ABSTRACT
Purpose: The objectives of this study were (1) to examine the effects of a 12-week exercise cessation period separating two 12-week exercise sessions on physical variables in an elderly institutionalized population and (2) to explore overall programme effectiveness.
Methods: The functional mobility of 25 elderly institutionalized adults participating in an existing exercise programme was examined using a one-group, interrupted time-series design. Functional mobility was evaluated at four time points during two cycles of a 12-week exercise programme, alternating with a 12-week period of no formal exercise.
Results: The primary outcome was the change in functional mobility scores, assessed at baseline, 12, 24 and 36 weeks. In both the higher-functioning (HF) group and the lower-functioning (LF) group, the cessation of exercise was associated with deterioration in physical function. The overall non-continuous nature of the programming under study also seemed to be detrimental to the physical function of the LF group.
Conclusions: Findings of this small-sample study support the need to modify common practices in exercise programming for older, institutionalized people.
Key Words: exercise cessation, exercise class, exercise programming, institutionalized elderly, physical function
RÉSUMÉ
Objectif : Les objectifs de cette étude étaient (1) de se pencher sur les effets d'une cessation de la pratique d'exercice physique durant 12 semaines sur des variables physiques au sein d'une population d'aînés en institution; et (2) d'explorer l'efficacité globale d'un programme d'exercices sur cette clientèle.
Méthode : La mobilité fonctionnelle de 25 aînés en institution dans le cadre d'un programme d'exercices a été étudiée en ayant recours à un modèle à un seul groupe, avec temps d'interruption. La mobilité fonctionnelle des sujets a été mesurée à quatre reprises au cours de deux cycles de 12 semaines pour le programme d'exercices, en alternance avec des périodes de 12 semaines sans période d'exercices organisée.
Résultats : Le premier résultat observé a été un changement dans les mesures de la mobilité fonctionnelle au début de l'étude, puis après 12, 24 et 36 semaines. Dans le groupe à haut fonctionnement (HF) comme dans le groupe à faible fonctionnement (FF), l'arrêt des séances d'activité physique a été associé à une détérioration de la fonction physique. La nature globale non continue du programme auquel s'est intéressée cette étude a également semblé nuire à la fonction physique du groupe FF.
Conclusions : Les conclusions de cette étude réalisée sur un petit échantillon appuient la nécessité de modifier les pratiques usuelles liées aux programmes d'exercices pour les aînés en institution.
Mots clés : aînés en institution, cessation de l'exercice, classe d'exercice, fonction physique, personnes âgées, programme d'exercices
INTRODUCTION
There is a wealth of evidence in the literature to support the role of exercise in the well-being of community-dwelling elderly people.1–6 Exercise and physical activity have been recognized by public-health organizations as necessary for physical and mental health and well-being through the lifespan, including in the elderly.7,8 In seniors, ageing is generally associated with a deterioration in physical and bio-psychosocial domains.9–12 It appears that environmental factors such as institutionalization negatively influence the trajectory of decline in function of the elderly; the trajectory of decline seems more pronounced in institutionalized populations than in community-dwelling elderly people.13,14 Rolland et al.15 demonstrated less decline in activities of daily living (ADL) associated with subjects who exercised than with those who did not exercise. Recent studies have shown the benefits of exercise and physical activity for shortness of breath and continence16,17 as well as for fall prevention.18 Formal recommendations by the American College of Sports Medicine (ACSM) and the American Heart Association (AHA) have existed since 1995 and have been updated in recent years,7,19 but these primarily address exercise prescription for community-dwelling older adults.
Despite the abundance of research supporting the value of exercise in elderly community-dwelling populations, there remains relatively little research examining the role of exercise and, specifically, the effect of exercise cessation in the institutionalized elderly. Exercise may slow the deterioration in physical and psychosocial domains in the elderly,4,14,20–23 but exercise strategies in nursing homes and other long-term care facilities are not standardized.24
Research has suggested that available programming should be increased substantially relative to what is recommended by guidelines.25 Gaps in availability of exercise programming are apparent in institutional settings. Non-continuous exercise programmes are common in both community and institutional settings; many exercise programme interventions that are offered to the institutionalized elderly are not run continuously throughout the year, contrary to current thought on training and wellness.26 Current research seems to indicate that exercise sessions of 8 to 12 weeks' duration can result in strengthening effects even in populations of quite elderly institutionalized people; however, a marked heterogeneity exists in the literature in how programmes themselves have been assessed as well as in the choice of specific outcome measures. Therefore, the purpose of this study was to answer the following research question and its corollary: In an institutionalized elderly population, is stopping an existing exercise programme associated with a deterioration in physical function? And, if so, are these losses regained with subsequent retraining? The null hypothesis proposed for this study was that there would be no significant deterioration in physical functional measures following exercise cessation of 12 weeks' duration.
