Abstract
Background
Patients with diabetes have an increased risk for allograft rejection, possibly related to peri-operative hyperglycaemia. Hyperglycaemia is also common following transplantation in patients without diabetes. We hypothesise that exposure of allograft tissue to hyperglycaemia could influence the risk for rejection in any patient with high sugars. To investigate the relationship of peri-operative glucose control to acute rejection in renal transplant patients without diabetes, all patients receiving their first cadaveric graft in a single center were surveyed and patients without diabetes receiving cyclosporin-based immunosuppression were reviewed (n = 230). Records of the plasma blood glucose concentration following surgery and transplant variables pertaining to allograft rejection were obtained. All variables suggestive of association were entered into multivariate logistic regression analysis, their significance analysed and modeled.
Results
Hyperglycaemia (>8.0 mmol/L) occurs in over 73% of non-diabetic patients following surgery. Glycaemic control immediately following renal transplantation independently predicted acute rejection (Odds ratio=1.08). 42% of patients with a glucose < 8.0 mmol/L following surgery developed rejection compared to 71% of patients who had a serum glucose above this level. Hyperglycaemia was not associated with any delay of graft function.
Conclusion
Hyperglycaemia is associated with an increased risk for allograft rejection. This is consistent with similar findings in patients with diabetes. We hypothesise a causal link concordant with epidemiological and in vitro evidence and propose further clinical research.
Background
Hyperglycaemia is common following renal transplantation [1]. Aside from patients with diabetes, many dialysis patients have impaired glucose tolerance [2] and much of the standard post-transplant management is diabetogenic. Recent data has demonstrated that patients with diabetes are at increased risk for allograft rejection [3]. We have recently described how glycaemic control correlates with allograft rejection in patients with diabetes, raising the possibility of a causal association between peri-operative hyperglycaemia and allograft rejection [4]. Transplantation is a unique situation where naive tissue may be suddenly subjected to a hostile hyperglycaemic environment. Acute rejection is thought to be initiated in the early postoperative period by antigen presentation, possibly in response to allograft inflammation and injury. Acute hyperglycaemia is known to enhance ischaemic injury [5], antigen presentation [6], apoptosis [7], and augment the inflammatory response [8]. We hypothesise that exposure of allograft tissue to hyperglycaemia could influence the risk for rejection, not only in diabetes, but in any patient with an elevated glucose. This study investigates the relationship of peri-operative hyperglycaemia to acute rejection in patients without diabetes.
Methods
A retrospective review was made of the records for each of the 365 patients who underwent their first cadaveric renal transplant (CD1) at The Queen Elizabeth Hospital, Adelaide, Australia between January 1990 and January 2000. Patients with primary graft failure, death without graft function or graft loss due to technical complications were excluded (n=25). All established diabetic patients were also excluded (n = 50) and are studied elsewhere [4]. Patients who did not carry the preoperative diagnosis of diabetes but who required insulin in the postoperative period or subsequently developed de novo or post-transplant diabetes mellitus were not designated as diabetic for the purposes of this study. All CD-1 patients commencing Cyclosporin A, mycophenolate or azathioprine, and/or prednisolone as their starting immunosuppression on an intention-to-treat basis were identified (n=230) and formed the primary study group. In this protocol [9], cyclosporin A (5 mg/kg/d with diltiazem or 8 mg/kg/d without diltiazem), mycophenolate (2 g/d) or azathioprine (2 mg/kg/d) were first given orally 6-8 hours after transplantation. Every patient received an intravenous bolus dose of 1 g of methylprednisolone prior to surgery and 500 mg on the following morning. Only CD1 patients sensitised to panel reactive lymphocytotoxic antigens (peak PRA > 50%) or with a positive T or B-cell cross-match received oral prednisolone (30 mg/d), commenced from day two after morning blood testing.
