Abstract
1 This is a manifesto for UK clinical pharmacology.
2 A clinical pharmacologist is a medically qualified practitioner who teaches, does research, frames policy, and gives information and advice about the actions and proper uses of medicines in humans and implements that knowledge in clinical practice. Those without medical qualifications who practise some aspect of clinical pharmacology could be described as, say, ‘applied pharmacologists’.
3 Clinical pharmacology is operationally defined as a translational discipline in terms of the basic tools of human pharmacology (e.g. receptor pharmacology) and applied pharmacology (e.g. pharmacokinetics) and how they are used in drug discovery and development and in solving practical therapeutic problems in individuals and populations.
4 Clinical pharmacologists are employed by universities, health-care services, private organizations (such as drug companies), and regulatory agencies. They are
• mentors and teachers, teaching laboratory science, clinical science, and all aspects of practical drug therapy as underpinned by the science of pharmacology; they write and edit didactic and reference texts;
• researchers, covering research described by the operational definition;
• clinicians, practising general medicine, clinical toxicology, other medical specialties, and general practice;
• policy makers, framing local, national, and international medicines policy, including formularies, licensing of medicines and prescribing policies.
5 The future of clinical pharmacology depends on the expansion and maintenance of a central core of practitioners (employed by universities or health-care services), training clinical pharmacologists to practise in universities, health-care services, private organizations, and regulatory agencies, and training other clinicians in the principles and practice of clinical pharmacology.
Keywords: clinical pharmacology, manifesto, definitions
Introduction
This is a manifesto for clinical pharmacology.
A manifesto is ‘a public declaration or proclamation … esp. a printed declaration, explanation, or justification of policy’; and in extended use ‘a book or other work … propounding a theory or argument’. [All the definitions I quote here, unless otherwise indicated, are taken from the Oxford English Dictionary [1].]
This manifesto consists of:
A definition of a clinical pharmacologist, with notes.
An intensional definition of clinical pharmacology and two other complementary definitions, visually presented – an extensional definition and an operational definition.
A description of what clinical pharmacologists should be expected to do.
A model of how expertise in clinical pharmacology should be disseminated.
It does not include details of how clinical pharmacologists should be trained, which deserves separate consideration.
This manifesto is based on the way that academic and health service clinical pharmacology is practised in the UK and recognizes the importance of basic pharmacology to the discipline. It also recognizes the importance of clinical pharmacology in commercial companies and regulatory authorities. Its perspective is for the most part a UK one; however, most of the discussion is relevant to clinical pharmacology wherever in the world it is practised, and it could be used to develop a manifesto elsewhere.
A definition of a clinical pharmacologist
I define a clinical pharmacologist as ‘a medically qualified practitioner who teaches, does research, frames policy, and gives information and advice about the actions and proper uses of medicines in humans and implements that knowledge in clinical practice.’ For notes on this definition see Box 1. For definitions related to other relevant terms, including terminology in drug safety and medication errors, see elsewhere [2–5].
Box 1 Notes on the definition of a clinical pharmacologist
Medically qualified practitioner In the UK and elsewhere there are academic practitioners who are regarded as clinical pharmacologists but who are not clinically qualified. I am reluctant to exclude them from my definition, because I recognize and value the contributions that they make. However, I strongly believe that one cannot be a fully fledged clinical pharmacologist unless equipped to practice clinical medicine, i.e. medically qualified, and that to describe those who are not is potentially harmful to the furtherance of the specialty. Those who are, for example, pharmacologists or pharmacists and who practise some aspect of clinical pharmacology could be described using a related term, such as ‘applied pharmacologists’. This also applies to those in drug companies who do clinical pharmacological studies, many of whom nowadays are not clinically qualified.
Teaches This term encompasses all pharmacological subjects from basic human pharmacology to applied pharmacology (as defined in the text). The place where the teaching is performed is not specified – in a laboratory or at the bedside; in a University, hospital, private institution or regulatory agency. Nor is the form that the teaching takes specified – by personal contact or distance learning, in small or large groups (tutorials, seminars or lectures), or through written materials (websites, journal articles, textbooks).
Policy Includes local, national, and international policy related to medicines.
Defining ‘clinical pharmacology’
Here I offer three different but related definitions of clinical pharmacology – intensional, extensional, and operational. Notes about different types of definition are given in Box 2.
Box 2 Notes on different types of definition
Descriptive definitions The simplest type of definition. A diary, for example, can be described as ‘a book prepared for keeping a daily record, or having spaces with printed dates for daily memoranda and jottings’. Descriptive definitions suffice when all one needs to do is to make a thing recognizable. However, they are usually inadequate for technical terms. For these we need something more – a stipulative definition.
Stipulative definitions Definitions that stipulate ‘what [a term] shall be used to mean’. A stipulative definition of a diary is ‘a daily record of events or transactions … specifically, a daily record of matters affecting the writer personally, or which come under his personal observation’. This definition does not describe a diary in its physical state but stipulates what it is used for and what it contains.
Intensional definitions Stipulative definitions come in different forms. Intensional definitions specify the necessary and sufficient attributes or qualities that make a thing a member of a specific set; they describe its essence.
