Abstract
AIM
This randomized, double-blind, crossover study compared post-prandial hormonal and metabolic effects of vildagliptin, (an oral, potent, selective inhibitor of dipeptidyl peptidase IV [DPP-4]) administered morning or evening in patients with type 2 diabetes.
METHODS
Forty-eight patients were randomized to once daily vildagliptin 100 mg administered before breakfast or before dinner for 28 days then crossed over to the other dosing regimen. Blood was sampled frequently after each dose at baseline (day −1) and on days 28 and 56 to assess pharmacodynamic parameters.
RESULTS
Vildagliptin inhibited DPP-4 activity (>80% for 15.5 h post-dose), and increased active glucagon-like peptide-1 compared with placebo. Both regimens reduced total glucose exposure compared with placebo (area under the 0–24 h effect–time curve [AUE(0,24 h)]: morning −467 mg dl−1 h, P = 0.014; evening −574 mg dl−1 h, P = 0.003) with no difference between regimens (P = 0.430). Reductions in daytime glucose exposure (AUE(0,10.5 h)) were similar between regimens. Reduction in night-time exposure (AUE(10.5,24 h) was greater with evening than morning dosing (−336 vs. −218 mg dl−1 h, P = 0.192). Only evening dosing significantly reduced fasting plasma glucose (−13 mg dl−1, P = 0.032) compared with placebo. Insulin exposure was greater with evening dosing (evening 407 µU ml−1 h; morning 354 µU ml−1 h, P = 0.050).
CONCLUSIONS
Both morning and evening dosing of once daily vildagliptin 100 mg significantly reduced post-prandial glucose in patients with type 2 diabetes; only evening dosing significantly decreased fasting plasma glucose. Although evening dosing with vildagliptin 100 mg tended to decrease night-time glucose exposure more than morning dosing, both regimens were equally effective in reducing 24 h mean glucose exposure (AUE(0,24 h)) in patients with type 2 diabetes.
Keywords: DPP-4, GLP-1, pharmacokinetics, type 2 diabetes, vildagliptin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Vildagliptin is an orally active, potent inhibitor of dipeptidyl peptidase IV and was developed for the treatment of type 2 diabetes.
In clinical trials, once or twice daily dosing with vildagliptin (up to 100 mg day−1) has been shown to reduce endogenous glucose production and fasting plasma glucose in patients with type 2 diabetes.
The comparative efficacy of vildagliptin under a morning vs. evening dosing regimen has not previously been determined.
WHAT THIS STUDY ADDS
Once daily dosing with vildagliptin 100 mg for 28 days improved glycaemic control in patients with type 2 diabetes independent of whether vildagliptin was administered in the morning or evening.
Morning or evening dosing with vildagliptin had similar effects on 24 h glycaemic control and plasma concentrations of the hormones insulin, glucagon and glucagon-like peptide 1.
Introduction
Dipeptidyl peptidase IV (DPP-4) inhibitors represent a new approach to the treatment of type 2 diabetes [1, 2]. DPP-4 inactivates the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide, which are released into the bloodstream in response to food intake and stimulate the secretion of insulin in a glucose-dependent manner [3]. GLP-1 has other potentially beneficial metabolic effects, including inhibition of glucagon release [4, 5], slowing of gastric emptying [6], and appetite suppression [7]. DPP-4 acts rapidly to inactivate GLP-1; hence GLP-1 has a very short half-life of approximately 2 min. In patients with type 2 diabetes, the incretin effects of GLP-1 are not reduced although GLP-1 secretion is impaired [8, 9]. Inhibition of DPP-4 activity enhances and prolongs the effects of GLP-1 and thus improves glycaemic control in patients with type 2 diabetes.
Vildagliptin (LAF237) is an orally active, potent and selective inhibitor of DPP-4, which has been developed for the treatment of type 2 diabetes [10–12]. Vildagliptin has been shown to increase post-prandial concentrations of active GLP-1 compared with placebo [13], improve β-cell function and insulin sensitivity [14], and reduce fasting and post-prandial plasma glucose concentrations and concentrations of glycosylated haemoglobin (HbA1c) [15–17] in studies of patients with type 2 diabetes. The clinical efficacy of vildagliptin at differing dosages has been demonstrated in long-term clinical trials in previously treated and untreated patients with type 2 diabetes [18–21].
Vildagliptin may be administered either once or twice daily; trials in patients with type 2 diabetes have compared once daily morning dosing [22, 23] and/or twice daily dosing [11, 15, 16, 23, 24] with placebo. In a study in patients with type 2 diabetes [11], twice daily administration of vildagliptin 10 mg, 25 mg and 100 mg led to average DPP-4 inhibition of 68%, 86% and 96%, respectively, over a 24 h period. High levels of inhibition were maintained throughout the 24 h period for the 25 mg and 100 mg doses; however, the lower 10 mg dose allowed significant recovery of DPP-4 activity to ∼30% inhibition. This suggests that the average DPP-4 inhibition over 24 h must exceed ∼70% to achieve clinically relevant antihyperglycaemic effects. These results are consistent with a study in mice in which the maximum glucose response occurred with ≥80% DPP-4 inhibition [25]. The sustained DPP-4 inhibition observed with vildagliptin irrespective of its short half-life is mostly attributed to the very high potency of DPP-4 inhibition by vildagliptin (IC50 = 4.5 nmol l−1). The peak plasma concentration after a single dose of vildagliptin 100 mg was ∼2000 nmol l−1 [10]. In patients with type 2 diabetes, a single 100 mg dose of vildagliptin given before the evening meal inhibited DPP-4 activity >95% for over 12 h and decreased endogenous glucose production throughout the overnight post-absorptive period. The reduction in fasting glucose was directly proportional to that in endogenous glucose production, suggesting that evening dosing may have additional benefit on reducing fasting plasma glucose by inhibiting more effectively endogenous glucose production [26].
