with CIA, we identified an increased number of fibrocytes, in contrast to the paws of control mice.
Conclusions. These data suggest that activated fibrocytes may influence the disease process in RA and may serve as surrogate markers for disease in the PB of affected patients.
Keywords: Rheumatoid arthritis, Peripheral blood, Stem cell, FACS, Collagen-induced arthritis
See page 617 for the editorial comment on this article (doi:10.1093/rheumatology/kep365)
Introduction
RA is a chronic inflammatory disease affecting 0.5–1% of the adult population worldwide [1]. Dramatic changes to the synovial micro-environment occur in RA, specifically, proliferation and transformation of synovial lining cells, pannus accumulation over articular cartilage, bone erosion and the infiltration of inflammatory cells. The aetiology of RA is complex, but breakdown of self-tolerance resulting in T cell-mediated autoimmune responses appears critical [2]. Hyperplastic synovial tissue (ST) supports the chronic inflammatory process, in part by driving continued leucocyte accumulation. Fibroblast-like synovial (FLS) cells synthesize and secrete many pro-inflammatory mediators [3] and, therefore, are critical effectors in regulating the inflammatory response in RA.
FLS cell hyperproliferation has been implicated in pannus formation [3]. However, direct evidence for hyperproliferation is lacking as few mitotic figures are observed and thymidine uptake occurs only in a percentage of cells [4]. While FLS cells do grow in culture, they divide relatively slowly ([5] and personal observation). The outgrowth of FLS cells that contributes to pannus formation may be a consequence of their decreased senescence and/or the fact that they retain telomerase activity [6]. Notably, FLS cells are not immortalized in vitro [6, 7]. The outgrowth of FLS cells in the ST of affected RA joints is, therefore, an enigma, and one possible explanation might be that FLS cells are recruited from the circulation.
Fibrocytes are a unique population of circulating progenitor cells, comprising 0.1–0.5% of the total circulating leucocyte population [8]. In vitro, fibrocytes express markers of both stromal and haematopoietic cells, including fibronectin, collagen, CD11b, CD34 and CD45RO [8, 9]. In vivo evidence indicates that fibrocytes contribute to the myofibroblast population in a murine wound healing model [10]. Fibrocytes secrete extracellular matrix components, thereby enhancing granulation formation. Additionally, fibrocytes express α-smooth muscle actin (α-SMA), are contractile and enhance wound contraction and healing. Notably, variable levels of α-SMA expressing cells are present in RA patients (1–30%) [11]. Fibrocytes function as antigen presenting cells [12] and can secrete chemokines, cytokines and angiogenic factors [8], suggesting that fibrocytes contribute to the inflammatory process. Fibrocytes have been implicated in influencing disease development in tumour biology, scleroderma, asthma and pulmonary fibrosis [8, 13–15]; however, the role of these cells in an autoimmune/inflammatory response, specifically in RA, remains largely ill-defined [16–18].
Recent advances in flow cytometry have expanded the number of parameters available to allow for the simultaneous detection of surface and intracellular epitopes to assess specific subsets of cells and functional activation in heterogeneous cell populations. Phospho-specific flow cytometry (phospho-flow) permits the quantification of phosphorylation levels of intracellular signalling proteins in individual cells, including rare cell populations [19–21]. The method is highly quantitative [20, 22], allowing for a novel network-based screen of complex populations in disease samples [19]. Phospho-flow has been successfully used to identify mutated signalling pathways in leukaemia [23], predict the responsiveness of leukaemic patients to chemotherapy [24] and to assay the effectiveness of drugs in blocking cellular signalling [25]. Here, we describe phospho-flow analysis of peripheral blood (PB) fibrocytes from healthy individuals and from RA patients with early and established disease. The data suggest that a signature phosphorylation profile in RA fibrocytes may be predictive of disease.
Methods
Patients
Informed consent was obtained from all study participants, and ethical approval was granted by the Mount Sinai Hospital, St Michael's Hospital and Sunnybrook and Women's College Health Sciences Centre ethics committee (Toronto, Ontario, Canada). Early RA (ERA) was defined as within the first year following the onset of symptoms with a minimum of three swollen joints. Both ERA and established RA patients were diagnosed according to the ACR 1987 revised criteria [26]. Sample collection involved confirmation of the diagnosis of RA using clinical, serological and radiological data (Tables 1 and 2). The study included a total of 4 patients with ERA, 12 patients with late-stage RA and 10 healthy controls (non-RA and non-OA). ERA patients had a median disease duration of <1 year and 18.8 ± 10.3 years for RA.
Table 1.
Summary of patient demographics and clinical parameters
| Demographics | Late RA, n = 2 | ERA, n = 4 | Late RA n = 10 |
|---|---|---|---|
| Age, mean (s.d.), years | 56.05 (1.03) | 50.25 (11.17) | 57.88 (10.73) |
| Male/female | 0/2 | 0/4 | 2/8 |
| RF+, % | 100 | 0a | 80b |
| Erosions, % | 100 | 25 | 100 |
| HAQ score, mean (s.d.) | 1.63 (0) | 0.72 (0.69) | 1.66 (0.54c) |
| DAS28, mean (s.d.) | 3.95 (1.51) | 4.45 (1.06) | NA |
| MDGA score, mean (s.d.) | 6.5 (0.71) | 4.75 (1.5) | NA |
| Pat Global, mean (s.d.) | 7 (0) | 4.5 (3) | 4.5 (2.69c) |
| Functional class, mean (s.d.) | 3.25 (0.35) | 1.75 (0.5) | NA |
| No. of tender joints, mean (s.d.) | 4.0 (5.66d) | 12.0 (4.24) | NA |
| No. of swollen joints, mean (s.d.) | 3.0 (4.24d) | 7.75 (3.30) | NA |
| ESR, mean (s.d.) | 49.5 (6.37) | 18.33 (14.98a) | 61.0 (11.43a) |
| CRP, mean (s.d.) | 53.38 (50.52) | 17.05 (10.54e) | 23.7 (22.3a) |
| Parenteral corticosteroids, % | 0 | 50 | NA |
| Prednisone, % | 0 | 50 | 30 |
| MTX, % | 50 | 50 | 70 |
| Failed biologicals | 1/2 | 0/4 | NA |
n = 3.
n = 5.
n = 7.