Extensional definitions Intensional definitions are not always entirely satisfactory. For example, the second definition of a diary (above) does not describe everything that may be contained in a diary, such as calendars, maps, lists of institutions and other information. In order to define a diary completely one would need to list all its contents, in what is called an extensional definition, one that consists of a list in which every object that is a member of a specific set is named. An intensional definition should provide an accurate description of the essence of a subject, but will give no information about its range or scope. An extensional definition, or what you might call a scoping definition, does just that.
Operational definitions An operational definition is one in which concepts are defined ‘in terms of the operations necessary to determine them’.
The essence of clinical pharmacology – an intensional definition
Clinical pharmacology, intensionally defined, is all aspects of the study and use of drugs in humans. This definition is slightly modified from a previous definition [6]. It stresses, as do the other definitions discussed below, how important the basic study of pharmacology is to clinical pharmacology.
The scope of clinical pharmacology – an extensional definition
The complete scope of clinical pharmacology is illustrated in Figure 1. The heart of it is a list of topics that the subject covers (second column from the right, blue). This list, which shows how principles are translated into practice (left-hand column, red), is based on the paradigm in the second column from the left (green), which shows how molecular mechanisms are translated into the clinical effects of medicines (‘from molecules to medicines’). In this paradigm, effects at the molecular level are translated into cellular or tissue effects, which produce organ effects, in turn resulting in effects in the individual; the sum of those individual effects can also be measured in the population. An example is given in Box 3, drawn from the pharmacology and clinical effects of cardiac glycosides.
Figure 1.
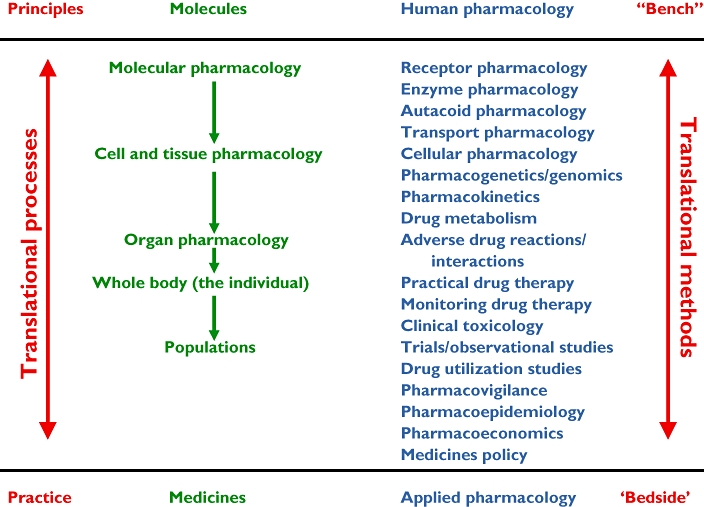
An extensional definition of clinical pharmacology in a framework that encompasses the topics covered by clinical pharmacology, from basic pharmacology in humans (human pharmacology) to all aspects of applied pharmacology in individuals and populations. Receptor pharmacology includes ligand–receptor binding studies and studies of second messengers. Cellular pharmacology includes all biophysical aspects of cell function. Autacoid pharmacology includes the effects of drugs on substances such as hormones, cytokines, and neurotransmitters. Practical drug therapy includes all aspects as practised at the bedside or in the clinic, including rational prescribing, medication errors, and adherence to therapeutic regimens. Clinical trials include meta-analysis and teleoanalysis. Medicines policy includes formulary construction, research planning and commissioning, pre- and post-marketing drug regulation, and advisory roles
Box 3 How the actions of cardiac glycosides are translated into pharmacological, physiological, and clinical effects (see Figure 1)
Molecular pharmacology Cardiac glycosides inhibit Na/K-ATPase
Cellular pharmacology Intracellular sodium and calcium concentrations change
Cellular physiology The electrophysiological properties of cardiac conducting cells change
Organ physiology Through this (and other actions) the ventricular rate in atrial fibrillation slows
Individual effects The signs and symptoms of that arrhythmia are relieved
Population effects A clinical trial of such therapy could show what the benefits and harm of such treatment would be in the affected population and how cost-effective it is
This paradigm gives a framework for the ways in which the pharmacological (including toxicological) properties of medicines can be studied:
Molecular pharmacology
pharmacodynamic effects mediated through receptors, autacoids, enzymes, transporters;
pharmacokinetics (e.g. protein binding).
Cellular and tissue pharmacology
pharmacodynamics (pharmacology, biochemistry, physiology);
pharmacokinetics (e.g. drug distribution) and [the biochemistry of] drug metabolism;
the pharmacodynamic and pharmacokinetic effects of genetic variants.
Organ pharmacology
pharmacokinetics (e.g. organ clearance);
pharmacodynamics;
adverse drug reactions and interactions.
Whole body (individual) pharmacology
pharmacodynamics;
adverse drug reactions and interactions;
practical drug therapy, including prescribing, n-of-1 studies, and monitoring therapy;
clinical toxicology;
psychological and behavioural factors that affect therapy (e.g. adherence).
Population pharmacology
randomized clinical trials and observational studies (such as case–control studies);
pharmacoepidemiology, including drug utilization studies;
pharmacovigilance;
pharmacoeconomics;
social factors that affect therapy;
medicines policy.