The serum blood glucose immediately following surgery (while still in theatre recovery) and fasting results from the following two mornings were obtained from laboratory records. Transplant records were obtained from a common database including donor age and gender, recipient age, gender, and race, body mass index (kg/m2), type of dialysis, ischaemic time, duration of operation, PRA, peak and current, HLA-A, -B, and -DR matching. Post-transplantation records of all (230) patients were examined for the presence of allograft rejection. Acute rejection was said to occur if biopsy-proven or clinical rejection occurred within 30 days of transplantation. If a biopsy was not performed, clinical rejection was retrospectively identified by a sustained rise by more than 10% from the predicted serum creatinine, responsive to adjunctive immunosuppressive therapy.
Statistical tests
All variables considered as possible predictors of acute rejection were entered into a multivariate logistic regression; initial bi-variable screening was not used. The full model incorporated seven categorical variables (donor gender, recipient gender, race, type of dialysis, sensitization/use of oral prednisolone, the use of azathioprine or mycophenolate, the presence of delayed graft function requiring dialysis) scored one, zero and ten continuous variables (donor age, recipient age, body mass index (kg/m2), warm and cold ischaemic times, duration of operation and post operative glucose concentrations immediately following and on the two mornings after surgery). HLA matching was also considered as a continuous variable (scored 0-6). No collinearity was present in the full data set. Variables were sequentially removed from the model using the likelihood ratio test with the significance level set at p=0.05. Functional form (in particular, non-linearity) of continuous variables in the final model was checked (i) graphically, using partial residual plots and (ii)formally, by both parametric and non-parametric means. Categorical ("cut-point") analysis of continuous variables was not primarily utilized except when testing for first order interactions. Model performance was assessed using indices of calibration: Hosmer-Lemershow χ2 test (p > 0.1) and discrimination: area under the receiver operating curve (ROC) curve (acceptable discrimination area > 0.7). Confidence intervals for the final model were computed using the bootstrap method (BCa, bias corrected and accelerated). The parameters of the final model were estimated as risk ratios (generalized linear model with binomial family and log link) to correct the odds ratio interpretation under conditions of common prevalence (>10%) in the study population. Uni-variate results are expressed as ± 95% confidence interval. Stata® statistical software, version 6.0 (1999) was used.
Results
Demographics
Two hundred and thirty patients were both not diabetic at the time of admission and received cyclosporin-based immunosuppression following successful transplantation. All (230) patients were reviewed in this study. Mean age of the recipient was 44.7 ± 1.7 years, and 60% of patients were men. 7 % of patients were Australian aboriginal. Mean donor age was 36.3 ± 2.2 years. Mean HLA match was 2.4 ± 0.2 and mismatch 2.9 ± 0.2 antigens. 18 % of patients were sensitised and therefore received oral prednisolone from day two. 33% received mycophenolate and 67% received azathioprine.
Hyperglycaemia
The mean blood glucose concentration immediately after surgery was 10.75 ± 0.56 mmol/L (median 9.90 mmol/L, range 5.0-25.6 mmol/L). The mean blood glucose concentration taken the following morning (mean = 12.3 ± 1.5 hours, range 7-16 hours after surgery) was 9.34 ± 0.43 mmol/L (median 8.60) and 8.0 ± 0.31 mmol/L on the morning of day-2. A glucose concentration of greater than 8.0 mmol/L was present in 73 % of patients in our study with more than 31% having a glucose greater than 11.2 mmol/L immediately after surgery. 51% of all patients had glucose levels greater than 8.0 mmol/L both after surgery and on the following morning. The incidence of hyperglycaemia had no relationship to pre-operative glucose levels, ischaemic time, duration of operation, donor weight or age. In addition, the amount of intra-operative or postoperative dextrose solutions received by patients did not influence the incidence of hyperglycaemia. Glycaemic control immediately following surgery correlated with control on subsequent days (R2=0.076).