Ste-Anne's Hospital (SAH) is a long-term care institution located in a suburb of Montreal that had a population in 2002 of approximately 500 resident veterans. An exercise intervention programme had been adopted consisting of 12 weeks of exercising in one of two groups (higher-functioning and lower-functioning), followed by a 12-week period with no formal exercise, followed in turn by another 12 weeks of exercise. This programming had been initiated based on literature available at the time. The objective of this study was to examine the effects of the 12-week exercise cessation period separating two 12-week exercise sessions on physical variables in SAH's elderly institutionalized population. The study also served to explore overall programme effectiveness related to physical performance.
METHODS
Research Design
This was a prospective observational study, using a one-group, interrupted time-series design for each of two exercise groups.27 Physical measures were taken at baseline and every 12 weeks over a 36-week period (i.e., four measurement sessions). This modified repeated-measures design mimics current clinical practice of assessing and reassessing patient status before and after therapeutic interventions.
Participants
The participants were all residents of SAH. A convenience sample of 29 residents was recruited for the study; 25 completed all four testing sessions. This sample represented 100% of the participants of two existing exercise groups that had run in previous years. The sample was divided according to functional mobility: residents who were able to walk independently with or without a mobility aid, such as a cane or a walker, were registered in the higher-functioning (HF) group (n=9); residents who required physical assistance to ambulate with a mobility aid such as a walker were assigned to the lower-functioning (LF) group (n=20). The mean age of participants in the HF group was 80.1 years (SD=2.8 years), while the mean age of participants of the LF group was 84.7 years (SD=4.0 years); see Table 1 for baseline characteristics of the participants. Inclusion criteria were physician referral to the exercise group, age greater than 70 years at the start of the study, consent of the resident to participate in the exercise group, and ability to understand oral or written instructions in either French or English. Exclusion criteria were cognitive impairment precluding participation in a group exercise setting or completion of testing, blindness, or acute or terminal illness. The study protocol was approved by the research ethics committees of SAH and McGill University, and participants signed an informed consent (in the case of cognitive impairment, participants assented to participate in the study and their health care proxy signed the informed consent). Sample-size and power calculations were deferred because of the preliminary nature of this small-sample study.
Table 1.
Baseline Characteristics of Study Participants
| HF group (n=9) | LF group (n=20) | ||
| Mean±SD or n (%) | Mean±SD or n (%) | ||
| Age (years) | 80.12±2.75 | 84.72±3.95 | |
| BMI39,40 | At risk (≤23.9) | 3 (33.3) | 4 (20.0) |
| Healthy range (24.0–27.0) | 3 (33.3) | 9 (45.0) | |
| Overweight (≥27.1) | 3 (33.3) | 7 (35.0) | |
| History of falls | None in the past 180 days | 8 (88.9) | 12 (60.0) |
| Fell within the past 30 days | 0 (0.0) | 7 (25.0) | |
| Fell between 1 and 6 months ago | 1 (11.1) | 1 (5.0) | |
| Physical Measures | |||
| 2MWT (m) | 88.9±25.2 | n/a | |
| Gait speed (m/s) | 0.72±0.2 | n/a | |
| TUG (sec) | 16.93±5.06 | n/a | |
| BBS (/56) | 46.33±4.8 | n/a | |
| PFMP (/63=%) | n/a | 75.88±15.2 | |
| Activities of daily living* | 0 | 4 (44.4) | 5 (25.0) |
| 1–3 | 3 (33.3) | 7 (35.0) | |
| 4–7 | 1 (11.1) | 1 (5.0) | |
| 8–21 | 1 (11.1) | 5 (25.0) | |
| 22–28 | 0 (0.0) | 1 (5.0) | |
| Daily decision making* | 0 (independent) | 3 (37.5) | 2 (11.8) |
| 1–2 (modified independence) | 5 (62.5) | 11 (64.7) | |
| 3–4 (moderately impaired) | 0 (0.0) | 3 (17.6) | |
| 5–6 (severely impaired) | 0 (0.0) | 1 (5.9) |
BMI=Body Mass Index; 2MWT=2-minute walk test; TUG=timed up-and-go; BBS=Berg Balance Scale; PFMP=Physiotherapy Functional Mobility Profile
From Minimum Data Set (MDS); lower scores indicate less problematic results.
