Abstract
AIM
To determine the pharmacokinetics, pharmacodynamics, safety and tolerability of multiple oral doses of ticagrelor, a P2Y12 receptor antagonist, in healthy volunteers.
METHODS
This was a randomized, single-blind, placebo-controlled, ascending dose study. Thirty-two subjects received ticagrelor 50–600 mg once daily or 50–300 mg twice daily or placebo for 5 days at three dose levels in two parallel groups. Another group of 16 subjects received a clopidogrel 300 mg loading dose then 75 mg day−1, or placebo for 14 days.
RESULTS
Ticagrelor was absorbed with median tmax 1.5–3 h, exhibiting predictable pharmacokinetics over the 50–600 mg dose range. Mean Cmax and AUC for ticagrelor and its main metabolite, AR-C124910XX, increased approximately dose-proportionately (approximately 2.2- to 2.4-fold with a twofold dose increase) over the dose range. Inhibition of platelet aggregation (IPA) with ticagrelor was greater and better sustained at high levels with ticagrelor twice daily vs. once daily regimens. Throughout dosing, more consistent IPA was observed at doses ≥300 mg once daily and ≥100 mg twice daily compared with clopidogrel. Mean IPA with ticagrelor ≥100 mg twice daily was greater and less variable (93–100%, range 65–100%) than with clopidogrel (77%, range 11–100%) at trough concentrations. No safety or tolerability issues were identified.
CONCLUSIONS
Multiple dosing provided predictable pharmacokinetics of ticagrelor and its metabolite over the dose range of 50–600 mg once daily and 50–300 mg twice daily with Cmax and AUC(0,t) increasing approximately dose-proportionally. Greater and more consistent IPA with ticagrelor at doses ≥100 mg twice daily and ≥300 mg once daily were observed than with clopidogrel. Ticagrelor at doses up to 600 mg day−1 was well tolerated.
Keywords: antiplatelet therapy, AZD6140, inhibition of platelet aggregation, P2Y12 antagonist, pharmacokinetics, ticagrelor
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The antiplatelet agent clopidogrel is currently the recommended treatment for acute coronary syndrome (ACS).
Inhibition of platelet aggregation (IPA) with clopidogrel is insufficient, which increases the risk for recurrent ischaemic events. Therefore, there is a need for antiplatelet agents with improved IPA.
Ticagrelor (AZD6140) is a new antiplatelet agent in clinical development for reduction of thrombotic events in patients with ACS.
WHAT THIS STUDY ADDS
This study assesses the optimal dosing schedule for ticagrelor in healthy volunteers and compares the degree of IPA with clopidogrel.
Our findings illustrate that the pharmacokinetics of ticagrelor are predictable and are associated with consistent inhibition of platelet activity.
IPA with ticagrelor was greater and better sustained at high levels with twice daily ticagrelor than once daily regimens.
Introduction
Current standard treatment for acute coronary syndrome (ACS) is clopidogrel in combination with aspirin [1]. Clopidogrel is the only thienopyridine antiplatelet therapy currently recommended by the National Institute for Health and Clinical Excellence (NICE) for the management of non-ST-segment elevation ACS in people at moderate-to-high risk of myocardial infarction or death [2]. However, clopidogrel has been shown to produce an overall low degree of inhibition of platelet aggregation (IPA) and wide response variability (approximately 15–48% of patients have a poor platelet inhibition response to clopidogrel) [3–6]. Furthermore, growing evidence suggests that persistence of enhanced platelet reactivity despite the use of clopidogrel is associated with adverse clinical outcomes, including an increased risk for recurrent ischaemic events [6–11]. Some of the limitations of clopidogrel have been addressed by prasugrel, a recently approved thienopyridine that has demonstrated more consistent platelet inhibition [12]. However, prasugrel was associated with significantly increased risk of major bleeding, including life-threatening and fatal bleeding [12, 13]. There is therefore still a need for new antiplatelet therapies for ACS that provide high and consistent IPA [14–17].
Ticagrelor (AZD6140) is the first reversibly binding oral P2Y12 receptor antagonist, which is in clinical development for the treatment of ACS [17–20]. In vitro studies indicate that ticagrelor exerts its antiplatelet activity by binding to the P2Y12 receptor at a site distinct from the adenosine diphosphate (ADP) binding site [21, 22], thereby inhibiting ADP-induced receptor signalling in a non-competitive manner [20]. Ticagrelor undergoes extensive hepatic metabolism, with one known active metabolite (AR-C124910XX) present at a concentration approximately 30% of the parent drug (R Teng et al., unpublished data). Unlike the thienopyridines, however, ticagrelor does not require metabolic activation to exert its effect, which may account for its fast onset of action. Based on mass balance data, renal clearance of ticagrelor is limited and elimination occurs mainly through metabolism in the liver and biliary excretion (R Teng et al., unpublished data).
Studies suggest that ticagrelor may provide improvements in IPA compared with clopidogrel [19, 23]. Compared with clopidogrel, ticagrelor offered a wider separation between antithrombotic effects and bleeding time in preclinical models and, in phase II studies, provided greater and more consistent inhibition of ADP-induced platelet aggregation without an increase in major plus minor bleeding. The efficacy and safety of ticagrelor has been evaluated in comparison with clopidogrel in a broad population of ACS patients, in the double-blind, randomized phase III PLATO (PLATelet inhibition and patient Outcomes) study [24, 25]. After 12 months, a lower proportion of patients receiving ticagrelor than clopidogrel reached the primary endpoint of death from vascular causes, myocardial infarction or stroke (9.8% vs. 11.7%; P < 0.001), with no increase in the rate of overall major bleeding and an increase in the rate of non-procedure-related bleeding [25].
Single ascending dose studies in healthy volunteers indicate that ticagrelor has linear pharmacokinetics and, at doses ranging from 100 to 400 mg, produces near-complete IPA at 2 h post-dose [26]. IPA gradually decreased with declining plasma concentrations starting around 12 h post-dose, indicating that the IPA associated with ticagrelor is concentration dependent and reversible.
The aims of this study were to evaluate the pharmacokinetics, pharmacodynamics and the safety and tolerability of multiple doses of ticagrelor dosed to steady-state in healthy volunteers and thereby establish the optimal dosing regimen for subsequent studies. As a comparator, a clopidogrel control arm was included (using the dose indicated for ACS in clinical practice) [27]. Although pharmacokinetic and pharmacodynamic data were collected, only pharmacodynamic data are reported.
Methods
Study population
The study enrolled healthy male and post-menopausal or surgically-sterile female volunteers between 18 and 65 years. Subjects were required to have a body mass index (BMI) between 18 and 30 kg m−2, with a bodyweight >50 kg, and to be in good health based on physical examination, vital signs, laboratory values, and 12-lead electrocardiography (ECG) and have a bleeding time of <4 min (by lancet method). Exclusion criteria included: a history or presence of conditions known to interfere with the absorption, distribution, metabolism, or excretion of drugs, presence of clinically significant abnormal values for prothrombin or activated partial thromboplastin time, a history of intolerance or hypersensitivity to drugs or their excipients similar in chemical structure to ticagrelor (such as adenine nucleoside antivirals and immunosuppressant drugs), aspirin or other nonsteroidal anti-inflammatory drug use within 2 weeks of enrolment or treatment with any medication other than hormone replacement therapy within 3 weeks of dosing, a history of drug or alcohol abuse and tobacco use greater than 10 cigarettes per day or 12.5 g of tobacco per week or inability to abstain from tobacco use during treatment.