One patient had multiple joint replacement surgeries affecting tender joint score/swollen joint score.
n = 2. NA: not available; DAS28: disease activity score 28; MDGA: Physician global assessment; Pat Global: Patient global assessment; Specimens from a distinct cohort of patients were analyzed using BD Powerblots.
Table 2.
Individual patient demographics and clinical parameters
| Demographics | RA #1 | RA #2 | ERA #1 | ERA #2 | ERA #3 | ERA #4 |
|---|---|---|---|---|---|---|
| Age, years | 55.32 | 56.78 | 47 | 36 | 57 | 61 |
| Male/female | F | F | F | F | F | F |
| RF+/− | Positive | Positive | Negative | NA | Negative | Negative |
| Erosions | Yes | Yes | No | Yes | No | No |
| HAQ score | 1.625 | 1.625 | 1.75 | 0.5 | 0.25 | 0.38 |
| DAS28-4 | 5.02 | 2.89 | 4.89 | 3.24 | 3.99 | 5.67 |
| MDGA | 6 | 7 | 6 | 6 | 4 | 3 |
| Pat Global | 7 | 7 | 7 | 7 | 3 | 1 |
| Functional class | 3.5 | 3 | 2 | 2 | 1 | 2 |
| No. of tender joints | 8 | 0a | 15 | 6 | 12 | 15 |
| No. of swollen joints | 6 | 0a | 8 | 4 | 7 | 12 |
| ESR | 45 | 54 | 14 | 6 | 6 | 35 |
| CRP | 89.1 | 17.65 | 9.6 | 0.8 | NA | 24.5 |
| No. of failed biologicals | 4 | 0 | 0 | 0 | 0 | 0 |
| Parenteral corticosteroids, % | No | No | No | Yes | No | Yes |
| Prednisone, % | No | No | Yes | No | No | Yes |
| MTX, % | No | Yes | Yes | Yes | No | No |
Values are given as mean s.e.m. *Patient had multiple joint replacement surgeries affecting TJS/SJS. NA: not available; DAS28; disease activity score 28; MDGA: Physician global assessment; Pat Global: Patient global assessment.
ST
RA ST samples (n = 11) were collected from 10 patients (one patient was sampled from both knee joints) with erosive, end-stage RA at the time of joint replacement surgery. All ST specimens were immediately transferred to research personnel for processing.
Blood collection
Human PB was collected into heparinized vacutainer tubes, and PB mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation at 800× g for 20 min. PBMCs were washed, counted, resuspended in 90% human serum (Irvine Scientific, Irvine, CA, USA) and 10% dimethyl sulfoxide (Sigma, St Louis, MO, USA) and frozen in liquid nitrogen to maintain cellular activation levels. Extensive lot testing was performed to identify a human serum batch that was suitable for these studies, i.e. to ensure that cell activation was not influenced [27]. This methodology has been employed successfully with immune cells harvested from patients with other autoimmune disorders [21, 28]. Blood samples from control healthy adults (University Health Network, Toronto) were handled and processed in parallel. Murine blood was collected by terminal cardiac puncture from anaesthetized mice and immediately fixed with BD fix and lyse buffer (BD Biosciences) at 37°C for 10 min. Samples were stored at −80°C. Murine PBMCs were permeabilized in BD Perm Buffer III (BD Biosciences, Mississauga, Ontario, Canada) according to the manufacturer's protocol.
Cell surface and intracellular phospho-specific flow cytometry
Human PBMCs were thawed at 37°C, washed twice and incubated for 1 h. PBMCs were then fixed in 2% paraformaldehyde at 37°C for 10 min and permeabilized with 90% ice-cold methanol for 15 min. PBMCs were stained with directly conjugated antibodies against CD45-PeCy7, CD14-Ax700 and CD34-PE (Becton-Dickinson; San Jose, CA, USA; or eBiosciences, San Diego, CA, USA) as previously described [21]. Intracellular stains consisted of anti-collagen I antibody (Millipore, Billerica, MA, USA or Rockland, Gilbertsville, PA, USA), prolyl hydroxylase antibody (5B5, Abcam, Cambridge, MA, USA), biotinylated anti-vimentin antibody (Thermo Scientific, Rockford, IL, USA) and phospho-specific proteins for p38 (pT180/Y202), p44/42 (pT201/Y202), STAT3 (pY705) and -5 (pS727) (BD Biosciences). Murine cells were stained for 30 min at room temperature and washed twice, followed by addition of secondary antibodies. Anti-rabbit-Alexa405 conjugate (Molecular Probes, Eugene, OR, USA) and APC-Cy7 streptavidin (eBiosciences) were incubated for an additional 30 min. 100 000–500 000 cells were acquired on an LSRII FACS machine (12 colours, BD Biosciences) with DiVa software (Becton-Dickinson). Data were analysed using Flojo software (Treestar, San Carlos, CA, USA).