Content of the Exercise Intervention
The exercise group was held twice weekly for both categories of resident abilities (HF and LF) for two 12-week periods. The content of the classes was unchanged from that of previous years; each session was approximately 75 minutes in duration and included seated warm-up exercises of the upper and lower extremities, resisted exercises of the upper and lower extremities in a seated position, and group interactions with a soft ball (ball toss) or an inflated balloon (wheelchair badminton) using the dominant hand and the non-dominant hand. Guided breathing exercises were also part of the group intervention; for those residents able to do so, supervised or assisted sit-to-stand movements were sometimes offered, depending on staff resources. In the HF group, supervised agility work and a four-step staircase were used when possible, again depending on resources.
Data Collection
Physical performance measures were selected for the subjects in the two groups relative to their abilities. Objective measures for participants in the HF group included the 2-minute walk test (2MWT)28 and gait speed.29 Physical function was assessed for the participants in the LF group using the Physiotherapy Functional Mobility Profile (PFMP) as well as a measure of activities of daily living (MDS-ADL long format) from the Minimum Data Set (MDS). Data were collected prior to commencing the exercise sessions at baseline (T1), after the first 12-week exercise intervention (T2), after the 12-week period of exercise cessation that followed (T3), and after the second and final 12-week exercise intervention (T4).
Outcome Measures
Higher-Functioning Group
The 2MWT is widely used in clinical practice and has demonstrated reliability and validity when used with elderly populations.28 The 2MWT has shown a moderate relationship with physiologic measures such as VO2max (r=0.45) and VO2/kg (r=0.55), as well as with gait speed (r=−0.61).28
Gait speed has been used extensively to describe walking ability in heterogeneous populations similar to those residing at SAH;28 it has been strongly recommended as an indicator of mobility and falls29 and has been recommended as a criterion measure for other mobility tests. Interrater and test–retest reliability estimates for gait speed are good to excellent (r=0.94–0.99; ICC=0.90–0.99).28
Lower-Functioning Group
The PFMP was developed by a team of clinicians as a measure of global mobility in adult institutionalized populations. It scores nine mobility items of increasing difficulty, progressing from bed mobility to unsupported sitting, standing, transfers, propelling a wheelchair, walking, and the ability to manage stairs. Items are scored from 1 (requiring total assistance) to 7 (independent), for a maximum possible score of 63. The measure has demonstrated high intrarater reliability (ICC=0.99).30,31 Interrater reliability estimates of the PFMP were very strong (ICC=0.97) for raters who were trained therapists,30 as was the case in this study. At the time of this study, the PFMP was the predominant measure of functional mobility used in the physical therapy department of SAH. Although the PFMP has been shown to be valid in acute and subacute mobility evaluations in geriatric populations upon admission to hospital,30 further psychometric studies have not yet been forthcoming on the specific validity of this practical tool in the long-term care setting. The scale has been shown to be reliably scored, and its validity has also been examined relative to existing measures.30
The MDS-ADL scale has been validated in the literature as an appropriate outcome measure for institutionalized populations.32,33 It is a 28-point ordinal scale summing seven items (bed mobility, transfers, locomotion, dressing, eating, toilet use, and personal hygiene) scoring degree of assistance required and frequency of assistance required each day. It is scored through observation of daily activities and care, by a trained clinical professional; scores range from 0 to 28, higher scores indicating greater dependence.32
Data Analysis
Physical measures across the four different testing times were compared separately for HF and LF groups using two single-factor repeated-measures analyses of variance (ANOVAs) with one independent variable (time). When significant differences were found, post hoc paired t-tests were performed. The analyses were performed on the scores of the 2MWT and gait speed for the HF group and on PFMP and MDS-ADL scores for the LF group. All tests were two-tailed, and the critical level of significance was 0.05. Analyses were completed using SPSS software (SPSS Inc., Chicago, IL).