All subjects provided written informed consent, and the study was approved by an Independent Ethics Committee and performed in accordance with guidelines established by the Declaration of Helsinki.
Study design and treatment
This phase I study was a sequential, parallel-group, placebo-controlled, ascending dose study (D5130C05239) performed at a single centre to examine the pharmacokinetics, pharmacodynamics, and safety and tolerability of ticagrelor. A clopidogrel control arm was included in the study to compare pharmacodynamic data with that of ticagrelor. Additionally, the carboxylic acid metabolite of clopidogrel was assayed to determine performance to published literature. Although due to the different dosing schedules and pharmacokinetic sampling times, it was not possible to allocate subjects blind to the ticagrelor and clopidogrel groups, placebo controls were used so subjects were unaware of whether they were receiving active or placebo treatments. In addition, ticagrelor was administered as a double-dummy, so subjects were unaware of whether they were receiving the once daily or twice-daily dosing regimen.
A total of 48 subjects were allocated to three groups (Figure 1), with assignment based on a strictly sequential randomization code. Two parallel groups of 16 subjects (groups A and B) received either placebo (n = 2) or ticagrelor (n = 14). Subjects received each ticagrelor dose (as tablets with 150 ml of water) for 5 days before escalation to the next dose level. A third group (group C) received either placebo (n = 2) or clopidogrel (n = 14) at the dose indicated for ACS in clinical practice [27].
Figure 1.
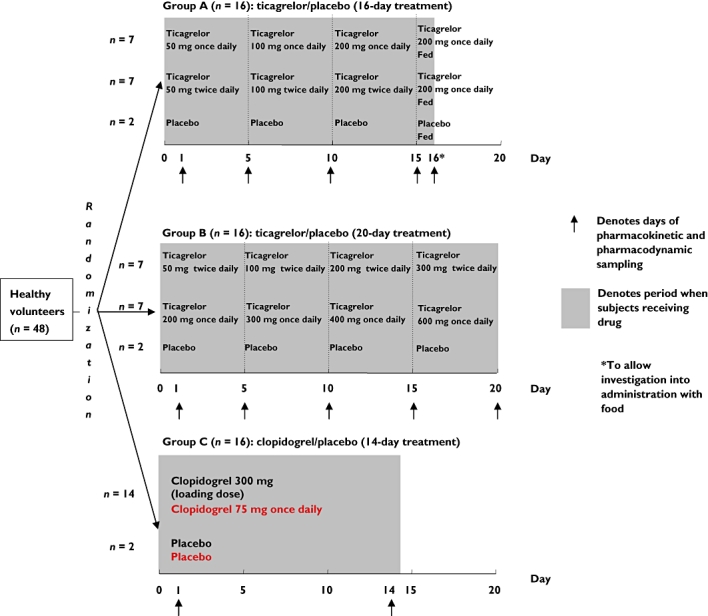
Study design
Group A received ticagrelor at doses of 50, 100 and 200 mg once daily (n = 7) or ticagrelor at doses of 50, 100 and 200 mg twice daily (n = 7) or placebo (n = 2) for a total duration of 16 days. Group B received ticagrelor at doses of 50, 100, 200 and 300 mg twice (n = 7) or ticagrelor at doses of 200, 300, 400 and 600 mg once daily (n = 7) or placebo (n = 2) for a total duration of 20 days. Before group B (scheduled to examine the higher ticagrelor doses) was initiated, Ggroup A was completed and the safety data reviewed before treatment of group B was allowed.
Early ticagrelor tablet formulations (50, 100 and 200 mg) were evaluated in this study and phase IIa studies [19, 28]. The phase III tablet formation (90 mg), which was also used in phase IIb and phase III PLATO studies, provided comparable ticagrelor exposure with the early tablet formulation (90 mg twice daily corresponded to 100 mg twice daily of the old formulation).
Group C received clopidogrel (n = 14) as an overencapsulated tablet at a 300 mg loading dose followed by 75 mg once daily for 14 days or placebo (n = 2) for a total duration of 14 days. Bioequivalence between the overencapsulated and intact tablets was demonstrated in a bioequivalence study in healthy subjects (AstraZeneca; data on file). To ensure blinding in those receiving ticagrelor once daily, subjects were administered placebo for the evening dose. Subjects were administered the morning dose of ticagrelor fasting and the evening dose with food. Clopidogrel doses were given in the fasting state (with 150 ml of water).
Blood sampling
Blood samples for pharmacokinetic (2–3 ml) and pharmacodynamic evaluation (4.5 ml) were collected at each dose level for the determination of ticagrelor and metabolite AR-C124910XX plasma concentrations. Pharmacokinetic sampling was performed on the days illustrated in Figure 1. Samples were collected on day 1 of the first dose level, and at day 5 at each ticagrelor dose level (corresponding to steady-state). After the pharmacokinetic sampling on day 5, subjects then received the next dose level. For clopidogrel, samples were collected on day 1 and at day 14.
Pharmacokinetic evaluations
Plasma concentrations of ticagrelor and AR-C124910XX were measured at 0 (pre-dose) and 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h after the morning dose on the days of sampling. After the final study dose, measurements were also made 24 h after dosing. Blood samples were collected into lithium heparin tubes, centrifuged at 1500 g at 4°C for 10 min within 30 min of sampling. The resultant plasma was transferred into a plain polypropylene tube (screw cap) and stored at or below −20°C until analyzed.
Plasma concentrations of ticagrelor and AR-C124910XX were determined using a validated liquid chromatography and mass spectrometry detection after protein precipitation (H Sillén et al., unpublished data). The method was validated using 0.1 ml plasma sample volumes for ticagrelor in the range of 1.0–500 ng ml−1 with a lower limit of quantification (LLOQ) of 1.0 ng ml−1 and for AR-C124910XX in the range of 2.5–500 ng ml−1 with a LLOQ of 2.5 ng ml−1. The two assays provided accurate and reproducible results, with the defined limits of accuracy (standard deviation <20%) and precision (coefficient of variation <20% at the LLOQ and <15% at all other levels up to the upper limit of quantification).
Pharmacokinetic parameters
All pharmacokinetic calculations were performed with WinNonlin 3.2 Enterprise (Pharsight, Mountain View, CA, USA). Statistical analyses were carried out with SAS version 8 (SAS Institute, Inc., Cary, NC, USA).
The primary pharmacokinetic parameters were: peak plasma concentration (Cmax); time to peak plasma concentration (tmax); area under the plasma concentration–time curve within the dosing interval (AUC(0,t)) on days 1 and 5, plasma elimination half-life (t1/2) and apparent plasma clearance (CL/F). Cmax was measured within 24 h after the last dose of once daily treatment and within 12 h after the last dose of twice daily treatment. tmax was defined as the time of first occurrence of Cmax. Individual ticagrelor and AR-C124910XX t1/2 values were calculated as 0.693/λz, where λz is the terminal-phase elimination rate constant estimated by least-squares regression analysis of the plasma concentration–time data obtained over the terminal log-linear phase. AUC(0,t) was calculated by the trapezoidal rule and CL/F was calculated as dose divided by AUC(0,t).
