Abstract
Background
There is limited research to support the effect of exercise adherence on clinical outcomes in patients with heart failure (HF). This secondary analysis was conducted on the intervention arm of an exercise training study in patients with HF to assess whether adherence and the dose of treatment exposure were associated with clinical outcomes, functional performance (maximum oxygen consumption [VO2], anaerobic threshold, and workload), and quality of life (QOL).
Methods
Seventy-one patients (average age, 54.0 ± 12.5 years; male, 66%; Caucasian, 66%; married, 61%; New York Heart Association class II III, 97.2%; and average ejection fraction, 26.4% ± 6.5%) were included in the current study. Patients with an increase ≥ 18% in the amount of exercise from baseline to 6 months, as measured by pedometers, were categorized as adherers (n = 38); patients who had no change or an increase in the amount of exercise of < 18% were categorized as nonadherers (n = 33).
Results
The 2 groups were significantly different in the composite endpoint of all-cause hospitalization, emergency room admissions, and death/urgent transplantation (hazard ratio, 0.31; confidence interval, 0.159–0.635; P < 0.001). Adherers had greater improvements in functional performance and QOL compared with nonadherers (P < 0.001).
Conclusion
These findings suggest that among patients with advanced HF, adherence to exercise is associated with more favorable clinical outcomes. There is also a positive dose-response relationship between the amount of exercise performed and improvement in functional performance and QOL.
Introduction
Chronic heart failure (HF) is a major cause of morbidity and mortality worldwide. By focusing on the multiple needs of patients with HF, exercise training offers opportunities to address the disability associated with this chronic, progressive disease. Exercise training in HF has been shown in a meta-analysis of randomized trials to improve exercise capacity, functional status, and quality of life (QOL).1–3 However, there is a paucity of research examining the beneficial effects of exercise training on clinical outcomes. One critical aspect in determining the efficacy of exercise training is the degree to which patients adhere to a given exercise prescription, particularly in the case of home-based exercise programs.4 Although much has been written about adherence to exercise in healthy adults, there has been limited examination or discussion of exercise adherence and its potential impact on outcomes in the HF population to date.4 Likewise, while adherence is critical in evaluating the impact of exercise training on outcomes, researchers and clinicians argue that an understanding of the effects of exercise training is affected by our limited ability to adequately and accurately measure adherence.5 Prior investigations have indicated that measuring adherence to lifestyle interventions such as exercise training poses a greater challenge than measuring adherence to pharmacologic or device therapies.6
Our research team conducted a randomized clinical trial to examine the effects of a home-based exercise training program and found no significant improvements in clinical outcomes at 1-year follow-up and no significant improvements in functional performance, QOL, and psychological states at 3 and 6 months in patients with HF.7 The lack of improvement in the majority of endpoints may reflect participants’ level of adherence to the exercise protocol. In addition, one of the greatest challenges in exercise training trials is distinguishing the exercise component from the other types of interventions patients may receive as a result of their participation. Due to the nature of exercise training interventions, participants in the training arm of these studies may receive additional attention by research staff, further complicating interpretation of results.
To explore the relationship between exercise adherence and clinical outcomes, a secondary analysis was conducted on the intervention arm of a home-based exercise study to determine whether patient adherence and the dose of treatment exposure (ie, amount of exercise) were associated with clinical outcomes. The specific aims of the study were to 1) determine whether adherence to the exercise training program is associated with improved clinical outcomes (hospital admissions, emergency department [ED] admissions, and death/urgent transplantations) over 12 months in patients with HF; and 2) compare the effect of a home-based exercise training program on functional performance (maximum oxygen consumption [peak VO2], anaerobic threshold, and workload) and QOL (physical, emotional, overall) at 6 months in adherers and nonadherers; and 3) examine changes in functional performance and QOL across different doses of exercise at 6 months.
Methods
Study Design and Participants
A complete description of the study design and methods of the parent study describing the effects of a 6-month, home-based exercise program has been published elsewhere.7,8 Briefly, patients were assigned to either an exercise group or a control group. Participants in the exercise group were asked to perform a graduated, low-level exercise protocol consisting of low-level aerobic exercise and resistive training. Participants in the control group were asked to maintain their usual level of daily activities, with no systematic exercise component. The inclusion criteria for the parent study and the substudy reported here were as follows: English-speaking, aged 18 to 80 years, advanced systolic HF, and New York Heart Association (NYHA) class II to IV.