RESULTS
Twenty-five participants completed all four evaluations. Reasons for non-completion were an acute psychotic episode (n=1, HF group) and death (n=3, LF group). In general, these participants were similar to the other members of their groups, although the participant who withdrew because of his psychosis was younger (75.8 yrs) than the mean of his group, while the three participants from the LF group who died were older (mean 87.1 yrs) than others in their group. The participants in the two groups differed as anticipated with respect to baseline characteristics, given the criteria for assignment to each of the two groups (see Table 1). Participants in the HF group showed fewer signs of frailty, such as requiring assistance with ADL (12.5% of HF participants required physical assistance in ADL, vs. 29.4% of LF participants) and decision making (none of the participants in the HF group had moderate or severe impairments in daily decision making, whereas 23.5% of participants in the LF group were ranked in these categories). There were no women in the HF group; the LF group had three female participants (88.0% of participants were male).
Physical Measures
Higher-Functioning Group
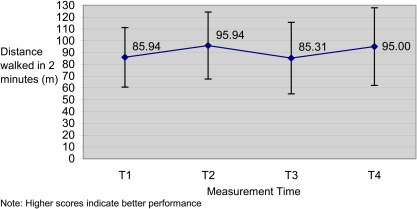
Functional ambulation abilities, as measured by the distance walked in 2 minutes (2MWT) (see Figure 1) and by gait speed (see Figure 2), were monitored in study participants from the HF group. On average, HF participants walked 85.9 m at baseline, 95.9 m after the first exercise period, 85.3 m after 12 weeks without the class, and 95.0 m at the end of the second exercise period. Repeated-measures ANOVA (RM-ANOVA) confirmed that the mean within-subject change over time was greater than could be expected to occur by chance across the four time points (F(3,21)=4.39, p=0.015). Post hoc tests indicated that the 10 m improvements from T1 to T2 and from T3 to T4 were both greater than expected by chance: T1 to T2 (F(1,7)=10.93, p=0.013) and T3 to T4 (F(1,7)=9.91, p=0.016). The decrease in mean scores from T2 to T3 was not significant (F(1,7)=5.2, p=0.06).
Figure 1.
The distance walked in 2 minutes was measured in HF group participants.
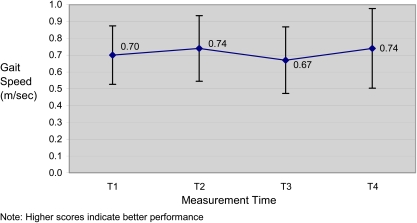
Figure 2.
The speed of ambulation (gait speed) was measured in HF group participants.
As shown in Figure 2, the direction of the changes in gait speed followed the same pattern as the direction of changes in the 2MWT: results improved with exercise and declined without exercise. RM-ANOVA of the gait speed scores suggested that the within-subject change was greater than that expected to occur by chance (F(3,21)=4.97, p=0.009). The improvement from T1 to T2 was 0.04 m/s (F(1,7)=7.23, p=0.031), and the improvement from T3 to T4 was 0.07 m/s (F(1,7)=8.86, p=0.021). Gait speed at T3 was 0.07 m/s slower after the period of exercise cessation (F(1,7)=6.09, p=0.043).
Lower-Functioning Group
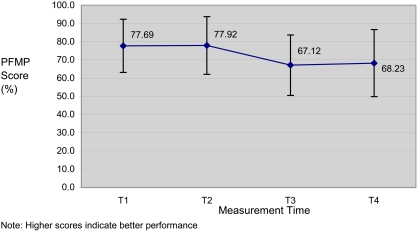
The physical performance of participants in the LF group was monitored using the PFMP (see Figure 3) and the MDS-ADL scale (see Figure 4). RM-ANOVA suggested that although sphericity could not be assumed, the PFMP scores did demonstrate within-subject differences greater than those expected to occur by chance (lower-bound F(3,48)=6.78, p<0.019).
