ABSTRACT
Purpose: Immobility and pain are modifiable risk factors for development of venous thromboembolism and pulmonary morbidity after major abdominal surgery (MAS). The purpose of this study was to investigate the effect of abdominal incision support with an elasticized abdominal binder on postoperative walk performance (mobility), perceived distress, pain, and pulmonary function in patients following MAS.
Methods: Seventy-five patients scheduled to undergo MAS via laparotomy were randomized to experimental (binder) or control (no binder) groups. Sixty (33 male, 27 female; mean age 58±14.9 years) completed the study. Preoperative measurements of 6-minute walk test (6MWT) distance, perceived distress, pain, and pulmonary function were repeated 1, 3, and 5 days after surgery.
Results: Surgery was associated with marked postoperative reductions (p<0.001) in walk distance (∼75–78%, day 3) and forced vital capacity (35%, all days) for both groups. Improved 6MWT distance by day 5 was greater (p<0.05) for patients wearing a binder (80%) than for the control group (48%). Pain and symptom-associated distress remained unchanged following surgery with binder usage, increasing significantly (p<0.05) only in the no binder group.
Conclusion: Elasticized abdominal binders provide a non-invasive intervention for enhancing recovery of walk performance, controlling pain and distress, and improving patients' experience following MAS.
Key Words: abdominal binder, abdominal surgery, Adapted Symptom Distress Scale, pain, 6-minute walk test
RÉSUMÉ
Objectif : L'immobilité et la douleur sont des facteurs de risque modifiables dans le développement de thrombo-embolie veineuse et de morbidité pulmonaire à la suite d'une intervention chirurgicale abdominale majeure. L'objectif de cette étude était d'investiguer les effets d'un soutien de l'incision abdominale à l'aide d'une bande abdominale élastique sur la marche (mobilité) postopératoire, sur la douleur perçue, la douleur et la fonction pulmonaire des patients à la suite d'une telle intervention.
Méthode : 75 sujets appelés à subir une chirurgie abdominale majeure par laparotomie ont été choisis de façon aléatoire pour faire partie du groupe expérimental (avec bande abdominale) et d'un groupe de contrôle (sans bande abdominale). De ce nombre, 60 (33 hommes et 27 femmes dont la moyenne d'âge était de 58±14,9 ans) ont participé à l'étude jusqu'à la fin. Les mesures préopératoires comprenaient un test de marche de 6 minutes (TM6), une mesure du degré de douleur perçu, de la douleur et de la fonction pulmonaire; ces mesures ont aussi été prises aux jours 1, 3 et 5 suivant l'intervention.
Résultats : L'intervention chirurgicale a été associée à des diminutions postopératoires marquées (p<0.001) dans la distance de marche (∼75–78 %, au jour 3) la capacité pulmonaire forcée (35 %, tous les jours), et ce, pour les deux groupes. L'amélioration du TM6 au jour 5 a toutefois été plus importante (p<0,05) chez les sujets portant une bande abdominale (80 %) que sur les sujets du groupe de contrôle (48 %). La douleur et les symptômes qui y sont associés sont demeurés inchangés après une intervention chirurgicale chez les sujets ayant porté une bande abdominale, mais ont augmenté de manière significative (p<0,05) chez les sujets sans bande abdominale uniquement.
Conclusion : Les bandes abdominales élastiques constituent une intervention non invasive qui améliore le retour à la marche, le contrôle de la douleur et influe aussi de manière positive sur l'expérience vécue par les patients à la suite d'une intervention chirurgicale abdominale majeure.
Mots clés : bande abdominale, douleur, échelle adaptée des symptômes de la douleur, intervention chirurgicale abdominale, test de marche de 6 minutes
INTRODUCTION
Postoperative pulmonary complications (PPCs) continue to be an important risk of major abdominal surgery (MAS, defined as abdominal or upper abdominal [between rib cage and umbilicus] surgery lasting 3 hours or more), accounting for approximately 25% of postoperative deaths occurring within 6 days of surgery.1,2 The reported incidence of PPCs ranges from 10% to 88%, depending on the definition used and the population studied.1–8 The incidence of PPCs also depends on the procedure and on individual patients' postoperative risk factors.1 Compared to peripheral surgery, thoracic and upper abdominal surgeries result in the highest incidence of PPCs.2,6,8,9 After open upper abdominal surgery (UAS), patients routinely develop a restrictive respiratory deficit characterized by a severe reduction (50–60%) in vital capacity and a lesser reduction (20%) in functional residual capacity,4,10–14 which does not fully recover within the first postoperative week, regardless of anaesthetic technique.14 This suppression of pulmonary function has been variously ascribed to generic intra-operative factors such as prolonged recumbent supine position,15 anaesthetic technique,16 and postoperative pain,17–19 as well as to UAS-specific respiratory muscle dysfunction.18,19
Efforts to prevent and/or manage PPCs have been directed at three main areas: (1) improvements in surgical and anaesthetic techniques, (2) identification of patient-related features associated with increased risk for PPCs, and (3) minimization of attendant modifiable risk factors in the postoperative period. The primary modifiable risk factors are shallow breathing, incision pain, and immobility. Breathing exercises and early mobilization are cornerstones of postoperative management.2,4,10,13,20–23 Early mobility following surgery is deemed crucial, since postoperative immobilization is widely held to contribute to cardiovascular instability,10 thromboembolic complications,24 and catabolism,10 in addition to pulmonary morbidity.25 Sitting out of bed has been shown to be associated with an increase in postoperative functional residual capacity,25 and it has been suggested that a programme of active enforced progressive mobilization can improve pulmonary function following colon resection.10
Although evidence supports the beneficial effects of early mobilization and deep breathing exercises, most patients who undergo abdominal surgery are reluctant to move and to take deep breaths. Possible reasons include pain and fear of damaging the surgical incision. While surgical patients have typically been encouraged to splint the incision with their hands or a pillow,26 any relief afforded by these techniques is at best temporary and unlikely to be sustained. Meisler27 proposed a “sternum harness” for the prevention of pain and sternal instability when coughing and mobilizing in patients following cardiac surgery.
The question of whether early mobilization and pain control of patients following MAS can be facilitated by the use of an elasticized abdominal binder that surrounds the abdomen and supports the incision has important implications for postoperative care. We were unable to locate any studies that systematically investigated the effect of maintained incision support on the postoperative course of patients undergoing MAS.
The purpose of the present study, therefore, was to investigate the effects of incision support using an elasticized abdominal binder on postoperative physical function (as measured by the 6-minute walk test [6MWT] distance) and perceived distress (as measured by the Adapted Symptom Distress Scale [ASDS-2]) in adult patients for 5 days following MAS. A secondary purpose was to describe the postoperative course in terms of the pain experience and pulmonary function.
This prospective randomized controlled trial (RCT) tested the hypotheses that the use of an elasticized abdominal binder would (1) improve postoperative physical function and (2) reduce postoperative symptom-associated perceived distress.
