Abstract
Background
A single serum creatinine measurement cannot distinguish acute kidney injury from chronic kidney disease or prerenal azotemia.
Objective
To test the sensitivity and specificity of a single measurement of urinary neutrophil gelatinase–associated lipocalin (NGAL) and other urinary proteins to detect acute kidney injury in a spectrum of patients.
Design
Prospective cohort study.
Setting
Emergency department of Columbia University Medical Center, New York, New York.
Participants
635 patients admitted to the hospital with acute kidney injury, prerenal azotemia, chronic kidney disease, or normal kidney function.
Measurements
Diagnosis of acute kidney injury was based on the RIFLE (risk, injury, failure, loss, and end-stage) criteria and assigned by researchers who were blinded to experimental measurements. Urinary NGAL was measured by immunoblot, N-acetyl-β-D-glucosaminidase (NAG) by enzyme measurement, α1-microglobulin and α1-acid glycoprotein by immunonephelometry, and serum creatinine by Jaffe kinetic reaction. Experimental measurements were not available to treating physicians.
Results
Patients with acute kidney injury had a significantly elevated mean urinary NGAL level compared with the other kidney function groups (416 μg/g creatinine [SD, 387]; P = 0.001). At a cutoff value of 130 μg/g creatinine, sensitivity and specificity of NGAL for detecting acute injury were 0.900 (95% CI, 0.73 to 0.98) and 0.995 (CI, 0.990 to 1.00), respectively, and positive and negative likelihood ratios were 181.5 (CI, 58.33 to 564.71) and 0.10 (CI, 0.03 to 0.29); these values were superior to those for NAG, α1-microglobulin, α1-acid glycoprotein, fractional excretion of sodium, and serum creatinine. In multiple logistic regression, urinary NGAL level was highly predictive of clinical outcomes, including nephrology consultation, dialysis, and admission to the intensive care unit (odds ratio, 24.71 [CI, 7.69 to 79.42]).
Limitations
All patients came from a single center. Few kidney biopsies were performed.
Conclusion
A single measurement of urinary NGAL helps to distinguish acute injury from normal function, prerenal azotemia, and chronic kidney disease and predicts poor inpatient outcomes.
A cute kidney injury is a common complication among ambulatory and hospitalized patients, and its incidence has increased by 11% in recent years (1). It is a rapidly progressive illness that independently predicts excess morbidity and mortality (2–4). Twenty percent to 60% of patients with acute kidney injury require dialysis (5), and mortality rates range from 15% in the community setting (2, 3) to 50% to 80% in the setting of multiorgan failure (6, 7) and more than 80% in the postoperative setting (8, 9). Less severe forms of acute kidney injury may also result in prolonged hospitalization (10). These characteristics contrast with those of other kidney diseases, such as chronic kidney disease, which is typified by an insidious decline in renal function and is usually nonprogressive during hospitalization. Acute kidney injury is also distinct from prerenal azotemia, a physiologic response of the kidney to various predisposing factors (volume depletion, diuretic use, renin–angiotensin blockade) that promptly resolve on fluid administration, regimen modification, or amelioration of the extrakidney organ malfunction (11).
It is critical to distinguish acute kidney injury from prerenal azotemia and chronic kidney disease at the time of patient presentation to rapidly manage associated illness. However, the initial measurement of serum creatinine, the standard marker of kidney function, does not distinguish acute kidney injury from prerenal azotemia (12) or chronic kidney disease. In addition, the initial measurement of serum creatinine cannot reflect the extent of injury because its accumulation always lags behind the insult (13). Even a large decline in glomerular filtration rate (GFR) may manifest as a small change in serum creatinine level, particularly in the initial 48 hours after acute kidney injury before steady-state equilibrium is reached (13, 14). Serum creatinine may also vary by age, race, sex, muscle mass, metabolism, nutritional status, comorbid conditions, hydration status, and medication use and consequently may not increase in proportion to the severity of the injury. As a result, the diagnosis of acute kidney injury currently requires measuring serum creatinine repeatedly and delaying maneuvers to prevent ongoing kidney damage, such as stopping use of nonsteroidal anti-inflammatory drugs, adjusting medication dosages, or correcting hemodynamic status. Even elevations of serum creatinine level that do not meet established criteria for acute kidney injury (15) are associated with excess mortality (16), prolonged hospitalization (17), functional decline (18), and greater financial costs (19), and this highlights the insensitivity of serum creatinine measurement as a diagnostic test. These limitations in the use of serum creatinine provide the rationale for the discovery of kidney proteins that are expressed at the onset of injury and are more sensitive and specific for the diagnosis of acute injury than current diagnostic tests.
Neutrophil gelatinase–associated lipocalin (NGAL) is secreted into the urine by the thick ascending limb of Henle and collecting ducts of the kidney (20, 21). At these sites, NGAL is likely to play a critical role in host defense by chelating iron–siderophore complexes that enhance microbial growth (22–24) or mediate oxidative damage. In some segments of the nephron, NGAL may also recycle the iron complexes by endocytosis (25, 26). Urinary NGAL is expressed in proportion to the degree of acute injury (27), whereas in chronic kidney disease, urinary NGAL is expressed in patients with progressive but not stable kidney failure (22). Volume depletion or diuretics do not increase urinary NGAL levels in mice (28), again reflecting the specificity of NGAL for ongoing tubular damage. These observations suggest not only that urinary NGAL detects acute kidney injury but also that its degree of expression might distinguish among acute kidney injury, prerenal azotemia, and chronic kidney disease. In addition, because NGAL is detectable before the accumulation of serum creatinine (20, 27, 29), NGAL might be used to diagnose acute kidney injury at patient presentation even when changes in serum creatinine level are incipient.