Acute rejection
Acute rejection occurred in 147 patients (64% overall) at a mean of 8.2 ± 0.8 days after transplantation. 88 patients (60%) had biopsy-proven rejection and had 59 patients (40%) had clinical rejection without biopsy confirmation. Six variables independently predicted allograft rejection including donor youth, the use of azathioprine (over mycophenolate), HLA-matching and the presence of delayed graft function (table 1). Sensitisation was inversely correlated with rejection in this study, probably as a result of the exclusive use of oral steroids in this group. In addition, serum glucose immediately following renal transplantation was independently associated with acute rejection. (Full model: Hosmer-Lemeshow χ2 = 4.93, p=0.76, area under the ROC curve = 0.75, Bootstrap 95% confidence interval 0.71-0.81). The initial relationship between serum glucose and probability of rejection was assumed to be linear. However an examination of partial residual plots suggested a non-linear relationship. The power of this relationship was identified using a non-linear power (Box-Tidwell) available in Stata statistical software [10]. This power function was subsequently used to generate a graph of probability of rejection versus glucose concentration using a fractional polynomial routine (fig 1). The only significant interaction, between glucose levels and the use of prednisolone/sensitisation (p=0.01, likelihood ratio test), was incorporated into this model (fig 2). This interaction was not able to be demonstrated using a different estimator (BinReg). The mean glucose levels immediately following surgery were 9.8 ± 0.78 mmol/L in patients without rejection and 10.8 ± 0.57 mmol/L in patients with rejection. 42% of patients with a glucose < 8.0 mmol/L following surgery developed rejection compared to 71% of patients who had a serum glucose above this level. While persistent hyperglycaemia on the morning after surgery also predicted rejection, it did not offer better discriminating power compared to that immediately following surgery.
Table 1.
Model predictors for allograft rejection
| Variable | Risk ratio | Standard Error | P>|z| | 95% confidence limits | |
| Age (years) | 0.993 | 0.002 | 0.009 | .9894094 | .9985157 |
| Immediate graft function | 0.782 | 0.101 | 0.060 | .6065589 | 1.009974 |
| Azathioprine/MMF | 1.159 | 0.118 | 0.147 | .9493317 | 1.416481 |
| PRA> 50% + Prednisolone | 0.595 | 0.104 | 0.003 | .4221672 | .8384729 |
| Number of HLA-matches | 0.944 | 0.023 | 0.016 | .9004449 | .9894315 |
| Glucose (mmol/L) | 1.015 | 0.004 | 0.000 | 1.008164 | 1.022061 |
Figure 1.
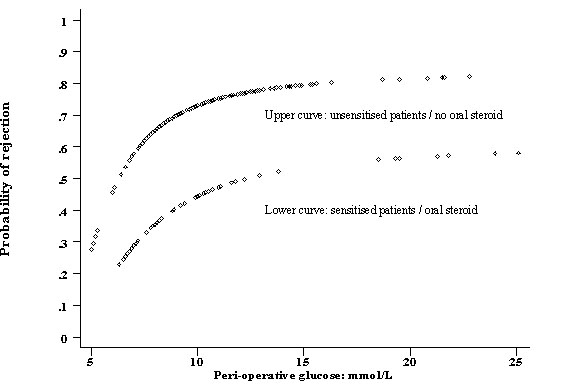
Fractional polynomial showing the probability of rejection in sensitised and un-sensitised CD1 patients versus the immediate post operative glucose level.
Figure 2.
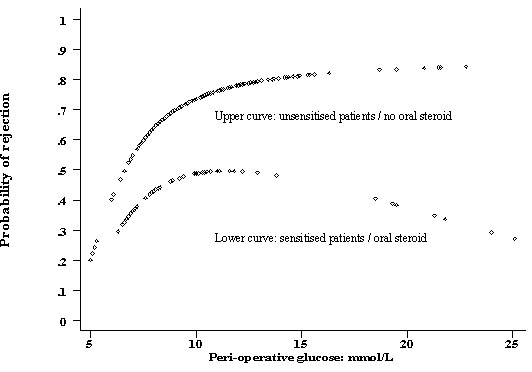
Fractional polynomial showing the probability of rejection in sensitised and un-sensitised CD1 patients versus the immediate post operative glucose level incorporating the interaction term.