Patients in the exercise group were instructed to use the hip-borne pedometer (Sportline Electronic Pedometer, Model 345®) each day on waking and were oriented to their use and application at the beginning of the study. The pedometers were designed to display an output proportional to the number of movements of a spring-loaded pendulum displaced by vertical acceleration of the hip during walking; a cumulative count proportional to the number of footsteps taken or distance traveled in miles was displayed and recorded at monthly intervals by nurses when they conducted their follow-up visits. Once the data were recorded, the pedometers were recalibrated. The reliability and validity of pedometers in measuring exercise activity in this population has been described in depth elsewhere.5
Because pedometers measure levels of physical activity, pedometer recordings were used in the current study to estimate adherence to the aerobic portion of the exercise protocol. In preliminary analyses of data involving fewer patients, our team utilized a conservative value of 10% improvement in pedometer scores to distinguish between adherers and nonadherers.5 However, to obtain more robust comparisons, the current analyses were conducted using the median percent improvement of 18% from the current sample to define adherers and nonadherers. Participants who showed improvements in distance walked ≥ 18% from baseline to 6 months were categorized as “adherers,” while patients with < 18% were categorized as “nonadherers.”
Procedures
The research protocol was reviewed and approved by the appropriate Institutional Review Board, and all participants signed a written informed consent. Demographic information (ie, age, gender, race/ethnicity, marital status, education, and current employment status) was collected through a simple self-administered form. Information pertaining to medical history and clinical status (eg, etiology of HF, NYHA class, ejection fraction, medications) was obtained through self-reports and verified by chart reviews. Clinical events, defined as a combination of all-cause hospitalization, ED admission, and all-cause mortality were tracked at monthly intervals over 12 months through self-report and medical record review.
The secondary outcome measures, functional performance and QOL, were assessed at baseline and 6 months. Functional performance was measured with the cardiopulmonary exercise test using a standard 15 W ramp protocol and included the following measurements: peak VO2 (highest VO2 observed during exercise), anaerobic threshold, and workload.9 Quality of life, defined as the degree to which aspects of patients’ physical, social, functional, and emotional well-being are impacted by health,10 was measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ). This disease-specific, 21-item tool asked participants to indicate the extent to which various symptoms they had experienced in the previous month had prevented them from living as they wanted. The reliability and validity of the functional performance and QOL measures have been described in depth elsewhere.7
Statistical Analysis
Data were analyzed using the SPSS® for Windows (version 13.0, SPSS, Inc., Chicago, IL).11 An unpaired Student’s t-test and Mann–Whitney U test were used to compare baseline characteristics between the groups. A Kaplan–Meier survival curve was constructed using time-dependent all-cause hospitalization, ED admission, and death/urgent transplantations as a composite endpoint to test the hypothesis that adherence would result in improved clinical outcomes at 12-month follow-up. The Cox model was also used to assess the consistency of the treatment effects by testing for interactions between treatment and prespecified baseline characteristics (Aim 1). For functional performance variables (peak VO2, anaerobic threshold, and workload), a 2-factor (before/after measure, group) analyses of variance (ANOVA) was used to evaluate intergroup differences in change and paired Student’s t-test to evaluate change within the group. To evaluate treatment effect on QOL variables, the Wilcoxon’s signed ranks test was used (Aim 2). To answer Aim 3, participants were divided into 4 groups based on the percent improvement (eg, amount increase) in exercise from baseline to 6 months: 1) no improvement (control); 2) < 18% improvement (below recommended levels); 3) 18% to 30% improvement (recommended levels); and 4) > 30% improvement (above recommended levels). Dose-response effects were evaluated with regression analysis to test for trends in functional performance and QOL change across groups of participants with varying doses of exercise. For statistically significant analysis of covariance (P < 0.05), pairwise comparisons between the 3 exercise groups and the control group were made using the Bonferroni correction for multiple testing. Results are presented as adjusted least squares means with 95% confidence intervals (CIs).