Because of the diagnostic ambiguities that occur with current tests at patient presentation, we conducted a prospective cohort study in an inner-city emergency department to determine the accuracy of urinary NGAL to identify acute kidney injury. We subsequently followed each participant’s hospital course to determine the relationship among the presenting level of NGAL and other urinary proteins, serum creatinine level, and patient outcome. Our hypothesis was that a single measurement of urinary NGAL is superior to conventional and novel biomarkers in predicting acute kidney injury and its comorbid conditions.
Methods
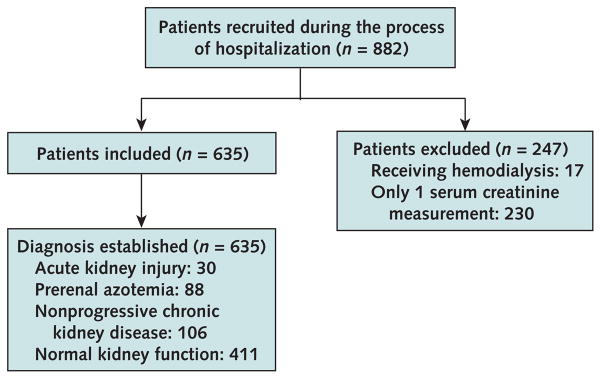
This study was approved by the Columbia University Medical Center Institutional Review Board, and informed consent was obtained before enrollment. We recruited consecutive patients 18 years and older who visited the Columbia University Medical Center emergency department between 6 a.m. and 12 a.m. from March to August 2007. We obtained the first sample of donated urine and blood. We excluded 17 patients who were receiving hemodialysis and 230 patients without subsequent creatinine measurements from further analysis (Figure 1).
Figure 1.
Study flow diagram.
Diagnosis of Kidney Disease
We defined altered kidney function by age- and sex-based criteria: men and women between 18 and 50 years of age with a serum creatinine level greater than 106 μmol/L (>1.2 mg/dL), men older than 50 years with a level greater than 88.4 μmol/L (>1.0 mg/dL), and women with a level greater than 70 μmol/L (>0.8 mg/dL). We estimated GFR by using the Modification of Diet and Renal Disease formula (30).
We determined baseline kidney function for 509 patients from a retrospective analysis of serum creatinine, medical history, and demographic characteristics recorded in the Columbia University Medical Center electronic records for the 1 to 12 months before admission. A prospective analysis of kidney function after hospital admission consisted of daily serum chemistry studies and renal ultrasonography. On the basis of the retrospective and prospective studies, a coordinator and an internist who were blinded to the experimental measurements independently assigned patients to 1 of 4 diagnostic categories (normal kidney function, nonprogressive chronic kidney disease, prerenal azotemia, or acute kidney injury); a nephrologist adjudicated the 24 cases of disagreement.
We defined normal kidney function as a baseline estimated GFR greater than 60 mL/min per 1.73 m2 and no transient or sustained increases in serum creatinine level or decreases in estimated GFR during the patient’s stay in the hospital.
We defined nonprogressive chronic kidney disease as a sustained and unchanging (<25% change from baseline) increase in serum creatinine level that met our criteria for altered kidney function and persisted for more than 3 months before hospitalization, reflecting a stably reduced estimated GFR of less than 60 mL/min per 1.73 m2 that is consistent with the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative definition of chronic kidney disease (31). In the absence of retrospective data, presumptive nonprogressive chronic kidney disease was diagnosed if patients had a sustained elevated serum creatinine level that did not change during hospitalization and reflected a stable estimated GFR of less than 60 mL/min per 1.73 m2 despite volume resuscitation.
We defined prerenal azotemia as a new-onset increase in serum creatinine level that satisfied our criteria for altered kidney function and either resolved within 3 days with treatment aimed at restoring perfusion, such as intravenous volume repletion or discontinuation of diuretics (in the setting of historical and laboratory data suggesting decreased renal perfusion), or was accompanied by fractional excretion of sodium less than 1% at presentation.
We defined acute kidney injury as a new-onset 1.5-fold increase in serum creatinine level or a 25% decrease in estimated GFR from baseline values that satisfied minimal RIFLE (risk for kidney dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage kidney disease) criteria for serum creatinine and GFR that was sustained for at least 3 days despite volume resuscitation. The RIFLE criteria provide a meaningful way to stratify patients at different stages of kidney failure on the basis of severity (risk, injury, failure) and outcome (loss and end-stage disease) (Appendix Table, available at www.annals.org) (15).
Appendix Table.
The RIFLE Criteria*
| Stage | Criteria | Urine Output Criteria |
|---|---|---|
| Risk for kidney dysfunction | Creatinine level 1.5 times baseline or GFR decrease >25% | <0.5 mL/kg per h for 6 h |
| Injury to the kidney | Creatinine level 2 times baseline or GFR decrease >50% | <0.5 mL/kg per h for 12 h |
| Failure of kidney function | Creatinine level 3 times baseline or GFR decrease >75% | <0.3 mL/kg per h for 24 h or anuria for 12 h |
| Loss of kidney function | Persistent acute kidney failure = complete loss of kidney function for >4 wk | – |
| End-stage kidney disease | End-stage kidney disease for >3 mo | – |
GFR = glomerular filtration rate; RIFLE = risk, injury, failure, loss, end-stage kidney disease.
We prospectively identified patient outcomes (nephrology consultation, intensive care admission, dialysis initiation, mortality) from electronic medical records.
