Abstract
AIM
This study examined the effect of co-administration of febuxostat, an investigational urate lowering therapy, and hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of febuxostat.
METHODS
Healthy subjects (36 healthy men and women) received single doses of febuxostat 80 mg alone and febuxostat 80 mg + hydrochlorothiazide 50 mg, separated by 7 days in an open-label, randomized, crossover fashion. Plasma concentrations of febuxostat and urinary and serum concentrations of uric acid were assessed.
RESULTS
Mean febuxostat Cmax, AUC(0–t), AUC(0–∞), t1/2,z, CL/F and Vss/F values for regimens co-administration/febuxostat alone were 2.9/2.9 µg ml−1, 9.3/9.1 µg ml−1 h, 9.6/9.3 µg ml−1 h, 6.5/6.1 h, 8.8/9.3 l h−1 and 45/44 l, respectively. Geometric mean ratios (co-administration : febuxostat alone) and their 90% confidence intervals for febuxostat plasma Cmax, AUC(0–t), and AUC(0–∞) were 1.00 (0.86, 1.17), 1.03 (0.98, 1.09), and 1.04 (0.98, 1.10), respectively; all of the 90% CIs were within the no effect range of 0.8 to 1.25. Serum uric acid Cmean,24h, Cmean,48h and CLR for both regimens co-administration/febuxostat alone were 216/203 µmol l−1, 218/202 µmol l−1 and 9.1/10.1 ml min−1, respectively. Although serum uric acid Cmean,24h and Cmean,48h values were higher and CLR values lower after co-administration compared with dosing of febuxostat alone, with the differences being statistically significant (P < 0.003), none of the differences (6.5%–9.5%) was considered clinically significant.
CONCLUSION
Dose adjustment for febuxostat is not necessary when it is administered with hydrochlorothiazide.
Keywords: antihypertensive, diuretic, gout, interaction, urate, uric acid
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Hyperuricaemia and gout frequently coexist with cardiovascular disorders such as hypertension and heart failure. The use of diuretics has been re-established as a first-line treatment for patients with hypertension and the effects of diuretics on serum uric acid may diminish the urate-lowering effects of febuxostat, a novel, potent, non-purine selective inhibitor of xanthine oxidase.
WHAT THIS STUDY ADDS
Co-administration of febuxostat 80 mg and hydrochlorothiazide 50 mg had no effect on the pharmacokinetics and did not have a clinically significant effect on the pharmacodynamics of febuxostat. Dose adjustment for febuxostat is not necessary when it is administered with hydrochlorothiazide.
Introduction
Approximately 6.1 million adults in the United States develop gout, with prevalence increasing with age for both men and women [1]. Hyperuricaemia (particularly, persistent asymptomatic hyperuricaemia with serum uric acid (sUA) concentrations ≥7 mg dl−1) is the most important determining risk factor for the development of gout [2]. Long-term management of chronic gout often requires urate-lowering therapy that includes xanthine oxidase (XO) inhibitors, uricosuric agents, and recombinant urate oxidases. XO inhibitors decrease the production of UA, whereas uricosuric agents and recombinant urate oxidases increase the elimination rate of UA. Febuxostat is a novel, potent, non-purine, selective inhibitor of XO that has activity against both the oxidized and reduced forms of XO [3].
In a large, multicentre trial in 760 patients with gout and hyperuricaemia (baseline sUA of ≥8.0 mg dl−1), febuxostat at a daily dose of 80 mg or 120 mg was more effective than allopurinol at the commonly used [4] fixed daily dose of 300 mg in lowering serum urate [5]. Absorption of febuxostat is rapid (tmax of approximately 1 h), and Cmax and AUC exhibit dose proportionality over the dose range of 10 to 120 mg. Febuxostat is highly bound (>99%) to plasma proteins [6] and undergoes extensive phase 1 and phase 2 metabolism to its acyl-glucuronide metabolite and to hydroxylated metabolites [7]. Less than 5% of the dose is excreted unchanged in urine [7].
Hyperuricaemia and gout frequently coexist with cardiovascular disorders such as hypertension and heart failure [8, 9]. Recently, the use of diuretics has been re-established as a first-line treatment for patients with hypertension following the results of the ALLHAT study [10]. Of concern is that the effects of diuretics on sUA may diminish the urate-lowering effects of febuxostat.