METHODS
Participants
Following approval by the Research Ethics Boards of the participating institutions (Hamilton Health Sciences / McMaster University and the University of Western Ontario), a convenience sample of adult patients scheduled for MAS was recruited by a research assistant from the preoperative clinic of the Digestive Diseases Program at McMaster University Medical Centre of Hamilton Health Sciences (HHS), Hamilton, Ontario, Canada. The primary reasons for referral were gastrointestinal malignancies and inflammatory bowel disease.
To be included in the study, participants had to be 19 years of age or older, undergoing MAS via laparotomy, able to understand the study requirements and provide informed consent prior to participation, and able to understand and follow written and/or oral instructions in English. Patients were excluded if they had any orthopaedic, neuromuscular, or circulatory disorder severe enough to preclude 6MWT evaluation. Following provision of informed consent, eligible participants were then given a three-digit code (starting at 100), which was entered into the Statistical Package for the Social Sciences (SPSS version 11.0, SPSS Inc., Chicago, IL) for computer-generated random allocation to either the experimental (binder) or the control (no binder) group.
Protocol
Basic demographic information was collected and baseline evaluations were performed at the time of the preoperative clinic to assess the primary (physical function and perceived distress) and secondary (pain and pulmonary function) dependent variables. Pain was assessed using the short-form McGill Pain Questionnaire (SF-MPQ), and pulmonary function (PFT) was measured by spirometry. All outcome measures (6MWT, ASDS-2, PFT, SF-MPQ) were repeated on the first (excluding 6MWT), third, and fifth postoperative days (PODs).
Starting on POD 1, patients in the experimental group were fitted with a binder that was applied prior to the first morning transfer and worn at all times when out of bed, including during ambulation. The elasticized binder was applied over the abdominal surgical incision, with the upper border not higher than the lower margin of the rib cage, ensuring minimal restriction of lateral costal expansion and diaphragmatic excursion. In addition, for participants who had stomas, drains, or other lines or tubes inserted during surgery, holes were cut in the binder to ensure that no pressure was applied over these devices. Patient comfort determined the tension of the binder; however, for maximal benefits to be derived from binder support, it was applied firmly (binder circumference 10–20% smaller than the patient's postoperative abdominal circumference measured at the level of the umbilicus). As soon as patients were positioned in a chair, the PFT, SF-MPQ, and ASDS-2 were completed. The same procedure was used, in the same order, on PODs 3 and 5, with the addition of the 6MWT; all tests were administered during the morning to control for any confounding variation induced by diurnal circadian rhythm. To ensure high interrater reliability, data were collected 7 days a week, primarily by the research assistant, with one of the study investigators (OC) as backup only as needed.
Both study groups received standard nursing and physiotherapy care postoperatively. Physiotherapy care included education about PPCs, bed exercises (hip, knee, and ankle movements), early transfer to chair (on day of surgery or POD 1) and ambulation,28 diaphragmatic breathing,5 and manual techniques as clinically indicated (manual vibration and/or percussion). All patients were seen by a physiotherapist once a day for approximately 20 minutes.
Outcome Measures
Physical Function
The 6MWT was chosen for the evaluation of overall physical function because it has been the most extensively studied of the multiple walk tests available and because it is currently recommended for use in both research and clinical settings.29 The 6MWT has excellent test–retest reliability (ICC 0.82–0.99),30 and has been shown to be predictive of success for surgical outcomes.31–33 The 6MWT was performed indoors, on a hard, level surface in a straight corridor, free of distractions. The test was administered according to a standardized protocol as recommended by the American Thoracic Society,34 including standard phrases of encouragement at 1-minute intervals during the conduct of the test to control for the influence of encouragement on test performance. Participants were required to “walk as far as possible” in 6 minutes but were allowed to stop and rest as required.
To ensure patient safety, blood pressure was measured immediately prior to and following walk testing, and heart rate and blood oxygen saturation (SpO2) were monitored continuously by finger pulse oximetry. Resting blood pressure >150/100 mmHg and/or heart rate >100 beats per minute precluded walk testing.34 Tests conducted in the postoperative period were interrupted if SpO2 dropped below 90% or if heart rate exceeded 125 beats per minute, as per routine clinical practice on the Digestive Diseases Unit at HHS. The 6MWT was administered twice in the preoperative clinic to establish a baseline;34 the second test was used for data analysis. On PODs 3 and 5, the test was administered once.
Perceived Distress
Patients' perceived distress was evaluated using the ASDS-2,35 a 31-item self-report instrument that measures perception of the occurrence of and distress associated with 14 symptoms: nausea; vomiting; pain; anorexia; trouble sleeping; fatigue; difficulty breathing; coughing; lacrimation; restlessness; and changes in ability to concentrate, body temperature, bowel elimination, and physical appearance. Each item is rated on a 5-point Likert-type scale (0=no occurrence or distress, 4=greatest occurrence or distress); a higher total score indicates greater perceived distress. Use of the ASDS-2 yields a total score for symptom experience (TSES) and subscores for symptom occurrence (SOS) and distress (SDS). The TSES is calculated by totalling the patient's responses to each of the 31 items (range: 0–124); the SOS is the sum of the patient's responses to 17 items (range: 0–68), while the SDS is the sum of the other 14 items (range: 0–56). Rhodes et al.35 reported the ASDS-2 to be a valid and reliable tool in patients who underwent upper abdominal surgery, with test–retest reliability of 0.92 (internal consistencies: α=0.91 for the total experience score, α=0.76 for total distress, α=0.90 for total occurrence).
Pain
Pain was assessed using the SF-MPQ,36 which consists of 15 adjectives that describe both sensory and affective properties of pain. These descriptors are rated on a 4-point intensity scale (0=none, 3=severe); a higher total score indicates more pain (range: 0–45). The SF-MPQ also includes a “present pain intensity” (PPI) index and a visual analogue scale (VAS) to evaluate the acuity of the pain. Melzack36 compared the SF-MPQ to the long-form MPQ and found that the two were highly correlated in post-surgical patients (r=0.71–0.87) and sensitive to changes in pain level after the administration of pain medications.
Pulmonary Function
Pulmonary function was assessed by portable spirometry in patients seated upright and wearing a nose clip while breathing through a pre-calibrated sterile disposable mouthpiece, using a computer-software-supported (Office Medic v4.5.1, QRS Diagnostic, LLC, Playmouth MN, USA) bi-directional flow sensor (PC Card Spirometer). After 2–3 tidal breaths, patients were required to inhale deeply to total lung capacity and then immediately exhale (without any pause) at a maximal rate until as much air as possible had been expelled from the lungs. Participants were encouraged to continue exhaling until the end test criteria of a minimum duration of 6 seconds and a volume change of less than 40 ml over 2 seconds were satisfied.37 Repeat maximal forced expiratory manoeuvres (usually 3–5) were performed according to American Thoracic Society standards37 until a reproducible response—two tests with the forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) within 5% and peak expiratory flow (PEFR) within 15%—was achieved.37 Peak values for the FEV1, FVC, and FEV1/FVC were recorded and used for statistical analysis.