Laboratory Measurements
We centrifuged the urine samples at 12 000 rpm for 10 minutes and stored the supernatants at −80 °C. Urinary NGAL (10 μL) was quantified by immunoblots with nonreducing 4% to 20% gradient polyacrylamide gels (Bio-Rad Laboratories, Hercules, California) and monoclonal (1:1000; Antibody Shop, BioPorto Diagnostics, Gentofte, Denmark) or rabbit polyclonal antibodies together with standards (0.2 to 10 ng) of human recombinant NGAL protein (32, 33). The measurement was reproducible to 0.4 ng/lane. We selected the immunoblotting procedure (run time, approximately 10 hours) instead of commercially available enzyme-linked immunosorbent assays (run time, approximately 4 hours) to authenticate monomeric NGAL. Urinary N-acetyl-β-D-glucosaminidase (NAG) activity was assayed by using an NAG kit (Roche Diagnostics, Mannheim, Germany), and α1-microglobulin and α1-acid glycoprotein were assayed by immunonephelometry with the Dade Behring BN ProSpec System (Dade Behring, Marburg, Germany) at Cincinnati Children’s Hospital, Cincinnati, Ohio; the intra-assay and interassay variation coefficients were less than 5%. Urinary creatinine was measured by using a QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, California). Urinary sodium was quantified by using the Olympus AU2700 analyzer (Olympus Imaging America, Center Valley, Pennsylvania). Urinary proteins were measured in absolute terms and normalized to urinary creatinine, resulting in similar test characteristics (data not shown). Serum creatinine, the reference standard, was measured in the Columbia University Medical Center Core Laboratory by using the Jaffe reaction.
Statistical Analysis
We used SPSS, version 13.0 (SPSS, Chicago, Illinois), and SAS, version 9.1 (SAS Institute, Cary, North Carolina). We compared continuous variables between groups by using analysis of variance and categorical variables by using chi-square tests, rejecting the null hypothesis at P < 0.05. Data are presented as the mean (SD). To determine the diagnostic test characteristics, we generated conventional receiver-operating characteristic (ROC) curves and used a nonparametric approach for correlated ROC curves to compare discriminatory power (34). We derived biomarker cutoff levels from ROC analysis to maximize sensitivity and specificity. We also determined likelihood ratios and 95% CIs for each biomarker (35, 36). We used the McNemar test to measure the association of each biomarker with defined clinical outcomes of nephrology consultation, ICU admission, initiation of hemodialysis, and inpatient death. To determine the association of biomarkers, demographic variables (age, sex, and race), comorbid conditions (diabetes, hypertension, congestive heart failure, and cirrhosis), and laboratory values (blood urea nitrogen level and leukocyte count) with any of the 4 clinical outcomes, we performed univariate logistic regression analysis. We included the parameters that were significantly associated with the combined clinical outcome in a multiple logistic regression model to identify the independent variables most associated with combined clinical outcomes, which was the dependent variable. Because urinary sodium concentrations were not measured for patients with normal kidney function, we tested fractional excretion of sodium to discriminate acute kidney injury from patients with other types of kidney dysfunction.
Role of the Funding Source
The study was funded by the Emerald Foundation; the National Institutes of Health; the March of Dimes; the Doris Duke Foundation; the National Center for Research Resources, a component of the National Institutes of Health (NIH); and NIH Roadmap for Medical Research. The funding source had no role in study design, data collection, analysis, interpretation, or presentation.
Results
Baseline Characteristics
We received urine and blood samples for biomarker measurements from 882 adults presenting to the emergency department for hospital admission. From these, we excluded 247 persons (Figure 1). We prospectively tracked kidney function in the remaining 635 patients by subsequent measurements of serum creatinine level while they were in the hospital. Approximately one half of the patients were male and one quarter were black. Mean age was 60.1 years (SD, 0.7). Thirty patients met our criteria for acute kidney injury (4.7%), 88 had prerenal azotemia (13.8%), 106 had nonprogressive chronic kidney disease (16.6%), and 411 (64.7%) had normal kidney function (Table 2). The primary causes of acute kidney injury included cardiogenic shock (40%), urinary obstruction diagnosed by renal ultrasonography, (16.7%), multiple myeloma (10%), sepsis (6.7%), hypertensive emergency (6.7%), nephrotoxicity from nonsteroidal anti-inflammatory drugs (6.7%), lupus nephritis (3.3%), biopsy-confirmed acute interstitial nephritis (3.3%), glomerulonephritis (3.3%), and rhabdomyolysis (3.3%). Previous baseline creatinine data were available for 509 (80%) patients, including 26 (87%) patients with acute kidney injury, 74 (84%) patients with prerenal azotemia, 94 (89%) patients with stable chronic kidney disease, and 315 (76%) patients with normal kidney function. Patients with acute kidney injury were similar to patients with chronic kidney disease in sex distribution, but they were younger and more than half were black. They also had the highest mean serum creatinine at baseline and presentation and the greatest mean increase in serum creatinine (when baseline data were available for comparison). In addition, patients with acute kidney injury had the highest levels of blood urea nitrogen and urinary leukocytes and the highest fractional excretion of sodium.
Table 2.