Discussion
Hyperglycaemia is common following transplantation in patients without diabetes. 73 % of patients in our study developed hyperglycaemia > 8.0 mmol/L following surgery which persisted beyond 12-hours in 51% of patients. Many patients with renal failure have so called "uraemic pre-diabetes" characterised by impaired glucose tolerance, insulin resistance and hyperinsulinism [2]. In addition, standard peri-transplant management is diabetogenic. Non-diabetic transplant recipients who are treated with steroids show insulin resistance comparable to diabetic patients [11]. Although all patients in our study received the same large doses of methylprednisolone, there was a wide range of glycaemic responses. Certainly, even a slow infusion of 5% dextrose can cause hyperglycaemia in a non-diabetic patient on steroids [12], but the use of intra-operative or peri-operative dextrose did not correlate with blood sugar levels in our study. Cyclosporin A may also affect insulin resistance [13], although it seems unlikely that any patient in this study would have received sufficient cyclosporin to produce hyperglycaemia the morning following transplantation.
Some have suggested hyperglycaemia is simply an epiphenomenon, acting as a marker for more extensive peri-operative insult [14]. However, blood glucose levels in our study did not correlate with intra-operative blood loss, duration of operation or cold ischaemic times. It is also possible that more extensive renal damage may lead to a greater rise in `stress' hormones. Some studies in myocardial infarction have shown a correlation between extent of ischaemic injury and glucose levels [14]. However immediacy of allograft function did not correlate with sugar levels in our study.
The Australia and New Zealand Dialysis and Transplant registry (ANZDATA) has reported that renal transplant patients with diabetes have more acute rejection [3]. We have previously demonstrated that amongst patients with diabetes, those with hyperglycaemia have enhanced rejection rates [4]. We report here for the first time, that peri-operative hyperglycaemia is also associated with an increased risk of allograft rejection in patients without diabetes. Patients with peri-operative hyperglycaemia had significantly more rejection than those who remained euglycaemic. Acute rejection is thought to be initiated in the early post-operative period by allograft injury and the inflammatory response to that injury. We hypothesise that early hyperglycaemia may directly increase the risk of allograft rejection by one of three broad mechanisms.
First, high sugar levels may exacerbate warm ischaemic damage, with the resulting tissue injury acting as trigger for rejection. It is known that hyperglycaemia, worsens renal ischaemic injury in experimental models [5], suggesting a direct role for glucose. The generation of lactate and reactive oxygen species are augmented by acute hyperglycaemia and re-perfusion injury may also be increased [15]. High sugars have a direct vasoconstrictor effect in non-diabetic renal vessels [16]. and result in endothelial dysfunction through hyper-osmolarity, oxidant formation, and protein kinase C (PKC) activation [17]. In addition, high sugars may also have a pro-coagulant effect [7]. Although no relationship between glucose levels and delayed graft function was observed in our study, some studies have shown the incidence of delayed graft function to be increased in patients with diabetes [18]. A state of relative insulin deficiency (ie. hyperglycaemia) may also result in reduced glucose uptake and increased lipolysis in ischaemic tissue, leading to the generation of toxic fatty acids [14]. This has prompted the use of insulin in hyperglycaemic patients to prevent ischaemic injury following myocardial infarction. However most patients with renal failure are hyperinsulinemic. It seems unlikely that this level of hyperinsulinism, regardless of hyperglycaemia, would be inadequate to suppress lipolysis in graft tissue.
Secondly, antigen presentation and co-stimulation are increased in hyperglycaemia. The expression of MHC class I and class II antigens on allograft cells are up-regulated by glucose-induced ischaemia/reperfusion injury and oxidative stress [19]. The production of chemokines that induce expression of MHC antigens are increased [8] and the tissue response to interferon-gamma is enhanced by the presence of high glucose concentrations [6]. Reactive oxygen species, potentiated by hyperglycaemia, are capable of activating peripheral dendritic cells [20]. Apoptosis, also enhanced by hyperglycaemia [7], can initiate re-perfusion-induced inflammation and tissue injury [21] as well as enhance antigen presentation [22] Expression of co-stimulatory molecules are up-regulated directly by hyperglycaemia [8,23] and indirectly, by glucose-enhanced ischaemia [24], and oxidative stress [25].