Data Analysis
Demographic information (age, body mass, and height) on study entry was analyzed using one-way analysis of variance (ANOVA) to compare baseline characteristics of study completers versus non-completers and of experimental versus control-group study completers. The chi-square statistic was used to compare categorical variables.38
Changes in all parametric measures (6MWT, ASDS-2, SF-MPQ, PFTs) of dependent variables across time (preoperative, PODs 1, 3, 5) were evaluated using two-way (group by time) repeated-measures ANOVA (one-factor repetition).38 A further analysis examining the change in 6MWT distance during the recovery period (POD 3 and 5) for the two groups was likewise performed using a two-way repeated-measures ANOVA (one-factor repetition). The Holm-Sidak method, which controls for multiple comparisons, was used for post hoc pair-wise comparisons.39 The Friedman test and Kruskal-Wallis one-way ANOVA by ranks test for non-parametric data were used to evaluate the effect of time and of the abdominal binder, respectively, on the PPI section of the SF-MPQ.38 Underlying assumptions were satisfied for the performed analyses. Descriptive tests were conducted using SPSS; the repeated-measures analyses were performed using SigmaStat (version 3.5, Systat Software Inc, Richmond, CA). The overall significance level was set at 0.05 for all analyses.38
RESULTS
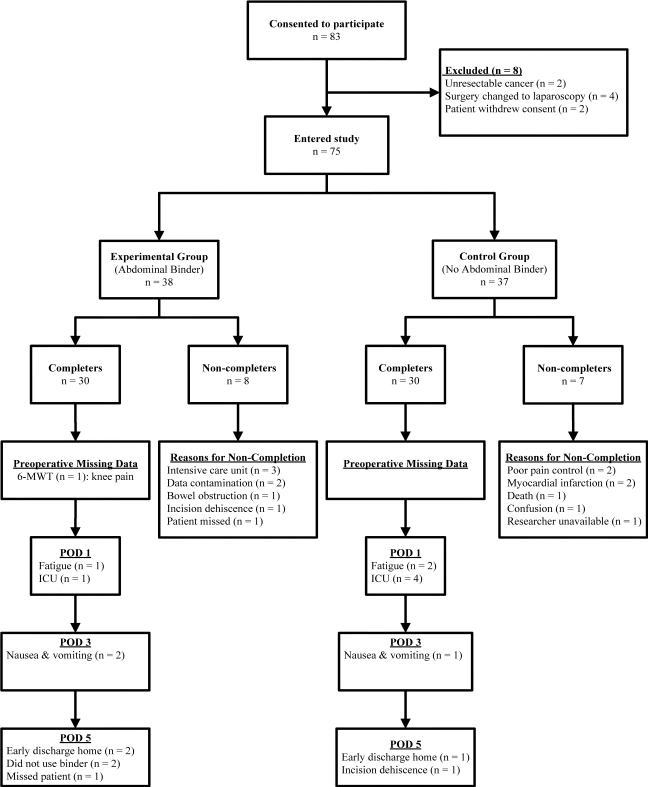
A total of 83 patients (38 women, 45 men) consented to participate, of whom eight were subsequently excluded because they did not meet the inclusion criteria. Participant recruitment, participation, and reasons for ineligibility are presented in Figure 1. Of the 75 remaining volunteers, 60 (33 men, 27 women) completed the research protocol in its entirety (completers), 30 in each of the experimental and control groups; 15 were unable to complete the protocol (non-completers) for various reasons (see Figure 1), none of which was a consequence of study participation. No differences were found in participant characteristics or baseline measurements between those who did and those who did not complete the study. Similarly, preoperative comparisons indicated no differences between the experimental and control groups on study entry for participant characteristics (see Table 1). Diagnoses and surgery-related information for study completers are described in Table 2. Duration of anaesthetic, but not duration of surgery, was significantly longer (p<0.05) for the no binder group.
Figure 1.
Flow diagram of study participants. For study completers, reasons (n) are provided for incomplete data on each measurement day. POD=postoperative day; 6MWT=6-minute walk test.
Table 1.
Participant Characteristics and Surgical Diagnoses for Binder (Experimental) and No Binder (Control) Groups
| Characteristics |
No Binder(n=30) |
Binder (n=30) |
p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (y) | 59 | (18.4) | 57 | (16.8) | 0.70 | |
| Female | 14 | (23) | 13 | (22) | ||
| Male | 16 | (27) | 17 | (28) | ||
| Mass (kg) | 78 | (15.4) | 77 | (14.2) | 0.45 | |
| Height (cm) | 169 | (8.6) | 171 | (8.4) | 0.40 | |
| BMI (kg•m−2) | 28 | (5.0) | 26 | (4.1) | 0.15 | |
| Surgical Diagnosis | Frequency | % of total | Frequency | % of total | ||
| Pancreatic cancer | 9 | (15.0) | 8 | (13.3) | ||
| Colon cancer | 7 | (11.7) | 9 | (15.0) | ||
| Liver cancer | 4 | (6.7) | 3 | (5.0) | ||
| Crohn's disease | 3 | (5.0) | ||||
| Ulcerative colitis | 1 | (1.7) | 2 | (3.3) | ||
| Other diagnoses | 9 | (15.0) | 5 | (8.3) | ||
BMI=body mass index
Table 2.
Surgery-Related Information for Binder (Experimental) and No Binder (Control) Groups
| Characteristics | No Binder(n=30) | No Binder(n=30) | ||
|---|---|---|---|---|
| Type of Surgery | Frequency | % | Frequency | % |
| Whipples procedure | 14 | (23.3) | 9 | (15) |
| Colectomya | 5 | (8.3) | 8 | (13.3) |
| APR | 5 | (8.3) | 5 | (8.3) |
| Splenectomy | 2 | (3.3) | 1 | (1.7) |
| Pancreatectomy | 1 | (1.7) | ||
| Other patient-specific cancer surgery | 3 | (5.0) | 7 | (11.7) |
| Duration of surgery (min) mean (SD) | 223 | (107.5) | 171 | (91.5) |
| Duration of anaesthetic (min) mean (SD) | 300 | (121.2) | 239 | (108.5)* |
| Type of analgesia, PODs 1–3b | ||||
| Epidural | 20 | (33.3) | 17 | (28.3) |
| PCA | 8 | (13.3) | 10 | (16.7) |
| Epidural to PCA | 2 | (3.3) | 3 | (5.0) |
| ASA score | ||||
| 1 (healthy) | 1 | (1.7) | ||
| 2 (mild to moderate systemic disorders) | 7 | (11.7) | 6 | (10.0) |
| 3 (severe systemic disorders) | 18 | (30.0) | 18 | (30.0) |
| 4 (severe systemic disorders threatening life) | 5 | (8.3) | 5 | (8.3) |
| LOS (days) | 14 | (18.4) | 10 | (7.6) |
Total, hemi-, or subtotal colectomy
Analgesia changed to oral medications on POD 3
p<0.05 binder versus no binder
APR=abdominal-peroneal resection; ASA=American Society of Anesthesiologists; PCA=patient-controlled analgesia; LOS=length of (hospital) stay
Physical Function
6MWT performance of completers is summarized in Table 3. Baseline 6MWT distance prior to surgery was similar for the two groups. There was a significant decrease in 6MWT distance following surgery for combined results of both groups (F(2,104)=605.3, p<0.001). Post hoc within-group analyses demonstrated that postoperative walk distance was significantly decreased from preoperative performance on PODs 3 and 5 for both groups (p<0.001, Table 3). Analysis of the postoperative period alone demonstrated improvement in walk performance from POD 3 to POD 5 (F(1,47)=97.56, p<0.001). Although walk distances were similar for the two groups on both PODs, there was a significant interaction between binder usage and time (F(1,47)=4.19, p<0.05): the improvement in walk performance on POD 5 was greater for those using the abdominal binder.