Test Characteristics of Novel Biomarkers and Standard Diagnostic Markers*
| Biomarker and Cutoff Value | Sensitivity |
Specificity |
Positive Likelihood Ratio† (95% CI) | Negative Likelihood Ratio† (95% CI) | Positive Predictive Value | Negative Predictive Value | AUC (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Value (95% CI) | Patients, n/n‡ | Value (95% CI) | Patients, n/n§ | ||||||
| NGAL | |||||||||
| 85 μg/g | 0.93 (0.78–0.99) | 28/30 | 0.98 (0.97–0.99) | 594/605 | 51.33 (28.36–92.91) | 0.07 (0.02–0.26) | 0.737 | 0.996 | 0.948 (0.881–1.000) |
| 130 μg/g | 0.90 (0.73–0.98) | 27/30 | 0.995 (0.99–1.00) | 602/605 | 181.50 (58.33–564.71) | 0.10 (0.03–0.29) | 0.900 | 0.995 | |
| NAG | |||||||||
| 1.0 U/g | 0.87 (0.69–0.96) | 26/30 | 0.32 (0.29–0.36) | 196/605 | 1.28 (1.10–1.49) | 0.41 (0.16–1.03) | 0.059 | 0.980 | 0.713 (0.618–0.809) |
| 4.5 U/g | 0.70 (0.51–0.85) | 21/30 | 0.63 (0.59–0.67) | 383/605 | 1.91 (1.48–2.47) | 0.47 (0.27–0.82) | 0.087 | 0.977 | |
| α1-Microglobulin | |||||||||
| 10 mg/g | 1.00 (0.88–1.00) | 30/30 | 0.53 (0.49–0.57) | 318/604 | 2.11 (1.94–2.30) | 0 (0–0.20) | 0.095 | 1.00 | 0.887 (0.840–0.934) |
| 35 mg/g | 0.80 (0.61–0.92) | 24/30 | 0.81 (0.77–0.84) | 487/604 | 4.13 (3.24–5.26) | 0.25 (0.12–0.51) | 0.173 | 0.987 | |
| α1-Acid glycoprotein | |||||||||
| 10 mg/g | 0.97 (0.83–1.00) | 29/30 | 0.48 (0.44–0.52) | 292/604 | 1.87 (1.69–2.07) | 0.07 (0.01–0.47) | 0.087 | 0.996 | 0.832 (0.772–0.893) |
| 21 mg/g | 0.87 (0.69–0.96) | 26/30 | 0.61 (0.57–0.65) | 367/604 | 2.21 (1.86–2.62) | 0.22 (0.09–0.55) | 0.099 | 0.989 | |
| Creatinine | |||||||||
| 124 μmol/L (1.4 mg/dL) | 0.93 (0.78–0.99) | 28/30 | 0.75 (0.71–0.78) | 451/602 | 3.72 (3.15–4.40) | 0.09 (0.02–0.34) | 0.187 | 0.995 | 0.921 (0.865–0.978) |
| 221 μmol/L (2.5 mg/dL) | 0.77 (0.58–0.90) | 23/30 | 0.93 (0.90–0.95) | 559/602 | 10.73 (7.57–15.22) | 0.25 (0.13–0.48) | 0/348 | 0.987 | |
| Fractional excretion of sodium | |||||||||
| 1.0% | 0.80 (0.61–0.92) | 24/30 | 0.44 (0.37–0.52) | 86/194 | 1.44 (1.15–1.79) | 0.45 (0.22–0.94) | 0.162 | 0.943 | 0.708 (0.611–0.806) |
All patient groups were used to calculate test characteristics for all markers except fractional excretion of sodium. We tested NGAL and NAG levels in 635 patients, α1-microglobulin and α1-acid glycoprotein levels in 634 patients, and creatinine levels in 632 patients. We measured fractional excretion of sodium only in the 244 patients with abnormal kidney function (acute kidney injury, chronic kidney disease, or prerenal azotemia). AUC = area under the receiver-operating characteristic curve; NAG = N-acetyl-β-D-glucosaminidase; NGAL = neutrophil gelatinase–associated lipocalin.
The likelihood ratio is the ratio of posttest odds to pretest odds corresponding to a test result. As such, it indicates the effect of a test result on the odds (or, equivalently, the probability) of a disease. A very large likelihood ratio or a likelihood ratio close to zero denotes a very good test; a likelihood ratio close to 1.0 indicates a poor test. The positive likelihood ratio is the likelihood ratio after a positive test result. The negative likelihood ratio is the likelihood ratio after a negative test result.
Patients with acute kidney injury who tested positive/all patients with acute kidney injury.
Patients without acute kidney injury who tested negative/all patients without acute kidney injury.
NGAL Level and Kidney Injury
The mean urinary NGAL concentration was significantly elevated in patients with acute kidney injury (Table 1), whereas patients with prerenal azotemia, nonprogressive chronic kidney disease, or normal kidney function had uniformly low urinary NGAL concentrations (Figure 2). Mean NAG, α1-microglobulin, and α1-acid glycoprotein levels showed the same pattern of association (Table 1).
Table 1.
Patient Characteristics, by Diagnostic Group*
| Characteristic | All Patients (n = 635) | Acute Kidney Injury (n = 30) | Prerenal Azotemia (n = 88) | Stable Chronic Kidney Disease (n = 106) | Normal Kidney Function (n = 411) | P Value† |