Approximately 30% of systemic UA is excreted into the GI tract, where it is then destroyed by bacterial uricases [11]. The remaining 70% of systemic UA is excreted into the urine [12]. While in the kidney, uric acid undergoes extensive reabsorption in the nephronal proximal tubule, with only 6%-12% of filtered UA finally excreted in urine [12]. Therefore, any medication that affects tubular reabsorption can influence sUA concentration [12]. All of the loop diuretics and thiazide diuretics increase the net reabsorption of UA, thus reducing its urinary excretion and increasing its serum concentration. Such increases in sUA can be seen within 24 h of the first dose following a high dose of diuretic; this response remains stable during prolonged administration [12]. Recent work has established that some drugs (e.g. allopurinol/oxipurinol, loop and thiazide diuretics, some uricosurics and salicylates) can alter urate renal transport via a multi-drug resistance protein (MRP4) in the apical membrane of the proximal tubule. Hydrochlorothiazide and furosemide can decrease urate transport by inhibiting MRP4 [13].
In addition, diuretic use and hypertension have both been shown to be independent risk factors for gout [14]. Therefore, diuretics have the potential to alter the urate-lowering efficacy of febuxostat through their direct pharmacodynamic effects. Diuretics also have the potential to attenuate urate-lowering efficacy through more indirect pharmacokinetic interactions. The purpose of this study was to examine the effect of a thiazide diuretic, hydrochlorothiazide 50 mg, on the pharmacokinetics and pharmacodynamics of febuxostat 80 mg.
Methods
Patient population
Healthy adult male and female subjects between the ages of 18 and 55 years with normal creatinine clearance (79–149 ml min−1) and a body mass index ≤30 kg m−2 were recruited for this study. Lactating or pregnant women, nicotine users, patients with histories of gout, hypertension, hypotension, xanthinuria, or hypersensitivities to hydrochlorothiazide or other sulfonamide derivatives; and subjects with any clinically significant abnormalities that could interfere with the evaluation of the study medication were excluded. The following medications/substances were discontinued prior to participation: nicotine (tobacco, nicotine patches, nicotine gum) for 3 months, over-the-counter medications (e.g. Moduretic, herbals, vitamins, dietary supplements) for 7 days, prescription medications or enzyme-altering agents (except for stable oral contraceptive and hormone replacement regimens) for 4 weeks and investigational drugs for 1 month. In addition, consumption of grapefruit-, caffeine-, and xanthine-containing products was prohibited during the study. The study was approved by an Institutional Review Board and written informed consent was obtained from each subject prior to enrollment.
Study design
This study was designed as a randomized, single-centre, single-dose, open-label, two-period crossover study. A total of 36 subjects were recruited and randomly assigned in equal numbers to one of two regimen sequence groups. Upon study completion, each subject had received both regimens in a crossover fashion (febuxostat 80 mg alone and febuxostat 80 mg + hydrochlorothiazide 50 mg) separated by 7 days. The hydrochlorothiazide dose was selected as the highest recommended dose for use in hypertension. Study medication was administered following an overnight fast (at least 10 h), and subjects continued to fast for 4 h post-dose.
In each period, blood samples for the determination of febuxostat plasma concentrations were collected pre-dose, and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h post-dose. Blood samples for the determination of sUA concentrations were collected pre-dose, and at 6, 12, 18, 24, and 48 h post-dose. Urine samples for the determination of UA were collected and pooled between 0 and 12 h post-dose, and between 12 and 24 h post-dose.
Safety was monitored through adverse event recording, vital signs, physical examination and laboratory evaluations.
Analytical methods
Plasma concentrations of febuxostat were determined using a validated high performance liquid chromatography method with fluorescence detection at excitation and emission wavelengths of 320 and 380 nm, respectively. In brief, after addition of the internal standard (2-naphthoic acid), plasma samples (0.5 ml) were deproteinized by the addition of 0.5 ml of acetonitrile, mixed, and centrifuged, and the resulting supernatant was acidified with 50 µl of glacial acetic acid. Febuxostat and the internal standard were resolved from the matrix components using a Phenomenex (Torrance, CA) Capcell Pak C18 column with a mobile phase composed of 0.032% glacial acetic acid in water : acetonitrile (55 : 45, v : v). The calibration curve range for febuxostat was linear from 0.01 to 20 µg ml−1 (r2 > 0.9988). The lower limit of quantification with a 0.5 ml plasma sample was 0.01 µg ml−1 for febuxostat. Quality control samples (at 0.03, 1, and 15 µg ml−1) analyzed with the study samples showed absolute deviations from the theoretical concentrations of 7.7% or less and coefficients of variation of less than 10% for febuxostat.