This study was designed to determine whether the time of dosing (morning dosing before breakfast compared with evening dosing before dinner) would influence the hormonal and metabolic responses to vildagliptin. The primary objective was to compare the pharmacodynamic effects of daily administration of vildagliptin 100 mg given in the morning or evening over 28 days in patients with type 2 diabetes. The study examined the effects of the two dosing regimens on mean plasma glucose and glucose excursions, plasma insulin, glucagon, active GLP-1 concentrations, and DPP-4 activity over 24 h. The pharmacokinetics, safety and tolerability of each dosing regimen were also assessed.
Methods
Study population
Men and women, aged 18–75 years, with type 2 diabetes diagnosed at least 3 months before screening, were eligible for enrolment in the study. For inclusion, patients had to have a fasting plasma glucose of 100–270 mg dl−1 (5.5–15.0 mmol l−1) at screening and baseline, HbA1c of 6.0–10.5% at screening and a body mass index of ≤40 kg m−2. Women were post-menopausal, or had to have been surgically sterilized or using adequate contraception for at least 6 months before screening. Patients whose diabetes was controlled by diet and exercise alone or who were able or willing to undergo a 3 week washout of anti-diabetic drugs were also eligible.
The exclusion criteria included type 1 diabetes, secondary forms of diabetes, complications of diabetes (e.g. ketoacidosis) within the preceding 6 months, an acute cardiovascular event within 6 months of screening, or treatment with any hypoglycaemic agent within 3 weeks of the start of the study or with anti-arrhythmics, β-adrenoceptor blockers, antihypertensive or lipid-lowering agents (unless on a stable dose for at least 3 months before screening), insulin (>4 weeks' treatment in the absence of an intercurrent illness), thiazolidinediones within 6 months before screening, or corticosteroids (>7 consecutive days' treatment) within 8 weeks before screening; fasting triglyceride concentrations above 450 mg dl−1 (5.1 mmol l−1) at screening. Thyroid hormone replacement was allowed if the dosage was stable for at least 3 months and thyroid stimulating hormone concentrations were within normal limits at screening.
The study was performed in compliance with good clinical practice and the ethical principles of the Declaration of Helsinki of the World Medical Association. Ethical approval was provided by the Research Consultants Review Committee Institutional Review Board, Austin, TX, USA. The study was conducted at Diabetes and Glandular Disease Research Associates in San Antonio, TX, USA. All participants provided written informed consent prior to study participation.
Study design
This was a randomized, double-blind, two-period crossover study, consisting of a 28 day screening period (day −28 to day −1) followed by two 28 day treatment periods. Following the initial screening visit, patients underwent a 21 day washout of all hypoglycaemic medications. Blood samples were collected on day −7 to assess the fasting plasma glucose concentrations for patients meeting the inclusion criteria; baseline assessments to confirm eligibility were performed on day −3 after a 10 h fast. On day −2, eligible patients began a 2 day, single-blind, run-in period during which they received placebo tablets twice daily.
Following the placebo run-in period, patients were randomized to either the morning dosing schedule (one vildagliptin 100 mg tablet in the morning 30 min before breakfast; one placebo tablet in the evening) or the evening dosing schedule (one placebo tablet in the morning, one vildagliptin 100 mg tablet in the evening 30 min before dinner) for 28 days (days 1–28). Patients who continued to meet all inclusion criteria then received the other treatment regimen for an additional 28 days (days 29–56). Randomization was performed by Novartis Drug Supply Management using a validated automated system.
Patients were domiciled at the study centre during all baseline assessments, placebo run-in period, randomization (day −4 to day 1), and all assessments at the end of each treatment period (days 26–29 and 54–57). Patients also returned to the study centre for safety assessments on days 14 and 42. When domiciled, patients received standardized meals at 08.00 h (breakfast), 12.00 h (lunch), and 18.00 h (dinner). Study medication was administered with 200 ml water 30 min before breakfast (after at least a 10 h fast) and 30 min before dinner. When not domiciled, medication was self-administered.
Pharmacokinetic measurements
Blood samples (1 ml) for plasma drug concentrations were collected into sodium or lithium heparin tubes at the following time points: 0 (pre-dose) and 0.5, 1, 1.5, 2, 3, 5, 7, 10, 10.5, 11, 11.5, 12, 13, 15, 17 and 24 h after the morning dose on days 28 and 56. Samples were centrifuged for 15 min at approximately 2500 rev min−1 at 3–5°C within 15 min of collection; the extracted plasma was frozen at −70°C or below until the analyses were performed.