CIA
CIA was induced in male DBA/1 mice (6–12 weeks, Taconic Farms, NY, USA or Jackson Laboratories, ME, USA) by injecting 100 µg chicken collagen II (CII) dissolved in 0.05 M acetic acid at a concentration of 4 mg/ml (Chondrex, Seattle WA, USA) and emulsified in 2 mg/ml complete Freund's adjuvant (CFA). Mice were immunized at the base of the tail with either 100 µg CII or phosphate buffered saline (PBS) (as a control) and were boosted 21 days later with CII or PBS in incomplete Freund's adjuvant (IFA). Animals were sedated with isofluorane, and up to 1 ml of PB was collected by terminal cardiac puncture on Days 0, 7, 14, 21, 22, 23 and 24 (prior to the onset of disease). Joints were collected from animals with swollen paws collected between Days 30 and 40. Clinical scores were defined as follows: 0, normal joint; 1, paw swelling only; 2, one joint of one limb along with paw swelling; 3, multiple joints on a limb involved; and 4, all joints involved or limb fusion. An arthritis score, range 0–16, is assigned to each mouse by summing the scores of each paw. The paws and knee joints of CIA mice were fixed in 10% formalin for routine histology and immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed on plastic- (Pathology Department, Mount Sinai Hospital, Toronto, Ontario, Canada) or paraffin-embedded joint tissue from mice with late-stage arthritis. All samples were fixed in 10% formalin and samples for routine histology were decalcified and processed (Histology Laboratory, Toronto General Research Institute, Toronto, Ontario, Canada). Plastic-embedded tissue was processed in ascending grades of alcohol and infiltrated with ascending grades of Spurr plastic resin and cut on a motorized Leica 2165 microtome using a tungsten carbide knife. Longitudinal sections were taken through the inflamed joints (paws) and stained for the presence of fibrocytes/myofibroblasts using anti-CD45 and anti-α-SMA. Briefly, sections were rinsed in xylene and dehydrated through a series of alcohol washes (100, 95, 70 and 50%), followed by a final wash in water. Antigen retrieval was performed in a microwave with 0.01 M citrate for 20 min. Sections were blocked in a solution of 2% gelatin/PBS for several hours or overnight at 4°C. Sections were stained with anti-CD45-PE (1:50), anti-α SMA-FITC (1 : 50) at 4°C overnight. Slides were examined under a confocal microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany).
FLS cell isolation and BD PowerBlot immunoarray analysis
FLS cells from ST from affected joints of RA patients were isolated, passaged and assessed as previously described [29]. RA FLS cell lysates were collected in lysis buffer (10 mM Tris, pH 7.4, 1 mM sodium orthovanadate, 1% SDS), sonicated and frozen at −80°C. Protein samples (200 µg) were resolved by electrophoresis on a 4–15% gradient SDS–polyacrylamide gel, and transferred onto an Immobilon-P membrane (Millipore) and analysed as previously described [30]. Briefly, the membrane was blocked for 1 h with blocking buffer (LI-COR, Lincoln, NE, USA) and clamped with a western blotting manifold (isolating 40 longitudinal channels). Antibody cocktails for either the full or phosphoprotein BD array were added to each channel and hybridized for 1 h at 37°C. The blot was washed and hybridized for 30 min at 37°C with secondary goat anti-mouse antibody conjugated to Alexa Fluor 680 fluorescent dye (Molecular Probes) and goat anti-rabbit antibody conjugated to IRDye 800 fluorescent dye (Rockland). The membrane was washed, dried and scanned at 700 and 800 nm with the Odyssey infrared imaging system (LI-COR). Densitometry analysis of detected bands was performed. Intensity readings were normalized to the sum intensity of all of the valid spots on a blot and then multiplied by 1 000 000, and molecular weight was determined from molecular weight markers. Triplicate readings for each sample were averaged.
Statistical analysis
Results are expressed as mean values ± s.e., unless otherwise indicated. The percentage of activated PB fibrocytes from RA patients and controls was evaluated by a Student's t-test. The number of PB fibrocytes and their activational state was assessed by multifactorial analysis of variance followed by Dunnett's multiple comparison test. Correlations were assessed using a two-tailed Pearson's correlation coefficient. The Wilcoxon signed rank test was used to confirm significant differences in BD PowerBlot data assuming a theoretical density value 0 for non-activated samples. P-values <0.05 were considered as statistically significant. Statistical tests were performed using GraphPad Prism software (San Diego, CA, USA).
Results
Immunophenotyping of PB fibrocytes
Fibrocytes express markers of both haematopoietic and stromal cells. Since the majority of the published studies report data with cultured fibrocytes, at the outset, we characterized the expression profile of freshly harvested circulating human PB fibrocytes. As reported for cultured fibrocytes, human PB fibrocytes were found to be CD45high, CD14+, CD34+, ColI+ and 5B5+ (Fig. 1, represented in orange). In all subsequent studies, we employed this signature surface marker profile to select for fibrocytes.
Fig. 1.
Characterization of human PB fibrocytes by multidimensional phospho-specific flow cytometry. Human PB was stained with anti-CD45-PeCy7, -CD34-PE, -CD14-A×700 and intracellular anti-ColI-A×488 and -prolyl hydroxylase-A×647 (5B5) antibodies, then examined by multi-channel phospho-specific flow cytometry, as described in ‘Methods’ section. Population staining is colour coded and the fibrocyte lineage, shown in orange, was identified as CD45high, CD14+, CD34+, ColI+ and 5B5+. Specific stains can be observed by comparing the row (y-axis, labelled on the right side) vs the column (x-axis, labelled along the top). A histogram of each population is shown at the bottom of each column.