For UA, the serum and urine sample analyses were performed using standard methods at the investigative site's clinical laboratory. In brief, UA concentrations for both serum and urine were determined by an enzymatic method with colorimetric assay, using a quinine di-imine dye (SYNCHRON LX® system), measured photometrically at 520 nm. Calibration for serum and urine UA was performed using the Synchron Multi Calibrator (URIC reagent) with linear analytical ranges of 0.5–12.0 mg dl−1 and 5.0–120 mg dl−1, respectively. Serum QC samples Level 1 and 3 (Triad®) analyzed with the study samples showed coefficients of variation of 1.4% or less. Urine QC sample Level 2 (Liquichek™) analyzed with the study samples showed coefficients of variation of 1.3% or less.
Pharmacokinetic assessments
Pharmacokinetic parameters of febuxostat were estimated using noncompartmental pharmacokinetic methods (linear trapezoidal method) with WinNonlin Professional Version 3.1 (Pharsight Co., Mountain View, CA). The following pharmacokinetic parameters were estimated: the peak plasma concentration (Cmax), the time to reach the peak concentration (tmax), the apparent terminal phase elimination rate constant (λz), the apparent terminal phase elimination half-life (t1/2), the area under the plasma concentration curve (AUC) from time zero to the last measurable concentration [AUC(0–t)], the AUC from time zero to infinity [AUC(0–∞)], oral clearance (CL/F), and the apparent steady state volume of distribution (Vss/F).
Pharmacodynamic assessments
Mean sUA concentrations over the 24- and 48-h post-dosing intervals (Cmean,24h and Cmean,48h, respectively) were calculated as serum AUC(0–24 h)/24 and AUC(0–48 h)/48, respectively. The sUA parameters AUC(0–24 h) and AUC(0–48 h) were calculated using noncompartmental pharmacokinetic methods (linear trapezoidal method) with WinNonlin Professional Version 3.1 (Pharsight Co., Mountain View, CA). The 24 h renal clearance of UA (CLR) was calculated by dividing the total amount UA excreted in urine over the 24 h (Ae24) post-dosing period by the sUA AUC(0–24 h).
Statistical methods
All statistical analyses were performed using SAS Version 8.2 on the UNIX operating system. Analyses of variance (anovas) were performed on febuxostat tmax, and the natural logarithms of Cmax, AUC(0–t), and AUC(0–∞), with factors for sequence, subjects nested within sequence, period and regimen. The factor of subjects nested within sequence was considered random and all other factors were fixed.
The effect of hydrochlorothiazide on the pharmacokinetics of febuxostat was assessed via geometric mean ratios and 90% confidence intervals (CIs) of the ratios for febuxostat + hydrochlorothiazide to febuxostat alone for Cmax, AUC(0–t) and AUC(0–∞). The geometric mean ratios were determined by exponentiating the difference between regimen natural logarithm least square means. Similarly, the CIs were determined by exponentiating the endpoints of 90% CIs for the difference of natural logarithm least square means obtained from the anova model estimates. A conclusion of no effect of hydrochlorothiazide on the pharmacokinetics of febuxostat was made if the 90% confidence intervals were completely contained within the interval of 0.80, 1.25 for Cmax, AUC(0–t) and AUC(0–∞).
Analyses of variance were performed on the pharmacodynamic parameters UA Cmean,24h, Cmean,48h, CLR and Ae24 with factors for sequence, subject nested within sequence, period and regimen. The factor of subject nested within sequence was treated as random and all other factors were considered fixed. A significance level of 0.05 was used for all tests.
Results
Subject population
A total of 36 subjects (20 men and 16 women) were randomized and received at least one dose of study drug. The mean age was 33 years (range: 19–51 years), the mean weight was 72.1 kg (47.2–91.3 kg), the mean height was 171 cm (147–185 cm), and the mean BMI was 24.6 kg m−2 (19.1–29.5 kg m−2) (Table 1). Four subjects prematurely discontinued the study: three subjects withdrew after receiving the first dose of study medication, and one subject withdrew after completing the blood collections for both treatment periods. Two of the four subjects withdrew for personal reasons and the remaining two subjects withdrew due to adverse events, which were not considered by the investigators to be related to the study medication. Therefore, 33 subjects (19 men and 14 women) were included in pharmacokinetic or pharmacodynamic analyses. Among these 33 subjects, three subjects took acetaminophen, and one subject took oral contraceptives; none of these medications was expected to have an effect on the study outcome.