The linearity of exposure to ticagrelor and AR-C124910XX vs. dose was assessed by fitting appropriate generaliszed linear models to AUC(0,12 h) for twice daily regimens, AUC(0,24 h) for once daily regimens, and Cmax for both once daily and twice daily regimens. Accumulation ratios, calculated as the ratios of AUC(0,t) at steady-state (day 5) to AUC(0,t) on day 1, were expressed as geometric mean (coefficient of variation, %).
Pharmacodynamic evaluation
Inhibition of platelet aggregation was measured in blood samples obtained at 0 (pre-dose), 4, 8, 12 and 24 h on day 1, and at day 5 of each subsequent dose level in subjects receiving ticagrelor, and at 36 and 48 h after the first dose on the final study day in group B. IPA was measured at 0 (pre-dose), 4, 8, 12, and 24 h on day 1 and day 14 in subjects receiving clopidogrel.
Aggregation was performed according to the Born method [29]. This method produced similar reproducibility and precision to that previously described [29]. Blood was centrifuged at 180–200 g for 10 min, and the platelet-rich plasma (PRP) collected. PRP platelet count was adjusted to 300 × 109 l−1 using autologous platelet-poor plasma (PPP). Aggregation was measured continuously by light transmittance using ADP at final concentrations of 5 and 20 µmol L−1 (model 490D or 540Vs aggregometers, Chrono-log Corporation, Havertown, PA, USA). Autologous PPP and unstimulated PRP were used as 100% and 0% aggregation references, respectively. The maximal and final aggregation responses were reported. Percentage IPA was calculated as 100 × ([mean pre-dose response − mean response during treatment visit]/mean pre-dose response). Percentage inhibition of optical aggregometry was summarized by treatment group and comparisons made between ticagrelor and clopidogrel.
Effect of food
To allow investigations into the food effect on pharmacokinetics and pharmacodynamics of ticagrelor, on day 16, subjects in group A received ticagrelor 200 mg with food. In this group, subjects received only a morning dose on the final day of the 16-day treatment period with a high-fat breakfast (Figure 1). The effect of food was then assessed by informal comparison of pharmacokinetic data obtained in the fasting and fed states in subjects receiving ticagrelor 200 mg once daily or twice daily in group A.
Bleeding times
Standard lancet bleeding times were assessed throughout the study, using the Simplate method at a distending venous pressure of 40 mmHg applied with a standard sphygmomanometer cuff. Blood was blotted in a systematic manner with a filter paper every 30 s noting the time (to nearest 15 s) when bleeding had ceased. For subjects taking ticagrelor, measurements were determined at enrolment, on admission (day 0), day 1, at 0 (pre-dose), 4 and 11 h after first dose, days 4 and 9, at 11 h post-dose, day 15, at 0, 4 and 11 h after the first dose and (group B only) day 20, at 0, 4 and 11 h after first dose. For group C, measurements were determined at enrolment, on admission, day 1, at 0, 4 and 11 h after first dose and days 1, 7 and 14 at 11 h post-dose. Bleeding time data were summarized by treatment group and comparisons were made between ticagrelor and clopidogrel groups.
Safety and tolerability
Safety evaluation included recording of adverse events, physical examination, assessment of vital signs, ECG, clinical chemistry and haematology assessments, and urinalysis. Samples for laboratory analysis were obtained prior to the first study dose, pre-dose at the start of each dose level, 48 h after the first dose on the last day of study dosing and at a follow-up visit 7 to 14 days after completion of study treatment. Safety results were summarized by treatment group.
Results
A total of 48 subjects (90% male, 92% Caucasian) were enrolled in the study. The mean age of the study population was 37 years (range 20–64 years), and mean weight was 75 ± 10.7 kg. Twenty-eight subjects received ticagrelor (groups A and B), and 14 subjects received clopidogrel (300 mg loading dose followed by 75 mg once daily). Two subjects in each group received placebo (groups A and B, ticagrelor placebo; group C, clopidogrel placebo). The mean age of subjects in groups A, B and C was 43 (± 17.7), 33 (± 10.6) and 35 (± 11.3) years, respectively; mean weight was 72 (± 11.5), 73 (± 8.3) and 81 (± 10.1) kg, respectively.
Pharmacokinetics of ticagrelor and AR-C124910XX
Calculated pharmacokinetic parameters and their variability are shown in Table 1.
Table 1.
Pharmacokinetics of ticagrelor and its main metabolite AR-C124910XX on day 1 and at end of dosing period (day 5) at doses of 50–600 mg once daily and 50–300 mg twice daily
| Cmax (ng ml−1) | tmax (h) | AUC(0,t) (ng ml−1 h) | CL/F (ml min−1) | t1/2 (h) | Accumulation ratios, Day 5 : Day 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor dose (mg) | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| Once daily dosing | |||||||||||
| Ticagrelor | |||||||||||
| 50 (n = 7) | 219 (60) | 222 (33) | 2 (1.5–6) | 3 (2–4) | 1,531 (40) | 1,877 (34) | 293 (37) | 417 (32) | 6.9 (16) | 7.7 (27) | 1.2 (15) |
| 100 (n = 7) | – | 557 (50) | – | 3 (1.5–4) | – | 4,295 (43) | – | 388 (43) | – | 7.8 (22) | – |
| 200 (n = 7, 14)* | 785 (27) | 1,030 (42) | 2 (1.5–4) | 2 (1.5–4) | 4,970 (29) | 7,884 (48) | 336 (32) | 423 (48) | 6.2 (12) | 8.2 (28) | 1.2 (16) |
| 300 (n = 7) | – | 1,354 (23) | – | 1.5 (1.5–2) | – | 10,611 (32) | – | 471 (32) | – | 7.8 (26) | – |
| 400 (n = 6) | – | 1,862 (12) | – | 1.5 (1–2) | – | 14,963 (26) | – | 446 (26) | – | 8.6 (35) | – |
| 600 (n = 6) | – | 2,958 (33) | – | 1.75 (1–3) | – | 24,088 (33) | – | 415 (33) | – | 13.1 (23) | – |
| AR-C124910XX | |||||||||||
| 50 (n = 7) | 57 (65) | 71 (40) | 4 (2–8) | 3 (3–6) | 542 (52) | 744 (40) | – | – | 9.4 (20) | 10.6 (36) | 1.4 (22) |
| 100 (n = 7) | – | 171 (50) | – | 4 (2–4) | – | 1,880 (42) | – | – | – | 11.3 (20) | – |
| 200 (n = 7, 14)* | 215 (36) | 293 (45) | 3 (1.5–4) | 3 (2–4.12) | 1,671 (40) | 3,053 (48) | – | – | 8.0 (20) | 12.5 (46) | 1.3 (15) |
| 300 (n = 7) | – | 362 (31) | – | 2 (1.5–3) | – | 3,941 (26) | – | – | – | 9.9 (8) | – |
| 400 (n = 6) | – | 508 (15) | – | 2 (2–3) | – | 5,577 (31) | – | – | – | 12.3 (39) | – |
| 600, day 5 (n = 6) | – | 791 (30) | – | 2.5 (1.5–3) | – | 8,962 (34) | – | – | – | 16.6 (19) | – |
| Twice daily dosing | |||||||||||
| Ticagrelor | |||||||||||
| 50 (n = 14) | 172 (38) | 251 (34) | 2 (1.5–4) | 3 (1–4) | 942 (37) | 1,682 (35) | 374 (58) | 511 (36) | 6.6 (3.3) | 6.7 (28) | 1.8 (27) |
| 100 (n = 13) | – | 626 (46) | – | 2 (1–6) | – | 4,108 (43) | – | 406 (45) | – | 6.7 (45) | – |
| 200 (n = 13) | – | 1,440 (27) | – | 2 (1.5–4) | – | 9,436 (30) | – | 362 (32) | – | 9.1 (62) | – |
| 300 (n = 7) | – | 1,936 (69) | – | 3 (2–4) | – | 14,203 (54) | – | 378 (55) | – | 8.6 (37) | – |
| AR-C124910XX | |||||||||||
| 50 (n = 14) | 55 (39) | 81 (28) | 2.5 (2–4) | 3 (1.5–6) | 336 (35) | 639 (29) | – | – | 6.4 (37) | 7.5 (25) | 1.9 (28) |
| 100 (n = 13) | – | 219 (49) | – | 3 (1.5–6) | – | 1,701 (47) | – | – | – | 9.2 (95) | – |
| 200 (n = 13) | – | 462 (48) | – | 3 (1.5–6) | – | 3,693 (49) | – | – | – | 12.4 (54) | – |
| 300 (n = 7) | – | 592 (42) | – | 3 (2–4) | – | 5,111 (31) | – | – | – | 10.7 (49) | – |
Data for tmax are median (range); all other data are geometric mean (coefficient of variation, %).