Plasma concentrations of vildagliptin were measured using an HPLC–MS/MS method [27, 28]. The assay consisted of a liquid–solid extraction on Oasis HLB 96-well extraction plates using an automated system, followed by HPLC using a XTerra MS C18 5 µm column (Waters Corporation, Milford, MA, USA) with isocratic elution using 40% mobile phase A (10 mmol l−1 ammonium acetate [adjusted to pH 8 with ammonia solution]–methanol [95:5 v : v]) and 60% mobile phase B (acetonitrile–methanol [10:90 v : v]) at a flow rate of 0.20 ml min−1. Detection was performed by MS/MS with electrospray ionization using an API 3000 (Applied Biosystems, Foster City, CA, USA) mass spectrometer in positive ion mode. The internal standard for this assay was [13C515N]vildagliptin. The masses for vildagliptin were precursor ion m/z 304 and product ion m/z 154, and for [13C515N]vildagliptin precursor ion m/z 310 and product ion m/z 160. The lower limit of quantification (LLOQ) for the assay was 2.0 ng ml−1. Within-study assay validation at nominal vildagliptin concentrations of 5.25, 400 and 900 ng ml−1 showed an assay precision (coefficient of variation [CV]) of 6.9% to 9.6% and a bias of −2.1% to 4.8%.
Pharmacokinetic parameters were calculated based on non-compartmental methods using WinNonlin Pro (Version 4.1, Pharsight Corp., Mountain View, CA, USA) and included Cmax (maximum plasma concentration), tmax (time to reach Cmax), t1/2 (half-life of the terminal disposition phase), AUC(0,τ) (area under the plasma concentration–time curve from time 0 to τ h), and AUC(0,∞) (area under the plasma concentration–time curve from time 0 extrapolated to infinity).
Pharmacodynamic measurements
Blood samples for assessment of pharmacodynamic parameters were collected on days −1, 28 and 56. Samples were collected for measurement of DPP-4 activity (1 ml blood collected into potassium EDTA tubes) pre-dose and at frequent intervals during the 24 h following the morning dose. Blood samples were collected for measurement of GLP-1 (2 ml blood collected into potassium EDTA tubes to which 0.1 ml of a 3 mmol l−1 diprotin A solution had been added), glucagon (2 ml blood collected into a serum separator vacutainer tube containing aprotinin), and glucose and insulin (2.5 ml blood collected into a serum separator vacutainer tube containing heparin) at frequent intervals during the 24 h following the morning dose. All samples were centrifuged for 15 min at approximately 2500 rev min−1 at 3–5°C within 15 min of collection; the extracted plasma was frozen at −70°C or below (−80°C or below for glucagon) until analysis was performed.
DPP-4 activity was determined using a fluorescent enzymatic assay as described previously [10, 28]. The LLOQ for DPP-4 activity was 0.24 mU ml−1 min−1. GLP-1 concentrations were measured by Novartis using the GLP-1 ELISA kit (Linco Research, Inc., St. Charles, MO, USA), with a LLOQ of 5 pmol l−1. Pharmacodynamic measurements of serum glucose, insulin and glucagon were measured by Northwest Lipid Research Laboratories (Seattle, WA, USA) using standardized procedures.
Plasma concentration–time profiles for each of the pharmacodynamic variables (DPP-4, GLP-1, glucagon, glucose and insulin) and the change from pre-dose value or baseline were calculated for each patient at each time point. Percentage inhibition of DPP-4 activity was calculated over time for each patient during each treatment period. The area under the effect–time curves from time 0 to 24 h (AUE(0,24 h)) was calculated for GLP-1, glucagon, glucose and insulin.
Safety and tolerability assessments
Safety and tolerability assessments included the monitoring and recording of all adverse events, and any concomitant medications used to treat adverse events. Physical examinations with vital signs and routine laboratory tests including blood chemistry and haematology, urine analysis and electrocardiogram were performed at regular intervals during the study.
Statistical analysis
The variability in glucose AUE(0,24 h) was estimated from the intra-patient CV for glucose AUE(0,14 h) from a previous study. Based on an intra-patient CV ≤0.30 for the primary pharmacodynamic endpoint (glucose AUE(0,24 h)), a sample size of 40 patients provided at least 87% power to detect a 15% difference between morning and evening dosing regimens.
All patients with evaluable pharmacodynamic assessments who completed the trial were included in the data analysis. Statistical comparisons of pharmacodynamic parameters were performed using analysis of variance, with sequence, period, and treatment as fixed effects and patient (sequence) as random effect. The estimated mean difference and associated 95% confidence intervals and P values for morning dosing compared with evening dosing were calculated using commercial software (SAS MIXED).
Results
Patient characteristics
A total of 48 patients with type 2 diabetes were enrolled; 39 patients completed the study. Four patients withdrew consent and five patients discontinued because of a protocol violation (n = 4) or an abnormal laboratory test result (n = 1) during the first treatment period. Baseline and demographic characteristics of enrolled subjects are summarized in Table 1. The majority of patients (n = 32) were of Hispanic ethnic origin.
Table 1.