Results
Seventy-one patients randomly assigned to the intervention arm of the original study had complete data at baseline and 6 months and were included in the subgroup analysis; 16 patients were excluded because they did not complete the 6-month follow-up. The sociodemographic and clinical characteristics of those included and excluded in this subgroup analysis were not significantly different. The current sample had been diagnosed with HF for a mean of 5.57 years (standard deviation [SD], 4.74; range, 1–18 years). The largest percentage (45%) had been diagnosed with HF in the past 1 to 5 years. Comparative analyses of sociodemographic and clinical characteristics of adherers and nonadherers at baseline are presented in Table 1. There were no significant differences in any of the baseline characteristics between the 2 groups, except for angiotensin-converting enzyme (ACE) inhibitor use; adherers were more likely to use ACE inhibitors than nonadherers (84% vs 60%; P = 0.039).
Table 1.
Sociodemographic and Clinical Characteristics at Baseline
| Total (N = 71) | Adherers (n = 38) | Nonadherers (n = 33) | P-Value | |
|---|---|---|---|---|
| Age, mean ± SD | 54 ± 12.5 | 54.6 ± 10.6 | 56.7 ± 11.7 | 0.683 |
| Ejection fraction, mean ± SD | 26.4 ± 6.8 | 26.7 ± 7.1 | 26.6 ± 6.0 | 0.381 |
| Male, n (%) | 47 (66.2) | 25 (65.8) | 22 (66.7) | 0.938 |
| Ethnicity, n (%) | 0.273 | |||
| White | 47 (66.2) | 22 (57.9) | 25 (75.7) | |
| Non-white | 24 (33.8) | 16 (42.1) | 8 (24.2) | |
| Education, n (%) | 0.344 | |||
| ≤ 12 | 26 (36.6) | 11 (28.9) | 15 (45.5) | |
| 12–16 | 29 (40.8) | 17 (44.7) | 12 (36.4) | |
| ≥ 16 | 16 (22.5) | 10 (26.3) | 6 (18.2) | |
| Married, n (%) | 43 (60.6) | 24 (63.2) | 19 (57.6) | 0.631 |
| Employed, n (%) | 20 (28.3) | 13 (34.2) | 7 (21.2) | 0.225 |
| Ischemic, n (%) | 30 (42.3) | 16 (42.1) | 14 (42.4) | 0.978 |
| NYHA class, n (%) | 0.406 | |||
| Class II | 56 (78.9) | 29 (76.3) | 27 (81.8) | |
| Class III | 13 (18.3) | 7 (18.4) | 6 (18.2) | |
| Class IV | 2 (2.8) | 2 (5.3) | 0 (0) | |
| Cardiac history, n (%) | ||||
| Hypertension | 32 (45.1) | 17 (44.7) | 15 (45.5) | 0.952 |
| Diabetes | 22 (31.0) | 14 (36.8) | 8 (24.2) | 0.252 |
| Dyslipidemia | 34 (47.9) | 20 (52.6) | 14 (42.4) | 0.390 |
| AICD | 25 (35.2) | 13 (34.2) | 12 (36.4) | 0.850 |
| Former smoker | 41 (57.7) | 19 (50.0) | 22 (66.7) | 0.156 |
| Current smoker | 8 (11.3) | 2 (5.3) | 6 (18.2) | 0.086 |
| History of CAD | 33 (46.5) | 18 (47.4) | 15 (45.5) | 0.872 |
| Cardiac medications, n (%) | ||||
| ACE inhibitors | 52 (73.2) | 32 (84.2) | 20 (60.6) | 0.039a |
| A ngiotensin receptor blockers | 13 (18.3) | 10 (26.3) | 3 (9.1) | 0.061 |
| β-Blockers | 50 (70.4) | 25 (65.8) | 25 (75.8) | 0.359 |
| Diuretics | 61 (85.9) | 31 (81.6) | 30 (90.9) | 0.260 |
| Spironolactone | 21 (29.6) | 11 (28.9) | 10 (30.3) | 0.901 |
| Digitalis | 49 (69.0) | 26 (68.4) | 23 (69.7) | 0.908 |
| Lipid-lowering agents | 37 (52.1) | 22 (57.9) | 15 (45.5) | 0.295 |
| Nitrates | 18 (25.4) | 8 (21.1) | 10 (30.3) | 0.372 |
P < 0.05.