Phospho-specific signalling in human fibrocytes
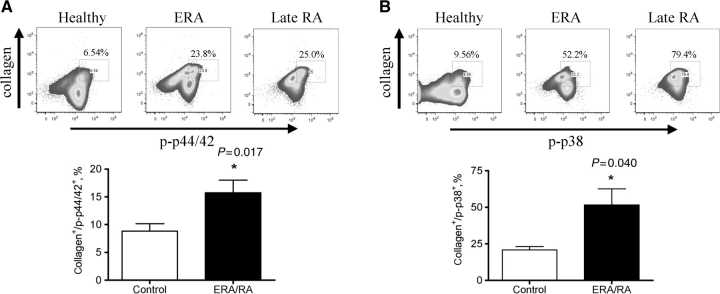
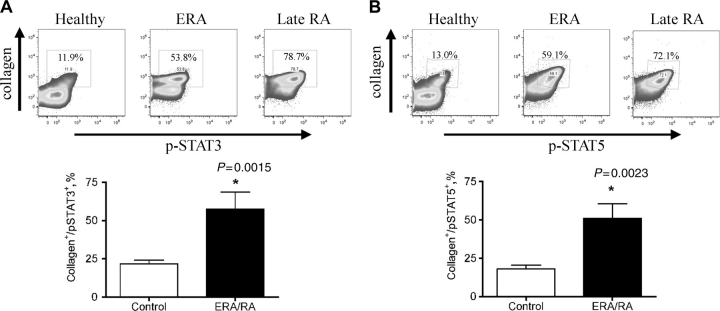
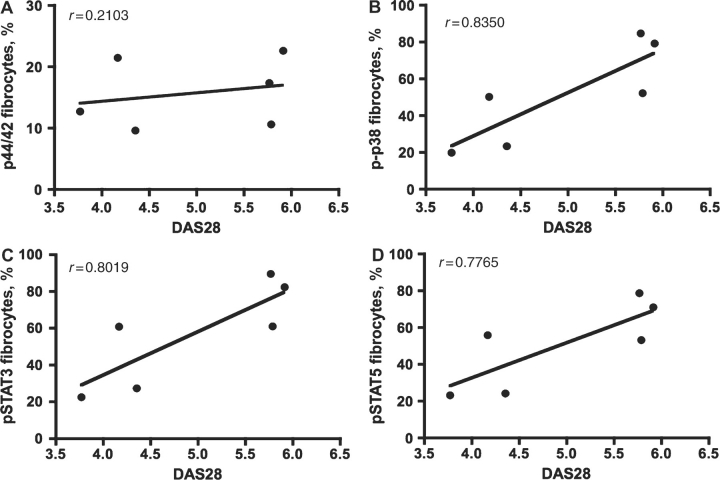
Fibrocytes are multipotent mesenchymal stem cells that circulate in the PB. Our working hypothesis was that activated fibrocytes may be recruited to the RA joint and contribute to pannus formation, potentially differentiating into FLS cells. To test this, PB was collected from healthy individuals, patients with ERA or established RA (>1 year) and fibrocyte cells were examined for phospho-specific activation of specific signalling effectors that would depict cell activation. Given the limited specimen availability, we investigated MAP kinase (MAPK) and signal transducer and activator of transcription (STAT) activation, since these signalling effectors are critical nodes in different signalling pathways and therefore are good candidates to serve as indicators of activation in this cell population. Fibrocytes from healthy individuals exhibited basal levels of signalling: phospho-p44/42 at 9.10 ± 3.78% (Fig. 2A), phospho-p38 at 21.11 ± 8.31% (Fig. 2B), phospho-STAT3 at 21.64 ± 9.11% (Fig. 3A) and phospho-STAT5 at 18.0 ± 2.54% (Fig. 3B). Circulating fibrocytes from patients diagnosed with ERA and established RA showed a significant (P < 0.05) increase in the percentage of fibrocytes expressing phospho-p44/42 (ERA/RA 15.73 ± 2.28%, Fig. 2A), phospho-p38 (ERA/RA 51.65 ± 11.06%, Fig. 2B), phospho-STAT3 (ERA/RA 57.38 ± 11.20%, Fig. 3A) and -5 (ERA/RA 51.08 ± 9.47%, Fig. 3B) compared with fibrocytes from healthy individuals. Interestingly, there was no significant difference in the activation status of PB fibrocytes from patients with ERA or established RA (phospho-p44/42: ERA mean 14.76 ± 2.81% vs established RA 17.68 ± 4.98%; phospho-p38: ERA mean 52.66 ± 12.58% vs established RA 49.63 ± 29.68%; phospho-STAT3: ERA mean 53.01 ± 11.18% vs established RA 47.20 ± 23.95%; and phospho-STAT5: ERA mean 59.73 ± 12.69% vs established RA 52.70 ± 29.70%). Moreover, there was no significant difference in the total number or percentage of circulating fibrocytes between the control or ERA/RA groups (data not shown). Notably, we observed a direct correlation between the extent of fibrocyte phosphorylation and disease activity score 28 (DAS28) for phospho-p38, phosph-STAT3 and -5 (Fig. 4B, C and D), but not for phospho-p44/42 (Fig. 4A).
Fig. 2.
MAPK phosphorylation in PB CD45+/CD34+/ColI+ fibrocytes from patients with ERA/established RA. Representative profile of PB CD45+/CD34+/CD14+ cells expressing ColI+ and (A) phospho-p44/42 or (B) phospho-p38 expression in healthy individuals (n = 10) and ERA/RA patient (n = 6) samples. A significant (P < 0.01) increase in (A) phospho-p44/42 and (B) phospho-p38 signalling in the fibrocyte population was observed in ERA/late-RA patients compared with healthy controls. Gates were determined using unstained, permeabilized cells. Data are shown as mean ± s.e.m.
Fig. 3.
STAT phosphorylation in PB CD45+/CD34+/ColI+ fibrocytes from patients with ERA/established RA. Representative profile of PB CD45+/CD34+/CD14+ cells expressing ColI+ and (A) phospho-STAT3 or (B) phospho-STAT5 expression in healthy individuals (n = 10) and ERA/RA patient (n = 6) samples. A significant (P < 0.01) increase in (A) phospho-STAT3 and (B) phospho-STAT5 signalling in the fibrocyte population was observed in ERA/late-RA patients compared with healthy controls. Gates were determined using unstained, permeabilized cells. Data are shown as mean ± s.e.m.
Fig. 4.