The various methods that clinical pharmacologists use to study these processes (such as ligand–receptor binding techniques, pharmacokinetic techniques, monitoring techniques, health economics) go ‘from bench to bedside’ (Figure 1, right-hand column, red).
This list of topics progresses from basic pharmacological studies in humans (human pharmacology), to its practical applications in individuals and populations (applied pharmacology). All of this together constitutes clinical pharmacology, thus extensionally defined.
The practice of clinical pharmacology – an operational definition
It is convenient to arrange this list of topics in a linear fashion, as shown in Figure 1. However, the repetition of certain items at each level, as listed above, shows that the linearity is imperfect. Furthermore, such an arrangement should not be taken to imply that that is how pharmacological science and practice actually progress. While it is true that the molecular actions of drugs are first translated into cellular effects and then into effects on organs and the whole body, that is not the way the subject is studied or necessarily analysed at the bedside. As every scientist knows, the linear narrative that constitutes the account of how the research was carried out is rarely if ever a true account of what actually happened.
In November 1944 President Roosevelt asked Vannevar Bush, Director of the Office of Scientific Research and Development, to advise the US Government on the further development of science in the anticipated aftermath of the Second World War. Bush reported in July 1945, outlining his vision [7], which became a dominating influence in scientific thinking [8]. Bush stressed the importance of basic research. Only by isolating basic research from ideas about its potential practical applications, he argued, would the former flourish and the latter be realized. Since then, the extent to which basic science and applied science individually contribute to technological developments has been hotly debated [9–12], but often with the implicit assumption that the progression is linear. Today, the concept of translational science, an idea that has not been well defined [13], is informed by this model, largely because of a strong emphasis on the role of the -omics, genomics, proteomics, metabolomics, and transcriptomics, which some have claimed are ‘the fundamentals of systems biology’ in a construct called ‘integrative genomics’ [14]. However, functions in biology emerge not in linear fashion but as a result of integration of different components of relevant systems at different levels, not merely the -omics [15]. Advances in clinical research often occur because of to-and-fro cross-talk between basic and clinical findings, in a manner that is familiar to those who have experienced the intellectual stimulus of collaboration across the scientific spectrum, whether they are clinical or non-clinical scientists. The importance of integrating pharmacology and clinical pharmacology cannot be overestimated.
A systems (or translational) approach to defining clinical pharmacology operationally is to depict it as a network (Figure 2). In this representation I have identified four discrete systems (the large ovals). These four systems are subdivided into two pairs. The top two systems are the basic tools of human pharmacology (left) and applied pharmacology (right); the bottom two are their practical applications in individuals (left) and populations (right). These systems are interconnected in many ways. For example, in pharmacokinetic–pharmacodynamic (PK/PD) studies the pharmacodynamic measurements that are used to investigate the way in which a system behaves can be taken from any level of drug action – molecular, cellular, organ, or whole body. At the applied end of the scale, the results of clinical trials and observational studies in large populations, including population dose–response curves and pharmacokinetics, inform clinical practice in the individual patient, and here the major feedback link is via evidence-based medicine, using techniques such as meta-analysis and teleoanalysis [16]; this in turn can inform basic science and pose further questions. For the sake of simplicity I have omitted some arrows from the diagram, for example the to-and-fro link between applied pharmacology and practical drug therapy. At the heart of all this, and providing missing links, are biomarkers, to which all aspects of pharmacology contribute; however, the diagram perforce simplifies these interactions – the different ways in which a relevant biomarker relates to the pathway that links the actions of a drug to its effects demand different, usually non-linear, models of such interactions [17]. Finally, drug development in all its pre- and post-marketing aspects hovers over the whole structure (the grey oval), feeding off all aspects of it. Indeed, one proposed definition of translational medicine in the context of pharmacology is ‘the application of biomedical research (pre-clinical and clinical), conducted to support drug development, which aids in the identification of the appropriate patient for treatment, the correct dose and schedule to be tested in the clinic and the best disease in which to test a potential agent’ [18].
Figure 2.
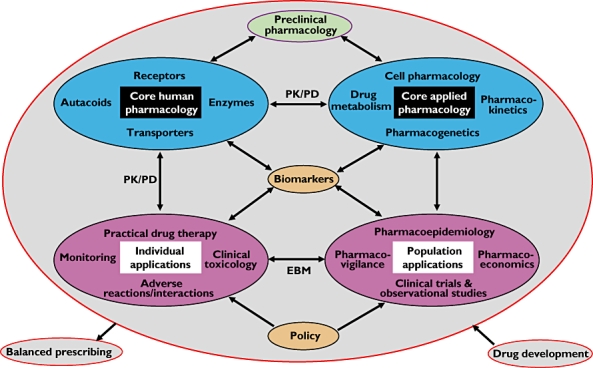
A non-linear operational definition of clinical pharmacology (see text for a full description); the sizes of the ovals do not reflect their relative importance
Pre-clinical pharmacology, indicated at the top of the diagram, could be subjected to similar analysis.
These two models (Figures 1 and 2) are not mutually exclusive. Each depicts an important aspect of what pharmacology means for both clinical and non-clinical scientists. Both models have something to say about the relation between clinical pharmacology and translational medicine. The extensional model in Figure 1 stresses the extensive scope of the subject. The operational model in Figure 2 shows how scientific and clinical developments go hand in hand and talk to each other, information from one area informing research in another, back and forth. It is neither entirely intensional nor extensional, but it does contain the essence of clinical pharmacology and shows how the subject is actually practised; it therefore figuratively constitutes an operational definition.