n = 7 for day 1, n = 14 for day 5.
Single dose pharmacokinetics
After a single dose (day 1), comparison of ticagrelor 50 mg and 200 mg once daily showed that the mean Cmax and AUC(0,t) for ticagrelor increased in proportion to dose. Ticagrelor was rapidly absorbed and the median tmax was 2 h. Mean ticagrelor t1/2 after a single dose ranged from 6.2 to 6.9 h (Table 1). Mean apparent clearance ranged from 293 to 374 ml min−1.
Comparison of the ticagrelor 50 mg and 200 mg once daily showed that the mean Cmax and AUC(0,t) of AR-C124910XX also increased in proportion to dose. Overall, exposure to the active metabolite AR-C124910XX was approximately 40% of exposure to ticagrelor. Formation of AR-C124910XX was rapid (day 1, median tmax 2.5–4.0 h over the range of ticagrelor doses). The mean elimination t1/2 of AR-C124910XX after a single dose ranged from 6.4 to 9.4 h (Table 1). Plasma concentrations of clopidogrel (300 mg loading dose followed by 75 mg once daily) were consistent with those previously reported [19, 23, 28].
Steady-state pharmacokinetics
Mean plasma concentration–time profiles of ticagrelor (Figure 2A,B) and AR-C124910XX (Figure 3A,B) at steady-state (day 5) demonstrated that after multiple dosing with both once daily and twice daily ticagrelor, increasing ticagrelor dose resulted in increases in the AUC(0,t) and Cmax. At day 5, median tmax of ticagrelor ranged from 1.5–3.0 h for once daily and 2–3 h for twice daily over the range of ticagrelor doses (Table 1). Mean apparent clearance was consistent over both once daily (range 388–471 ml min−1) and twice daily dosing (range 362–511 ml min−1). Mean ticagrelor t1/2 determined at steady-state ranged from 7.7 to 13.1 h with once daily dosing and from 6.7 to 9.1 h with twice daily dosing.
Figure 2.
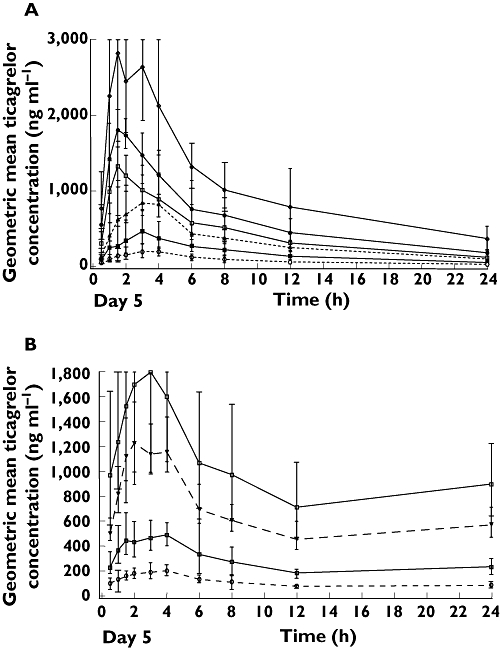
Geometric mean (± 95% CI) plasma concentrations of ticagrelor after A) ticagrelor once daily dosing and B) ticagrelor twice daily dosing on day 5. (A) 50 mg once daily ( ); 100 mg once daily (
); 100 mg once daily ( ); 200 mg once daily (
); 200 mg once daily ( ); 300 mg once daily (
); 300 mg once daily ( ); 400 mg once daily (
); 400 mg once daily ( ); 600 mg once daily (
); 600 mg once daily ( ). (B) 50 mg twice daily (
). (B) 50 mg twice daily ( ); 100 mg twice daily (
); 100 mg twice daily ( ); 200 mg twice daily (
); 200 mg twice daily ( ); 300 mg twice daily (
); 300 mg twice daily ( )
)
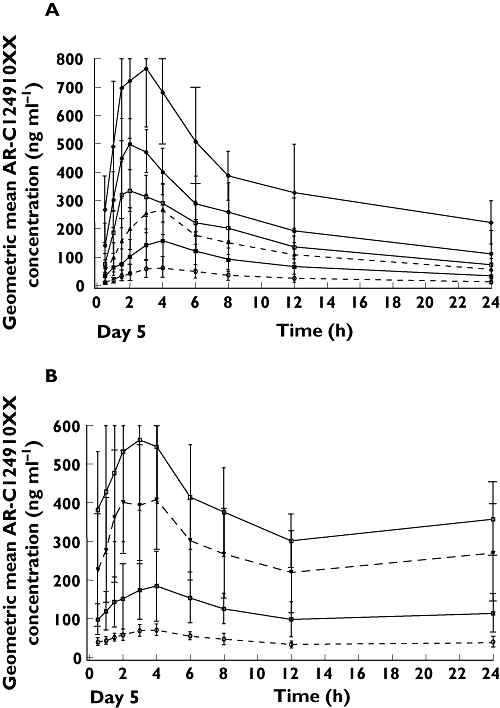
Figure 3.
Geometric mean (± 95% CI) plasma concentrations of AR-C124910XX after A) ticagrelor once daily dosing and B) ticagrelor twice daily dosing on day 5. (A) 50 mg once daily ( ); 100 mg once daily (
); 100 mg once daily ( ); 200 mg once daily (
); 200 mg once daily ( ); 300 mg once daily (
); 300 mg once daily ( ); 400 mg once daily (
); 400 mg once daily ( ); 600 mg once daily (
); 600 mg once daily ( ). (B) 50 mg twice daily (
). (B) 50 mg twice daily ( ); 100 mg twice daily (
); 100 mg twice daily ( ); 200 mg twice daily (
); 200 mg twice daily ( ); 300 mg twice daily (
); 300 mg twice daily ( )
)
After multiple dosing, exposure to AR-C124910XX was approximately 40% of exposure to ticagrelor (Table 1). Formation of AR-C124910XX was again rapid (median tmax 2.0–4.0 h over the range of once daily and twice daily ticagrelor doses). The mean elimination t1/2 of AR-C124910XX at steady-state ranged from 7.5 to 12.4 h (twice daily and once daily dosing).