Figure 3.
Scores of the PFMP were compared in LF group participants.
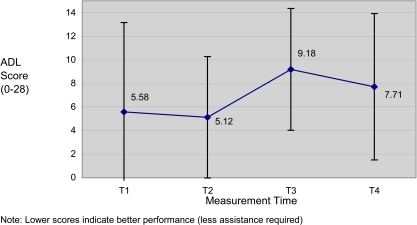
Figure 4.
Scores on the ADL scale were examined in LF group participants.
Post hoc comparisons illustrated that the deterioration in PFMP scores related to exercise cessation (T3 vs. mean of T1 and T2) was significant (F(1,16)=7.52, p=0.014). At the end of the 36-week study period, PFMP scores recorded after retraining (T4) as compared with the mean of the previous three measurement times (T1, T2, T3) were lower than expected by chance (F(1,16)=6.13, p=0.025).
The MDS-ADL scores followed a similar pattern to the PFMP scores over time. RM-ANOVA of the MDS-ADL scores supported the anticipated deterioration following cessation of exercise: the score at T3 (after cessation), when compared with the previous two measurement times (T1 and T2), indicated that a significant deterioration (F(1,16)=4.51, p=0.05) occurred following a 12-week period of cessation of exercise. Tests of within-subject contrasts indicated that the differences between T2 and the two later measurement times were also significant (F(1,16)=9.27, p=0.008), indicating that the score after the first training session was the highest and that participants' scores deteriorated thereafter.
DISCUSSION
Results of this study suggest that a 12-week period of exercise cessation during a 36-week exercise programme is detrimental to the physical functioning abilities of older adults residing in institutions. However, higher-functioning participants differed from lower-functioning participants in their response to exercise cessation, to exercise itself, and to the overall non-continuous nature of exercise programming.
For participants in the HF group, physical function improved following exercise and deteriorated following exercise cessation. The available literature, which for the most part documents studies of younger elderly community-dwelling populations, supports this finding.12,34–38 The observed deterioration in functional status was both statistically and clinically important. For example, HF participants walked an average of 10 m less in 2 minutes following the 12-week cessation period; in practical terms, 10 m represented the distance between bed and toilet for two-thirds of SAH residents at the time of the study.
HF participants appeared able to recover the physical abilities lost during exercise cessation following the second 12-week retraining session. In the first 12-week exercise training period, seven of the HF group participants attended at least 60% of the scheduled classes; in the second 12-week exercise period, eight participants attended at least 60% of the scheduled classes.
In contrast to the HF group, participants in the LF group did not display improvement in physical function following either of the two 12-week exercise sessions; however, their physical function deteriorated following exercise cessation. The physical decline of LF participants was measured by a drop of close to 7 points (10.8%) in functional mobility (PFMP) scores. Although this decrease did not speak directly to specific functional decline, it illustrated an alarming trend. Unlike participants in the HF group, participants in the LF group appeared unable to recover from the 12-week exercise cessation period; for this frailer group, physical performance deteriorated following the cessation period, and that decline persisted following the second 12-week retraining session. In the first 12-week exercise training period, 10 of the LF group participants attended at least 60% of the scheduled classes; in the second 12-week exercise period, only 6 participants attended at least 60% of the scheduled classes. This suggests that increased support for more dependent populations may be required in order to achieve optimal adherence to exercise programming. These findings also suggest that for more frail populations, continuous exercise may be a critical factor in maintaining physical abilities.
LIMITATIONS
The selection of participants for this study was based on convenience, and the sample was representative of institutionalized residents in an existing exercise programme intervention. We examined potential confounding variables for the relationship of interest; however, the small number of participants did not permit multivariate analysis.
This study was completed using testing protocols and equipment that are readily available in most clinical physical therapy settings in North America, permitting a greater degree of applicability for practising clinicians. However, the challenge to this clinical approach was that the protocols and equipment used may have lacked the refinement and precision of tools available in a non-clinical setting such as a laboratory.
An additional challenge was that the primary researcher had no influence on the content of the exercise programme or its frequency. Canada's physical activity guidelines for older adults26 recommend more frequent exercise than that provided in the two exercise groups; the exercise content, frequency, and adherence were likely suboptimal. It is therefore not surprising that a decline in physical function was observed in both HF and LF groups.