What clinical pharmacologists do
To begin again: a clinical pharmacologist is a medically qualified practitioner who teaches, does research, frames policy, and gives information and advice about the actions and proper uses of medicines in humans, and implements that knowledge in clinical practice. This definition contains four separate elements that constitute the activities of a clinical pharmacologist. I designate them by a simple mnemonic – MRCP. This is usually taken to stand for Member[ship] of the Royal College of Physicians, a qualification possessed by many UK clinical pharmacologists. However, in this case it stands for Mentoring, Research, Clinical work, and Policy [19]. These activities can be carried out in any of the spheres in which clinical pharmacologists are to be found – university departments, health-care services, drug companies and contract research organizations, and regulatory agencies.
Mentoring
Mentor was a character in the Odyssey, in whose likeness the goddess Athena appeared to Odysseus' son Telemachus, acting as his guide and tutor. The term entered English in the 18th century, strongly influenced by François de Salignac de la Mothe-Fénelon's novel Les Aventures de Télémaque (1699), in which the name was used for a counsellor.
A mentor is ‘a person who offers support and guidance to another’ and more specifically ‘an experienced person in a company, college, etc., who trains and counsels new employees or students’. To mentor is ‘to advise or train (someone, esp. a younger and less experienced colleague)’.
Teaching is the most important aspect of mentoring. Teaching in academic clinical pharmacology includes laboratory science and clinical science, in which case it encompasses not only the pharmacological aspects, but such subjects, where relevant, as biochemistry, physiology, statistics, and clinical medicine. Teaching also includes all aspects of practical drug therapy as underpinned by the science of pharmacology [20]. Those taught include research students, both clinical and non-clinical, medical students and doctors in training, senior colleagues in other specialties, pharmacists, and nurses. The importance of such teaching to the health of the nation cannot be overstated: teaching balanced prescribing [21], including the avoidance of medication errors, contributes to maximizing benefits and minimizing harm [22].
Another important part of a clinical pharmacologist's scholarly activity is the preparation of teaching materials, including journal articles, didactic textbooks, reference books, and e-learning materials, conveniently listed here under the heading of teaching, although sometimes also part of the framing of policy.
However, there is more to mentoring than simply teaching; it also includes sponsoring, protecting, and promoting recognition of one's junior colleagues, all of which are part and parcel of a clinical pharmacologist's mentoring duties [23]. In academic practice mentorship reportedly has important effects on personal development, career guidance, career choice, and research productivity, including publication and grant success, although hard evidence supporting this is scanty [24]. Although a mentor usually guides a younger or less experienced colleague, the definition does not exclude what has been termed ‘peer mentoring’, which may be important for professional and personal development.
Research
I have outlined the research activities of clinical pharmacologists above, stressing the translational aspects of clinical pharmacology. Not only do academic clinical pharmacologists deal with drug-related problems at any level, from molecular pharmacology to drug therapy in populations, and including all aspects of toxicology; there are also no boundaries to the types of clinical research that they, and their counterparts in drug companies, can undertake, since their interests span all medical specialties in which drug therapy is involved. This means that collaborative research is common; my own résumé includes collaborations with basic pharmacologists and pharmacists; with anaesthetists, cardiologists, epidemiologists, gastroenterologists, general practitioners, nephrologists, neurologists, obstetricians, psychiatrists, and surgeons; with biochemists and physiologists; with engineers, an economist, and a philosopher. Much original research in drug discovery and development goes on in drug companies [25]; clinical pharmacologists play important roles in drug companies and contract research organizations, taking part in all phases of drug development, including pharmacoeconomic assessments.
The methods that are used in this research are multifarious, and in addition to the tools of pharmacology include biochemical, physiological, genetic, statistical, and epidemiological techniques. They may also involve thought experiments, including definition of terms [2–4] and classification of systems [26, 27].
Clinical work
Clinical pharmacologists are physicians and expert prescribers. A physician is ‘a person who is trained and qualified to practise medicine; esp. one who practises medicine as opposed to surgery’. That implies membership of the Royal College of Physicians in the UK and equivalent accreditation elsewhere. Not all clinical pharmacologists practice clinical medicine, but those who are employed in academic departments or health-care services mostly work as physicians in general medicine or clinical toxicology. Many have a special clinical interest, such as hypertension, asthma, or epilepsy, which may feed their research. Some are clinical toxicologists, dealing with poisoning, drugs of abuse, and the toxicology of non-therapeutic drugs and chemicals. In out-patient clinics clinical pharmacologists manage general medical problems as well as patients with specific drug-related problems. They will often receive written or telephoned requests, from general practitioners or hospital colleagues, for information and advice about drug-related problems in patients who do not merit direct referral, particularly in these days of increasing financial parsimony.
Clinical expertise is also important in the design and conduct of drug trials, at all phases of drug development, whether in academic departments, drug companies or contract research organizations, and in understanding their implications in drug regulation and pharmacovigilance.