Table 1.
Demographics and baseline characteristics for all subjects enrolled and for the PK/PD population
| Demographic characteristics | All subjects n = 36 | PK/PD population n = 33 | ||
|---|---|---|---|---|
| Gender [n (%)] | ||||
| Male | 20 | (56%) | 19 | (58%) |
| Female | 16 | (44%) | 14 | (42%) |
| Race [n (%)] | ||||
| Caucasian | 22 | (61%) | 21 | (64%) |
| Black | 7 | (19%) | 7 | (21%) |
| Hispanic | 6 | (17%) | 4 | (12%) |
| Asian | 1 | (3%) | 1 | (3%) |
| Age (years) [Mean (Range)] | 33 | (19–51) | 33 | (19–51) |
| Weight (kg) [Mean (Range)] | 72.1 | (47.2–91.3) | 73.0 | (47.2–91.3) |
| Height (cm) [Mean (Range)] | 171 | (147–185) | 171 | (152–185) |
| BMI (kg m−2) [Mean (Range)] | 24.6 | (19.1–29.5) | 24.7 | (19.1–29.5) |
Pharmacokinetic results
As shown in Figure 1, administration of febuxostat 80 mg alone and co-administration of febuxostat 80 mg and hydrochlorothiazide 50 mg resulted in similar febuxostat plasma concentration profiles.
Figure 1.
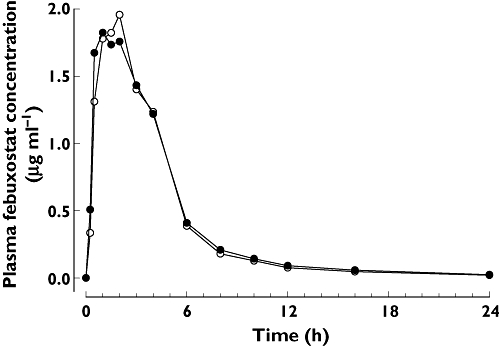
Mean plasma concentration–time profiles of febuxostat. Febuxostat alone ( ); Febuxostat + hydrochlorthiazide (
); Febuxostat + hydrochlorthiazide ( )
)
Pharmacokinetic results for febuxostat are provided in Table 2. Mean febuxostat Cmax, tmax, AUC(0–t), AUC(0–∞), t1/2, CL/F and Vss/F following co-administration appeared to be similar to values after the administration of febuxostat alone. Following febuxostat + hydrochlorothiazide co-administration and febuxostat alone; the respective peak plasma concentration mean values (Cmax) were 2.9 and 2.9 µg ml−1 and the estimated total exposure mean values [AUC(0–∞)] were 9.6 and 9.3 µg ml−1 h.
Table 2.
Arithmetic mean (±SD) febuxostat pharmacokinetic parameters following single dosing with febuxostat 80 mg alone and in combination with a single 50 mg dose of hydrochlorothiazide
| Parameter | Febuxostat 80 mg alone n = 33 | Febuxostat 80 mg + hydrochlorothiazide 50 mg n = 33 | ||
|---|---|---|---|---|
| tmax (h) | 2.0 | (1.28) | 1.9 | (1.43) |
| Cmax (µg ml−1) | 2.9 | (1.44) | 2.9 | (1.38) |
| AUC(0–t) (µg ml−1 h) | 9.1 | (2.57) | 9.3 | (2.56) |
| AUC(0–∞) (µg ml−1 h) | 9.3 | (2.61) | 9.6 | (2.57) |
| CL/F (l h−1) | 9.3 | (2.53) | 8.8 | (2.01) |
| Vss/F (l) | 44 | (16.0) | 45 | (17.0) |
| t1/2 (h) | 6.1 | (1.57) | 6.5 | (2.18) |
Statistical analyses of the relative bioavailability of febuxostat + hydrochlorothiazide compared with febuxostat alone showed that the 90% CIs for the ratios of central values for Cmax, AUC(0–t) and AUC(0–∞) were all contained within the interval of 0.80 to 1.25 (Table 3). This indicated that the two treatments were bioequivalent with respect to maximum and total systemic exposure to febuxostat.