Abbreviations: ACE, angiotensin-converting enzyme; AICD, automatic implantable cardioverter-defibrillator; CAD, coronary artery disease; NYHA, New York Heart Association; SD, standard deviation.
Clinical Events
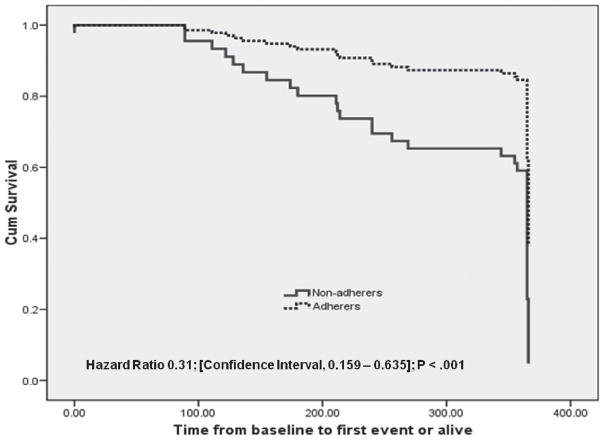
Differences in clinical endpoints between adherers and nonadherers are shown in Table 2. After adjusting for age, gender, HF severity, and comorbidities, significant differences were noted between the 2 groups in the composite endpoint (hazard ratio [HR], 0.31 [95% CI, 0.131–0.635]; P < 0.001) (Figure 1). There were 11 (28.9%) all-cause hospitalizations and 5 (13.2%) ED visits among adherers, and 20 (60.6%) all-cause hospitalizations and 15 (45.5%) ED visits among nonadherers (HR, 0.47 [95% CI, 0.226–0.987]; P = 0.041) and (HR, 0.29 [95% CI, 0.105–0.800]; P = 0.010), respectively. Seventy-one percent of the adherers were never hospitalized during the 1-year follow-up compared with only 39% among nonadherers; likewise, a greater percentage of nonadherers had multiple hospital admissions compared with adherers (36% vs 13%; P = 0.021). There were 2 (5.3%) deaths among adherers and 10 deaths (30.3%) among nonadherers (HR, 0.41 [95% CI, 0.275–0.992]; P = 0.045).
Table 2.
Differences in Clinical Outcomes Between Control and Exercise Groups at 1-year Follow-up
| Total Sample (N = 71) | Adherers (n = 38) | Nonadherers (n = 33) | P-Value | |
|---|---|---|---|---|
| Patients hospitalized during 1-year follow-up, n (%) | 31 (43.7) | 11 (28.9) | 20 (60.6) | 0.007 |
| No. of hospitalizations during 1-year follow-up | 0.021 | |||
| None, n (%) | 40 (56.3) | 27 (71.1) | 13 (39.4) | |
| 1, n (%) | 14 (19.7) | 6 (15.8) | 8 (24.2) | |
| > 1, n (%) | 17 (23.9) | 5 (13.2) | 12 (36.4) | |
| Patients admitted to ED, n (%) | 20 (28.2) | 5 (13.2) | 15 (45.4) | 0.003 |
| Deaths/urgent transplantations, n (%) | 12 (16.9) | 2 (5.3) | 10 (30.3) | 0.005 |
| Combined endpoint,a n (%) | 41 (57.7) | 11 (28.9) | 30 (90.9) | 0.000 |
All-cause hospitalization, ED admission, death/urgent transplantation.
Abbreviation: ED, emergency department.
Figure 1.
Kaplan–Meier curves illustrating the time to first event among adherers and nonadherers.