Further analysis of the dose proportionality of the pharmacokinetics of ticagrelor and AR-C124910XX showed that the pharmacokinetics were predictable. Mean Cmax and AUC(0,t) (AUC(0,12 h) for twice daily dosing and AUC(0,24 h) for once daily dosing) for ticagrelor and AR-C124910XX increased dose proportionately (Figure 4A,B). With a twofold increase in ticagrelor dose, 2.37- and 2.32-fold increases were seen in the AUC(0,t) of ticagrelor and AR-C124910XX, with approximately a 2.2-fold increase in Cmax with a doubling of the dose.
Figure 4.
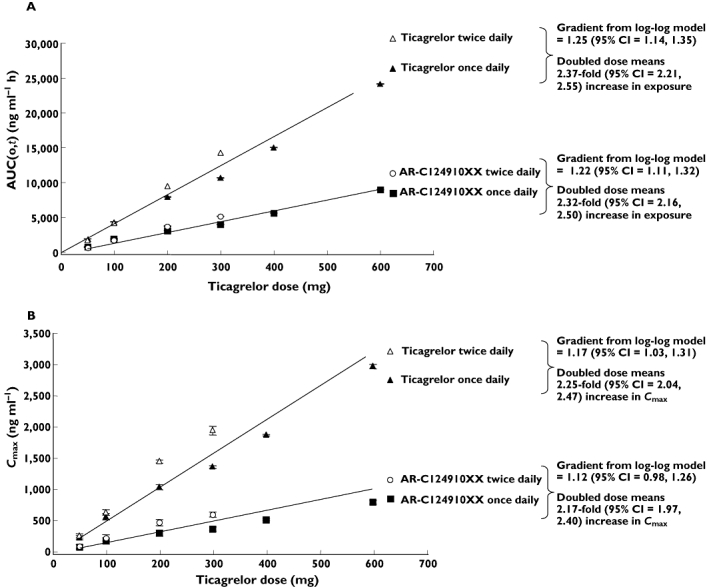
Analysis of pharmacokinetic dose-proportionality: effect of increasing ticagrelor dose (administered either as once daily or twice daily) on A) AUC(0,t) of ticagrelor and AR-C124910XX and B) Cmax of ticagrelor and AR-C124910XX. Data expressed as geometric mean ± 95% CV gradient determined from log-log model
Accumulation ratios (geometric mean [coefficient of variation, %]), expressed as the ratios of AUC(0,t) on day 5 to AUC(0,t) on day 1, were 1.2 for ticagrelor 50 mg and 200 mg once daily and 1.8 for 50 mg twice daily. For AR-C124910XX, accumulation ratios were 1.4, 1.3 and 1.9, respectively (Table 1).
Pharmacodynamics
The pattern of results was similar irrespective of whether IPA was assessed from the final extent of aggregation (observed at the end of the platelet response) or from the maximal extent of aggregation. Hence, only final extent of IPA responses (which tends to be more sensitive to P2Y12 receptor antagonists as it is mediated primarily by the P2Y12 receptor) [30] are shown.
Peak mean final extent IPA (80–100%, 20 µm ADP) at all doses of ticagrelor was observed at the first post-dose measurement (4 h) on day 1 (Figure 5A) and day 5 (Figure 5B,C). At all ticagrelor doses, substantial IPA was seen, although increasing levels of inhibition and duration of response were seen with increasing once daily and twice daily doses (Figure 5C,B, respectively). IPA was greater and more consistently sustained at high levels over the dosing interval with ticagrelor twice daily than with once daily dosing (Figure 5B,C, respectively).
Figure 5.
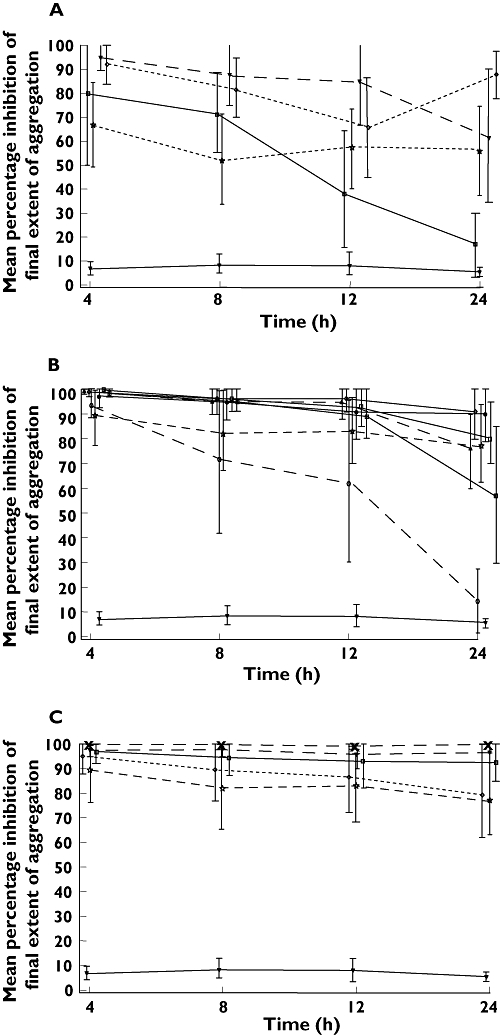
Mean % IPA (± 95% CI) over time at final extent (aggregation (optical aggregometry, 20 µm ADP) after ticagrelor (multiple doses) and clopidogrel (loading dose 300 mg followed by 75 mg once daily) after a single dose (day 1) (A) and at steady-state (ticagrelor, day 5; clopidogrel, day 14) (B and C). Figure 5B shows ticagrelor once daily doses; Figure 5C shows ticagrelor twice daily doses. Footnote: data were collected at timepoints 4, 8, 12 and 24 h, but are offset to avoid overlap. (A) 50 mg twice daily (◊); 200 mg once daily ( ); Clopidogrel 75 mg once daily (
); Clopidogrel 75 mg once daily ( ); 50 mg once daily (
); 50 mg once daily ( ); Placebo (
); Placebo ( ). (B) 600 mg once daily (
). (B) 600 mg once daily ( ); 400 mg once daily (
); 400 mg once daily ( ); 300 mg once daily (
); 300 mg once daily ( ); Clopidogrel 75 mg once daily (
); Clopidogrel 75 mg once daily ( ); 200 mg once daily (
); 200 mg once daily ( ); 100 mg once daily (
); 100 mg once daily ( ); 50 mg once daily (
); 50 mg once daily ( ); Placebo (
); Placebo ( ). (C) 300 mg twice daily (
). (C) 300 mg twice daily ( ); 200 mg twice daily (
); 200 mg twice daily ( ); 100 mg twice daily (
); 100 mg twice daily ( ); 50 mg twice daily (◊); Clopidogrel 75 mg once daily (
); 50 mg twice daily (◊); Clopidogrel 75 mg once daily ( ); Placebo (
); Placebo ( )
)
Ticagrelor twice daily doses resulted in higher mean IPA than the equivalent once daily dosing. At trough plasma drug concentrations (24 h, day 5), mean IPA (final extent) was greater with ticagrelor 100 mg twice daily (93%) compared with ticagrelor 200 mg once daily (76%); mean IPA was 97% with ticagrelor 200 mg twice daily compared with 400 mg once daily (90%); mean IPA was 100% with ticagrelor 300 mg twice daily compared with 600 mg once daily (91%).