Fibrocyte activation correlates with DAS28. Dot plot of the correlation of DAS28 with the percentage of (A) p44/42 (r = 0.2103; P = 0.5119), (B) p-p38 (r = 0.8350; P = 0.007), (C) pSTAT3 (r = 0.8019; P = 0.0017) and (D) pSTAT5 (r = 0.7765; P = 0.003) activation in PB fibrocytes in RA patients.
Phospho-specific signalling in murine fibrocytes
In order to further characterize fibrocyte activation in the context of phosphorylation activation at specific stages of arthritis development, we employed a mouse CIA model of RA. In time course studies, phospho-activation of STAT5 in circulating fibrocytes was examined by phospho-flow cytometry. This analysis permitted evaluation of fibrocyte activation prior to the onset of symptoms, generally detectable in mice around Day 28 post-immunization. An increase in the total number of circulating fibrocytes was observed in the circulation 7 days after mice were immunized (Day 0: 66 741 ± 18 990 vs Day 7: 142 512 ± 17 923), which subsequently declined (Fig. 5A). Likewise, we observed a significant increase in the total number of phospho-STAT5-positive circulating fibrocytes 7 days post-immunization (Day 0: 16 675 ± 3882 vs Day 7: 55 430 ± 13 275; Fig. 5B). By Day 21 following the initial immunization, the total number of phospho-STAT5-positive circulating fibrocytes returned to baseline. Boosting the mice with CII in IFA on Day 21 had no effect on the decline in phospho-STAT5-positive fibrocytes in the circulation. Interestingly, the percentage of fibrocytes in the circulation increased by Day 7 post-immunization (1.48%) compared with basal levels (0.31%) (data not shown).
Fig. 5.
Circulating fibrocytes are activated during early stages of CIA. Murine PB was stained with anti-CD45 PeCy7, -CD34-Alexa 647, -CD14 PeCy5.5 or -CD11b PeCy5, -ColI Alexa 405 and/or anti-CD3 Alexa700 and anti-vimentin APC-Cy7 and visualized on a BD LSRII flow cytometer. (A) The total number of CD45+/ColI+/α-SMA+ fibrocytes per millilitre blood on Days 0, 7, 14, 21, 22, 23 and 24 post-immunization with collagen is shown. (B) The total number of pSTAT5-positive/CD45+/ColI+/α-SMA+ fibrocytes per millilitre blood on Days 0, 7, 14, 21, 22, 23 and 24 post-immunization with collagen is shown. Data are shown as mean ± s.e.m. of duplicate experiments (Day 0, n = 9; Day 7, n = 10; Day 14, n = 9; Day 21, n = 10; Day 22, n = 4; Day 23, n = 10; and Day 24, n = 5) and the range of fibrocyte and pSTAT5-positive cells in normal mice are shown by the dashed lines.
Localization of fibrocytes/myofibroblasts in affected joints
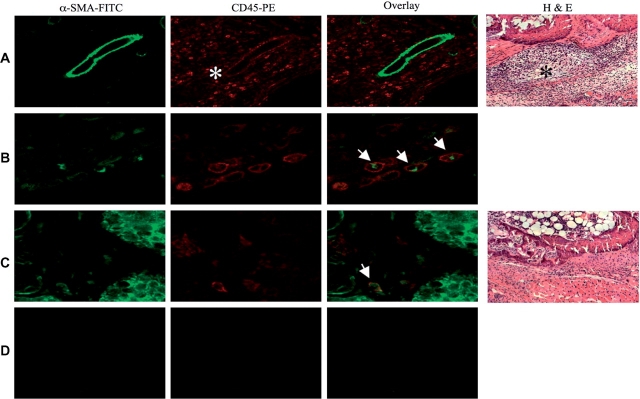
Immunohistochemistry was performed on joints harvested from mice with CIA with clinical scores ⩾3. Profound inflammatory infiltrates with CD45+/SMA+ fibrocytes localized within the inflammatory infiltrate surrounding affected joints were identified (Fig. 6A and B). Few CD45+/SMA+ cells were observed in mice immunized with adjuvant alone (Fig. 6C and D).
Fig. 6.
CD45 and α-SMA double positive cells are found in the inflammatory infiltrate in CIA. Mice were immunized with either 100 µg CII or PBS in CFA at Day 0 and boosted on Day 21 with CII or PBS in IFA. Joints were collected from CIA animals with a clinical score >3. Haematoxylin and eosin (H & E) staining showing profound inflammatory infiltrate (asterisks) in the CII immunized mice (A, 200× magnification) and no obvious inflammation in control-immunized animals (C, 200× magnification). Immunohistochemistry of plastic embedded joints from CIA or control mice were stained with α-SMA-FITC (green) and CD45-phycoerythrin (red). (A and B) Double positive α-SMA/CD45+ fibrocytes from CIA mice [(A), magnification 200×, (B) magnification 400×; smooth muscle, open arrow and infiltrating leucocytes, asterisks] or control-immunized mice (C, magnification 400×) are shown. (D) Isotype control (magnification 200×).
Phosphorylation activation of signalling effectors in RA ST FLS cells
Activated PB fibrocytes may migrate to affected RA joints to become resident ST FLS cells. In an earlier publication, we provided the evidence that RA ST FLS cells exhibit elevated gene expression consistent with an inflammatory response [29]. Accordingly, using stored cells from 11 of the RA ST FLS cell aliquots from our earlier publication [29], we employed the BD PowerBlot immunoarray as a high-throughput western blot screen for phosphorylated signalling effectors and identified phospho-p38, phospho-STAT1, -3 and -5 levels in the majority of the samples (Fig. 7).
Fig. 7.
Phospho-signalling profile in RA ST FLS cells. Cell lysates (n = 11) were prepared from ST FLS cells from patients with end-stage RA, at the time of joint replacement, as described in ‘Methods’ section, then analysed by BD PowerBlot. Values represent the average intensity of triplicate readings. Statistical significance was determined using the Wilcoxon signed rank test with P < 0.05 (asterisks).