Functional Performance and QOL
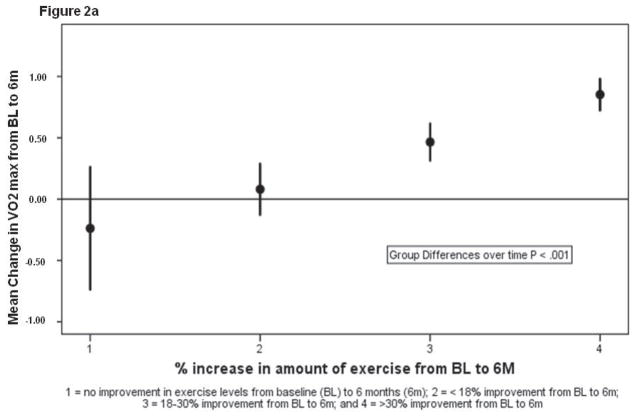
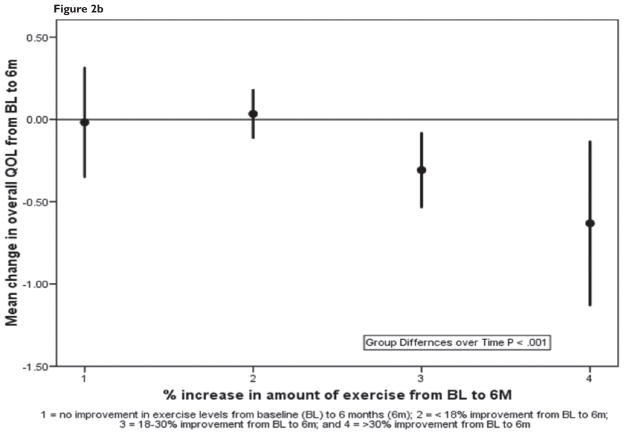
Table 3 summarizes the baseline and 6-month data for functional performance and QOL for adherers and nonadherers. Group differences over time were significant for all 6 measures (P < 0.001–0.012). Figures 2a and 2b summarize mean changes in peak VO2 and QOL, respectively, in the 4 dose-response groups; a positive linear trend across groups was statistically significant for both measures, and exercise dose was a significant independent predictor of change for peak VO2 and QOL (P < 0.001). An analysis of covariance indicated that patients who demonstrated 18% to 30% and > 30% improvement in exercise levels showed significant improvements compared with participants with no change (P < 0.001); however, there were no statistical differences between patients with < 18% change in exercise levels compared with patients with no change.
Table 3.
Comparison of Functional Performance and QOL Between Adherers and Nonadherers at Baseline and 6 Months
| Variable | Adherers (n =38) |
Nonadherers (n = 33) |
P Time | P (TxG) | ||
|---|---|---|---|---|---|---|
| Baseline | 6-Month | Baseline | 6-Month | |||
| Functional Performance | ||||||
| Peak VO2 (mL/kg/min), mean ± SD | 13.0 ± 3.1 | 14.3 ± 3.3 | 14.5 ± 3.5 | 13.9 ± 2.8 | 0.051 | 0.001 |
| VO2 (mL/kg/min) at anaerobic threshold, mean ± SD | 9.2 ± 3.1 | 9.9 ± 2.7 | 10.9 ± 3.8 | 8.8 ± 2.5 | 0.091 | 0.001 |
| W, mean ± SD | 97.0 ± 28.7 | 106.8 ± 29.1 | 100.8 ± 35.3 | 91.7 ± 38.2 | 0.899 | 0.003 |
| QOL (MLHFQ)a | ||||||
| Total score | 44.1 ± 20.3 | 35.7 ± 19.7 | 49.0 ± 20.8 | 52.6 ± 23.2 | 0.062 | 0.000 |
| Physical score | 19.7 ± 10.1 | 15.9 ± 9.2 | 21.2 ± 8.8 | 23.2 ± 9.9 | 0.134 | 0.000 |
| Mental score | 11.0 ± 6.9 | 9.4 ± 7.0 | 12.7 ± 6.2 | 14.5 ± 7.2 | 0.925 | 0.012 |
Higher score indicates worse QOL.
Abbreviations: MLHFQ, Minnesota Living with Heart Failure Questionnaire; QOL, quality of life; SD, standard deviation; VO2, maximum oxygen consumption; W, workload.
Figure 2.
Figure 2a. Mean change in least square means (95% CI) in peak VO2 for the 4 groups of participants with varying doses of exercise.
Figure 2b. Mean change in least square means (95% CI) in quality of life for the 4 groups of participants with varying doses of exercise.