IPA levels with ticagrelor doses of ≥300 mg once daily and ≥100 mg twice daily were higher and more sustained over the measured dose interval than those with clopidogrel (300 mg loading dose followed by 75 mg once daily; Figures 5B,C, respectively). At trough plasma drug concentrations (24 h, day 5), mean IPA (final extent) was 82–91% (range 61–100%) for ticagrelor ≥300 mg twice daily and 93–100% (range 65–100%) for ticagrelor ≥100 mg twice daily, compared with 77% (range 11–100%) after 14 days of clopidogrel. IPA with clopidogrel was in line with expectations based on previously reported data [19, 23, 28].
Figure 6 indicates that increasing plasma concentrations of ticagrelor were associated with increasing IPA. At lower ticagrelor plasma concentrations, the extent of IPA was considerable, ranging from (0–100%). A similar relationship was seen with AR-C124910XX plasma concentrations and IPA (data not shown).
Figure 6.
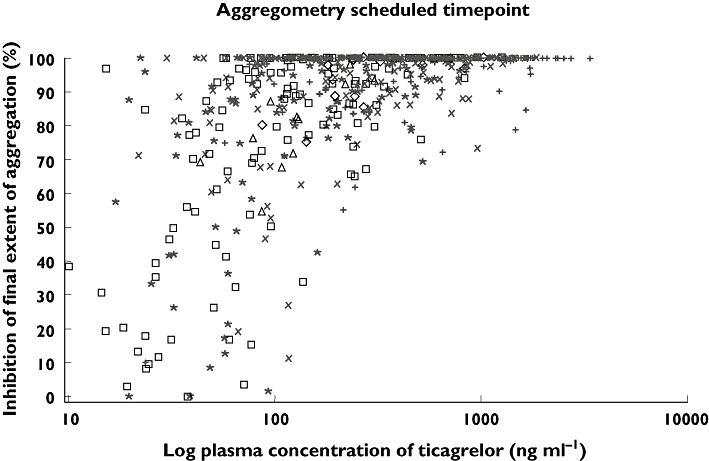
Effect of log ticagrelor plasma concentration on % IPA (final extent, 20 µm ADP). Data shown over a restricted plasma concentration range (0–1000 ng ml−1); key shows actual time (post-dose) of aggregometry measurement. 4 h (+); 8 h (×); 12 h (*); 24 h (□); 36 h (◊); 48 h (▵)
Effect of food
Administration of ticagrelor 200 mg (once daily or twice daily) with food resulted in an approximately 25% increase in ticagrelor exposure (Table 2). For ticagrelor 200 mg once daily, AUC(0,t) increased 20%, from 10 603 ng ml−1 h (fasting state) to 12 768 ng ml−1 h (fed state). Also, Cmax increased 17% when administered with food. For subjects receiving ticagrelor 200 mg twice daily, AUC(0,t) increased 31%, from 10 160 ng ml−1 h (fasting state) to 13 335 ng ml−1 h (fed state). Cmax increased 11% when administered with food. Administration of ticagrelor 200 mg (once daily or twice daily) with food had no apparent effect on exposure to AR-C124910XX compared with fasting administration. Administration of food with ticagrelor had no apparent effect on the final extent of IPA (Table 2).
Table 2.
Effect of administration with food compared with fasting on pharmacokinetics of ticagrelor and AR-C124910XX and % IPA (Final Extent, 20 µm ADP) after administration of ticagrelor 200 mg once daily or bid
| Pharmacokinetics | Mean % IPA (range) | ||||||
|---|---|---|---|---|---|---|---|
| AUC(0,τ) (ng ml−1 h) | Cmax(ng ml−1) | tmax(h) | 4 h | 8 h | 12 h | 24 h | |
| Once daily ticagrelor | |||||||
| 200 mg, fasting, day 5 (n = 7) | 10 603 (41) | 1304 (44) | 3.00 (1.50–4.00) | 99 (97–100) | 98 (93–100) | 98 (91–100) | 89 (67–100) |
| 200 mg, fed, Day 1 (n = 7) | 12 768 (42) | 1525 (39) | 3.00 (1.33–4.00) | 100 (100–100) | 95 (86–100) | 97 (89–100) | 80 (34–100) |
| Twice daily ticagrelor | |||||||
| 200 mg, fasting, day 5 (n = 6) | 10 160 (26) | 1583 (26) | 2.00 (2.00–4.00) | 97 (85–100) | 98 (89–100) | 96 (79–100) | 96 (76–100) |
| 200 mg, fed, day 1 (n = 6) | 13 335 (14) | 1763 (24) | 4.00 (3.00–12.00) | 96 (79–100) | 95 (73–100) | 95 (69–100) | 94 (66–100) |
| AR-C124910XX (after once daily dosing) | |||||||
| 200 mg, fasting, day 5 (n = 7) | 4 164 (35) | 385 (35) | 4.00 (2.00–4.12) | – | – | – | – |
| 200 mg, fed, day 1 (n = 7) | 4 079 (39) | 358 (23) | 4.00 (1.83–6.00) | – | – | – | – |
| AR-C124910XX (after twice daily dosing) | |||||||
| 200 mg, fasting, day 5 (n = 6) | 4 423 (71) | 555 (65) | 3.50 (2.00–4.00) | – | – | – | – |
| 200 mg, fed, day 1 (n = 6) | 4 887 (56) | 568 (52) | 5.00 (3.00–12.00) | – | – | – | – |
Data for tmax are median (range); all other pharmacokinetic data are geometric mean (coefficient of variation, %).
Bleeding times
Median baseline bleeding time was 165 s (range 128–263 s). Compared with baseline, median bleeding times increased with ticagrelor by approximately 1.1- to 3.3-fold, compared with a 1.1- to 1.2-fold increase with placebo, and a 1.5- to 1.9-fold increase with clopidogrel. On two occasions, individual bleeding times at scheduled timepoints exceeded 30 min (on day 4 in one subject receiving ticagrelor 100 mg twice daily and on day 14 in one subject receiving clopidogrel 75 mg once daily). Other bleeding times measured for these subjects were less than 10 min. There was no clear dose or plasma concentration relationship with bleeding times (data not shown).
Safety and tolerability
Thirty subjects in groups A and B completed study treatment, with two discontinuing due to adverse events. All subjects in group C completed study treatment.
Adverse events observed with ticagrelor, administered at various dosing schedules, were comparable with those observed with once daily clopidogrel. Study treatments were well tolerated with no serious, severe, or dose-related adverse events reported. The most common adverse events considered possibly related to study treatment were myalgia in three subjects receiving ticagrelor, and gingival bleeding in two subjects on clopidogrel, and one on ticagrelor. No subjects in the study reported dyspnoea.