|---|---|---|---|---|---|---|
| Mean age (SD), y | 60.1 (18.3) | 58.1 (16.7) | 65.1 (16.4) | 71.2 (14.5) | 56.3 (18.5) | <0.001 |
| Women, % | 49.0 | 53.3 | 48.9 | 42.5 | 50.4 | 0.50 |
| Black race, % | 27.1 | 36.7 | 31.8 | 33.0 | 23.8 | 0.091 |
| Mean systolic blood pressure (SD), mm Hg | 131.3 (29.0) | 129 (31.1) | 114 (31.0) | 141 (29.9) | 132 (26.7) | <0.001 |
| Mean diastolic blood pressure (SD), mm Hg | 74.8 (15.8) | 71.9 (18.5) | 66 (17.8) | 76 (16.3) | 76 (14.5) | <0.001 |
| Mean hematocrit (SD), % | 37.2 (6.7) | 30.9 (6.6) | 35.1 (8.4) | 36.2 (6.2) | 38.3 (6.0) | <0.001 |
| Mean serum albumin level (SD), g/L | 39 (14) | 33 (07) | 40 (33) | 37 (06) | 39 (06) | 0.039 |
| Mean blood urea nitrogen level (SD), mmol/L | 9.3 (9.0) | 25.7 (21.2) | 17.1 (11.6) | 12.6 (6.6) | 5.6 (2.4) | <0.001 |
| Mean serum leukocyte count (SD), cells × 109/L | 9.7 (5.7) | 10.5 (5.3) | 11.4 (7.3) | 9.1 (4.3) | 9.4 (5.8) | 0.21 |
| Mean serum creatinine level (SD) | ||||||
| Baseline | <0.001 | |||||
| μmol/L | 106 (79.6) | 194.5 (177) | 133 (79.6) | 168 (97.2) | 79.6 (18) | |
| mg/dL | 1.2 (0.9) | 2.2 (2.0) | 1.5 (0.9) | 1.9 (1.1) | 0.8 (0.2) | |
| Emergency department presentation | <0.001 | |||||
| μmol/L | 124 (159) | 495 (486) | 212 (124) | 177 (133) | 79.6 (18) | |
| mg/dL | 1.4 (1.8) | 5.6 (5.5) | 2.4 (1.4) | 2.0 (1.5) | 0.9 (0.2) | |
| Change from baseline to presentation‡ | <0.001 | |||||
| μmol/L | 26.5 (141) | 318 (495) | 88.4 (115) | 0.884 (17.7) | 1.77 (8.84) | |
| mg/dL | 0.3 (1.6) | 3.6 (5.6) | 1.0 (1.3) | 0.01 (0.2) | 0.02 (0.1) | |
| Urine studies | ||||||
| Mean fractional excretion of sodium (SD), % | – | 6.9 (9.1) | 1.7 (1.9) | 3.5 (5.1) | – | <0.001 |
| Mean specific gravity (SD) | 1.014 (0.008) | 1.012 (0.006) | 1.014 (0.007) | 1.012 (0.007) | 1.015 (0.008) | <0.001 |
| Mean leukocyte count (SD), cells/HPF | 11.4 (40.7) | 37.4 (68.2) | 12.4 (29.7) | 14 (74.6) | 8.2 (25.6) | 0.052 |
| Mean erythrocyte count (SD), cells/HPF | 11.2 (47.5) | 38.1 (147) | 16.0 (44.4) | 11.4 (36.7) | 7.6 (28.8) | 0.103 |
| Biomarkers§ | ||||||
| Mean NGAL level (SD), μg/g creatinine | 37.6 (125) | 416 (387) | 30.1 (92.0) | 22.5 (41.1) | 15.5 (15.3) | <0.001 |
| Mean NAG level (SD), U/g creatinine | 9.2 (21.8) | 24.8 (31.7) | 11.1 (17.8) | 13.0 (18.3) | 6.7 (21.9) | <0.001 |
| Mean α1-microglobulin level (SD), mg/g creatinine | 29.9 (57.4) | 129 (114) | 44.5 (66.7) | 38.1 (52.1) | 17.4 (39.8) | <0.001 |
| Mean α1-acid glycoprotein level (SD), mg/g creatinine | 45.7 (99.5) | 201 (274) | 73.3 (103) | 32.6 (71.5) | 31.8 (65.2) | <0.001 |
| Clinical outcome | ||||||
| Nephrology consultation, % | 5.1 | 70.8 | 5.6 | 4.7 | 0.0 | <0.001 |
| Dialysis initiation, % | 2.0 | 30.3 | 2.8 | 2.2 | 0.0 | <0.001 |
| ICU admission, % | 6.1 | 33.4 | 13.6 | 4.7 | 2.9 | <0.001 |
| Mortality, % | 2.0 | 13.9 | 5.6 | 0.9 | 0.7 | <0.001 |
HPF = high-power field; ICU = intensive care unit; NAG = N-acetyl-β-D-glucosaminidase; NGAL = neutrophil gelatinase–associated lipocalin.
Analysis of variance was performed on log-transformed values; however, raw values are presented here.
Average change in creatinine was calculated only for patients whose baseline creatinine level was known.
Values are normalized to the urinary creatinine concentration.
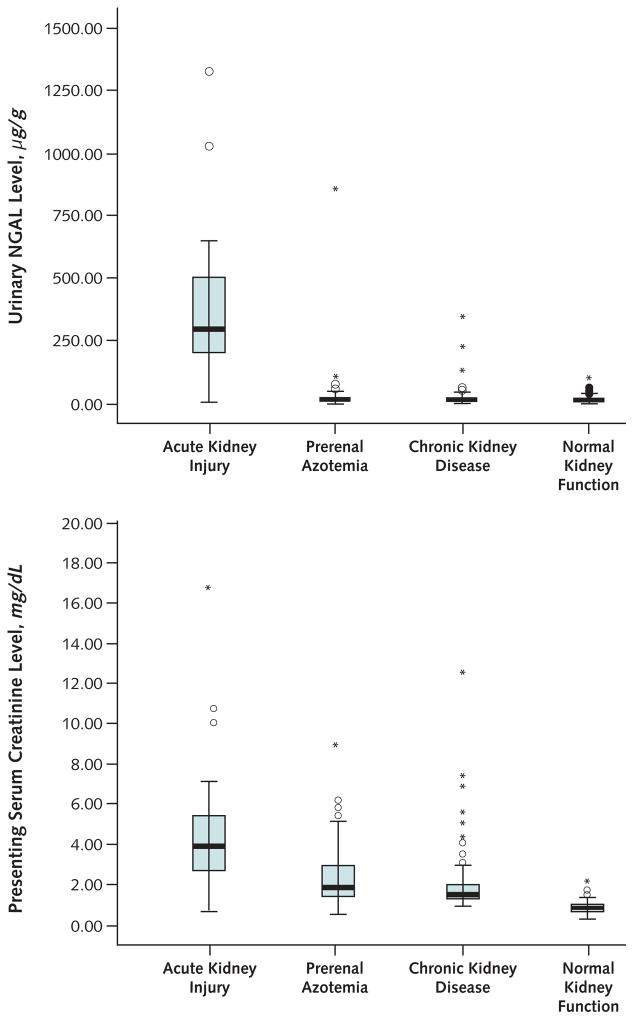
Figure 2. Box plots of urinary neutrophil gelatinase–associated lipocalin (NGAL) and serum creatinine levels, by diagnostic group.
Top. Patients with acute kidney injury had markedly elevated mean urinary NGAL levels compared with patients who had other forms of kidney dysfunction. Little overlap was present, except in 3 patients with chronic kidney disease and 1 patient with prerenal azotemia who had high urinary NGAL levels. Bottom. Patients with acute kidney injury had significantly elevated mean serum creatinine levels compared with patients who had other forms of kidney dysfunction, but values overlapped among the different categories of kidney function. To convert mg/dL to μmol/L, multiply by 88.402.