Table 3.
Relative bioavailability of febuxostat following single dosing with febuxostat 80 mg in combination with a single 50 mg dose of hydrochlorothiazide
| Pharmacokinetic parameter | Geometric mean ratio (febuxostat 80 mg + hydrochlorothiazide 50 mg : febuxostat 80 mg alone) n = 33 | 90% confidence interval |
|---|---|---|
| Cmax | 1.00 | (0.86, 1.17) |
| AUC(0–t) | 1.03 | (0.98, 1.09) |
| AUC(0–∞) | 1.04 | (0.98, 1.10) |
Pharmacodynamic results
Following co-administration of febuxostat and hydrochlorothiazide, mean 24 and 48 h sUA concentrations (Cmean,24h and Cmean,48h, respectively) were higher than those with administration of febuxostat alone. The Cmean,24h was 216 µmol l−1 with febuxostat + hydrochlorothiazide and 203 µmol l−1 with febuxostat alone, a 6.5% difference. The Cmean,48h was 218 µmol l−1 with febuxostat + hydrochlorothiazide and 202 µmol l−1 with febuxostat alone, a 7.9% difference (Table 4). Although these differences for sUA concentration between the two regimens were statistically significant (P < 0.001), the differences in the means were considered not clinically relevant. The 24-h renal clearance of UA (CLR) and the total amount of UA excreted in urine (Ae24) were 9.5% and 4.4% lower, respectively, for co-administration relative to febuxostat alone. This difference for CLR was statistically significant (P = 0.003) but not clinically relevant, and the difference for Ae24 was neither clinically nor statistically significant.
Table 4.
Arithmetic mean (±SD) pharmacodynamic parameters for uric acid following single dosing with febuxostat 80 mg alone and in combination with a single 50 mg dose of hydrochlorothiazide
| Parameter | n | Febuxostat 80 mg alone | Febuxostat 80 mg + hydrochlorothiazide 50 mg | P value* |
|---|---|---|---|---|
| Serum uric acid | ||||
| Cmean,24h (µmol l−1) | 30 | 203 (71.4) | 216 (77.3) | <0.001 |
| Cmean,48h (µmol l−1) | 30 | 202 (67.8) | 218 (76.1) | <0.001 |
| Urine uric acid | ||||
| Ae24 (mg) | 33 | 454 (114.3) | 434 (82.8) | 0.129 |
| CLR (ml min−1)† | 30 | 10.1 (2.84) | 9.1 (2.79) | 0.003 |
P values from anova with terms for sequence, subject (sequence), period and regimen. A significance level of 0.05 was used for all tests.
The 24-h renal clearance of uric acid.
Safety results
All available data for the 36 enrolled subjects were used for safety assessments. The overall incidence of adverse events was no different between the two regimens (Table 5). The incidence of neurological signs and symptoms (e.g. dizziness) was numerically greater for subjects treated with febuxostat + hydrochlorothiazide; however, the incidence of headaches was similar between the two regimens. Also of note, known side effects for hydrochlorothiazide include dizziness.
Table 5.