Discussion
In the present study, we have compared the phospho-signalling profiles of a rare population of PB fibrocytes from RA patients with fibrocytes from healthy individuals. Most of the information relating to PB fibrocytes has been obtained from in vitro cultured cells, where differentiation occurs [8]. Few studies have examined circulating fibrocytes in vivo [13, 31] and their activation status. Based on in vitro and in vivo data, it has been suggested that the combination of collagen production and CD45, CD11b, CD13 or CD34 expression is sufficient to discriminate fibrocytes from other leucocytes in the circulation [32]. We confirm that expression of CD45, CD34 and ColI is sufficient to distinguish the PB fibrocyte population. This circulating CD45+/ColI+ fibrocyte population exhibits a signature phospho-specific activation in ERA, which is similar to the phospho-profile identified in fibrocytes from patients with longstanding disease. Additionally, the phospho-activation profile of PB fibrocytes from RA patients is similar to that observed in the ST FLS cells from affected joints. In a mouse model of arthritis, CIA, we observed both an increase in fibrocyte numbers and their phospho-STAT5 activation, conspicuously prior to the onset of symptoms. Taken together, our data suggest that PB fibrocyte activation may have a role in the early activation/initiation of RA.
Previously, we have shown that RA ST FLS cells express genes that denote an inflammatory response, and also genes unique to embryonic limb development [29]. This has led us to hypothesize that a multipotent progenitor-like cell, such as the fibrocyte, may be involved in RA pathogenesis, specifically as either a progenitor of the ST FLS cell or as has been reported for wound healing in burn patient tissue, as a cell that activates fibroblasts to proliferate [31]. Comparing the phosphorylation profiles of PB fibrocytes with ST FLS cells, we observed phospho-STAT3, -5 and phospho-p38 activation in both populations. Given these similarities, we speculate that activated PB fibrocytes may be recruited to affected joints where they may contribute to pannus formation and influence FLS cell differentiation and/or proliferation. Direct interactions between activated fibrocytes and resident fibroblasts may generate FLS cell differentiation. The relationship of the kinetics of PB fibrocyte activation to FLS cell activation in affected joints is the subject of our ongoing investigations.
Pannus formation is central to the process of joint destruction. The FLS cell layer expands, albeit in the absence of obvious evidence for excessive proliferation. Stem cell recruitment and differentiation may contribute to pannus formation, although de-differentiation or transformation of FLS cells is an alternative explanation. While there is a paucity of data for PB fibrocyte trafficking in RA, these cells are capable of migrating into many inflammatory sites [15, 33]. Certainly, circulating endothelial progenitor cells migrate into RA joints [34] and PB fibrocytes express the cognate chemokine receptors for many chemokine ligands found in RA SF (CXCR4, CCR3, CCR5 and CCR7). In the CIA model we employed, we observed increased numbers of circulating fibrocytes following collagen immunization, which would be consistent with bone marrow mobilization and trafficking to inflammatory sites. Increased numbers of circulating cells are observed in several diseases where there is a subsequent recruitment to affected sites, fibrocytes in idiopathic pulmonary fibrosis [31] and endothelial progenitor cells during the onset of CIA [35]. Elevated levels of G-CSF are found in the serum and SF of RA patients [36], and G-CSF can mobilize CD34+ cells from the bone marrow [37, 38]. Although we did not observe elevated numbers of fibrocytes in our human RA samples, this process may be transient and because of the limited patient sampling, missed. In as much as the PB fibrocyte population that constitutes a minor population in the circulation, fibrocytes constitute 10% of the leucocytes infiltrating into wound chambers [39], and 1–30% of the synovial fibroblasts from affected joints of RA patients express α-SMA [11]. Here we provide evidence that α-SMA-positive fibrocytes accumulate in the inflammatory infiltrate in affected paws of mice with CIA, where they may influence the disease process.
Clinical information revealed that the ERA patients in this study, who were within 1 year of diagnosis, had lower HAQ, MDGA and functional class scores than the late-RA cohort, corroborating our approach to distinguishing ERA from late RA. Notably, FACS analysis of PB fibrocytes revealed activation of MAPK and STAT signalling effectors in both ERA and established RA. We specifically included ERA patient specimens to assess whether fibrocyte activation occurs early in the disease process and whether differences in the activation profiles might distinguish early and established disease. Since the levels of fibrocyte phosphorylation activation were equivalent in established vs ERA patient specimens, we infer that fibrocyte activation likely occurs early in the pathogenesis of RA. Further scrutiny of other signalling effectors may discriminate early from established disease. Our ERA patient cohort had moderate to high DAS28, and we observed a positive correlation between fibrocyte activation and DAS28. DAS are strong predictors of radiological progression and future disability, and the correlation of fibrocyte activation with DAS may implicate fibrocytes in joint destruction.
Accordingly, in a subsequent series of mouse studies, we examined the time course of phosphorylation activation of STAT5 in circulating fibrocytes in a CIA model of arthritis. We observed early phospho-STAT5 activation 7 days after CII immunization. Intriguingly, phosphorylation of STAT5 was transient in this mouse CIA model, at early time points in pathogenesis. Notably, all mice immunized to develop CIA were sampled prior to the development of symptoms; whereas, all of the ERA patients were symptomatic and fulfilled ACR criteria for RA at the time that their PB was collected for fibrocyte analysis. Thus, differences in the kinetics of fibrocyte activation between our mouse studies and the human samples may be reflective of both of the species differences and the timing of sampling. Additionally, the ERA patients were taking medications, potentially influencing the phosphorylation status of signalling effectors. Nevertheless, the data suggest that phosphorylation activation of fibrocytes is an early process in RA.