Table 3.
6-Minute Walk Test (6MWT) Distance (m) for Binder (Experimental) and No Binder (Control) Groups
|
Preoperative |
POD 3 |
POD 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | n | Distance | 95% CI | n | Distance | 95% CI | n | Distance | 95% CI |
| mean (SD) | mean (SD) | mean (SD) | |||||||
| No binder | 30 | 443 (68.4)§ | 417–468 | 29 | 99 (68.7) | 73–125 | 27 | 147 (62.2) | 122–172 |
| Binder | 29 | 435 (105.6)§ | 395–475 | 27 | 110 (59.4) | 87–134 | 25 | 198 (88.8)* | 161–234 |
p<0.001 preoperative versus POD 3 and 5
p<0.05 change in walk distance POD 5 compared to POD 3 for binder versus no binder
POD=postoperative day
Perceived Distress
The total symptom experience (TSES), distress (SDS), and occurrence (SOS) scores of participants are presented in Table 4. Prior to surgery, all ASDS-2 scores were similar in both study groups (see Table 4). Following surgery, TSES, SOS, and SDS scores increased significantly compared to preoperative measures for combined results of the two groups (F(3,154)=16.53, p<0.001; F(3,154)=22.65, p<0.001; F(3,154)=7.87, p<0.001, respectively). Post hoc within-group analyses, however, demonstrated a dissimilar pattern of change in ASDS-2 scores. Participants in the no binder group reported a significant increase (p<0.05) in TSES, occurrence of symptoms (SOS), and degree of associated distress (SDS) across all three postoperative measurement days compared to preoperative scores (see Table 4). Conversely, patients who used the abdominal binder had a significant (p<0.05) increase in the TSES only on POD 1 and reported increased occurrence of symptoms (SOS) on PODs 1 and 3 but not on POD 5. Despite increased occurrence of symptoms, postoperative symptom-associated distress (SDS) scores for the binder group remained unchanged from preoperative levels.
Table 4.
Adapted Symptom Distress Scale (ASDS-2) Scores for Binder (Experimental) and No Binder (Control) Groups
|
Preoperative |
POD 1 |
POD 3 |
POD 5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | |
| mean (SD) | mean (SD) | mean (SD) | mean (SD) | |||||||||
| TSES | ||||||||||||
| No binder | 29 | 25 (20.6) | 17–33 | 23 | 41 (19.9)* | 32–50 | 29 | 44 (20.1)* | 37–52 | 26 | 41 (22.4)* | 32–50 |
| Binder | 30 | 27 (20.0) | 19–34 | 28 | 34 (13.8)* | 29–39 | 30 | 37 (15.4) | 31–43 | 24 | 31 (13.8) | 25–37 |
| SOS | ||||||||||||
| No binder | 29 | 15 (10.7) | 11–19 | 23 | 26 (9.8)* | 22–30 | 29 | 26 (10.2)* | 22–30 | 26 | 24 (12.3)* | 19–29 |
| Binder | 30 | 15 (10.4) | 11–19 | 28 | 22 (7.7)* | 19–25 | 30 | 23 (8.6)* | 20–26 | 24 | 19 (7.3) | 16–22 |
| SDS | ||||||||||||
| No Binder | 29 | 10 (10.4) | 6–14 | 23 | 15 (10.8)* | 11–20 | 29 | 18 (10.9)* | 14–23 | 26 | 17 (10.8)* | 12–21 |
| Binder | 30 | 12 (10.2) | 8–15 | 28 | 12 (7.2) | 9–15 | 30 | 14 (7.5) | 11–17 | 24 | 12 (7.6) | 8–15 |
p≤0.05 versus preoperative scores
POD=postoperative day; TSES=total symptom experience score; SOS=symptom occurrence score; SDS=symptom distress score
Pain
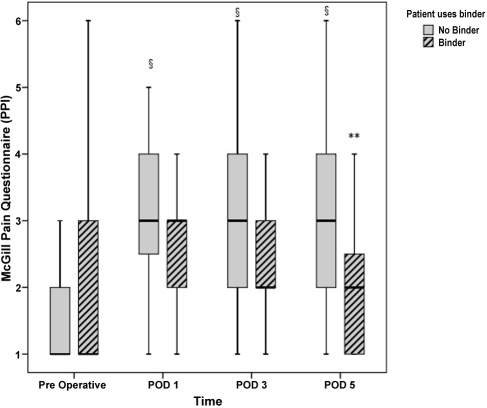
Results of the SF-MPQ total pain, sensory, and affective sub-scales and VAS scores are presented in Table 5, and PPI scores in Figure 2. All preoperative pain scores were similar for both study groups. Following surgery there were significant increases in total pain (F(3,158)=8.44, p<0.001), sensory sub-scale (F(3,158)=9.15, p<0.001), affective sub-scale (F(3,158)=3.54, p<0.05), and VAS (F(3,148)=13.08, p<0.001) scores relative to preoperative measures for combined results of the two groups. Post hoc within-group analyses demonstrated that these postoperative SF-MPQ scores were not significantly different from preoperative scores for patients who wore the abdominal binder; conversely, all scores increased significantly across PODs 1, 3, and 5 compared to preoperative scores for patients in the no binder group (see Table 5). Between-group post hoc analyses demonstrated that sensory sub-scale pain scores on POD1 (p<0.05) and VAS scores on PODs 1 (p<0.01) and 5 (p<0.05) were significantly lower for patients who wore a binder than for those who did not. Similarly, postoperative PPI scores were significantly increased from preoperative levels on PODs 1, 3 and 5 for patients in the no binder group (p<0.001), but not for those in the binder group (see Figure 2). Furthermore, pain intensity was significantly higher for patients in the no binder group than for those in the binder group on POD 5 (p<0.05; see Figure 2).
Table 5.