Policy
Under this heading I include local, national, and international policy related to medicines. These activities take many forms, such as formulary development, medicines licensing, prescribing policies, and development of guidelines. Most are undertaken part time, such as membership or chairmanship of committees, a selection of which is given in Box 4; clinical pharmacologists serve on all of these. In drug companies work that can be listed under this heading includes preparation and assessment of company dossiers during drug development, pharmacovigilance, assessment of the benefit to harm balance (‘risk-benefit’ analysis) and the development of risk management policies.
Box 4 UK committees and organizations to which clinical pharmacologists make important contributions
Local Drug and Therapeutics Committees
The Medicines and Healthcare products Regulatory Agency (MHRA)
The Commission on Human Medicines
The National Institute of Health and Clinical Excellence (NICE) and its Technology Appraisal Committees
The Scottish Medicines Consortium (SMC)
The All Wales Medicines Strategy Group
Committees of the British National Formulary (BNF), including the Joint Formulary Committees of the BNF and the BNF for Children
Subcommittees of the British Pharmacopoeia Commission
The Herbal Medicines Advisory Committee
Government and charitable grant-giving bodies (e.g. the Health Technology Appraisal Programme)
Some clinical pharmacologists hold permanent positions in regulatory authorities, such as the MHRA, NICE, and the European Medicines Agency (EMEA). A few carry out research on medicines policy.
Clinical pharmacologists are also often called upon to give advice outside clinical medicine and areas of medicines policy, and some have set up individual consultancies to provide advice in a wide range of areas related to drug discovery, development, and use. These activities include consultation by drug companies about drug development and advice in legal cases, such as patent disputes or criminal cases involving medicines or drugs of abuse. They may also be called upon by the media to comment on drug-related events of public interest.
Doing the work
Asking a clinical pharmacologist to outline a typical week is fruitless – the work is so varied that no week is typical. However, in Table 1 I have listed some of the activities that an academic clinical pharmacologist might undertake in any atypical week. Table 2 contains similar details for an NHS clinical pharmacologist, Table 3 for one working in a regulatory authority, and Table 4 for one working in a drug company.
Table 1.
Possible activities of an academic clinical pharmacologist in any atypical week
| Day | am | pm |
|---|---|---|
| Monday | Ward round (business and undergraduate teaching) – 25 patients with a wide range of medical conditions, some drug-induced | Chair Drug and Therapeutics Committee (lunch-time); out-patients clinic (general medicine and drug-related problems); advise colleagues on designing a pharmacokinetic study of a new drug |
| Tuesday | NICE technology appraisal committee: management of osteoporosis | NICE technology appraisal committee: a new treatment for multiple sclerosis |
| Wednesday | Research with NHS colleague; scrutinize proofs of the next issue of the British National Formulary; acute take round (evening) | |
| Thursday | Write papers, edit international encyclopaedia on adverse drug reactions | General medical grand round; radiology meeting; student case presentations |
| Friday | Ward round (business and undergraduate teaching); research meeting | Research; library; applied pharmacology in the Rose & Crown with PhD students |
| Weekend and evenings | Clinical work, writing, editing, preparation for committees/consultancies | |
Table 2.
Possible activities of an NHS clinical pharmacologist in any atypical week
| Day | am | pm |
|---|---|---|
| Monday | Ward round (business and undergraduate teaching); library | Chair Drug and Therapeutics Committee (lunch-time); out-patients clinic (general medicine, new and returning patients) |
| Tuesday | Research with academic colleague; scrutinize proofs of the next issue of the British National Formulary; acute take round (evening) | |
| Wednesday | Lecture (e.g. advanced medicine course); other postgraduate teaching; library | NICE technology appeals committee: a new treatment for Alzheimer's disease |
| Thursday | Write papers; library | General medical grand round; radiology meeting; student case presentations |
| Friday | Ward round (business and undergraduate teaching) | Out-patients clinic (hypertension, new and returning patients) |
| Weekend and evenings | Clinical work, writing, editing, preparation for committees/consultancies | |
Table 3.
Possible activities of a clinical pharmacologist working in a regulatory authority in any atypical week
| Day | am | pm |
|---|---|---|
| Monday | Plan a week's dossier assessment work from drug companies; continue assessment of current case work; start new applications against agreed targets | |
| Tuesday | Meeting with a pharmaceutical company for an advisory session on a proposed application | Continue with scheduled assessments |
| Wednesday | Attend a unit meeting to discuss projects, share problems, receive new assignments | Continue with scheduled assessments |
| Thursday | Attend a meeting of the Commission on Human Medicines; present an advisory paper on a drug application/pharmacovigilance problem; respond to questions; contribute to discussion | |
| Friday | Visit EMEA as part of a specific working party on (for example) pharmacovigilance | |
| Weekend and evenings | Try to keep up with an ever-growing pile of dossiers for assessment | |
Table 4.