Thirdly, hyperglycaemia induces an exaggerated inflammatory response to ischaemia/reperfusion and rejection [8]. The acute phase response is increased by insulin deficiency [14]. Production of Nuclear factor kappa B (NFkB) is enhanced in the presence of high glucose levels [24], leading to up-regulation of both cellular and humoral effectors of inflammation. Expression of adhesion molecules including ICAM-1 and VGEF are increased by hyperglycaemia [26]. In addition, increased expression of CD18, VCAM-1, E-selectin [27] and the phosphorylation of PCAM-1 [28] combine to enhance the adhesion and trans-endothelial migration of monocytes. Reactive oxygen species, generated in hyperglycaemia, lead to the induction of proinflammatory cytokines [29] and both the production and activity of these cytokines including TNF-α and interferon-gamma [6] may be enhanced by high glucose levels. Augmented production of TGFβ-1 in hyperglycaemia also suppresses the production of IL-10 [30].
Marked hyperglycaemia following steroid and surgery also suggests the presence of the insulin resistance syndrome. This metabolic milieu (to which graft tissue would be newly exposed) is characterised by hypertension, dyslipidaemia, hyperinsulinism and increased levels of circulating advanced glycation end-products (AGE), leptin, TNF-alpha, IL-1, IL-6, and IL-12.[31,32] These may act, by themselves or in combination with hyperglycaemia, to up-regulate allograft injury or rejection. Patients with the insulin resistance syndrome also possess abnormalities of the innate immune system including an augmented cytokine responsiveness that may predispose to rejection [32]. A recent study has shown that pre-transplant serum C-reactive protein (CRP), a recognised marker of inflammatory responsiveness also independently predicts allograft rejection [33]. Peri-operative hyperglycaemia could therefore identify such allo-responsive patients without being causal. At the same time, it would enable better targeting of immuno-therapy. However, the fact that better sugar control reduced rejection rates in patients with diabetes (who presumably all have this milieu) suggests that hyperglycaemia is more than just an marker of an occult diabetic state. In addition, short-term intensive glycaemic control rapidly results in normalization of immune function and markers of inflammation [34].
Patients on triple therapy in this study had substantially less rejection than patients on double therapy. This is consistent with other published work [9]. The reduced probability of rejection in patients who received oral steroids patients appeared to be greatest in those with very high glucose levels (Fig 2). This may be an effect both of the relatively small numbers of patients with very high glucose levels (at the tail) and of our modeling strategy. The interaction was not demonstrated using a different estimator. However, if real, this may also the underscore the need for more potent immunotherapy in hyperglycaemic patients who have an increased risk for allograft rejection.
Conclusions
This, and previous studies by us, have shown that patients with early hyperglycaemia, whether diabetic or not, have an increased risk for allograft rejection. While hyperglycaemia is not the only risk for allograft rejection, it is both common and eminently susceptible to intervention. There are sound reasons why sugars should be tightly controlled following transplantation in patients with diabetes. Further, we believe the prevention of early hyperglycaemia and attention to insulin resistance may also serve to reduce allograft injury and decrease rejection episodes in patients without diabetes. Further research is needed to determine if such interventions can improve transplant outcomes.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/content/backmatter/1471-2369-1-1-b1.pdf
Contributor Information
Merlin C Thomas, Email: mdorbell@hotmail.com.
John Moran, Email: john.moran@nwahs.sa.gov.au.
Timothy H Mathew, Email: tim.mathew@nwahs.sa.gov.au.
Graeme R Russ, Email: grame.russ@nwahs.sa.gov.au.
M Mohan Rao, Email: mohan.rao@nwahs.sa.gov.au.
References
- Pourmand G, Ebrahimi MR, Mehrsai AR, Taheri M. Patient blood glucose levels before and after kidney transplantation. Transplant Proc, . 2000;32 (3):566–568. doi: 10.1016/s0041-1345(00)00892-7. [DOI] [PubMed] [Google Scholar]
- Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998 May;53(5):1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- Australia and New Zealand Dialysis and Transplant Registry. ANZDATA Registry Report 1999. (Edited by Disney APS, Russ GR, Walker R, Sheil AGR, Collins J, Herbert K, Kerr P). Adelaide, South Australia. . 1999.