Short-Form McGill Pain Questionnaire (SF-MPQ) Scores for Binder (Experimental) and No Binder (Control) Groups
|
Preoperative |
POD 1 |
POD 3 |
POD 5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | |
| mean (SD) | mean (SD) | mean (SD) | mean (SD) | |||||||||
| Total Score | ||||||||||||
| No binder | 30 | 5.3 (8.2) | 2.3–8.4 | 25 | 14.5 (11.3)§ | 9.9–19.2 | 29 | 12.7 (10.0)§ | 8.9–16.5 | 27 | 13.3 (10.9)§ | 8.9–17.6 |
| Binder | 30 | 7.8 (11.2) | 3.6–12 | 29 | 10.5 (7.8) | 7.6–13.5 | 30 | 11.1 (9.1) | 7.7–14.5 | 24 | 8.7 (8.1) | 5.3–12.1 |
| Sensory | ||||||||||||
| No binder | 30 | 3.6 (6.0) | 1.3–5.9 | 25 | 11.1 (9.0)§ | 7.4–14.8 | 29 | 9.4 (7.5)§ | 6.6–12.3 | 27 | 10.0 (7.5)§ | 7.1–13.0 |
| Binder | 30 | 5.4 (8.4) | 2.3–8.5 | 29 | 7.3 (6.3)* | 4.9–9.7 | 30 | 8.2 (7.1) | 5.6–10.9 | 24 | 6.6 (6.3) | 3.9–9.2 |
| Affective | ||||||||||||
| No binder | 30 | 1.8 (2.7) | 0.7–2.8 | 25 | 3.5 (2.7)‡ | 2.4–4.6 | 29 | 3.3 (3.1)† | 2.1–4.5 | 27 | 3.2 (3.6)† | 1.8–4.7 |
| Binder | 30 | 2.4 (3.4) | 1.1–3.7 | 29 | 3.2 (2.3) | 2.4–4.1 | 30 | 2.8 (3.0) | 1.7–4.0 | 24 | 2.1 (2.6) | 1.0–3.2 |
| VASa | ||||||||||||
| No binder | 29 | 12 (21.5) | 4–21 | 23 | 48 (29.2)§ | 36–61 | 28 | 36 (25.8)§ | 26–46 | 23 | 34 (25.7)§ | 23–45 |
| Binder | 30 | 23 (30.6) | 12–34 | 29 | 31 (28.5)** | 20–42 | 28 | 26 (28.5) | 16–38 | 23 | 18 (19.1)* | 10–26 |
VAS numbers represent % of 10 cm scale
p≤0.05
p<0.01
p≤0.001 vs. preoperative
p≤0.05
p<0.01 binder vs. no binder
POD=postoperative day; VAS=visual analogue scale
Figure 2.
Box plot distribution for the short-form McGill Pain Questionnaire, present pain intensity (PPI) before and after surgery: bold solid box line is median, top of box is upper quartile (QU 75%), bottom of box is lower quartile (QL 25%), and whiskers correspond to inner fences. Upper and lower inner fences are drawn to the “outermost” observed data points within one step (1.5×the interquartile (QU—QL) range) above QU 75% and below QL 25% respectively. Binder group medians: preoperative=1, POD 1=3, POD 3=2; no binder group medians: preoperative=1. §p<0.001 PODs 1, 3, 5 versus preoperative; **p<0.01 binder versus no binder
Lung Function
Pulmonary function data are presented in Table 6. Nausea and vomiting were the main reasons for patients' not participating in postoperative forced-spirometry testing. Baseline preoperative pulmonary function was similar for both groups. Postoperatively, participants in both groups demonstrated an approximately 35% reduction in mean FVC and FEV1 on POD 1 that was not recovered by POD 5 (F(3,133)=75.33 and F(3,133)=60.31, p<0.001, respectively). The postoperative FEV1/FVC ratio did not change relative to the preoperative ratio in either group. There was, however, a significant group effect (F(1,133)=4.26, p<0.05): post hoc between-group analysis demonstrated a significantly higher FEV1/FVC ratio on POD 3 for patients who wore a binder than for those who did not.
Table 6.
Pulmonary Function Test Results for Binder (Experimental) and No Binder (Control) Groups
|
Preoperative |
POD 1 |
POD 3 |
POD 5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | n | Score | 95% CI | |
| mean (SD) | mean (SD) | mean (SD) | mean (SD) | |||||||||
| FVC (l) | ||||||||||||
| No binder | 29 | 3.6 (1.11)§ | 3.2–4.1 | 19 | 2.3 (1.14) | 1.7–2.8 | 23 | 2.4 (1.46) | 1.8–3 | 25 | 2.5 (1.07) | 2–3 |
| Binder | 29 | 3.6 (1.24)§ | 3.2–4.1 | 24 | 2.3 (1.13) | 1.8–2.7 | 27 | 2.2 (1.06) | 1.8–2.6 | 22 | 2.4 (0.82) | 2.1–2.8 |
| FEV1 (l) | ||||||||||||
| No binder | 29 | 2.8 (0.94)§ | 2.4–3.2 | 19 | 1.7 (0.85) | 1.3–2.1 | 23 | 1.8 (1.13) | 1.3–2.3 | 25 | 1.9 (0.83) | 1.5–2.2 |
| Binder | 29 | 2.9 (0.92)§ | 2.6–3.3 | 24 | 1.8 (0.87) | 1.4–2.1 | 27 | 1.7 (0.69) | 1.5–2 | 22 | 1.9 (0.62) | 1.6–2.2 |
| FEV1/FVC (%) | ||||||||||||
| No binder | 29 | 76 (10.7) | 72–80 | 19 | 73 (14.9) | 66–81 | 23 | 77 (14.8) | 71–83 | 25 | 76 (12.2) | 71–81 |
| Binder | 29 | 82 (10.2) | 78–86 | 24 | 80 (15.0) | 74–87 | 27 | 84 (13.7)* | 79–90 | 22 | 80 (13.9) | 74–86 |
p≤0.001 preoperative vs. PODs 1, 3, 5
p<0.05 binder vs. no binder
POD=postoperative day; FVC=forced vital capacity; l=litres; FEV1=forced expiratory volume in 1 second
DISCUSSION
This randomized controlled trial was unique in systematically investigating the effects of maintained incision support with an elasticized abdominal binder on a 5-day postoperative course of mobilizing patients after MAS. Furthermore, to our knowledge, the 6MWT and ASDS-2 had not previously been administered repeatedly in the acute postoperative period to evaluate change in physical function and emotional well-being.
Physical Function
Following surgery, subjects experienced a marked reduction in 6MWT performance (∼75–78%) on POD 3, irrespective of binder use. In contrast, walk distance recovered to 45% of preoperative levels by POD 5 (an 80% increase from POD 3) for subjects wearing a binder but only to 33% of preoperative levels (a 48% increase from POD 3) for those who did not. This suggests that binder use may be beneficial in facilitating early postoperative mobilization, which is considered crucial in reducing the risk of venous thromboembolism24 and pulmonary morbidity.10,25
Following MAS, many patients are reluctant to move because they fear damaging their incision and because of movement-associated increases in pain. Our research hypothesis of the beneficial effect of an abdominal binder on physical function was predicated on the assumption that maintained support of the entire abdominal wall would improve patients' comfort and potentially assist in allaying their fears. Meisler27 had previously suggested that wearing a “sternum harness” helped to reduce incision pain and improve compliance with deep-breathing exercises in individuals following cardiac surgery. Notably, participants randomized to wearing an abdominal binder prior to transferring and whenever out of bed experienced significantly less pain than those not wearing a binder and reported no increases in symptom distress during the postoperative course, providing evidence to support our assumption. These results, while suggestive that wearing an abdominal binder after surgery may mitigate some of the immediate postoperative decline in walk-test performance, were underpowered to detect between-group differences in walk distance during initial ambulation (on POD 3) following surgery.