Subject baseline characteristics
| Characteristic | Patients (n = 48) |
|---|---|
| Age (years) | 57.3 ± 8.9 |
| Ethnicity, n (%) | |
| Caucasian | 14 (29.2) |
| Hispanic | 32 (66.7) |
| Asian | 1 (2.1) |
| Native American | 1 (2.1) |
| Male/female, n | 26/22 |
| Body weight (kg) | 84.4 ± 13.7 |
| Height (cm) | 164.6 ± 9.5 |
| BMI (kg m−2) | 31.1 ± 4.0 |
| Fasting plasma glucose (mg dl−1) | 159.8 ± 42.2 |
| HbA1c (%) | 7.9 ± 1.1 |
Data are mean ± SD, unless otherwise stated. BMI, body mass index; HbA1c, glycosylated haemogloblin.
Pharmacokinetics
Steady-state plasma concentration–time profiles were similar whether vildagliptin was administered in the morning or evening (Figure 1). Steady-state pharmacokinetic parameters are summarized in Table 2. Cmax tended to be slightly lower when vildagliptin was administered in the evening. The slightly longer t1/2 observed following administration of vildagliptin in the morning probably reflected the different plasma sampling schedule during the day and night.
Figure 1.
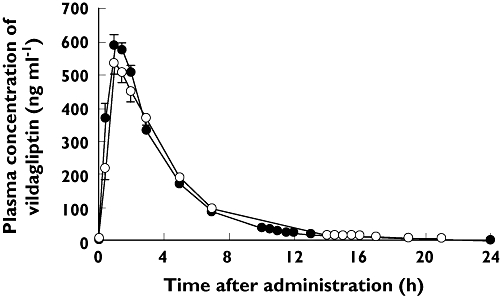
Plasma concentration–time profiles for vildagliptin following morning dosing (vildagliptin 100 mg in the morning; placebo in the evening; n = 36) or evening dosing (vildagliptin 100 mg in the evening; placebo in the morning; n = 36). Data are mean ± SEM. Morning dose ( ); Evening dose (
); Evening dose ( )
)
Table 2.
Steady-state pharmacokinetic parameters for vildagliptin 100 mg following morning or evening dosing
| Parameter | Morning dosing (n = 36) | Evening dosing (n = 36) |
|---|---|---|
| Cmax (ng ml−1) | 684 ± 187 | 578 ± 152 |
| AUC(0,7 h) (ng ml−1 h) | 2062 ± 438 | 1977 ± 527 |
| AUC(0,∞) (ng ml−1 h) | 2414 ± 561 | 2427 ± 692 |
| tmax (h) | 1.00 (0.50–2.00) | 1.00 (1.00–3.00) |
| t1/2 (h) | 3.43 ± 1.58 | 2.45 ± 0.53 |
Data are mean ± SD except for tmax values, which are median (range). Cmax, maximum plasma concentration; AUC(0,7 h), area under the plasma concentration–time curve from time 0 to 7 h; AUC(0,∞), area under the plasma concentration–time curve from time 0 extrapolated to infinity; tmax, time to reach Cmax; t1/2, terminal elimination half-life.
Pharmacodynamics
DPP-4 activity and GLP-1 concentrations
Both morning and evening dosing of vildagliptin inhibited plasma DPP-4 activity by ≥90% during the first 5.5 h after dosing, by ≥80% up to 15.5 h after dosing and by ≥45% up to 24 h after dosing. DPP-4 inhibition was greater during the daytime following morning dosing and greater during the night-time following evening dosing (Figure 2A). DPP-4 inhibition as a function of time after dosing was similar after morning and evening dosing regimens.
Figure 2.
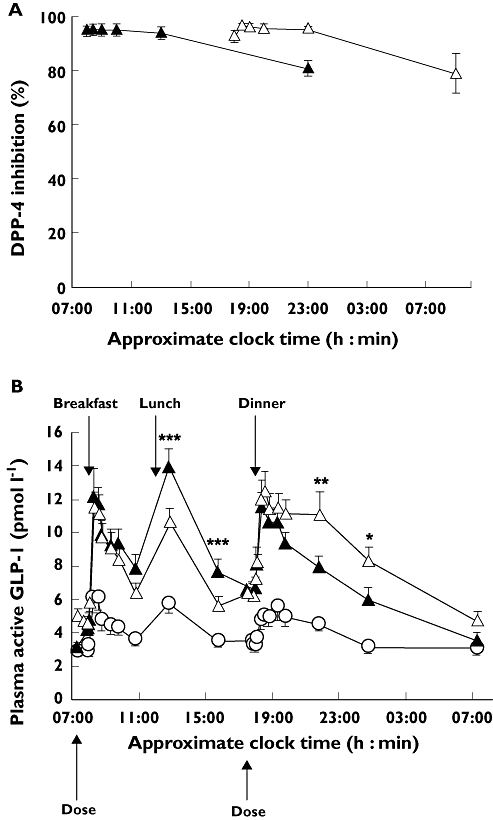
A) Dipeptidyl peptidase IV (DPP-4) inhibition and B) mean plasma concentration–time profiles for active glucagon-like peptide-1 (GLP-1) after 28 days of morning (A, n = 38; B, n = 37) or evening (A, n = 38; B, n = 37) administration of vildagliptin 100 mg and during the placebo run-in (n = 44). Data are mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.005 for vildagliptin 100 mg vs. placebo. (A) Morning dose ( ); Evening dose (
); Evening dose ( ); (B) Run-in (
); (B) Run-in ( ); Morning dose (
); Morning dose ( ); Evening dose (
); Evening dose ( )
)
Active GLP-1 concentrations following morning and evening dosing exceeded 200 pmol l−1 in one patient. GLP-1 data were therefore analysed with and without inclusion of this patient. As there were no meaningful differences between the results, this patient was excluded from GLP-1 data. Figure 2B (patient excluded) shows the mean 24 h profile of active GLP-1 plasma concentrations during the placebo run-in period and after 28 days of morning or evening vildagliptin dosing. Both treatment regimens significantly increased GLP-1 concentrations compared with placebo. GLP-1 concentrations during the day were higher with morning than with evening dosing, while GLP-1 concentrations in the evening and overnight period were higher with evening dosing (Figure 2B). Twenty-four hour GLP-1 exposure (AUE(0,24 h)) did not differ significantly between the two regimens (morning 187 ± 12 pmol l−1 h; evening 196 ± 14 pmol l−1 h; P = 0.315). Fasting concentrations of GLP-1 were significantly higher in the morning with evening dosing than with morning dosing (5.0 ± 0.5 vs. 3.1 ± 0.3 pmol l−1; P < 0.001).