Discussion
The present study was conducted to analyze the effect of exercise adherence on clinical outcomes, functional performance, and QOL in a cohort of 71 patients with advanced systolic HF who were randomly assigned to the treatment arm of a home-based exercise training program. Data from the parent study showed no significant difference between experimental and control groups at 12 months on the combined endpoint of all-cause hospitalizations, ED admissions, and death/heart transplantation.7 Likewise, data from a recent large multicenter trial (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training [HF-ACTION]) showed that exercise training was safe but provided a nonsignificant reduction in the risk for the primary endpoint of all-cause mortality or all-cause hospitalizations in a cohort of patients with left ventricular systolic dysfunction.12 The investigators of HF-ACTION speculated that the lack of significant findings could be attributed to issues of patient adherence and crossover.
The current findings showed that patients who adhered to the low-level, home-based exercise program demonstrated a significant difference in the composite endpoint of all-cause hospitalizations, ED admissions, and death/urgent transplantation compared with patients who did not adhere to the protocol. Data also showed a significant difference between the 2 groups on individual clinical outcomes; adherers had lower hospital admission rates, decreased incidence of multiple admissions, reduced number of ED visits, and fewer deaths. Our findings support prior speculations that adherence to the exercise prescription or training protocol may be vital for exercise to be a maximally effective treatment.4
Data showed that adherence to exercise training in patients with advanced systolic HF was associated with improvements in functional performance. The HF literature is replete with evidence to support these findings. A number of small randomized studies examining the effects of exercise training on exercise performance demonstrated improvements in peak VO2 after training.1,3,6,13–15 A pooled analysis of the data from these studies confirmed exercise training was associated with a 10% to 18% increase in peak VO214,16,17 and increased maximal cardiac output, regional blood flow, and stroke volume, as well as improvements in diastolic filling rates, reduction in sympathetic activity, and a partially reversed activation of the neurohormonal system with decreased levels of proinflammatory cytokines.3 These peripheral and central changes potentially slow the progression of the HF syndrome and likely lead to improvements in survival and reduction in hospitalizations that we observed in our study.
Our data also showed a relationship between the adherence to exercise and improvements in functional performance and QOL. Improved QOL is routinely cited as a benefit of regular exercise; a meta-analysis of 9 studies showed that exercise training was associated with significant improvements in the MLHFQ score at 9.7 points (28% improvement), which is considered to be a clinically meaningful difference.15 Interestingly, only one of the studies included in the meta-analysis could demonstrate a significant positive correlation between change in functional performance and change in QOL.3 While most cross-sectional studies have observed that higher levels of activity are associated with higher QOL scores,16,18,19 investigators have reported improvements in functional performance without improvement in QOL.3,20,21 Many of the mixed or negative results of past home exercise studies may be related to crossover between groups or lack of adherence to the exercise protocol in the experimental group.
Early studies of exercise in patients with HF were conducted in outpatient or rehabilitation facilities that allowed for the measurement of adherence.16 Exercise adherence has been expressed in some studies as the total number of training sessions attended19 or as time spent at > 60% maximum heart rate. However, over the past decade, investigators have tested exercise protocols based in the home or community, recognizing that structured outpatient exercise programs pose significant economic barriers.1,3,19 The challenge to these protocols is how to measure exercise adherence conducted away from a structured research setting. To date, little attention has been devoted to measuring adherence with valid and reliable measures.4 There is a lack of uniformity across trials with regard to how adherence was measured and what, if any, interventions were used to improve adherence. Exercise adherence must be measured accurately to better understand the results of clinical trials and to interpret the impact of a dose response on clinical outcomes. Future investigations are needed to evaluate standardized instruments that can be used easily and are cost-effective (eg, wireless health networks).
The study is limited because of the small sample size and the fairly homogeneous sample (ie, participants were predominantly male; enrolled from a tertiary care HF clinic; average age 55 years), which limits generalizability of the results to all HF populations. As in all observational studies, these findings do not imply causation, and it is easy to hypothesize that people with a higher perceived QOL or higher social support are more like to be physically active or more likely to increase their level of exercise. However, other factors could explain why they exercised and why they did better. It has not been established whether adherence caused the better outcomes. Because adherence was defined by pedometers, results provided in this study only reflect data from patients who had pedometer data compared with those who did not (16 [18%] of the 87 patients randomly assigned to the intervention) limits the comparison of outcomes for the entire cohort. Furthermore, there is strong evidence that adherence to a therapy is a potent marker of better outcomes, even if the therapy is not efficacious. A large randomized controlled trial showed that adherence to candesartan and adherence to placebo were associated with similar adjusted HRs,22 which suggests that the current study may likely reflect the same phenomenon, thus diminishing its significance in this greater context. Finally, because adherence was measured based on pedometer data, we can only assume adherence to the aerobic component of the exercise protocol; adherence to the resistive component of the exercise protocol is not available. Nevertheless, to our knowledge, this is one of the first demonstrations in patients with advanced HF participating in a randomized controlled trial that exercise adherence is associated with reductions in hospital admissions, ED visits, and death, as well as significant improvements in functional performance and QOL that were sensitive to exercise dose.