On average, walk-test distance improved by 88 m in the experimental group and 48 m in the control group between POD 3 and POD 5. The magnitude of change necessary to be clinically meaningful in this population (responsiveness) remains to be determined. It is noteworthy, however, that the observed changes for the binder group did exceed the minimum clinically important difference of 54 m in 6MWT performance identified by Redelmeier et al. as necessary for patients with chronic lung disease to perceive a change in walking ability.40
Perceived Distress
Patients in both study groups reported a significant increase in the occurrence of symptoms following surgery. The increased symptom frequency was not surprising, since the ASDS-2 assesses 14 symptoms that are commonly experienced following abdominal surgery.35 It has been suggested that symptom occurrence elicits simultaneous responses of fear and coping behaviours, which will either decrease or escalate perceived distress, depending on the adequacy of the coping mechanisms.35 Particularly noteworthy in the present investigation was the divergent postoperative perceived symptom distress reported by the two participant groups. Specifically, despite increased occurrence of symptoms following surgery, postoperative distress levels for patients who wore an abdominal binder remained unchanged from preoperative levels; conversely, symptom distress was increased significantly across the entire postoperative period for patients in the control group. The mechanism by which using an abdominal binder alleviates distress remains to be clarified, but it may include allaying fears or providing an effective coping strategy. Regardless, these results provide evidence that maintained incision support with an abdominal binder was beneficial in improving the postoperative experience.
Pain
In the current investigation, self-reported pain levels further reflected the important difference in the postoperative course between the experimental and control groups identified by the ASDS-2. Notably, patients who wore binders reported unchanged pain levels following surgery, in contrast to those in the control group, for whom pain measures were significantly higher (p≤0.05) across the entire postoperative period.
The hypothesis that maintained abdominal support would improve patient comfort and reduce postoperative distress was based in part on the potential for reducing the acute increases in incision pain that occur during movement out of bed. One possibility is that by providing sufficient circumferential compression to reduce incision stresses during transfers and ambulation, the binder produced the lower pain scores reported by patients in the binder group; another is that the sensory input provided by the binder when in contact with the skin “closed the gate” on pain generated at the surgical site.41 The importance of controlling movement-associated incision pain was also emphasized by Shea et al.,42 who reported that postoperative abdominal incision pain was greater during ambulation (range: 5.5–8.5 on a 0–10 rating scale) than at rest (range: 2.5–4.5). Similarly, Meisler27 found that providing incision support with a “sternum harness” after cardiac surgery resulted in acute reductions in VAS pain scores during ambulation and coughing. The unchanged symptom distress scores reported by the binder group and the significantly higher PPI scores reported by patients in the no-binder group suggest that maintained incision support with a binder may reduce both pain and associated distress in the immediate postoperative period.
Lung Function
As expected based on the literature, a postoperative restrictive respiratory deficit, characterized by proportional reductions in FEV1 and FVC values with relative preservation of the FEV1/FVC ratio,4,10–12,14,22 was observed for all patients in the current investigation. Vital capacity was reduced by approximately 35% on POD 1 for both groups, with no significant improvement noted by POD 5. Both Olsen et al.12 and Basse et al.10 have likewise reported a 35–40% reduction in FVC levels in patients following abdominal surgery, while a 60% reduction in FVC with a prolonged recovery period (minimal recovery by POD 8) has been reported after thoracoabdominal resection.43
The suppression of pulmonary function noted following open abdominal surgery does not fully recover within the first postoperative week, regardless of anaesthetic technique.14 Therefore, inhibition of pulmonary function has been attributed in part to diaphragmatic dysfunction specific to upper abdominal surgery18,19 and to nasogastric (NG) tube stimulation,44,45 as well as to prolonged intra-operative recumbent supine positioning15 and postoperative pain.17–19 Patients in the current investigation, irrespective of binder usage, demonstrated parallel reductions in postoperative pulmonary function. Notably, subjects who wore a binder reported unchanged pain levels following surgery, which suggests that the intra-operative recumbent supine position (>4 hours for patients in both groups), the NG tube, and/or actual organ manipulation during surgery are more likely than pain to be the major contributors to the observed postoperative respiratory suppression.
In this trial, elasticized binders were specifically chosen over rigid support because of concerns about restricting diaphragmatic and lower ribcage excursion, which would further increase the risk of postoperative atelectasis. These concerns were alleviated by the fact that the suppression of pulmonary function was of no greater magnitude for patients in the binder group than for those in the no binder group or than that reported elsewhere.4,10,11,14
Anaesthesia
Duration of anaesthesia has previously been demonstrated, in a multivariate-analysis study, to be an important risk factor for the development of PPCs following general elective surgery;45 specifically, the mean duration of anaesthesia was longer for those with PPCs (480 min) than for those without (309 min). Patients in this study's control group (no binder) underwent anaesthesia for a significantly longer time (p<0.05). Therefore, we questioned the contribution that duration of anaesthesia might make to PPCs in our study. However, mean anaesthetic duration (300 min) for the control group in the current investigation was notably lower than that associated with increased risk of PPCs by Mitchell et al.45 In addition, postoperative reductions in pulmonary function were of equivalent magnitude, and occurrence of postoperative symptoms was similar, for both control and experimental groups, which suggests that the specific contribution of anaesthetic duration to group differences in postoperative outcomes was unlikely to be major.
Clinical Implications
Immobility and pain have been identified as potentially modifiable risk factors for the development of PPCs; therefore, early mobilization and optimization of pain control are primary foci of postoperative health care. In addition, an improved overall symptom experience for patients, important to client-centred care, may contribute to enhancing recovery after MAS. The enhanced recovery of 6MWT performance from POD 3 to POD 5, unchanged symptom-associated distress despite increased symptom occurrence, and the experience of pain reported by patients in the experimental group suggest that binders can assist in encouraging early mobilization and optimizing pain control. Furthermore, the use of a binder may provide patients with another coping strategy to better manage the stresses associated with their hospitalization. Patients in our study were fully compliant with binder use, and, in fact, some expressed a desire to wear the binder even when in bed. Importantly, no adverse affects on either the skin or abdominal drainage were noted in association with binder use.
This study also demonstrated that the 6MWT can be used safely following MAS, adding to its usefulness as a research tool and in measuring clinical progress. The ASDS-2 can provide input related to changes in patients' distress levels, offering insight into their postoperative experience. The ASDS-2 can thus be used to identify stresses in different groups of patients or changes over time, which may be helpful in developing a holistic approach to providing medical, physical, and emotional postoperative care.