- Thomas MC, Mathew TH, Russ GR, Rao MM. Peri-operative hyperglycaemia and increased allograft rejection following renal transplantation in patients with diabetes (abstract) Kid Int. 2000.
- Podrazik RM, Natale JE, Zelenock GB, D'Alecy LG. Hyperglycaemia exacerbates and insulin fails to protect in acute renal ischaemia in the rat. J Surg Res. 1989 Jun;46(6):572–578. doi: 10.1016/0022-4804(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Pavlovic D, van de Winkel M, van der Auwera B, Chen MC, Schuit F, Bouwens L, Pipeleers D. Effect of interferon-gamma and glucose on major histo-compatibility complex class I and class II expression by pancreatic beta- and non-beta-cells. J Clin Endocrinol Metab. 1997 Jul;82(7):2329–2336. doi: 10.1210/jcem.82.7.4055. [DOI] [PubMed] [Google Scholar]
- Min C, Kang E, Yu SH, Shinn SH, Kim YS. Advanced glycation end products induce apoptosis and pro-coagulant activity in cultured human umbilical vein endothelial cells. Diabetes Res Clin Pract. 1999 Dec;46(3):197–202. doi: 10.1016/s0168-8227(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Panes J, Kurose I, Rodrigez-Vaca D, Anderson DC, Miyasaka M, Tso P, Granger DN. Diabetes exacerbates inflammatory responses to ischaemia-reperfusion. Circulation. 1996;93:161–167. doi: 10.1161/01.cir.93.1.161. [DOI] [PubMed] [Google Scholar]
- Elias TJ, Bannister KM, Clarkson AR, Russ GR, Mathew TH, Barratt LJ, Faull RJ. Excellent long-term graft survival in low risk, primary renal allografts treated with prednisolone-avoidance immunosuppression. Clin Transplant. 2000 Apr;14(2):157–161. doi: 10.1034/j.1399-0012.2000.140210.x. [DOI] [PubMed] [Google Scholar]
- Roysten P, Ambler GG. Sg112: Non-linear regression models involving power or exponential functions of co-variates. . In The Stata Technical Bulletin Reprint, v9 Newton HJ College Station; Tx: Stata Corporation. 1999. pp. 173–180.
- Ekstrand AV. Effect of steroid therapy on insulin sensitivity in insulin-dependent diabetic patients after kidney transplantation. J DiabetComplications. 1991 Oct-Dec;5(4):244–248. doi: 10.1016/0891-6632(91)90084-3. [DOI] [PubMed] [Google Scholar]
- Chee YC. Blood glucose levels in non-diabetics on intravenous dextrose infusions. Ann Acad Med Singapore. 1985 Apr;14(2):294–296. [PubMed] [Google Scholar]
- Kutkuhn B, Hollenbeck M, Heering P, Koch M, Voiculescu A, Reinhard T, Grabensee B. Development of insulin resistance and elevated blood pressure during therapy with cyclosporine A. Blood Press. 1997 Jan;6(1):13–17. doi: 10.3109/08037059709086440. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000 Mar 4;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabet Med. 1997;14(suppl3):S45–9. doi: 10.1002/(sici)1096-9136(199708)14:3+<s45::aid-dia444>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- Walczyk MH, Pulliam J, Bennett WM. Effects of hyperglycaemia and mannitol infusions on renal hemodynamics in normal subjects. Am J Med Sci. 1990 Oct;300(4):218–224. doi: 10.1097/00000441-199010000-00004. [DOI] [PubMed] [Google Scholar]
- Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycaemia. J Vasc Surg. 1998 Oct;28(4):687–694. doi: 10.1016/s0741-5214(98)70095-3. [DOI] [PubMed] [Google Scholar]
- Troppmann C, Gillingham KJ, Benedetti E, Almond PS, Gruessner RW, Najarian JS, Matas AJ. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995 Apr 15;59(7):962–968. doi: 10.1097/00007890-199504150-00007. [DOI] [PubMed] [Google Scholar]
- Goes N, Urmson J, Ramassar V, Halloran PF. Ischaemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation. 1995 Feb 27;59(4):565–572. [PubMed] [Google Scholar]