To determine whether NGAL could predict acute kidney injury better than other biomarkers, we performed an ROC analysis. Table 2 shows the area under the ROC curve (AUC) for each biomarker and sensitivities, specificities, and positive and negative likelihood ratios. Whereas the AUC for NGAL did not significantly differ from that for serum creatinine (P = 0.60) or α1-microglobulin (P = 0.100), the AUC for NGAL was significantly higher than that for α1-acid glycoprotein (P = 0.003) or NAG (P value <0.001). At either of 2 cutoff values (>85 μg/g creatinine or >130 μg/g creatinine), a positive urinary NGAL level had a stronger correlation with acute kidney injury than levels of serum creatinine or other biomarkers. A negative urinary NGAL level was also highly associated with the absence of acute kidney injury at both cutoff values. Figure 2 shows box plots for urinary NGAL and serum creatinine measurements, stratified by each type of kidney dysfunction. Although NGAL values overlapped very little among patient categories, serum creatinine values overlapped substantially.
NGAL Level and Clinical Outcome
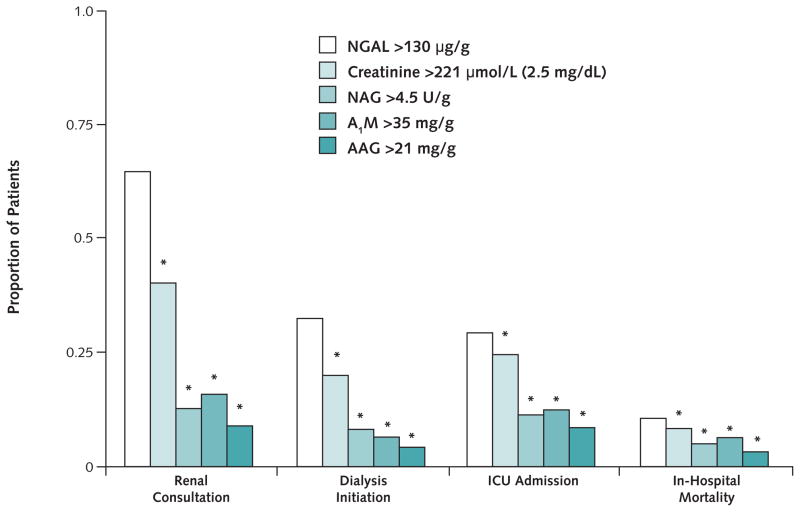
To determine the association between clinical outcomes and biomarkers, we used ROC analysis to choose cutoffs that maximized both sensitivity and specificity for acute kidney injury (Figure 3). Of the 31 patients with a urinary NGAL level of at least 130 μg/g creatinine on presentation to the emergency department, 64.5% required nephrology consultation, 32.3% required initiation of dialysis, 29.3% required ICU admission, and 9.7% died during hospitalization. A urinary NGAL level greater than 130 μg/g creatinine identified more patients who received nephrology consultation, dialysis initiation, or ICU admission than did other biomarkers (P < 0.001 for NGAL compared with all biomarkers) (Figure 3).
Figure 3. Kidney injury biomarkers versus clinical outcome.
Measurements are normalized per gram of creatinine. Bars show the proportion of patients with biomarker levels above the selected cutoffs and clinical outcome. The total number of patients whose levels were above the cutoff was 66 for creatinine, 31 for neutrophil gelatinase–associated lipocalin (NGAL), 133 for N-acetyl-β-D-glucosaminidase (NAG), 143 for α1-microglobulin (A1M), and 294 for α1-acid glycoprotein (AAG). *P < 0.001 compared with NGAL.
We used univariate logistic regression to evaluate the association of conventional and novel biomarkers, laboratory values, demographic variables, and comorbid conditions with clinical events. Urine and serum biomarker levels, blood urea nitrogen levels, and serum leukocyte count were significantly associated with the composite clinical outcome; however, age, sex, race, and comorbid conditions were not. In multiple logistic regression analysis (Table 3), levels of NGAL, serum creatinine, and blood urea nitrogen and serum leukocyte count were all predictive of poor outcome.
Table 3.
Multivariate Analysis of Acute Kidney Injury Biomarker Levels Derived from Receiver-Operating Characteristic Curve Analysis*
| Model Parameters | Odds Ratio (95% CI) |
|---|---|
| NGAL level (>130 μg/g) | 24.70 (7.69–79.42) |
| α1-Microglobulin level (>35 mg/g) | 1.85 (0.80–4.31) |
| α1-Acid glycoprotein level (>21 mg/g) | 0.741 (0.33–1.69) |
| NAG level (>4.5 U/g) | 1.07 (0.52–2.18) |
| Presenting creatinine level >221 μmol/L (>2.5 mg/dL) | 6.03 (2.25–16.14) |
| Blood urea nitrogen level (per mg/dL increase) | 1.01 (1.00–1.03) |
| Serum leukocyte count (per cells × 109/L increase) | 1.05 (1.01–1.10) |
Regression analysis of biomarkers as predictors of combined clinical outcomes (nephrology consultation, intensive care unit admission, dialysis initiation, or mortality). NAG = N-acetyl-β-D-glucosaminidase; NGAL = neutrophil gelatinase–associated lipocalin.
Identification of NGAL Expression Site
To investigate whether urinary NGAL was derived from degranulated leukocytes, we correlated NGAL with markers of neutrophils. We found a high correlation between NGAL and myeloperoxidase assayed in serum neutrophils (R2 = 0.9; P < 0.001). However, we found no correlation between urinary NGAL and urinary leukocyte counts (Spearman correlation coefficient, 0.15; P = 0.060), urinary myeloperoxidase (Spearman correlation co-efficient, 0.007; P = 1.0), or serum leukocytes (Spearman correlation coefficient, −0.02; P = 0.70).