LIMITATIONS
The strength of this study is its design, a prospective, randomized controlled trial, with consistency of data assured by having data collected primarily by a single research assistant (RA), who was neither a study investigator nor a member of the patient-care team. A limitation, however, was that neither the subjects nor the RA who collected the data could be blinded to binder usage. Blinding to binder usage was not possible, as the RA often assisted with the application of the binder to ensure that lines (catheters, wound drains) were not tangled prior to moving the patients. Consequently, the results may have been influenced by the subjects' expectations of the binder's effectiveness and possibly by the greater attention provided to them by staff (albeit limited to a few minutes) while applying the binder. The potential of a placebo effect should thus be considered in interpreting the unchanged postoperative pain and symptom-related distress reported by the binder group.
The trial was further limited by being underpowered to detect between-group differences in walk distance in the immediate postoperative period; this is explained in part by the fact that study enrolment was halted after 24 months because of funding limitations associated with a longer than anticipated recruitment period. The majority of study participants were diagnosed with cancer shortly before requiring surgery; many prospective participants, perhaps feeling overwhelmed by their new medical diagnosis, chose not to participate. In addition, the study was conducted in a large teaching hospital in which there were many concurrent trials. Further contributing to the lack of between-group power was the lesser magnitude of improvement with binder use relative to the magnitude of the surgery-associated reduction (75–78%) in walk performance.
CONCLUSION
This RCT was unique in (1) systematically investigating the effects of maintained incision support with an elasticized abdominal binder on the postoperative course of patients undergoing MAS, (2) documenting the course of change in 6MWT performance and the ASDS-2 during the immediate 5-day postoperative period, and (3) evaluating the sensitivity of the ASDS-2 to between-group differences in the postoperative experience in response to a symptom-management intervention (binder vs. no binder). Results from this study suggest that the use of an elasticized abdominal binder after major abdominal surgery can enhance the speed of postoperative recovery of walk performance. Furthermore, the study demonstrated that circumferential support of abdominal incisions results in less distress from common postoperative symptoms, with an important reduction in pain, which suggests a less stressful experience after surgery. Importantly, the safety of postoperative binder use was attested by the results of the pulmonary function tests, which demonstrated that the elasticized abdominal binders did not adversely affect lung function. In view of the noted beneficial effects on enhancement of early mobilization, pain control, and emotional distress, and in the absence of adverse side effects, study results suggest that use of an elasticized abdominal binder warrants consideration by clinicians in the postoperative management of individuals undergoing major abdominal surgery.
KEY MESSAGES
What Is Already Known on This Subject
Postoperative pulmonary morbidity and thromboembolic complications continue to be important risks of major abdominal surgery performed via laparotomy. The beneficial effects of deep-breathing exercises and early mobilization are well established, yet most patients are reluctant to engage in either, likely because of movement-associated pain and fear of damaging their incisions. Although patients are typically encouraged to splint the incision with their hands, the effect of maintained incision support with an abdominal binder on the postoperative course of such patients had not previously been investigated systematically.
What This Study Adds
This study provides important new evidence that maintained circumferential abdominal incision support is beneficial in enhancing early mobilization and in improving patients' postoperative experience by optimizing pain control and alleviating symptom-associated emotional distress. Importantly, the safety of binder use and the postoperative administration of the 6MWT to evaluate change and progress in walk performance were demonstrated. The study further contributes first-time information in support of the utility of the ASDS-2 as a more holistic measure for offering insight into, and distinguishing between-group differences in, patients' postoperative health care experience in response to a symptom-management intervention.
Cheifetz O, Lucy SD, Overend TJ, Crowe J. The effect of abdominal support on functional outcomes in patients following major abdominal surgery: a randomized controlled trial. Physiother Can. 2010;62:242–253.
References
- 1.Arozullah AM, Conde MV, Lawrence VA. Preoperative evaluation for postoperative pulmonary complications. Med Clin North Am. 2003;87(1):153–73. doi: 10.1016/s0025-7125(02)00151-7. doi: 10.1016/S0025-7125(02)00151-7. [DOI] [PubMed] [Google Scholar]
- 2.Brooks-Brunn JA. Postoperative atelectasis and pneumonia. Heart Lung. 1995;24:94–115. doi: 10.1016/S0147-9563(05)80004-4. [PubMed] [Google Scholar]
- 3.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564–71. doi: 10.1378/chest.111.3.564. doi: 10.1378/chest.111.3.564. [DOI] [PubMed] [Google Scholar]
- 4.Christensen EF, Schultz P, Jensen OV, Egebo K, Engberg M, Grøn I, et al. Postoperative pulmonary complications and lung function in high-risk patients: a comparison of three physiotherapy regimens after upper abdominal surgery in general anesthesia. Acta Anaesth Scand. 1991;35:97–104. doi: 10.1111/j.1399-6576.1991.tb03255.x. doi: 10.1111/j.1399-6576.1991.tb03255.x. [DOI] [PubMed] [Google Scholar]
- 5.Denehy L, Carroll S, Ntoumenopoulos G, Jenkins S. A randomized controlled trial comparing periodic mask CPAP with physiotherapy after abdominal surgery. Physiother Res Int. 2001;6:236–50. doi: 10.1002/pri.231. [DOI] [PubMed] [Google Scholar]
- 6.Kips JC. Preoperative pulmonary evaluation. Acta Clin Belg. 1997;52:301–5. doi: 10.1080/17843286.1997.11718592. doi: 10.1002/pri.231. [DOI] [PubMed] [Google Scholar]
- 7.Mackay MR, Ellis E. Physiotherapy outcomes and staffing resources in open abdominal surgery patients. Physiother Theory Pract. 2002;18:75–93. doi: 10.1080/09593980290058463. [Google Scholar]
- 8.Smetana GW. Preoperative pulmonary evaluation. N Engl J Med. 1999;230:937–44. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445–51. doi: 10.1378/chest.104.5.1445. doi: 10.1378/chest.104.5.1445. [DOI] [PubMed] [Google Scholar]
- 10.Basse L, Raskov HH, Jakobsen DH, Sonne E, Billesbølle P, Hendel HW, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Brit J Surg. 2002;89:446–53. doi: 10.1046/j.0007-1323.2001.02044.x. doi: 10.1046/j.0007-1323.2001.02044.x. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen NT, Lee SL, Goldman C, Fleming N, Arango A, McFall R, et al. Comparison of pulmonary function and postoperative pain after laparoscopic versus open gastric bypass: a randomized trial. J Am Coll Surg. 2001;192:469–77. doi: 10.1016/s1072-7515(01)00822-5. doi: 10.1016/S1072-7515(01)00822-5. [DOI] [PubMed] [Google Scholar]