Conclusion
Our data showed improvements in clinical outcomes, functional performance, and QOL among patients assigned to the intervention arm who adhered to the exercise training protocol. These findings support prior research that adherence to the exercise prescription or training protocol is vital for exercise to be a maximally effective treatment. Further research aimed at exploring the factors that influence adherence to exercise in HF are needed to promote our understanding of factors that predict whether an exercise program will be successful in increasing physical activity levels.
Acknowledgments
The authors would like to acknowledge the funding source: American Heart Association Western Division (NCR, 133-09, PI, K. Dracup). The primary author also received support from the National Heart, Lung, and Blood Institute (1R01HL093466-01) and from the University of California, Los Angeles, Resource Centers for Minority Aging Research/Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under NIH/NIA Grant P30-AG02-1684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institute on Aging or the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Lorraine S. Evangelista, RN, PhD, Michele A. Hamilton, MD, Gregg C. Fonarow, MD, and Kathleen Dracup, RN, DNSc disclose no conflicts of interest.
References
- 1.Lloyd-Williams F, Mair FS, Leitner M. Exercise training and heart failure: a systematic review of current evidence. Br J Gen Pract. 2002;52(474):47–55. [PMC free article] [PubMed] [Google Scholar]
- 2.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.McKelvie R. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- 4.Barbour K, Miller NH. Adherence to exercise training in heart failure: a review. Heart Fail Rev. 2008;13(1):81–89. doi: 10.1007/s10741-007-9054-x. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista LS, Dracup K, Erickson V, McCarthy WJ, Hamilton MA, Fonarow GC. Validity of pedometers for measuring exercise adherence in heart failure patients. J Card Fail. 2005;11(5):366–371. doi: 10.1016/j.cardfail.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Whellan DJ, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Dracup K, Evangelista LS, Hamilton MA, et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J. 2007;154(5):877–883. doi: 10.1016/j.ahj.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Evangelista LS, Doering LV, Lennie T, et al. Usefulness of a home-based exercise program for overweight and obese patients with advanced heart failure. Am J Cardiol. 2006;97(6):886–890. doi: 10.1016/j.amjcard.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow G, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30(3):725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 10.Bennett SJ, Pressler ML, Hays L, Firestine LA, Huster GA. variables and hospitalization in persons with chronic heart failure. Prog Cardiovasc Nurs. 1997;12(4):4–11. [PubMed] [Google Scholar]
- 11.SPSS User’s Guide Version 13.0. 13. Chicago, IL: SPSS Inc; 2006. [Google Scholar]
- 12.O’Conner CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Piepoli MF, Davos C, Francis DP, Coats AJ ExTramatch Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piña IL, Apstein CS, Balady GJ, et al. American Heart Association Committee on exercise, rehabilitation, and prevention. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 16.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 17.McKelvie R, Kroon KT, Roberts R, et al. Effects of exercise training in patients with heart failure: The Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144(1):23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 18.Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L ELVD-CHF Study Group. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108(5):554–559. doi: 10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- 19.Tyni-Lenné R, Dencker K, Gordon A, Jansson E, Sylvén C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3(1):47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 20.Jónsdóttir S, Andersen KK, Sigurosson AF, Sigrosson SB. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006;8(1):97–101. doi: 10.1016/j.ejheart.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Keteyian SJ, Brawner CA, Schairer JR, et al. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J. 1999;138(2 pt 1):233–240. doi: 10.1016/s0002-8703(99)70106-7. [DOI] [PubMed] [Google Scholar]
- 22.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2007;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]