Glucose
Both morning and evening vildagliptin dosing regimens decreased plasma glucose concentrations throughout the day and overnight compared with concentrations observed during the placebo run-in period (Figure 3A). Both dosing regimens significantly improved glycaemic control, reducing the 24 h mean glucose concentration by 19.5 mg dl−1 with morning dosing (P = 0.014 relative to placebo run-in) and by 23.9 mg dl−1 with evening dosing (P = 0.003 relative to placebo run-in) although there was no significant difference in the 24 h mean glucose concentration between the two dosing regimens (P = 0.43). Plasma glucose concentrations in the morning and afternoon were similar with both dosing regimens, while there was a strong trend towards lower glucose concentrations in the evening with evening dosing. The largest between-regimen difference in glucose concentrations (16 mg dl−1) was seen 2 h after the evening dose but was not statistically significant (P = 0.057).
Figure 3.
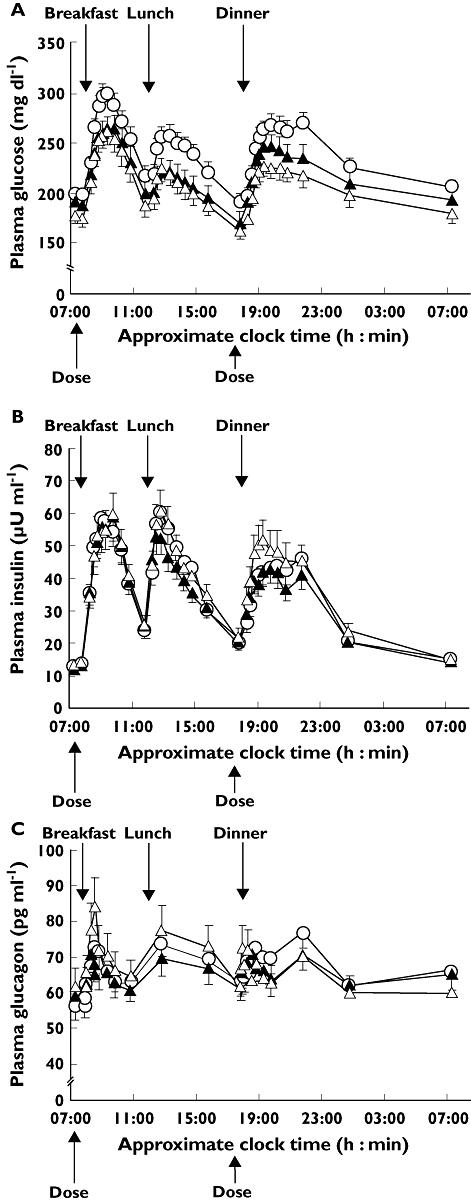
Mean plasma concentration–time profiles for A) glucose, B) insulin and C) glucagon after 28 days of morning (n = 38) or evening (n = 38) administration of vildagliptin 100 mg, and during the placebo run-in (n = 45). Data are mean ± SEM. Run-in ( ); Morning dose (
); Morning dose ( ); Evening dose (
); Evening dose ( )
)
Compared with the placebo run-in period, each treatment regimen reduced 24 h glucose exposure (AUE(0,24 h)): morning −467 mg dl−1 h, P = 0.014; evening −574 mg dl−1 h, P = 0.003); the average reduction in glucose over 24 h was 20 mg dl−1 after morning dosing and 24 mg dl−1 after evening dosing. Differences between dosing regimens in 24 h glucose exposure and average glucose concentration were not statistically significant (Table 3). Compared with placebo, the reduction in daytime glucose exposure (AUE(0,10.5 h)) was similar with morning and evening dosing (average daytime exposure 213.7 mg dl−1 h and 207.3 mg dl−1 h with evening dosing; Table 3); however, the reduction in night-time glucose exposure (AUE(10.5,24 h)) was numerically greater with evening compared with morning dosing (evening −336 mg dl−1 h; morning −218 mg dl−1 h; P = 0.192). Mean fasting plasma glucose was 195 ± 8 mg dl−1 during the placebo run-in period. After 28 days of vildagliptin treatment, mean fasting plasma glucose tended to be lower with evening than with morning dosing but the difference was not statistically significant (evening 178 ± 9 vs. morning 192 ± 9 mg dl−1, P = 0.130). However, mean fasting plasma glucose was significantly decreased compared with the placebo run-in period for evening dosing (−13 mg dl−1, P = 0.032), but not morning dosing (−5 mg dl−1, P = 0.374).