Possible activities of a clinical pharmacologist in a drug company in any atypical week
| Day | am | pm |
|---|---|---|
| Monday | Work on experimental medicine study protocol | Teleconference with FDA to discuss design of a drug–drug interaction study during phase III development |
| Tuesday | Project team meeting to design early-phase development plan for a new chemical entity | |
| Wednesday | Present development proposals to internal multidisciplinary management group | Review report of a serious adverse event from a current study; speak to investigator to obtain additional information; prepare report and discuss with safety department |
| Thursday | Work on a study report for a recently completed phase II study | Meet with external academic expert to discuss design of experimental medicine study |
| Friday | Chair dose escalation meeting for first-in-man study currently in progress | Review headline data from recently completed phase II study |
| Weekend and evenings | On call for medical advice for current clinical studies; prepare abstracts/manuscripts of completed studies | |
A model for the dissemination of expertise in clinical pharmacology
During the 1960s the specialty of clinical pharmacology and therapeutics (CPT) grew to the point where the Royal College of Physicians of London (1969) [28] and the World Health Organization (1970) [29] thought it worth reporting on the future of the subject [30]. The specialty grew in the UK, although not to the extent that those reports had proposed. Then, starting in 1993, the numbers of consultant clinical pharmacologists began to fall. There are currently an estimated 50–60 in all, insufficient to fulfil all the tasks outlined above. However, in the last 4 years there has been increasing awareness outside of the specialty that more clinical pharmacologists are needed, not least in order to deliver essential teaching in medical schools, but also to train specialists, both to maintain a critical mass and to provide staff for drug companies, and to carry out high-class translational research; one can confidently expect that the numbers will again start to increase [31]. Nevertheless, it is unlikely that there will ever be enough posts to fulfil the latest recommendations of the Royal College of Physicians [32], namely that ‘[acknowledging] the shortage of trained individuals and their importance to the future use of drugs in the NHS … the workforce requirement for consultants in clinical pharmacology [in the UK] is approximately 200 whole-time equivalents … [requiring] an expansion of at least 10% per annum over the next decade’ and that ‘there is a strong case to be made for having one whole-time equivalent clinical pharmacologist in every large district general hospital for acute medicine’.
Welcome though these recommendations are, a more realistic expectation would be as outlined in Figure 3. It is essential to maintain a core of dedicated academic clinical pharmacologists (inner ring, yellow and mauve); a realistic target in the UK would be about 100 such individuals, performing the various activities outlined above (‘MRCP’), with clinical duties mainly in general medicine and toxicology. Even so, that would not be enough to fulfil all requirements, particularly teaching. The shortfall in the health-care services should be made good by a cadre of core clinical pharmacologists working outside academic clinical pharmacology departments (middle ring, orange and blue), trained in both clinical pharmacology and a second medical specialty, the latter being the career specialty, such as cardiology, geriatrics, or general practice [33, 34]. Others in this group would fulfil the urgent need for clinical pharmacologists in drug companies and contract research organizations and in regulatory authorities.
Figure 3.
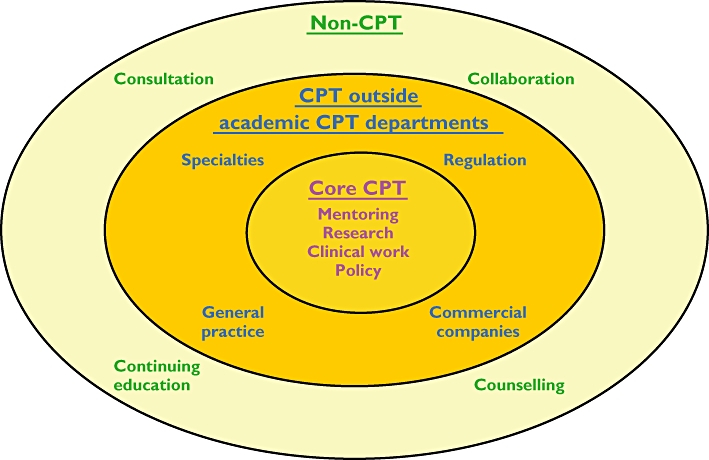
A diagrammatic representation of how expertise in clinical pharmacology should be created and disseminated in a clinical environment
As the only clinical specialty that still includes a substantial amount of research training, clinical pharmacology is an attractive option for those who want to pursue a career in academic medicine in no matter what specialty, and should be one of the disciplines used in training Foundation Year doctors on special research programmes [35, 36] and in subsequent Academic Clinical Fellowships [37]. That there is interest in clinical pharmacology as a training ground is evidenced by the large disparity between the number of core clinical pharmacologists in the UK (currently about 50–60) and the number of medical practitioners registered with the General Medical Council [38] as having been trained in CPT – currently about 200. Of the latter, who include the former, 27 are retired, some work in drug companies, contract research organizations, or regulatory agencies and some have gone abroad; however, at least 45 are employed in other medical specialties, including general medicine, cardiology, geriatrics, respiratory medicine, endocrinology, rheumatology, gastroenterology, and public health. Such individuals contribute to teaching and research while in training and then carry their pharmacological expertise into other disciplines.
Those in the two core sectors in this model are in a position to influence the use of medicines in the wider medical and non-medical communities (outer ring, cream and green) by research collaboration, continuing education, clinical consultation, and counselling (‘peer mentoring’).
Conclusion
It is important to distinguish between clinical pharmacology as a discipline in all its aspects (Figure 1) and the clinical pharmacologists who practise it. This is why my definition of a clinical pharmacologist includes the requirement of a medical qualification.