- 12.Fagevik Olsén M, Josefson K, Lönroth H. Chest physiotherapy does not improve the outcome in laparoscopic fundoplication and vertical-banded gastroplasty. Surg Endosc. 1999;13:260–3. doi: 10.1007/s004649900958. doi: 10.1007/s004649900958. [DOI] [PubMed] [Google Scholar]
- 13.Fagevik Olsén M, Nordgren IH, Lönroth H, Lundholm K. Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. Brit J Surg. 1997;84:1535–8. doi: 10.1111/j.1365-2168.1997.02828.x. doi: 10.1007/s004649900958. [DOI] [PubMed] [Google Scholar]
- 14.Schwenk W, Böhm B, Witt C, Junghans T, Gründel K, Muller JM. Pulmonary function following laparoscopic or conventional colorectal resection. AMA Arch Surg. 1999;134:6–12. doi: 10.1001/archsurg.134.1.6. doi: 10.1111/j.1365-2168.1997.02828.x. [DOI] [PubMed] [Google Scholar]
- 15.Skootsky SA, Smith MI. Preoperative evaluation, medical management, and critical care. In: Berek JS, Hacker NF, editors. Practical gynecologic oncology. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 16.Watson CB. Respiratory complications associated with anesthesia. Anesthesiol Clin N A. 2002;20:513–7. doi: 10.1016/s0889-8537(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 17.Ford GT, Rosenal TW, Clergue F, Whitelaw WA. Respiratory physiology in upper abdominal surgery. Clin Chest Med. 1993;14:237–52. doi: 10.1136/thx.54.5.458. [PubMed] [Google Scholar]
- 18.Siafakas NM, Mitrouska I, Bourus D, Georgopoulos D. Surgery and the respiratory muscles. Thorax. 1999;54:458–65. doi: 10.1136/thx.54.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassilakopoulos T, Mastora Z, Katsaounou P, Doukas G, Klimopoulos S, Roussos C, et al. Contribution of pain to inspiratory muscle dysfunction after upper abdominal surgery. Am J Resp Crit Care. 2000;161:1372–5. doi: 10.1164/ajrccm.161.4.9907082. [DOI] [PubMed] [Google Scholar]
- 20.Brooks D, Crowe J, Kelsey CJ, Lacy JB, Parsons J, Solway S. A clinical practice guideline on peri-operative cardiorespiratory physical therapy. Physiother Can. 2001;53:9–25. [Google Scholar]
- 21.Ntoumenopoulos G, Greenwood K. Effects of cardiothoracic physiotherapy on intrapulmonary shunt in abdominal surgical patients. Aust J Physiother. 1996;42:297–303. doi: 10.1016/s0004-9514(14)60394-9. [DOI] [PubMed] [Google Scholar]
- 22.Fagevik Olsén M, Lönroth H, Bake B. Effects of breathing exercises on breathing patterns in obese and non-obese subjects. Clin Physiol. 1999;19:251–7. doi: 10.1046/j.1365-2281.1999.00167.x. doi: 10.1046/j.1365-2281.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 23.Westerdahl E, Lidmark B, Eriksson T, Friberg O, Hedenstierna G, Tenling A. Deep-breathing exercises reduce atelectasis and improve pulmonary function after coronary artery bypass surgery. Chest. 2005;128:3482–8. doi: 10.1378/chest.128.5.3482. doi: 10.1378/chest.128.5.3482. [DOI] [PubMed] [Google Scholar]
- 24.Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, et al. Prevention of venous thromboembolism. Chest. 2001;119:132S–75S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen KG, Holte K, Kehlet H. Effects of posture on postoperative pulmonary function. Acta Anaesth Scand. 2003;47:1270–5. doi: 10.1046/j.1399-6576.2003.00240.x. doi: 10.1046/j.1399-6576.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Pryor JA, Prasad SA. Physiotherapy for respiratory and cardiac problems: adults and pediatrics. 3rd ed. Edinburgh: Churchill Livingstone; 2002. [Google Scholar]
- 27.Meisler P. The sternum support harness for the treatment of sternotomy pain and the prevention of sternal instability. Cardiopulm Phys Ther J. 2000;11:63–8. [Google Scholar]
- 28.Mackay MR, Ellis E, Johnston C. Randomised clinical trial of physiotherapy after open abdominal surgery in high-risk patients. Aust J Physiother. 2005;51:151–9. doi: 10.1016/s0004-9514(05)70021-0. [DOI] [PubMed] [Google Scholar]
- 29.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systemic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–70. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 30.Finch E, Brooks D, Stratford PW, Mayo NE. Physical rehabilitation outcome measures: a guide to clinical decision making. 2nd ed. Hamilton, ON: BC Decker; 2002. [Google Scholar]
- 31.Holden DA, Rice TW, Stelmach K, Meeker DP. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest. 1992;102:1774–9. doi: 10.1378/chest.102.6.1774. doi: 10.1378/chest.102.6.1774. [DOI] [PubMed] [Google Scholar]
- 32.Szekely LA, Oelberg DA, Wright C, Johnson DC, Wain J, Trotman-Dickenson B, et al. Preoperative predictors of operative morbidity and mortality in COPD patients undergoing bilateral lung volume reduction surgery. Chest. 1997;111:550–8. doi: 10.1378/chest.111.3.550. doi: 10.1378/chest.111.3.550. [DOI] [PubMed] [Google Scholar]
- 33.Kadikar A, Maurer J, Kesten S. The six-minute walk test: a guide to assessment for lung transplantation. J Heart Lung Transpl. 1997;16:313–9. [PubMed] [Google Scholar]
- 34.American Thoracic Society, ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Resp Crit Care. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes VA, McDaniel RW, Homan SS, Johnson M, Madsen R. An instrument to measure symptom experience: symptom occurrence and symptom distress. Cancer Nurs. 2000;23:49–54. doi: 10.1097/00002820-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 37.American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis. 1987;136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 38.Portney LG, Watkins MP. Foundations of clinical research, applications for practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 39.Ludbrook J. On making multiple comparisons in clinical and experimental pharmacology and physiology. Clin Exp Pharmacol Physiol. 1991;18:379–92. doi: 10.1111/j.1440-1681.1991.tb01468.x. doi: 10.1111/j.1440-1681.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 40.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Resp Crit Care. 1997;155:1278–82. doi: 10.1164/ajrccm.155.4.9105067. doi: 10.1097/00008483-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 42.Shea RA, Brooks JA, Dayhoff NE, Keck J. Pain intensity and postoperative pulmonary complications among the elderly after abdominal surgery. Heart Lung. 2002;31:440–9. doi: 10.1067/mhl.2002.129449. [DOI] [PubMed] [Google Scholar]
- 43.Fagevik Olsén M, Wennberg E, Johnsson E, Josefson K, Lönroth H, Lundell L. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. Brit J Surg. 2002;89:1228–34. doi: 10.1046/j.1365-2168.2002.02207.x. doi: 10.1046/j.1365-2168.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- 44.Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: a systematic review of blinded studies. Am J Med. 2002;112:219–25. doi: 10.1016/s0002-9343(01)01082-8. doi: 10.1016/S0002-9343(01)01082-8. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell CK, Smoger SH, Pfeifer MP, Vogel RL, Pandit MK, Donnelly PJ, et al. Multivariate analysis of factors associated with postoperative pulmonary complications following general elective surgery. AMA Arch Surg. 1998;133:194–8. doi: 10.1001/archsurg.133.2.194. doi: 10.1001/archsurg.133.2.194. [DOI] [PubMed] [Google Scholar]