Two subjects receiving ticagrelor discontinued treatment due to adverse events. One subject was withdrawn from the study due to elevated alanine (ALT) and aspartate transaminase (AST) concentrations (ALT 266 IU l−1 and AST 141 IU l−1), which were three times the upper limit of normal following 4 days of treatment with ticagrelor 300 mg once daily. By day 24, transaminase concentrations were below 100 IU l−1, and were normal at 10-day follow-up in this subject. There was no associated rise in bilirubin or alkaline phosphatase, and no other causative aetiology was determined. A second subject on ticagrelor withdrew due to asthma/bronchitis, considered unrelated to study treatment. Another subject had a clinically relevant increase in ALT and AST concentrations (110 IU l−1 and 64 IU l−1, respectively) on study day 15 following 4 days of treatment with ticagrelor 200 mg twice daily. This adverse event was mild in severity, and the subject continued treatment. Transaminase concentrations improved in this subject during 300 mg once daily dosing and were resolved in 14 days. Baseline levels for both subjects with elevated transaminase levels were within the upper limit of normal. A causal relationship between the study drug and the increase in levels was considered reasonably possible. No other clinically relevant changes in vital signs, ECG, or laboratory values were observed.
Discussion
This multiple ascending dose study showed that the pharmacokinetics of ticagrelor are linear and stationary over the dose range of 50–600 mg once daily and 50–300 mg twice daily. The pharmacokinetics of ticagrelor and its metabolite behaved in a predictable manner with Cmax and AUC(0,t) increasing approximately dose-proportionally with increasing dose (approximately 2.2- to 2.4-fold with a twofold increase in dose). The t1/2 and tmax of ticagrelor and its active metabolite AR-C124910XX were independent of ticagrelor dose, as was the CL/F of ticagrelor. Consistent with the results of other pharmacokinetic studies over a range of ticagrelor doses [31], ticagrelor demonstrates a predictable and linear pharmacokinetic profile that would make it easy to use in clinical practice.
The finding that the magnitude of IPA was associated with plasma ticagrelor concentrations is to be expected, since ticagrelor exerts its antiplatelet activity by binding directly to the P2Y12 receptor. This mechanism is in contrast to the thienopyridines, clopidogrel and prasugrel, that must undergo metabolic conversion in order to exert activity. Our pharmacokinetic data showed that the absorption of ticagrelor was rapid, with mean tmax of 2 h. This finding is consistent with previous data showing that the onset of the antiplatelet effect of ticagrelor occurs within approximately 30 min [19, 32, 33]. As the first measurement of IPA was not performed until 4 h, our study was not suitable to characterize fully the early onset of the effect of ticagrelor, but our data support previous findings that the onset of peak inhibitory activity is faster with ticagrelor than with the standard clopidogrel dose [14, 19, 23, 33]. IPA was not measured with clopidogrel until day 14 in our study, since even with a loading dose, clopidogrel does not achieve full platelet activity for 4–8 days [34].
In addition to the rapid onset of effect, in vitro binding studies have shown that ticagrelor binds reversibly to the P2Y12 receptor [21, 22]. Thus, a more rapid recovery of function of existing platelets would be expected, compared with the irreversibly binding thienopyridines (whose recovery of platelet function depends on regeneration of platelets) [35]. Indeed, the European Society of Cardiology guidelines recommend that coronary artery bypass surgery should be postponed for 5 days in patients pre-treated with clopidogrel [1]. Therefore, antiplatelet agents with rapid recovery of platelet function would provide greater flexibility for surgery.
The plasma concentrations of clopidogrel and associated degree of IPA observed in our study were consistent with the expected effects based on published results with clopidogrel at the 300 mg loading dose followed by 75 mg once daily [19, 23, 28]. The superior antiplatelet activity of ticagrelor at doses ≥50 mg twice daily over the standard clopidogrel dose is also consistent with previously published data [19, 23, 28]. In the randomized, double-blind, phase IIb DISPERSE2 trial in 900 patients with ACS, ticagrelor 90 mg twice daily over 12 weeks produced a level of platelet inhibition that was almost double that of standard treatment with clopidogrel [28]. A pharmacodynamic sub-study of DISPERSE2 in 89 patients showed that after 4 weeks, both ticagrelor 90 mg twice daily and ticagrelor 180 mg twice daily demonstrated a greater mean IPA (79% and 95%, respectively; 4 h final aggregation response) than standard clopidogrel treatment (64%) [23]. In addition, a double-blind, randomized, phase II trial in 200 patients with stable atherosclerosis demonstrated that both ticagrelor 100 mg and 200 mg twice daily in combination with aspirin (75–100 mg once daily) had superior antiplatelet efficacy to clopidogrel 75 mg once daily plus aspirin over 28 days of treatment [19].
Our finding that the extent of IPA over the dosing interval was greater with ticagrelor doses of ≥50 mg twice daily than with the standard dose of clopidogrel (300 mg loading dose followed by 75 mg once daily) is in agreement with previous data showing that clopidogrel only moderately inhibits platelet aggregation in response to ADP [19, 36]. In addition, we showed that the range of responses with clopidogrel at steady-state with standard dosing was variable. We acknowledge the large variability in all IPA measurements as well as the small number of patients in this study arm (n = 14), but these data are consistent with the wide variability in responses to clopidogrel (approximately 15–48% of patients have a poor platelet inhibition response) [3–6].
One concern with superior inhibition of platelet activity is the potential hazard of additional bleeding. However, in our study, there were no notable differences in bleeding time between any ticagrelor dosing regimen and clopidogrel (300 mg loading dose followed by 75 mg once daily) based on median bleeding times for each regimen. Moreover, phase II and phase III data showed that the increased level of platelet inhibition was not associated with any substantial increase in bleeding with ticagrelor, compared with clopidogrel [25, 28]. Although ticagrelor did show an increase in minor bleeding compared with clopidogrel, there was no difference in major bleeding.
The superior responses with ticagrelor twice daily dosing compared with once daily administration support the selection of a twice daily regimen as the optimal dosing regimen for subsequent phase II and phase III studies [25, 28]. To accelerate further the onset of antiplatelet activity, ticagrelor is given as a loading dose of 180 mg followed by 90 mg twice daily (selected due to its tolerability and significantly improved IPA over clopidogrel [23, 28]), which has been compared with clopidogrel in the PLATO trial [25]. As a result of the improved potency of ticagrelor over clopidogrel, the degree of IPA with ticagrelor in the present study was greater than with clopidogrel, consistent with the efficacy results observed in the PLATO study.
Administration with food resulted in a small increase in exposure to ticagrelor, though there was no apparent effect on exposure to the metabolite AR-C124910XX. Due to the exploratory nature of the food effect evaluation in this study, no definitive conclusions regarding the magnitude of the effect of food can be estimated from these data. However, in a previous study designed specifically to assess the effect of food on ticagrelor pharmacokinetics with the proposed commercial tablet formulation, consumption of a standard high-fat meal resulted in a 21% increase in ticagrelor AUC(0,∞) and a 22% decrease in AR-C124910XX Cmax, with no effect on ticagrelor Cmax or AR-C124910XX AUC(0,∞) [37]. As these changes are considered of minimal clinical significance, these preliminary results suggest that ticagrelor can be administered with or without food. Consequently, ticagrelor was administered in the clinical development studies without consideration of food intake.