Discussion
In a sample of patients presenting to an inner-city emergency department, a single measurement of urinary NGAL distinguished acute kidney injury from other forms of kidney dysfunction and predicted excess morbidity after hospital admission. Logistic regression analysis demonstrated that NGAL was a better predictor of nephrology consultation, dialysis, ICU admission, and death than conventional or novel biomarkers of acute kidney injury.
Studies in children and adults have shown that urinary NGAL is rapidly and massively expressed in well-defined cases of acute kidney injury (20, 25, 28, 29, 37–39). For example, in a prospective study of children receiving cardiopulmonary bypass, urinary NGAL levels greater than 50 μg/L 2 hours after surgery had 100% sensitivity and 98% specificity for the subsequent diagnosis of acute kidney injury (AUC, 99.8%) (21). In contrast, even 1 to 3 days after surgery, acute kidney injury could not be diagnosed by using criteria based on serum creatinine level (such as a 50% increase). These findings were confirmed in a prospective study of adults who had cardiac surgery (29), in whom the urinary NGAL level increased rapidly after surgery (postoperative AUC, 74% at 3 hours and 80% at 18 hours). Similarly, in a prospective study of children receiving intensive care (39), urinary NGAL increased 48 hours before a diagnosis based on serum creatinine level was possible. Neutrophil gelatinase–associated lipocalin has also been evaluated as a biomarker of acute injury in kidney transplantation, and multiple studies have shown a significant correlation between NGAL levels and delayed graft function (37, 38). In each of these studies, patients were intentionally chosen to eliminate comorbid conditions and confounding variables and to test a specific type of injury with unequivocal timing (for example, after surgery) (27, 29, 40, 41).
In contrast to these studies, we demonstrate that the urinary NGAL level identifies acute kidney injury in a broad patient sample with different mechanisms of injury. In addition, we demonstrate that the urinary NGAL level remained highly diagnostic even when the timing of injury was unknown, making NGAL potentially diagnostic of kidney disease for many clinical presentations.
Interest in identifying kidney proteins that permit risk assessment, early diagnosis of injury, or surveillance of acute kidney function is intense, and a panel of biomarkers is probably required. Candidates include interleukin-18, a proinflammatory cytokine that is induced in the proximal tubule and whose levels peak 6 to 12 hours after acute disease but do not change in chronic renal disease or prerenal azotemia (42, 43). The urinary interleukin-18 level can increase 24 hours before the serum creatinine level in acute ischemia (AUC, 73%) (44). The serum cystatin C level also anticipates acute kidney injury (AUC, 97%) 1 to 2 days before the serum creatinine level (AUC, 82%) (45), and an increased urinary cystatin C level has predicted the need for dialysis (AUC, 75%) earlier than serum creatinine (46). However, in a study of 202 patients, cystatin C did not outperform serum creatinine in the diagnosis of acute injury or prediction of clinical outcomes (47). Urinary levels of kidney injury molecule-1, derived from the proximal tubule, can distinguish ischemic injury from prerenal azotemia and chronic kidney disease (48). In a case–control study of 20 patients with acute kidney injury and 20 control patients, kidney injury molecule-1 levels peaked 12 hours after injury (AUC, 83%) (49) and predicted a combined end point of dialysis or death in hospitalized patients (AUC, 61%) (50). In a cross-sectional study of 20 children, NAG identified acute kidney injury 12 hours after insult (AUC, 69%) (49). Urinary α1-microglobulin increased 8-fold after tubular damage, and patients nearing a requirement for dialysis after acute injury also had increased urinary α1-microglobulin levels (46). α1-Acid glycoprotein increased 5-fold within 2 hours of cardiopulmonary surgery in patients who developed acute kidney injury (51, 52).
Together, these studies demonstrate that several proteins can detect acute kidney injury early in its course, although the AUCs for these biomarkers tended to be lower than that for NGAL. In a direct comparison, NGAL was better than NAG, α1-acid glycoprotein, or α1-microglobulin at detecting acute injury and predicting clinical outcomes, even after inclusion of other biomarkers and indicators of poor outcome in a multivariate analysis.
Our study has several limitations. Although biopsy is the gold standard to identify the mechanism of kidney damage, it is seldom used when the patient first presents; we used current clinical criteria to diagnose kidney disease instead. Second, we excluded 28% of the patients because they lacked follow-up serum creatinine measurement or had end-stage kidney disease. Third, although we recruited patients from 6 a.m. to midnight, potentially producing a cohort biased toward medical conditions associated with acute referrals, we believe the effect to be minimal because the mean 12- to 18-hour length of stay in our emergency department allowed us to enroll admitted patients regardless of when they first presented. Finally, we studied patients from a single center, and our findings require validation in other groups of patients.
In summary, NGAL is a host defense protein that distinguishes acute kidney injury from other types of kidney dysfunction. Although our patients came from an urban hospital, our inclusion of a broad sample with many types of illnesses, selected only on the basis of presentation to the emergency department and inpatient disposition, highlights the specificity of urinary NGAL level for diagnosis of acute kidney injury and prediction of compromised patient outcomes.
Context
Studies in surgical patients suggest that urinary neutrophil gelatinase–associated lipocalin (NGAL) may be an effective test for diagnosing acute kidney injury.
Contribution
The authors measured markers of renal function in 635 consecutive adults who were admitted to the emergency department of 1 urban hospital; of these patients, 30 had a final clinical diagnosis of acute kidney injury. When urinary NGAL was at least 130 μg/g creatinine, the test was highly discriminatory for acute kidney injury (sensitivity, 0.90; specificity, 0.995; positive likelihood ratio, 181.5; and negative likelihood ratio, 0.10).
Caution
Patients were from 1 setting in 1 hospital. Kidney diagnoses were clinical, not biopsy-proven.
Implication
Urinary NGAL is a promising test because of its large effect on the probability of acute kidney injury.
—The Editors
Acknowledgments
The authors thank Dean Lee Goldman, Columbia University, for critical review. This work would not have been possible without the generous support of the Emerald Foundation.