All adverse events
| Adverse event | Febuxostat 80 mg alone n = 34 | Febuxostat 80 mg + hydrochlorothiazide 50 mg n = 35 | Overall n = 36 | |||
|---|---|---|---|---|---|---|
| Any event | 14 | (41%) | 15 | (43%) | 23 | (64%) |
| Vision blurred | 0 | (0%) | 1 | (3%) | 1 | (3%) |
| Dyspepsia, eructation | 1 | (3%) | 1 | (3%) | 2 | (6%) |
| Abdominal pain | 2 | (6%) | 0 | (0%) | 2 | (6%) |
| Nausea and vomiting | 2 | (6%) | 3 | (9%) | 5 | (14%) |
| Upper respiratory tract infections | 2 | (6%) | 2 | (6%) | 4 | (11%) |
| Sunburn | 1 | (3%) | 0 | (0%) | 1 | (3%) |
| Abnormal liver function test | 1 | (3%) | 0 | (0%) | 1 | (3%) |
| Myalgia | 0 | (0%) | 1 | (3%) | 1 | (3%) |
| Muscle cramp | 1 | (3%) | 0 | (0%) | 1 | (3%) |
| Somnolence | 2 | (6%) | 0 | (0%) | 2 | (6%) |
| Headache | 4 | (12%) | 4 | (11%) | 6 | (17%) |
| Dizziness | 2 | (6%) | 4 | (11%) | 6 | (17%) |
| Menstruation with increase bleeding | 0 | (0%) | 1 | (3%) | 1 | (3%) |
| Vaginal laceration | 0 | (0%) | 1 | (3%) | 1 | (3%) |
| Dermatitis, contact | 0 | (0%) | 1 | (3%) | 1 | (3%) |
| Orthostatic hypotension | 1 | (3%) | 1 | (3%) | 2 | (6%) |
All adverse events were mild to moderate in severity with the exception of a vaginal wall tear, which occurred during the washout period between regimens, and was considered not related to study drug by the investigator. There were no deaths reported. Two subjects prematurely discontinued due to adverse events, one of the two subjects discontinued due to pharyngitis, and the other subject discontinued due to the previously discussed vaginal wall tear. Neither of these adverse events leading to withdrawal was considered related to study drug. The subject (20 year old male) who experienced the pharyngitis adverse event also experienced a single transient elevation in liver function tests (ALT and AST), which was considered by the investigator possibly related to the study drug; however, an alternate aetiology of intercurrent illness (viral syndrome) and concomitant medication (Tylenol, Nyquil, and Augmentin) use were cited by the investigator.
Co-administration of febuxostat and hydrochlorothiazide showed notable changes in urinary electrolytes, none of which was considered clinically significant. No clinically meaningful changes from baseline were observed for serum concentrations of sodium, chloride, potassium, or bicarbonate. Group mean reductions in systolic blood pressure were observed. These reductions were unlikely to be the result of co-administration with hydrochlorothiazide, as this diuretic exerts its antihypertensive effect only with chronic use. In addition, no clinically meaningful mean changes from baseline were observed for any orthostatic or other vital signs. No trends were noted for other laboratory tests or vital signs, physical examinations or ECGs.
Discussion
The present study demonstrated that the overall rate and extent of absorption of a single dose of febuxostat 80 mg was not affected by co-administration with a single dose of hydrochlorothiazide 50 mg in healthy adult subjects. This lack of effect of hydrochlorothiazide on the pharmacokinetics of febuxostat is consistent with the metabolic characteristics of the two medications. Hydrochlorothiazide does not undergo biotransformation, and is primarily excreted unchanged in urine. In addition, hydrochlorothiazide is not known to affect the uridine diphosphoglucuronyl transferase enzyme or cytochrome P450 enzymes involved in the elimination of febuxostat [6]. Therefore, no effect on the biotransformation of febuxostat was expected.
Hydrochlorothiazide is known to decrease the renal clearance and subsequently increase serum concentrations of UA [12, 13]. In this study, co-administration of febuxostat and hydrochlorothiazide modestly decreased the renal clearance of UA (9.5% reduction), potentially due to a hydrochlorothiazide-induced increase in the net reabsorption of UA in the renal proximal tubules. This decrease in renal clearance of UA caused a slight increase in the mean sUA concentrations over 24 and 48 h post-dosing intervals (6.5% and 7.9%, respectively). These slight increases in sUA concentrations, despite being statistically significant, were not considered clinically significant. Therefore, diuretics are not expected to diminish significantly the overall urate-lowering effect of febuxostat.
A limitation of this study is its design as a single dose drug–drug interaction study in healthy subjects with normal renal function. However, since hydrochlorothiazide does not accumulate significantly following multiple oral dosing, the extent of its effect after multiple dosing on both the pharmacokinetics and pharmacodynamics of febuxostat is expected to resemble that after single dosing with hydrochlorothiazide. Therefore, the effects of a single dose of hydrochlorothiazide may be extrapolated to multiple dose conditions. Similarly, the percent change in the renal clearance of UA caused by hydrochlorothiazide in hyperuricaemic subjects is not expected to be substantially different from that in healthy subjects, suggesting that the findings from this study may also be applicable to hyperuricaemic patients.