Data from the present study indicate that treatment with oral ticagrelor at doses up to 600 mg day−1 was well tolerated in healthy volunteers. Ticagrelor had a similar adverse event profile to clopidogrel, with no serious, severe, or dose-related adverse events reported. There were transient increases in transaminase concentrations in two subjects receiving ticagrelor (300 mg once daily and 200 mg twice daily). However, increased liver transaminase concentrations have not been reported in other studies with ticagrelor [19, 28]. While the potential for an effect of high plasma concentrations of ticagrelor on hepatic function in those patients cannot be ruled out, the doses received by these two patients were higher than would be used in clinical practice. Notably, in the PLATO study carried out in over 18 000 patients [25], transaminase elevations were not identified as one of the major laboratory abnormalities with 90 mg twice daily ticagrelor. No clinically important effects on other laboratory parameters, vital signs or ECG were observed. Phase III studies in a greater number of subjects and in patients with ACS will clarify these findings.
In conclusion, our findings illustrate that the pharmacokinetics of ticagrelor are predictable, and are associated with consistent inhibition of platelet activity, to a greater extent than clopidogrel. The rapid onset of activity of ticagrelor coupled with a fast rate of platelet recovery may offer several advantages to patients with ACS.
Acknowledgments
The authors wish to thank Dr Thierry Duvauchelle and his team for conducting the studies and Dr Gary Peters for his medical contributions. We also thank Joe Hirsch, from BioScience Communications and Patrick Hoggard from Gardiner Caldwell Communications, who provided medical writing support funded by AstraZeneca.
Competing interests
KB and RT are current employees of AstraZeneca.
REFERENCES
- 1.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Task force for diagnosis and treatment of non-ST-segment elevation acute coronary syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Clinical Excellence (NICE) Post myocardial infarction secondary prevention in primary and secondary care for patients following a myocardial infarction: full guideline. May 2007. Available at http://www.nice.org.uk/nicemedia/pdf/CG48FullGuideline.pdf (last accessed 3 August 2009.
- 3.Gurbel PA, Bliden KP. Durability of platelet inhibition by clopidogrel. Am J Cardiol. 2003;91:1123–5. doi: 10.1016/s0002-9149(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 4.Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003;89:783–7. [PubMed] [Google Scholar]
- 5.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–51. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–16. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 8.Geisler T, Langer H, Wydymus M, Göhring K, Zürn C, Bigalke B, Stellos K, May AE, Gawaz M. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J. 2006;27:2420–5. doi: 10.1093/eurheartj/ehl275. [DOI] [PubMed] [Google Scholar]
- 9.Heptinstall S. Variable therapeutic effectiveness of clopidogrel in acute coronary syndromes. J Thromb Haemost. 2006;4:539–41. doi: 10.1111/j.1538-7836.2005.01777.x. [DOI] [PubMed] [Google Scholar]
- 10.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention. Is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–66. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 11.Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, Moschi G, Gori AM, Abbate R, Antoniucci D. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312–17. doi: 10.1016/j.jacc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antmanet EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 13.Eli Lilly and Company. Effient™ (prasugrel) Prescribing Information. Available at http://pi.lilly.com/us/effient.pdf (last accessed 28 January 2010.
- 14.Tantry US, Bliden KP, Gurbel PA. AZD6140. Expert Opin Invest Drugs. 2007;16:225–9. doi: 10.1517/13543784.16.2.225. [DOI] [PubMed] [Google Scholar]
- 15.Gurbel PA, Tantry US. Drug insight: clopidogrel nonresponsiveness. Nat Clin Pract Cardiovasc Med. 2006;3:387–95. doi: 10.1038/ncpcardio0602. [DOI] [PubMed] [Google Scholar]
- 16.Tantry US, Bliden KP, Gurbel PA. Resistance to antiplatelet drugs: current status and future research. Expert Opin Pharmacother. 2005;6:2027–45. doi: 10.1517/14656566.6.12.2027. [DOI] [PubMed] [Google Scholar]
- 17.van Giezen JJJ. Optimizing platelet inhibition. Eur Heart J Suppl. 2008;10(Suppl. D):D23–D29. [Google Scholar]
- 18.van Giezen JJJ, Humphries RG. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost. 2005;31:195–204. doi: 10.1055/s-2005-869525. [DOI] [PubMed] [Google Scholar]
- 19.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–47. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 20.Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC, Chapman D, Dixon J, Guile SD, Humphries RG, Hunt SF, Ince F, Ingall AH, Kirk IP, Leeson PD, Leff P, Lewis RJ, Martin BP, McGinnity DF, Mortimore MP, Paine SW, Pairaudeau G, Patel A, Rigby AJ, Riley RJ, Teobald BJ, Tomlinson W, Webborn PJ, Willis PA. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett. 2007;17:6013–18. doi: 10.1016/j.bmcl.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 21.van Giezen JJJ, Berntsson P, Wissing B-M. Direct oral P2Y12 receptor antagonist AZD6140 potently and reversibly binds to the rh-P2Y12 receptor expressed on CHO-K1 cells. Arterioscler Thromb Vasc Biol. 2008;28:e-72. Abstract P207. [Google Scholar]
- 22.Nilsson L, van Giezen JJJ, Greasley PJ. Evidence for distinct ligand binding sites on recombinant P2Y12 receptors. Circulation. 2006;114(Suppl. II):248. Abstract 1313. [Google Scholar]
- 23.Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P, Cannon CP. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–6. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 24.James S, Åkerblom A, Cannon C, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. doi: 10.1016/j.ahj.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 26.Teng R, Butler K. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect, in healthy subjects. Can J Clin Pharmacol. 2008;15:e426. Abstract 9. [Google Scholar]
- 27.Sanofi-Aventis. PLAVIX® (clopidogrel) prescribing information. May 2009. Available at http://packageinserts.bms.com/pi/pi_plavix.pdf (last accessed April 2010.
- 28.Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, Storey RF DISPERSE-2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 Trial. J Am Coll Cardiol. 2007;50:1844–51. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 29.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 30.Labarthe J, Théroux P, Angioï M, Ghitescu M. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–45. doi: 10.1016/j.jacc.2005.02.092. [DOI] [PubMed] [Google Scholar]
- 31.Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–96. doi: 10.1007/s00228-009-0778-5. [DOI] [PubMed] [Google Scholar]
- 32.Peters G, Robbie G. Single dose pharmacokinetics and pharmacodynamics of AZD6140 – an oral reversible ADP receptor antagonist. Haematologica. 2004;89(Suppl. 7):14–15. [Google Scholar]
- 33.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson AK. Platelet ADP receptor antagonists: ticlopidine and clopidogrel. Best Pract Res Clin Haematol. 2004;17:55–64. doi: 10.1016/j.beha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.van Giezen JJJ, Berntsson P, Wissing B-M. Direct oral P2Y12 receptor antagonist AZD6140 potently and reversibly binds to the rh-P2Y12 receptor expressed on CHO-K1 cells. Arterioscler Thromb Vasc Biol. 2008;28:e-72. Abstract P207. [Google Scholar]
- 36.Thebault JJ, Kieffer G, Lowe GD, Nimmo WS, Cariou R. Repeated-dose pharmacodynamics of clopidogrel in healthy subjects. Semin Thromb Hemost. 1999;25(Suppl. 2):9–14. [PubMed] [Google Scholar]
- 37.Butler K, Mitchell PD, Teng R. No significant food effect on the pharmacokinetics of ticagrelor (AZD6140), the first reversibly binding oral P2Y12 receptor antagonist, in healthy subjects. Presented at: 110th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics; March 18–21, 2009; Washington, DC.