CONCLUSION
The findings of this study should be considered tentative and should be interpreted in light of the small convenience sample. Nonetheless, the results appear to support a relationship between enhanced physical performance and exercise and between decreased physical performance and exercise cessation.
KEY MESSAGES
What Is Already Known on This Subject
Exercise and physical activity are necessary for physical health and wellness through the lifespan, including in the elderly. Non-continuous intermittent exercise programmes are commonly offered to the institutionalized elderly. Published research on the effectiveness of exercise programming in institutionalized elderly populations is limited.
What This Study Adds
We measured physical function in a sample of elderly institutionalized veterans who participated in a non-continuous, intermittent exercise programme. The results of this study suggest that decline in physical function is related to exercise cessation and that the more frail segment of this population is more at risk than the less frail elderly for decline in physical performance with intermittent programming.
Marshall SC, Berg K. Cessation of exercise in the institutionalized elderly: effects on physical function. Physiother Can. 2010;62:254–260.
References
- 1.Buchner DM. Physical activity to prevent or reverse disability in sedentary older adults. Am J Prev Med. 2003;25:214–5. doi: 10.1016/s0749-3797(03)00188-0. doi: 10.1016/S0749-3797(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 2.Fiatarone Singh MA. Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. J Gerontol A-Biol. 2002;57:262–82. doi: 10.1093/gerona/57.5.m262. [DOI] [PubMed] [Google Scholar]
- 3.Frank JS, Patla AE. Balance and mobility challenges in older adults: implications for preserving community mobility. Am J Prev Med. 2003;25:157–63. doi: 10.1016/s0749-3797(03)00179-x. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–74. doi: 10.1056/NEJMoa020423. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 5.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? a critical review of the scientific evidence. Am J Prev Med. 2003;25:129–36. doi: 10.1016/s0749-3797(03)00176-4. doi: 10.1016/S0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 6.Seguin R, Nelson ME. The benefits of strength training for older adults. Am J Prev Med. 2003;25:141–9. doi: 10.1016/s0749-3797(03)00177-6. doi: 10.1016/S0749-3797(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 7.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sport Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 8.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. J Am Med Assoc. 1995;273:402–7. doi: 10.1001/jama.273.5.402. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 9.Bosco C, Komi PV. Influence of aging on the mechanical behavior of leg muscles. Eur J Appl Physiol. 1980;45:209–19. doi: 10.1007/BF00421329. doi: 10.1007/BF00421329. [DOI] [PubMed] [Google Scholar]
- 10.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–8. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 11.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A-Biol. 1997;52A:B267–76. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 12.Vorhies D, Riley BE. Deconditioning. Clin Geriatr Med. 1993;9:745–63. [PubMed] [Google Scholar]
- 13.McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. J Gerontol A-Biol. 2002;57A:M569–75. doi: 10.1093/gerona/57.9.m569. [DOI] [PubMed] [Google Scholar]
- 14.Stewart AL. Conceptual challenges in linking physical activity and disability research. Am J Prev Med. 2003;25:137–40. doi: 10.1016/s0749-3797(03)00187-9. doi: 10.1016/S0749-3797(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 15.Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–65. doi: 10.1111/j.1532-5415.2007.01035.x. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 16.Bo M, Fontana M, Mantelli M, Molaschi M. Positive effects of aerobic physical activity in institutionalized older subjects complaining of dyspnea. Arch Gerontol Geriatr. 2005;43:139–45. doi: 10.1016/j.archger.2005.10.001. doi: 10.1016/j.archger.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ouslander JG, Griffiths PC, McConnell ES, Riolo L, Kutner M, Schnelle JF. Functional incidental training: a randomized, controlled crossover trial in Veterans Affairs nursing homes. J Am Geriatr Soc. 2005;53:1091–100. doi: 10.1111/j.1532-5415.2005.53359.x. doi: 10.1111/j.1532-5415.2005.53359.x. [DOI] [PubMed] [Google Scholar]