It is possible for many types of individual, medically qualified or not, to contribute to different aspects of the discipline, but each does it in a different way. For example, basic pharmacologists have important roles in different aspects of drug discovery and development; much innovative work in pharmacokinetic theory has been contributed by academic pharmacists, and clinical pharmacists can contribute to practical drug utilization. However, only those with a medical qualification can practise the discipline across the whole waterfront of its activities (Figure 1), since many of the items in the list relate directly to medicine as it is practised at the bedside or in the clinic, and an understanding of their complex inter-relationships (Figure 2) requires medical expertise and extensive experience of prescribing.
An important perspective of this manifesto is the need to train clinical pharmacologists who are equipped to work in academic departments, in drug companies or contract research organizations, and in regulatory authorities, to treat patients, to teach, and to provide information and advice in numerous settings in which policy decisions need to be made. We need to reverse the downward trend of such training that has occurred in the last 20 years [31]. This training is best done, initially at least, in academic departments of pharmacology and clinical pharmacology, although it can be, and often is, continued in CPT settings outside academic departments, as depicted in the middle area in Figure 3. Without bedside experience this training cannot be properly fulfilled.
I prefer to refer to those who contribute to the discipline but are not medically qualified as applied pharmacologists, since even at a time when the word ‘clinical’ has been arrogated by others, such as clinical pharmacists, clinical nurse assistants, clinical psychologists and so on, who may not be medically qualified, the word ‘clinical’ strongly implies medicine at the bedside or in the clinic. Without medical training there are many areas in which such individuals cannot fulfil all the aspects of the discipline of clinical pharmacology and are therefore not clinical pharmacologists.
This point was firmly brought home by Dollery, in a review of the history of clinical pharmacology, in which he outlined the fundamental problem of defining clinical pharmacology [39]. Is it, he asked, a laboratory discipline dealing with biomarkers, pharmacokinetics, drug metabolism, and genetics, based on human samples? Or a desk discipline dealing with design and evaluation of clinical trials, drug utilization on local and national levels, clinical guidelines for drug use, and pharmacovigilance? Or a hands-on clinical discipline dealing with patient care, experimental studies of old and new drugs, clinical investigation of adverse reactions and interactions, and consultancy services to other clinicians? The answer to this compound question is that clinical pharmacology is all of those things and more.
As laboratory researchers, clinical pharmacologists rank with other basic scientists as contributors to drug discovery and development.
As reviewers and interpreters of data about medicines they stand beside epidemiologists and statisticians as contributors to drug development and understanding drug action.
As clinicians they teach their students, inform and advise their colleagues, and complement the activities of their colleagues in other clinical specialties as contributors to practical drug therapy.
As policy makers they complement the contributions of their colleagues in all fields related to the use of medicines.
However, clinical pharmacologists are the only specialists who bring all of those attributes together in the study of the actions of medicines and the application of the basic science of pharmacology to practical drug therapy. And they are, par excellence, medical practitioners and expert prescribers.
Acknowledgments
I am grateful to a large number of pharmacologists and clinical pharmacologists, too many to name individually, in academic departments, the NHS, regulatory agencies, and commercial companies for helpful input at several stages in the drafting of this manifesto.
Competing interests
JKA is a President Emeritus of the British Pharmacological Society; however, the opinions expressed here are personal and may not be shared by other members of the Society. No funding was received for the preparation of this document.
REFERENCES
- 1.Oxford English Dictionary [online] http://ezproxy.ouls.ox.ac.uk:2118/entrance.dtl.
- 2.Laurence DR, Carpenter JR. A Dictionary of Pharmacology and Allied Topics. 2nd. Amsterdam: Elsevier; 1998. [Google Scholar]
- 3.Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28:851–70. doi: 10.2165/00002018-200528100-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug Saf. 2006;29:1011–22. doi: 10.2165/00002018-200629110-00001. [DOI] [PubMed] [Google Scholar]
- 5.Aronson JK. Medication errors: definitions and classification. Br J Clin Pharmacol. 2009;67:599–604. doi: 10.1111/j.1365-2125.2009.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurence D, Carpenter J. A Dictionary of Pharmacology and Clinical Drug Evaluation. London: UCL Press; 1994. [Google Scholar]
- 7.Bush V. Science – the Endless Frontier. A Report to the President on A Program for Postwar Scientific Research. Washington, DC: Office of Scientific Research and Development; 1945. [Google Scholar]
- 8.Stokes DE. Pasteur's Quadrant. Basic Science and Technological Innovation. Washington, DC: Brookings Institution Press; 1997. [Google Scholar]
- 9.Comroe JH, Jr, Dripps RD. Scientific basis for the support of biomedical science. Science. 1976;192:105–11. doi: 10.1126/science.769161. [DOI] [PubMed] [Google Scholar]
- 10.Smith R. Comroe and Dripps revisited. Br Med J (Clin Res Ed) 1987;295:1404. doi: 10.1136/bmj.295.6610.1404. Erratum: 1988; 296: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies SC, Scarffe H. London: The Wellcome Trust Publishing Department; 2001. Putting NHS research on the map. An analysis of scientific publications in England, 1990–97. Available at http://www.wellcome.ac.uk/stellent/groups/corporatesite/@policy_communications/documents/web_document/wtd003193.pdf (last accessed 29 December 2009. [Google Scholar]
- 12.Grant J, Green L, Mason B. From bedside to bench: Comroe and Dripps revisited. 2003. HERG Research Report No. 30. August Available at http://www.brunel.ac.uk/3289/Herg1/RR30.pdf (last accessed 29 December 2009.