Asymptomatic synovitis precedes clinical disease in RA [40]. Whether activated PB fibrocytes migrate into joints to contribute to this synovitis is unknown. In the mouse CIA model of arthritis, by Day 10 post-immunization joints exhibit synovial hyperplasia, and there is evidence of infiltrating mesenchymal cells, albeit in the absence of an inflammatory infiltrate [16]. There is evidence that the mesenchymal cells originate directly from the bone marrow and not from the circulation. Fibrocytes lose expression of haematopoietic markers following their differentiation into myofibroblasts [32] and, therefore, it is difficult to track the fate of these cells following their extravasation into affected joint tissue. Certainly, fibrocyte activation in the circulation and recruitment into joints in the preclinical phase would be consistent with previous observations in humans, monkeys and rats, where microscopic joint inflammation is observed early on in disease [16, 40].
Elevated levels of a number of cytokines are found in the serum of RA patients, including TNF-α, IL-1, -2, -6, -10 and GM-CSF [41–43]. Serum cytokine levels generally correlate with disease severity. MAPKs are positive regulators of pro-inflammatory cytokine production in arthritis, including IL-1, -6, -12, -23 and TNF [44]. Herein, we provided the evidence for elevated levels of phospho-MAPKs p42/44 and p38 in the circulating fibrocytes; this signalling may culminate in the production of pro-inflammatory cytokines. IL-1β activates cultured PB fibrocytes to secrete TNF-α, chemokines and haematopoietic growth factors [45]. Furthermore, since IL-2, -6, CCL2 and -4 levels are elevated in the serum from patients with RA and activate their respective cognate receptors to invoke STAT activation, fibrocyte cell surface expression of these receptors may contribute to fibrocyte activation.
Fibrocytes are multipotent progenitor cells capable of differentiating along multiple lineages. Therefore, activation of PB fibrocytes may be the prelude of cellular differentiation. Fibrocytes differentiate into α-SMA-expressing cells when cultured with T cells, or upon TGF-β stimulation [33]. Notably, TGF-β activates stress-activated protein kinase/Jun-amino-terminal kinase and p44/42 in fibrocytes [46], and elevated levels of TGF-β are present in RA ST [47]. TGF-β, in the context of an inflammatory cytokine milieu, supports de novo differentiation of IL-17-producing T cells [48] and synovial fibroblasts in affected joints [49]. The implications are that activated fibrocytes may be recruited to affected joints, where the cytokine environment could influence their differentiation into FLS cells. Inhibitors of fibrocyte differentiation (serum amyloid P and aggregated IgG) are found in discrete regions of affected joints, the exception being pannus [9, 50]. Therefore, specific sites within the inflamed joint may favour the differentiation of fibrocytes along specific lineages. Additionally, patients with ERA exhibit a distinctive and transient Th2 cytokine profile [51]. T cell contact induces fibrocyte differentiation in vitro [33], and the Th2 cytokines IL-4 and -13 promote fibrocyte differentiation [52], suggesting that the early stages of RA may favour fibrocyte differentiation. Establishing the cellular localization and activation status of infiltrating fibrocytes may help elucidate the mechanisms involved in pannus formation in RA.
Activated fibrocytes infiltrating the synovium of RA joints might influence many facets of joint disease. Their specific role in FLS cell pannus outgrowth, either as the precursor cells of FLS cells, or as the activated cytokine secreting cells that promote resident FLS cell activation, remains unclear. Fibrocytes secrete chemotactic chemokines that recruit CD4+ T lymphocytes, critical mediators of the persistent inflammation in RA [12]. Moreover, activated fibrocytes exhibit enhanced gene expression for a number of pro-inflammatory cytokines, suggesting that they may amplify an inflammatory response. Fibrocytes constitutively express M-CSF, required for the differentiation of osteoclasts. M-CSF, together with IL-1, -6 and PGE2, up-regulates receptor activator from nuclear factor K B ligand (RANKL) expression on synovial fibroblasts, thereby promoting bone resorption by osteoclasts [53]. In addition, TNF suppresses the maturation of osteoblasts [54], preventing the formation of new bone. Viewed altogether, we infer from our data that phosphorylation activation of specific signalling effectors in circulating fibrocytes in RA patients may contribute to both the induction phase and the persistent inflammation in RA. Moreover, given the evidence in the mouse CIA model of the appearance of activated circulating fibrocytes prior to the onset of symptoms of disease, phospho-activation of PB fibrocytes may serve as a surrogate marker for early diagnosis. Our ongoing studies are directed at validating the diagnostic and prognostic potential of fibrocytes for ERA and exploring the potential of targeted inhibitors as a therapeutic intervention strategy.

Acknowledgements
The authors gratefully acknowledge Susan Dubbin for co-ordinating patient sample collection, and Dennis Mitchell, Mileidys Alvarez, Thomas Burian and Ramtin Rahbar for their excellent technical assistance. O.D.P. was supported by Bristol-Meyer Squibb Irvington Institute Fellow and the Dana Foundation.
Funding: This work was supported by grants from Genome Canada, Canadian Institute of Health Research (MOP79321), the Arthritis Society (RG06/065) and the National Heart, Lung and Blood Institute (N01-HV-28183I). E.N.F. and K.A.S. are supported by Canada Research Chairs.