- 18.Kato M, Izumi K, Hiramatsu T, Shogenji M. Development of an exercise program for fall prevention for elderly persons in a long-term care facility. Kango Tenbo. 2006;3:107–17. doi: 10.1111/j.1742-7924.2006.00057.x. [Google Scholar]
- 19.Sheppard L, Senior J, Park CH, Mockenhaupt R. The National Blueprint Consensus Conference summary report: strategic priorities for increasing physical activity among adults aged greater than 50. Am J Prev Med. 2003;25:209–13. doi: 10.1016/s0749-3797(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 20.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians: effects on skeletal muscle. J Am Med Assoc. 1990;263:3029–34. doi: 10.1001/jama.263.22.3029. [PubMed] [Google Scholar]
- 21.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 22.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655–62. doi: 10.1046/j.1532-5415.2002.50159.x. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 23.Gill TM, Allore H, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. J Am Med Assoc. 2004;292:2115–24. doi: 10.1001/jama.292.17.2115. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 24.Morris JN, Fiatarone MA, Kiely DK, Belleville-Taylor P, Murphy K, Littlehale S, et al. Nursing rehabilitation and exercise strategies in the nursing home. J Gerontol A-Biol. 1999;54A:M494–500. doi: 10.1093/gerona/54.10.m494. [DOI] [PubMed] [Google Scholar]
- 25.Hughes SL, Williams B, Molina LC, Bayles C, Bryant LL, Harris JR, et al. Characteristics of physical activity programs for older adults: results of a multisite survey. Gerontologist. 2005;45:667–75. doi: 10.1093/geront/45.5.667. [DOI] [PubMed] [Google Scholar]
- 26.Health Canada. Canada's physical activity guide to healthy active living for older adults. Ottawa: Health Canada; 1999. [Google Scholar]
- 27.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- 28.Finch E, Brooks D, Stratford PW, Mayo NE. Physical rehabilitation outcome measures: a guide to enhanced clinical decision making. Hamilton, ON: BC Decker; 2002. [Google Scholar]
- 29.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–22. doi: 10.1046/j.1532-5415.2003.51104.x. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 30.Platt W, Bell B, Kozak J. Physiotherapy Functional Mobility Profile: a tool for measuring functional outcome in chronic care clients. Physiother Can. 1998;50:47–52. 74. [Google Scholar]
- 31.Brosseau L, Couroux N, Marion M, Theriault J, Laferrière L. Intra- and inter-rater reliability and factorial validity studies of the Physiotherapy Functional Mobility Profile (PFMP) in acute care patients. Physiother Theory Pract. 1999;15:147–54. doi: 10.1080/095939899307694. [Google Scholar]
- 32.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A-Biol. 1999;54A:M546–53. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter GI, Hastie CL, Morris JN, Fries BE, Ankri J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC Geriatr. 2006;6 doi: 10.1186/1471-2318-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Häkkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol. 2000;83:51–62. doi: 10.1007/s004210000248. doi: 10.1007/s004210000248. [DOI] [PubMed] [Google Scholar]
- 35.Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter EJ, Fozard JL, et al. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A-Biol. 2000;55A:B152–7. doi: 10.1093/gerona/55.3.b152. [DOI] [PubMed] [Google Scholar]
- 36.Hauer K, Rost B, Rütschle K, Opitz H, Specht N, Bärtsch P, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J Am Geriatr Soc. 2001;49:10–20. doi: 10.1046/j.1532-5415.2001.49004.x. doi: 10.1046/j.1532-5415.2001.49004.x. [DOI] [PubMed] [Google Scholar]
- 37.Elliott KJ, Sale C, Cable NT. Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Brit J Sport Med. 2002;36:340–5. doi: 10.1136/bjsm.36.5.340. doi: 10.1136/bjsm.36.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith K, Winegard K, Hicks AL, McCartney N. Two years of resistance training in older men and women: the effects of three years of detraining on the retention of dynamic strength. Can J Appl Physiol. 2003;28:462–74. doi: 10.1139/h03-034. doi: 10.1139/h03-034. [DOI] [PubMed] [Google Scholar]
- 39.Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–4. doi: 10.1001/archinte.160.17.2641. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- 40.Kergoat M-J. La dénutrition protéino-énergétique comme élément de fragilité chez la personne âgée. Clinicien. 1998;13(3):84–104. [Google Scholar]