Grant Support: From the Emerald Foundation; the National Institutes of Health (grants DK55388, DK58872, and DK73462); the March of Dimes; the Doris Duke Foundation; the National Center for Research Resources, a component of the NIH (grant UL1 RR024156); and NIH Roadmap for Medical Research.
Footnotes
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the NIH.
Potential Financial Conflicts of Interest: Honoraria: P. Devarajan (Biosite, Abbott). Grants pending: P. Devarajan (Abbott, Biosite). Patents pending: T.L. Nickolas (NGAL for diagnosis of chronic renal failure), K. Mori (NGAL for diagnosis of chronic renal failure), P. Devarajan (NGAL for diagnosis of acute renal failure and NGAL for diagnosis of chronic renal failure), J. Barasch (NGAL for diagnosis of acute renal failure and NGAL for diagnosis of chronic renal failure). Cincinnati Children’s Hospital Medical Center and Columbia University have received licensing fees from Biosite and Abbott Diagnostics for technology to use NGAL as a biomarker of acute renal failure.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available by written agreement from Dr. Nickolas (tln2001@columbia.edu).
Author Contributions: Conception and design: T.L. Nickolas, M.J. O’Rourke, J. Yang, N. Barasch, K. Mori, J. Giglio, P. Devarajan, J. Barasch.
Analysis and interpretation of the data: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, P.A. Canetta, N. Barasch, C. Buchen, F. Khan, K. Mori, J. Giglio, P. Devarajan, J. Barasch.
Drafting of the article: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, P.A. Canetta, N. Barasch, C. Buchen, K. Mori, J. Giglio, P. Devarajan, J. Barasch.
Critical revision of the article for important intellectual content: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, P.A. Canetta, K. Mori, J. Giglio, P. Devarajan, J. Barasch.
Final approval of the article: T.L. Nickolas, M.J. O’Rourke, J. Yang, M.E. Sise, P.A. Canetta, N. Barasch, C. Buchen, F. Khan, K. Mori, J. Giglio, P. Devarajan, J. Barasch.
Provision of study materials or patients: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, N. Barasch.
Statistical expertise: T.L. Nickolas, K. Mori.
Obtaining of funding: T.L. Nickolas, J. Barasch.
Administrative, technical, or logistic support: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, N. Barasch, C. Buchen, F. Khan, K. Mori, J. Giglio, J. Barasch.
Collection and assembly of data: T.L. Nickolas, M.J. O’Rourke, M.E. Sise, N. Barasch, C. Buchen, F. Khan.
References
- 1.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–42. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 2.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996;24:192–8. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. Am J Kidney Dis. 1991;17:191–8. doi: 10.1016/s0272-6386(12)81128-0. [DOI] [PubMed] [Google Scholar]
- 4.Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multi-center, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 5.Pascual J, Orofino L, Liaño F, Marcén R, Naya MT, Orte L, et al. Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc. 1990;38:25–30. doi: 10.1111/j.1532-5415.1990.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–11. [PubMed] [Google Scholar]
- 7.Maher ER, Robinson KN, Scoble JE, Farrimond JG, Browne DR, Sweny P, et al. Prognosis of critically-ill patients with acute renal failure: APACHE II score and other predictive factors. Q J Med. 1989;72:857–66. [PubMed] [Google Scholar]
- 8.Novis BK, Roizen MF, Aronson S, Thisted RA. Association of preoperative risk factors with postoperative acute renal failure. Anesth Analg. 1994;78:143–9. doi: 10.1213/00000539-199401000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Zanardo G, Michielon P, Paccagnella A, Rosi P, Caló M, Salandin V, et al. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994;107:1489–95. [PubMed] [Google Scholar]
- 10.Fischer MJ, Brimhall BB, Lezotte DC, Glazner JE, Parikh CR. Uncomplicated acute renal failure and hospital resource utilization: a retrospective multi-center analysis. Am J Kidney Dis. 2005;46:1049–57. doi: 10.1053/j.ajkd.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 12.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 13.Lameire N, Hoste E. Reflections on the definition, classification, and diagnostic evaluation of acute renal failure [Editorial] Curr Opin Crit Care. 2004;10:468–75. doi: 10.1097/01.ccx.0000144939.24897.71. [DOI] [PubMed] [Google Scholar]
- 14.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–41. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 18.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 22.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 23.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 24.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34:474–80. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 27.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 29.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 32.Bundgaard JR, Sengeløv H, Borregaard N, Kjeldsen L. Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem Biophys Res Commun. 1994;202:1468–75. doi: 10.1006/bbrc.1994.2096. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 35.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 36.Agresti A. Categorical Data Analysis. 2. Hoboken, NJ: J Wiley; 2002. [Google Scholar]
- 37.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 38.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–45. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 39.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone DH, Al-Badawi H, Conrad MF, Stoner MC, Entabi F, Cambria RP, et al. PJ34, a poly-ADP-ribose polymerase inhibitor, modulates renal injury after thoracic aortic ischemia/reperfusion. Surgery. 2005;138:368–74. doi: 10.1016/j.surg.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–8. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 42.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 43.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 44.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–52. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 45.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 46.Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–8. doi: 10.1373/clinchem.2003.027763. [DOI] [PubMed] [Google Scholar]
- 47.Ahlström A, Tallgren M, Peltonen S, Pettilä V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62:344–50. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 48.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 49.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–9. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen MT, Harris N, Kathman T, Dent C, Devarajan P. Novel early biomarkers of acute kidney injury [Abstract] J Am Soc Nephrol. 2006;17:49A. [Google Scholar]
- 52.de Vries B, Walter SJ, Wolfs TG, Hochepied T, Räbinä J, Heeringa P, et al. Exogenous alpha-1-acid glycoprotein protects against renal ischemia-reperfusion injury by inhibition of inflammation and apoptosis. Transplantation. 2004;78:1116–24. doi: 10.1097/01.tp.0000138096.14126.ca. [DOI] [PubMed] [Google Scholar]