Table 3.
Effects on plasma glucose and plasma insulin following morning or evening dosing of vildagliptin 100 mg
| Active treatment period | |||||
|---|---|---|---|---|---|
| Placebo run-in (n = 40) | Morning dosing (n = 38) | Evening dosing (n = 38) | Least-squares mean difference between morning dosing and evening dosing (95% CI) | P value | |
| Glucose (mg dl-1 h) | |||||
| Total (AUE(0,24 h)) | 5540 ± 264 | 5136 ± 271 | 4870 ± 241 | 120 (−185, 425) | 0.4296 |
| Daytime (AUE(0,10.5 h)) | 2493 ± 113 | 2244 ± 121 | 2177 ± 114 | 6 (−136, 148) | 0.9276 |
| Night-time (AUE(10.5,24 h) | 3132 ± 132 | 2891 ± 152 | 2693 ± 129 | 116 (−61, 293) | 0.1918 |
| Insulin (µU ml-1 h) | |||||
| Total (AUE(0,24 h)) | 772 ± 52 | 747 ± 60 | 828 ± 69 | −56 (−117, 5) | 0.0707 |
| Daytime (AUE(0,10.5 h)) | 403 ± 26 | 393 ± 33 | 421 ± 33 | −18 (−52, 17) | 0.3016 |
| Night-time (AUE(10.5,24 h)) | 368 ± 27 | 354 ± 29 | 407 ± 36 | −40 (−79, −0.1) | 0.0495 |
Data are presented as mean ± SEM unless otherwise stated. AUE, area under the effect–time curve; CI, confidence interval.
Insulin and glucagon
Figure 3B shows mean plasma insulin concentration as a function of time after 28 days of morning or evening dosing with vildagliptin and after placebo run in. Evening dosing tended to increase post-prandial insulin concentrations following lunch and dinner more than morning dosing, although differences in change from baseline were statistically significant only at 1.25 h post-dinner (37 vs. 26 µU ml−1, P = 0.018). While total insulin exposure (AUE(0,24 h)) tended to be greater with evening than with morning dosing (Table 3, P = 0.071), the only statistically significant difference between the two regimens was an 11% increase in night-time insulin exposure (AUE(10.5,24 h), P = 0.050) with evening dosing.
Figure 3C shows plasma glucagon concentration as a function of time after morning or evening dosing with vildagliptin for 28 days and after placebo run in. Morning administration of vildagliptin tended to reduce plasma glucagon concentrations throughout the day, whereas evening administration tended to reduce glucagon concentrations in the evening and overnight. However, AUE(0,24 h) for glucagon did not differ significantly between morning and evening dosing (1569 ± 86 vs. 1595 ± 101 ng l−1 h, P = 0.651), and was similar to that observed during the placebo run-in (1615 ± 76 ng l−1 h).
Safety and tolerability
Vildagliptin was generally well tolerated whether administered in the morning or the evening. During morning dosing, 18 patients reported 36 adverse events, 22 of which were suspected to be related to study medication. During evening dosing, 18 patients reported 42 adverse events, 23 of which were suspected to be related to study medication. In addition, 13 patients reported 23 adverse events during the placebo run-in period. Most events were mild or moderate in severity. The most frequently reported adverse events during active treatment were headache (11.1–14.3%) and pharyngolaryngeal pain (7.1–8.9%). No hypoglycaemic events were reported during the study, and there were no discontinuations due to adverse events. One serious adverse event was reported in a patient who was hospitalized with severe hyperglycaemia 9 days after completion of the study. The cause was unknown and was not suspected to be related to study treatment.
Discussion
The results of this study demonstrated that once daily dosing with vildagliptin 100 mg for 28 days either in the morning or evening improved glycaemic control in patients with type 2 diabetes. Evening dosing tended to reduce glucose exposure to a greater extent than morning dosing. These effects seem to be explained by differences in daytime inhibition of DPP-4 activity compared with night-time inhibition of DPP-4 activity and the resulting effects on insulin and glucagon. Importantly, the 24 h glycaemic control and hormonal responses were similar between dosing regimens in patients with type 2 diabetes.
The pharmacokinetic results of the present study confirmed that vildagliptin was rapidly absorbed after either morning or evening dosing with peak plasma concentrations observed approximately 1 h after administration. The observed short elimination half-life (3.43 ± 1.58 h with morning dosing; 2.45 ± 0.53 h with evening dosing) was also consistent with observations in healthy volunteers [29]. The slightly longer elimination half-life observed following administration of vildagliptin in the morning probably reflected the different plasma sampling schedule during the day and night. A previous study, in healthy volunteers administered with vildagliptin 100 mg, showed that food had no clinically relevant effects on vildagliptin pharmacokinetics, hence no differences in pharmacokinetics related to the timing of meals would be expected for morning vs. evening dosing [30].