- Ratault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26(1-2):232–238. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- Daemen MARC, Veer CV, Denecker G, Heemskerk VH, Wolfs TGAM, et al. Inhibition of apoptosis by ischaemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M. Ricciardi-Castagnoli P, Rugarli C, Manfredi AA Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998 Nov 1;161(9):4467–4471. [PubMed] [Google Scholar]
- Moriatsu Takada, Anil Chandraker, Kari C Nadeau, Mohamed H Sayegh, Nicholas L. Tilney The role of the B7 co-stimulatory Pathway in Experimental Cold ischaemia/reperfusion injury. J Clin Invest. 1997;100(5):1199–1203. doi: 10.1172/JCI119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycaemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes1. 1999 Apr;48(4):855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- Temaru R, Urakaze M, Satou A, Yamazaki K, Nakamura N, Kobayashi M. High glucose enhances the gene expression of interleukin-8 in human endothelial cells, but not in smooth muscle cells: possible role of interleukin-8 in diabetic macroangiopathy. Diabetologia. 1997 May;40(5):610–616. doi: 10.1007/s001250050723. [DOI] [PubMed] [Google Scholar]
- Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leucocyte-endothelial interaction is augmented by high glucose in an NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami S, Yamashita S, Kihara S, Kameda-Takemura K, Matsuzawa Y. High concentration of glucose induces expression of intercellular adhesion molecules-1 in human umbilical vein endothelial cells. Atherosclerosis. 1998;138(1):35–41. doi: 10.1016/s0021-9150(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Rattan V, Shen Y, Sultana C, Kumar D, Kalra VK. Glucose-induced transmigration of monocytes is linked to phosphorylation of PECAM-1 in cultured endothelial cells. Am J Physiol. 1996 Oct;271(4 Pt 1):E711–7. doi: 10.1152/ajpendo.1996.271.4.E711. [DOI] [PubMed] [Google Scholar]
- Manduteanu I, Voinea M, Serban G, Simionescu M. High glucose induces enhanced monocyte adhesion to valvular endothelial cells via a mechanism involving ICAM-1, VCAM-1 and CD18. Endothelium. 1999;6(4):315–324. doi: 10.3109/10623329909078498. [DOI] [PubMed] [Google Scholar]
- Reinhold D, Ansorge S, Schleicher ED. Elevated glucose levels stimulate transforming growth factor-beta 1 (TGF-beta 1), suppress interleukin IL-2, IL-6 and IL-10 production and DNA synthesis in peripheral blood mononuclear cells. Horm Metab Res. 1996 Jun;28(6):267–270. doi: 10.1055/s-2007-979789. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Padyukov L, Strandvik B, Wramner L. The immune system of the hunter-gatherer meets poverty and excess. Lakartidningen. 2000 Apr 12;97(15):1823–1826. [PubMed] [Google Scholar]
- Fernandez-Real JM, Ricart W. Insulin resistance and inflammation in an evolutionary perspective: the contribution of cytokine genotype/phenotype to thriftiness. Diabetologia. 1999 Nov;42(11):1367–1374. doi: 10.1007/s001250051451. [DOI] [PubMed] [Google Scholar]
- Perez RV, Brown DJ, Katznelson SA, Dubin JA, Muller HG, Chang T, Rudich SM, McVicar JP, Kaysen GA. Pre-transplant systemic inflammation and acute rejection after renal transplantation. Transplantation. 2000 Mar 15;69(5):869–874. doi: 10.1097/00007890-200003150-00034. [DOI] [PubMed] [Google Scholar]
- Albertini JP, Valensi P, Lormeau B, Aurousseau MH, Ferriere F, Attali JR, Gattegno L. Elevated concentrations of soluble E-selectin and vascular cell adhesion molecule-1 in NIDDM. Effect of intensive insulin treatment. Diabetes Care. 1998 Jun;21(6):1008–1013. doi: 10.2337/diacare.21.6.1008. [DOI] [PubMed] [Google Scholar]