Due to the long elimination half-life of systemic UA, the use of percent change in renal clearance of UA (CLR) instead of percent change in sUA may be a more accurate measure of the overall effect of hydrochlorothiazide from this single dose study for extrapolation to multiple dose conditions. Based on the % decrease in CLR of 9.5% from our study under single dose conditions in healthy subjects, one would expect an approximately 10% (i.e. 100 × [1-(100/90.5)]) increase in sUA in multiple dosing conditions. In a multiple dose study in 3693 stepped care participants in the ‘Hypertension Detection and Follow-up Program’ [15], among men and women with higher serum uric acid concentrations (upper quartile sub-population with sUA = 458 ± 40 µmol l−1 and 403 ± 45 µmol l−1, respectively), following multiple dosing for 1 year with chlorthalidone or other thiazide diuretics, the mean sUA also increased by approximately 11–12% (51 ± 77 µmol l−1 and 47 ± 88 µmol l−1, respectively).
The lack of a clinically significant pharmacodynamic interaction between febuxostat and hydrochlorothiazide suggests that febuxostat may be an effective treatment for patients who develop gout while on diuretics. In fact, diuretic use has been established as an independent risk factor for the development of gout [14]. One epidemiological study of elderly subjects (≥65 years) found that the relative risk of initiation of urate lowering therapy was higher in subjects receiving thiazide (alone or in combination). This increase was seen for all thiazide dose groups studied (<25 mg day−1, 25–49 mg day−1, ≥50 mg day−1) with the magnitude of the risk increasing along with dose [16]. In addition, febuxostat has a superior clinical pharmacokinetic profile for patients with other characteristics typically associated with gout and/or hyperuricaemia, requiring no dose adjustments based on age and gender and in subjects with mild-severe renal dysfunction or mild-moderate hepatic dysfunction [17–25].
Finally, the lack of a pharmacokinetic effect or a clinically significant pharmacodynamic effect of hydrochlorothiazide on febuxostat suggests that febuxostat may be a viable option for hyperuricemic gout patients who require diuretics. In addition, febuxostat 80 mg co-administered with hydrochlorothiazide 50 mg under single dose conditions was found to be safe and well-tolerated by healthy subjects. The results from this study indicate that no adjustment in the dose of febuxostat is necessary when it is co-administered with hydrochlorothiazide.
Acknowledgments
The authors would like to thank Thomas Marbury, MD, for performing the clinical study. The authors would also like to thank Eric Lloyd, MS, for his statistical assistance; Patricia MacDonald, RN, NP, and Nancy Joseph-Ridge, MD, for their review of the manuscript; Jeanne Zemaitis and Jennifer Jaworski, MS, for their assistance in preparation of the manuscript; and MDS Pharma Services for performing the bioanalytical sample analyses. All authors were employees of TAP Pharmaceutical Products Inc. at the time of the study (TAP Pharmaceutical Products Inc. is now part of Takeda Global Research & Development Center, Inc.). Data from this study (C03-059) have been presented previously: Grabowski BA, Khosravan R, Wu J-T, Lademacher C, Vernillet L. Effect of hydrochlorothiazide on pharmacokinetics and pharmacodynamics of febuxostat [abstract]. Arthritis Rheum 2005; 52: S103-4.
Competing interests
J-TW is an employee of Takeda Global Research & Development Center and holds stocks of Takeda. LV was an employee of TAP Pharmaceutical Products Inc at the time of the study. CL was employed by Takeda from 2002–2008. There are no other competing interests to declare.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs. 2004;64:2399–416. doi: 10.2165/00003495-200464210-00003. [DOI] [PubMed] [Google Scholar]
- 3.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–47. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Sarawate CA, Patel PA, Schumacher HR, Yang W, Brewer KK, Bakst AW. Serum urate levels and gout flares: analysis from managed care data. J Clin Rheumatol. 2006;12:61–5. doi: 10.1097/01.rhu.0000209882.50228.9f. [DOI] [PubMed] [Google Scholar]