Disclosure statement: O.D.P. is an employee of Tocagen, is a co-founder and stock owner of Nodality, has pending or issued patents related to the phospho-flow technology and was a consultant for BD Biosciences during the course of this work. All other authors have declared no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Panayi GS. T-cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1997;9:236–40. doi: 10.1097/00002281-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–28. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Nykanen P, Helve T, Kankaanpaa U, Larsen A. Characterization of the DNA-synthesizing cells in rheumatoid synovial tissue. Scand J Rheumatol. 1978;7:118–22. doi: 10.3109/03009747809098848. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs RA, Perrett D, Axon JM, Herbert KE, Scott DL. Rheumatoid synovial cell proliferation, transformation and fibronectin secretion in culture. Clin Exp Rheumatol. 1995;13:717–23. [PubMed] [Google Scholar]
- 6.Tsumuki H, Hasunuma T, Kobata T, Kato T, Uchida A, Nishioka K. Basic fgf-induced activation of telomerase in rheumatoid synoviocytes. Rheumatol Int. 2000;19:123–8. doi: 10.1007/s002960050115. [DOI] [PubMed] [Google Scholar]
- 7.Yudoh K, Matsuno H, Nezuka T, Kimura T. Different mechanisms of synovial hyperplasia in rheumatoid arthritis and pigmented villonodular synovitis: the role of telomerase activity in synovial proliferation. Arthritis Rheum. 1999;42:669–77. doi: 10.1002/1529-0131(199904)42:4<669::AID-ANR9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid p. J Immunol. 2003;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Mattey DL, Dawes PT, Nixon NB, Slater H. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis. 1997;56:426–31. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to cxcl12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R. Cd34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch. 2002;440:298–303. doi: 10.1007/s004280100530. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 16.Marinova-Mutafchieva L, Williams RO, Funa K, Maini RN, Zvaifler NJ. Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 2002;46:507–13. doi: 10.1002/art.10126. [DOI] [PubMed] [Google Scholar]
- 17.Zvaifler NJ. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther. 2006;8:210. doi: 10.1186/ar1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luyten FP. Mesenchymal stem cells in arthritis. In: Firestein GP, Panayi G, Wollheim F, editors. Rheumatoid arthritis. UK: Oxford University Press; 2006. [Google Scholar]
- 19.Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004;110:206–21. doi: 10.1016/j.clim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 21.Perez OD, Krutzik PO, Nolan GP. Flow cytometric analysis of kinase signaling cascades. Methods Mol Biol. 2004;263:67–94. doi: 10.1385/1-59259-773-4:067. [DOI] [PubMed] [Google Scholar]
- 22.Krutzik PO, Nolan GP. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods. 2006;3:361–8. doi: 10.1038/nmeth872. [DOI] [PubMed] [Google Scholar]
- 23.Irish JM, Anensen N, Hovland R, et al. Flt3 y591 duplication and bcl-2 overexpression are detected in acute myeloid leukemia cells with high levels of phosphorylated wild-type p53. Blood. 2007;109:2589–96. doi: 10.1182/blood-2006-02-004234. [DOI] [PubMed] [Google Scholar]
- 24.Irish JM, Hovland R, Krutzik PO, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–28. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Krutzik PO, Crane JM, Clutter MR, Nolan GP. High-content single-cell drug screening with phosphospecific flow cytometry. Nat Chem Biol. 2008;4:132–42. doi: 10.1038/nchembio.2007.59. [DOI] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Perez O, Mitchell D, Nolan GP, Campos R, Gao G, Li L. Multiparameter analysis of intracellular phosphoepitopes in immunophenotyped cell populations by flow cytometry. Current Protocols in Cytometry. 2005;6:1–22. doi: 10.1002/0471142956.cy0620s32. [DOI] [PubMed] [Google Scholar]
- 28.Perez OD, Mitchell D, Jager GC, et al. Leukocyte functional antigen 1 lowers t cell activation thresholds and signaling through cytohesin-1 and jun-activating binding protein 1. Nat Immunol. 2003;4:1083–92. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 29.Galligan CL, Baig E, Bykerk V, Keystone EC, Fish EN. Distinctive gene expression signatures in rheumatoid arthritis synovial tissue fibroblast cells: correlates with disease activity. Genes Immun. 2007;8:480–91. doi: 10.1038/sj.gene.6364400. [DOI] [PubMed] [Google Scholar]
- 30.Stanton RJ, McSharry BP, Rickards CR, Wang EC, Tomasec P, Wilkinson GW. Cytomegalovirus destruction of focal adhesions revealed in a high-throughput western blot analysis of cellular protein expression. J Virol. 2007;81:7860–72. doi: 10.1128/JVI.02247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 32.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 33.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 34.Ruger B, Giurea A, Wanivenhaus AH, et al. Endothelial precursor cells in the synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2004;50:2157–66. doi: 10.1002/art.20506. [DOI] [PubMed] [Google Scholar]
- 35.Kurosaka D, Yasuda J, Yoshida K, et al. Kinetics of circulating endothelial progenitor cells in mice with type ii collagen arthritis. Blood Cells Mol Dis. 2005;35:236–40. doi: 10.1016/j.bcmd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura H, Ueki Y, Sakito S, et al. High serum and synovial fluid granulocyte colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:713–18. [PubMed] [Google Scholar]
- 37.Jensen GS, Hart AN, Zaske LA, et al. Mobilization of human cd34+ cd133+ and cd34+ cd133(-) stem cells in vivo by consumption of an extract from aphanizomenon flos-aquae–related to modulation of CXCR4 expression by an l-selectin ligand? Cardiovasc Revasc Med. 2007;8:189–202. doi: 10.1016/j.carrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–20. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 40.Kraan MC, Versendaal H, Jonker M, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–8. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2:706–9. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki M, Kawabe Y, Nakamura H, et al. Elevated serum cytokine levels in a rheumatoid arthritis patient with large granular lymphocyte syndrome. Rheumatology. 2001;40:592–3. doi: 10.1093/rheumatology/40.5.592. [DOI] [PubMed] [Google Scholar]
- 43.Tetta C, Camussi G, Modena V, Di Vittorio C, Baglioni C. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990;49:665–7. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology. 2008;47:409–14. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 45.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–25. [PubMed] [Google Scholar]
- 46.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–31. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 47.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–10. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 48.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Pohlers D, Beyer A, Koczan D, Wilhelm T, Thiesen HJ, Kinne RW. Constitutive upregulation of the transforming growth factor-beta pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2007;9:R59. doi: 10.1186/ar2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–51. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–33. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–31. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]