- 13.Dougherty ER. Translational science: epistemology and the investigative process. Curr Genomics. 2009;10:102–9. doi: 10.2174/138920209787847005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrowski J, Wyrwicz LS. Integrating genomics, proteomics and bioinformatics in translational studies of molecular medicine. Expert Rev Mol Diagn. 2009;9:623–30. doi: 10.1586/erm.09.41. [DOI] [PubMed] [Google Scholar]
- 15.Noble D. The Music of Life. Biology Beyond the Genome. Oxford: Oxford University Press; 2006. [Google Scholar]
- 16.Wald NJ, Morris JK. Teleoanalysis: combining data from different types of study. BMJ. 2003;327:616–8. doi: 10.1136/bmj.327.7415.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronson JK. Biomarkers and surrogate endpoints in monitoring therapeutic interventions. In: Glasziou P, Irwig L, Aronson JK, editors. Evidence-Based Medical Monitoring: from Principles to Practice. Oxford: Wiley-Blackwell; 2008. pp. 48–62. [Google Scholar]
- 18.Johnstone D. Translational science – a sexy title for pre-clinical and clinical pharmacology. pA2 Online. 2006;4:2. Available at http://www.pa2online.org/articles/article.jsp?volume=5&issue=&article=54 (last accessed 29 December 2009. [Google Scholar]
- 19.Aronson JK. FitzPatrick Lecture, Royal College of Physicians, London, 2007. Clinical pharmacology: a suitable case for treatment. Available at https://admin.emea.acrobat.com/_a45839050/p74648562/last accessed 29 December 2009.
- 20.Maxwell S, Walley T BPS Clinical Section Committee. Teaching safe and effective prescribing in UK medical schools: a core curriculum for tomorrow's doctors. Br J Clin Pharmacol. 2003;55:496–503. doi: 10.1046/j.1365-2125.2003.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62:629–32. doi: 10.1111/j.1365-2125.2006.02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Likic R, Maxwell SR. Prevention of medication errors: teaching and training. Br J Clin Pharmacol. 2009;67:656–61. doi: 10.1111/j.1365-2125.2009.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambunjak D, Marui A. Mentoring: what's in a name? JAMA. 2009;302:2591–2. doi: 10.1001/jama.2009.1858. [DOI] [PubMed] [Google Scholar]
- 24.Sambunjak D, Straus SE, Marui A. Mentoring in academic medicine. A systematic review. JAMA. 2006;296:1103–15. doi: 10.1001/jama.296.9.1103. [DOI] [PubMed] [Google Scholar]
- 25.Rang H, editor. Drug Discovery and Development. Technology in Transition. Edinburgh: Churchill Livingstone; 2005. [Google Scholar]
- 26.Aronson JK, Ferner RE. Joining the DoTS. New approach to classifying adverse drug reactions. BMJ. 2003;327:1222–5. doi: 10.1136/bmj.327.7425.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferner RE, Aronson JK. EIDOS: a mechanistic classification of adverse drug effects. Drug Saf. 2010;33:13–23. doi: 10.2165/11318910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Clinical Pharmacology Committee. Clinical Pharmacology in the Reorganised National Health Service. London: Royal College of Physicians; 1969. revised 1975. [Google Scholar]
- 29.WHO Study Group. Clinical Pharmacology. Scope, Organization, Training. Geneva: Wld Hlth Org Techn Rep Series; 1970. no. 446. [PubMed] [Google Scholar]
- 30.Aronson JK. On being 30. Br J Clin Pharmacol. 2004;57:1–5. [Google Scholar]
- 31.Aronson J. Clinical pharmacology in the UK – a great instauration. Br J Clin Pharmacol. 2010;69:111–7. doi: 10.1111/j.1365-2125.2009.03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Advisory Appointment Committees. Consultant Physicians Working with Patients. The Duties, Responsibilities and Practice of Physicians. 4th. London: Royal College of Physicians; 2008. Available at http://www.rcplondon.ac.uk/pubs/brochure.aspx?e=253 (last accessed 29 December 2009. [Google Scholar]
- 33.Walley T. Rational prescribing in primary care – a new role for clinical pharmacology? Br J Clin Pharmacol. 1993;36:11–2. doi: 10.1111/j.1365-2125.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mant D. Clinical pharmacology and primary care. Pharmacol Matters. 2009;2:20–2. [Google Scholar]
- 35.UK Clinical Research Collaboration. Clinical Academic Careers for Doctors and Dentists. Available at http://www.ukcrc.org/workforcetraining/doctorsanddentists (last accessed 29 December 2009.
- 36.Stanley AG, Williams B, Gallen D. A novel foundation-year-two post in academic medicine. J R Soc Med. 2005;98:10–3. doi: 10.1258/jrsm.98.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Academy of Medical Sciences. National Clinician Scientist scheme. Available at http://www.acmedsci.ac.uk/p57.html (last accessed 29 December 2009.
- 38.General Medical Council. The Medical Register. Available at http://www.gmc-uk.org/doctors/medical_register.asp (last accessed 31 August 2009.
- 39.Dollery CT. Clinical pharmacology – the first 75 years and a view of the future. Br J Clin Pharmacol. 2006;61:650–65. [Google Scholar]