Both vildagliptin dosing regimens produced significant improvements in 24 h plasma glucose exposure compared with baseline. Improvements tended to be greater with evening than morning dosing, particularly during the overnight period, although the differences were not statistically significant. The improvements relative to baseline observed in the current study (AUE(0,24 h): morning −467 mg dl−1 h; evening −574 mg dl−1 h) were similar to those seen in a 4 week study of vildagliptin 100 mg administered once daily in the morning in patients with type 2 diabetes (AUE(0,24 h): −626 mg dl−1 h) [22]. The reductions from baseline in fasting plasma glucose were also larger with evening than morning dosing in the current study (−13 mg dl−1vs. −5 mg dl−1), but were smaller than the reductions of approximately 18 mg dl−1 observed in longer studies (12–24 weeks) with vildagliptin 100 mg once daily in the morning in patients with type 2 diabetes [16, 23]. The trend towards greater reduction in fasting plasma glucose with evening dosing may be related to the greater decreases in glucose exposure overnight. Excessive overnight hepatic glucose production is an important factor in determining fasting plasma glucose in patients with worsening type 2 diabetes [31].
In a preliminary study in 16 patients with type 2 diabetes, the reduction in fasting glucose of 14 mg dl−1 following a single evening dose of vildagliptin 100 mg was directly related to the treatment-induced decrease in overnight endogenous glucose production [26]. After vildagliptin treatment, the rate of overnight insulin release remained elevated whereas plasma glucagon release was significantly suppressed [26]. In the present study, insulin exposure over 24 h did not differ significantly with vildagliptin therapy compared with placebo (administered during the run-in period), irrespective of the time of dosing. However, evening dosing was associated with significantly greater night-time insulin exposure than morning dosing. The ratio AUE(0,24 h) of insulin to AUE(0,24 h) of glucose was higher during vildagliptin treatment than during the run-in period in this study, indicating greater insulin release at any given glucose concentration. The relatively small changes observed in insulin concentrations despite significant reductions in glucose concentrations were in line with findings from previous studies with vildagliptin [15, 22, 26]. These results were consistent with vildagliptin therapy enhancing β-cell responsiveness to glucose. A 4 week study in patients with type 2 diabetes showed that vildagliptin therapy increased insulin secretion at a given glucose concentration [24]. In a pooled analysis of vildagliptin monotherapy in patients with type 2 diabetes, robust and consistent improvements in both fasting and meal-test-derived measures of β-cell function were seen after 6 months' treatment [14].
As expected, vildagliptin inhibited DPP-4 activity leading to an increased active GLP-1 concentration when compared with baseline following both morning and evening dosing regimens. As DPP-4 inhibition is a function of vildagliptin concentration, DPP-4 inhibition was greater during the daytime following morning dosing and greater during the night-time following evening dosing. The DPP-4 inhibition vs. time profile as a function of time after vildagliptin administration was similar between both regimens. Maximum inhibition of DPP-4 activity was observed 30 min after vildagliptin dosing, and more than 80% inhibition was sustained for up to 15.5 h after dosing. As GLP-1 is secreted in response to a meal, the increases in GLP-1 activity were most pronounced after each meal, with peak concentrations observed after 30 min. It should be noted that the duration of DPP-4 inhibition with vildagliptin is prolonged relative to the plasma half-life of the drug. This reflects the fact that a vildagliptin plasma concentration of 15 ng ml−1 is sufficient to achieve 90% DPP-4 inhibition [10]; administration of vildagliptin 100 mg once daily or 50 mg twice daily maintains plasma vildagliptin concentrations sufficient to achieve ≥70% average DPP-4 inhibition throughout the day. These results are consistent with the DPP-4 and GLP-1 profiles observed with vildagliptin in previous studies [22, 26]. It is important to note that there is no evidence for circadian variation in DPP-4 concentrations that might influence the effects of morning or evening dosing of vildagliptin. A single study in mice performed before the physiological function of DPP-4 was known suggested that there may be ultradian variation in concentrations of DPP-4 [32], but there is no published evidence of similar variation in humans.
Vildagliptin therapy was well tolerated following either morning or evening administration. The incidence of adverse events was low, and most were mild or moderate in intensity. These findings are consistent with the safety and tolerability profile reported with vildagliptin treatment in previous clinical studies in patients with type 2 diabetes [15, 16, 23]. Indeed, in studies lasting up to 24 weeks, the overall incidence of adverse events at daily vildagliptin doses up to 100 mg was similar to that observed with placebo [16, 23]. In the current study, there were no reports of hypoglycaemic events with vildagliptin treatment. This is consistent with the low hypoglycaemic potential of agents that act by increasing GLP-1 activity, as GLP-1-induced insulin release is glucose-dependent [3].