- 5.Becker M, Schumacher HR, Jr, Wortmann R, MacDonald P, Eustace D, Palo W, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 6.Kondo S, Nishimura S, Mochizuki T, Taniguchi K, Hoshide S, Nagao T, Ishii S, Kiyoki M. Metabolic fate of [14C]-TEI-6720, a novel xanthine oxidase inhibitor: tissue distribution after oral administration in rats, protein binding and metabolism in vivo and in vitro[abstract] Drug Metab Rev. 1995;8:56. [Google Scholar]
- 7.Becker MA, Kisicki J, Khosravan R, Wu J, Mulford D, Hunt B, MacDonald P, Joseph-Ridge N. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004;23:1111–16. doi: 10.1081/NCN-200027372. [DOI] [PubMed] [Google Scholar]
- 8.Riedel AA, Nelson M, Wallace K, Joseph-Ridge N, Cleary M, Fam AG. Prevalence of comorbid conditions and prescription medication use among patients with gout and hyperuricemia in a managed care setting. J Clin Rheumatol. 2004;10:308–14. doi: 10.1097/01.rhu.0000147049.12220.32. [DOI] [PubMed] [Google Scholar]
- 9.Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am. 2006;32:275–93. v–vi. doi: 10.1016/j.rdc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic [errata in: JAMA. 2003; 289(2):178 and JAMA. 2004; 291(18):2196] JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 11.Koay ESC, Walmsley N. A Primer of Chemical Pathology. Hackensack, NJ: World Scientific; 1996. [Google Scholar]
- 12.Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. 2003;17:397–414. doi: 10.1023/b:card.0000015855.02485.e3. [DOI] [PubMed] [Google Scholar]
- 13.El-Sheikh AAK, van den Heuvel JJMW, Koenderink JB, Russel FGM. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008;155:1066–75. doi: 10.1038/bjp.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 15.Langford HG. Is thiazide-produced uric acid elevation harmful? Analysis of data from the Hypertension Detection and Follow-up Program. Arch Intern Med. 1987;147:645–9. doi: 10.1001/archinte.147.4.645. [DOI] [PubMed] [Google Scholar]
- 16.Gurwitz JH, Kalish SC, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Thiazide diuretics and the initiation of anti-gout therapy. J Clin Epidemiol. 1997;50:953–9. doi: 10.1016/s0895-4356(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 17.Hoshide S, Takahashi Y, Ishikawa T, Kubo J, Tsuchimoto M, Komoriya K, Ohno I, Hosoya T. PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids. 2004;23:1117–18. doi: 10.1081/NCN-200027377. [DOI] [PubMed] [Google Scholar]
- 18.Mayer MD, Khosravan R, Vernillet L, Wu J-T, Joseph-Ridge N, Mulford DJ. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther. 2005;12:22–34. doi: 10.1097/00045391-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Khosravan R, Kukulka MJ, Wu J-T, Joseph-Ridge N, Vernillet L. The effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. J Clin Pharmacol. 2008;48:1014–24. doi: 10.1177/0091270008322035. [DOI] [PubMed] [Google Scholar]
- 20.Khosravan R, Wu J-T, Lademacher C, Grabowski BA, Vernillet L. Effect of febuxostat on pharmacokinetics and pharmacodynamics of warfarin [abstract] J Clin Pharmacol. 2005;45:1084. [Google Scholar]
- 21.Khosravan R, Erdman K, Vernillet L, Wu JT, Joseph-Ridge N, Umeda S, Mulford D. Effect of febuxostat on pharmacokinetics of desipramine, a CYP2D6 substrate, in healthy subjects [abstract] Clin Pharmacol Ther. 2005;77:P43. [Google Scholar]
- 22.Khosravan R, Grabowski BA, Mayer MD, Wu J-T, Joseph-Ridge N, Vernillet L. The effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. J Clin Pharmacol. 2006;46:88–102. doi: 10.1177/0091270005282634. [DOI] [PubMed] [Google Scholar]
- 23.Khosravan R, Wu J-T, Joseph-Ridge N, Vernillet L. Pharmacokinetic interactions of concomitant administration of febuxostat and NSAIDs. J Clin Pharmacol. 2006;46:855–66. doi: 10.1177/0091270006289848. [DOI] [PubMed] [Google Scholar]
- 24.Khosravan R, Grabowski B, Wu JT, Joseph-Ridge N, Vernillet L. Effect of food or antacid on febuxostat pharmacokinetics and pharmacodynamics in healthy subjects. Br J Clin Pharmacol. 2008;65:355–63. doi: 10.1111/j.1365-2125.2007.03016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khosravan R, Mayer M, Wu J-T, Joseph-Ridge N, Vernillet L. Effect of concomitant administration of febuxostat and colchicine on pharmacokinetics of febuxostat and colchicine at steady state [abstract] Arthritis Rheum. 2005;52:S102–3. [Google Scholar]