In conclusion, treatment with vildagliptin at 100 mg once daily for 28 days led to a significant reduction in glucose excursion over 24 h after both morning and evening dosing. Although evening dosing of vildagliptin 100 mg was similarly effective in glucose lowering as morning dosing in patients with type 2 diabetes, evening dosing may be slightly more effective for night-time glucose reduction. Both regimens were equally effective in reducing post-prandial glucose in patients with type 2 diabetes.
Acknowledgments
The authors thank Dr Joelle Campestrini and Dr Jannick Denouel, Novartis Pharma S.A.S., Rueil-Malmaison, France, for carrying out the bioanalytical assessments.
All authors participated in the collection, analysis and interpretation of data, approved the final version of the manuscript for submission and meet ICMJE criteria for authorship. Editorial assistance with collating and incorporating comments from all authors to produce a final draft manuscript for submission was provided by Dr Ann Taylor (Oxford PharmaGenesis™ Ltd); this role was funded by Novartis.
Competing interests
This study was supported by Novartis Pharmaceuticals Corporation. Y-L.H., Y.Z., M.L.-S., J.F. and W.P.D. are employees of Novartis and hold Novartis stock. J.V. is an employee of Novartis. S.L.S. has received research funding from Novartis.
REFERENCES
- 1.Pratley RE, Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007;23:919–31. doi: 10.1185/030079906x162746. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–70. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 3.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 4.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–22. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 5.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I (7–36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–6. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 6.Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ, Hellstrom PM. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910–6. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 7.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 8.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YL, Wang Y, Bullock JM, Deacon CF, Holst JJ, Dunning BE, Ligueros-Saylan M, Foley JE. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47:633–41. doi: 10.1177/0091270006299137. [DOI] [PubMed] [Google Scholar]
- 11.He YL, Serra D, Wang Y, Campestrini J, Riviere GJ, Deacon CF, Holst JJ, Schwartz S, Nielsen JC, Ligueros-Saylan M. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2007;46:577–88. doi: 10.2165/00003088-200746070-00003. [DOI] [PubMed] [Google Scholar]
- 12.Villhauer EB, Brinkman JA, Naderi GB, Burkey BF, Dunning BE, Prasad K, Mangold BL, Russell ME, Hughes TE. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem. 2003;46:2774–89. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- 13.Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan LM, Dunning BE, Foley JE, Rizza RA, Camilleri M. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–80. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- 14.Pratley RE, Schweizer A, Rosenstock J, Foley JE, Banerji MA, Pi-Sunyer FX, Mills D, Dejager S. Robust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naive patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab. 2008;10:931–8. doi: 10.1111/j.1463-1326.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 15.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–8. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- 16.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–8. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, Wang Y, Foley JE. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25:435–41. doi: 10.1111/j.1464-5491.2008.02391.x. [DOI] [PubMed] [Google Scholar]
- 18.Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE. Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008;10:675–82. doi: 10.1111/j.1463-1326.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 19.Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–56. doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 20.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–5. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca V, Baron MA, Shao Q, Dejager S. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40:427–30. doi: 10.1055/s-2008-1058090. [DOI] [PubMed] [Google Scholar]
- 22.Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–84. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- 23.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose-response. Diabetes Obes Metab. 2005;7:692–8. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 24.Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–94. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin -7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–51. doi: 10.1021/jm0493156. [DOI] [PubMed] [Google Scholar]
- 26.Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF, Cusi K, Mari A, Foley JE, DeFronzo RA. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–55. doi: 10.1210/jc.2006-1882. [DOI] [PubMed] [Google Scholar]
- 27.He YL, Ligueros-Saylan M, Sunkara G, Sabo R, Zhao C, Wang Y, Campestrini J, Pommier F, Dole K, Marion A, Dole WP, Howard D. Vildagliptin, a novel dipeptidyl peptidase IV inhibitor, has no pharmacokinetic interactions with the antihypertensive agents amlodipine, valsartan, and ramipril in healthy subjects. J Clin Pharmacol. 2008;48:85–95. doi: 10.1177/0091270007307880. [DOI] [PubMed] [Google Scholar]
- 28.Serra D, He YL, Bullock J, Riviere GJ, Balez S, Schwartz S, Wang Y, Ligueros-Saylan M, Jarugula V, Dole WP. Evaluation of pharmacokinetic and pharmacodynamic interaction between the dipeptidyl peptidase IV inhibitor vildagliptin, glyburide and pioglitazone in patients with type 2 diabetes. Int J Clin Pharmacol Ther. 2008;46:349–64. doi: 10.5414/cpp46349. [DOI] [PubMed] [Google Scholar]
- 29.He YL, Barilla D, Ligueros-Saylan M, Riviere GJ, Campestrini J, Pratapa P. The pharmacokinetics and DPP-4 inhibition of LAF237 in healthy volunteers. J Clin Pharmacol. 2004;44:1212. [Google Scholar]
- 30.Sunkara G, Sabo R, Wang Y, He YL, Campestrini J, Howard D, Dole WP. Dose proportionality and the effect of food on vildagliptin, a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. J Clin Pharmacol. 2007;47:1152–8. doi: 10.1177/0091270007304313. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387–95. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 32.Balschun D, Schuh J. Dipeptidyl-peptidase IV (DP IV): rhythmic changes of serum activity in mice. Pharmazie. 1983;38:424. [PubMed] [Google Scholar]


