Abstract
The polytopic transmembrane protein, Niemann-Pick C1-Like 1 (NPC1L1), is enriched in the apical membrane of small intestine absorptive enterocytes where it mediates extracellular sterol transport across the brush border membrane. It is essential for intestinal sterol absorption and is the molecular target of ezetimibe, a potent cholesterol absorption inhibitor that lowers blood cholesterol in humans. NPC1L1 is also highly expressed in human liver. The hepatic function of NPC1L1 may be to limit excessive biliary cholesterol loss. NPC1L1-dependent sterol uptake seems to be a clathrin-mediated endocytic process and is regulated by cellular cholesterol content. Recently, NPC1L1 inhibition has been shown to have beneficial effects on components of the metabolic syndrome, such as obesity, insulin resistance, fatty liver, in addition to atherosclerosis.
Keywords: Ezetimibe, Intestinal cholesterol absorption, Hypercholesterolemia, ABCG5/ABCG8
Introduction
Elevated blood cholesterol levels contribute to atherosclerotic coronary heart disease. Cholesterol homeostasis in the body is mainly balanced by its intestinal absorption, endogenous biosynthesis, and biliary/intestinal excretion. While much is known about cholesterol biosynthesis and its regulation [1,2], the mechanisms for cholesterol absorption and excretion were poorly understood until recently. Early in the 21st century, the heterodimer of two ATP-binding cassette (ABC) transporters G5 and G8 (ABCG5/G8) was demonstrated to be crucial for biliary and perhaps intestinal secretion of cholesterol and noncholesterol sterols [3–6]. In 2004, Niemann-Pick C1-like 1 (NPC1L1) was shown to play an essential role in intestinal cholesterol absorption [7]. Discovery of NPC1L1 has greatly enhanced our understanding of whole-body cholesterol metabolism, and specifically intestinal cholesterol absorption, a process that can be blocked by the potent cholesterol absorption inhibitor ezetimibe. A growing body of data is implicating a role for NPC1L1 and NPC1L1-dependent intestinal cholesterol absorption in metabolic diseases such as nonalcoholic fatty liver disease, insulin resistance, diabetes, and obesity in addition to atherosclerotic coronary heart disease. This review will examine the recent studies on the role of NPC1L1 in sterol transport and diseases.
NPC1L1 as an intestinal cholesterol transporter
Cholesterol in the intestinal lumen is mainly derived from biliary secretion and dietary intake. Intestinal epithelial sloughing and direct secretion of cholesterol from enterocytes may also partially contribute to the luminal cholesterol pool where cholesterol is solubilized in micelles enriched with bile acids and phospholipids. Intestinal cholesterol absorption is an integrated process, including at least 3 major steps: 1) solubilization in micelles, 2) transport across the apical membrane of absorptive enterocytes, and 3) mobilization to chylomicrons for secretion into the lymph and blood via the basolateral membrane of enterocytes. In humans, the fractional intestinal cholesterol absorption ranges from 29–80% [8]. Despite this large variation, intestinal cholesterol absorption is a major pathway controlling whole-body cholesterol homeostasis, and therefore represents an attractive drug target.
The search for intestinal cholesterol absorption inhibitors led to the development of ezetimibe (commercially known as Zetia) by Schering-Plough Research Institute and Merck Co. (New Jersey, USA). The drug was approved by the U.S. Food and Drug Administration to treat hypercholesterolemic patients before its molecular target was known. Because ezetimibe inhibition of intestinal cholesterol absorption in animals results in a compensatory upregulation of endogenous cholesterol biosynthesis [9,10], the drug is often used in combination with a statin that blocks cholesterol synthesis by inhibiting the rate limiting enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase [11]. Ezetimibe monotherapy or coadministration with a statin efficiently lowers plasma total cholesterol and low-density lipoprotein cholesterol (LDL-C) in humans [10]. The mechanism for cholesterol transport across the apical membrane of enterocytes was thought to be passive diffusion before the discovery of ezetimibe [12]. The pharmacological potency of ezetimibe demonstrated that specific proteins rather than passive diffusion are implicated in this process. In the search for molecular targets of ezetimibe, Altmann and colleagues at Schering-Plough Research Institute (New Jersey, USA) identified NPC1L1, an apically-localized sterol transporter in the small intestine, using a genome-wide bioinformatics screening approach [7]. They further showed that genetic deletion of NPC1L1 in mice reduces intestinal cholesterol absorption to the levels seen in ezetimibe-treated mice, and that ezetimibe treatment results in no further reduction of intestinal cholesterol absorption in mice lacking NPC1L1 [7,9]. This study clearly demonstrated that NPC1L1 is in the ezetimibe-sensitive pathway and definitively established the critical role of NPC1L1 in intestinal cholesterol absorption.
Conserved domains in NPC1L1 protein
NPC1L1 is a polytopic transmembrane protein of 1332 amino acids. It shares sequence homology with Niemann-Pick C1 (NPC1) [13], a protein that is mutated in the lipid storage disorder Niemann-Pick disease type C1 [14,15] (Figure 1). Like its homolog, NPC1L1 was predicted to have a typical signal peptide and 13 membrane-spanning domains [7,13,16–18], and this prediction is consistent with recent experimental data [19]. NPC1L1 also has a conserved N-terminal “NPC1” domain, and extensive N-linked glycosylation sites [7,13,17] (Figure 1). Interestingly, the crystal structure of the cysteine-rich N-terminal domain of NPC1 exposed a sterol-binding pocket [20]. Because the N-terminus of NPC1L1 encompasses a similar cysteine-rich globular domain, it is likely that this region also binds sterols [21].
Figure 1. Amino acid sequence and predicted topological structure of human Niemann–Pick C1-like 1 (NPC1L1).

The conserved N-terminal “NPC1” domain is shown with red residues. Two potential YXXØ endocytic motifs are outlined in blue squares. Residues in dark circles denote the sterol-sensing domain. The luminal portion of NPC1L1 also has extensive N-linked glycosylation sites which are highlighted in green. The N-terminal 21 amino acids are assumed to be the signal peptide and are not shown in this figure.
Five of the 13 membrane-spanning helices of NPC1L1 constitute a predicted sterol sensing domain (SSD) encompassing ~180 amino acids [7,13] (Figure 1). This SSD is conserved in several other membrane proteins involved in cholesterol transport, metabolism, or regulation [22], including NPC1 [13–15]; 3-hydroxy-3-methylglutaryl CoA reductase, the rate-limiting enzyme in the cholesterol biosynthetic pathway [1,23]; sterol regulatory element-binding protein (SREBP)-cleavage activating protein (SCAP), a protein that controls the transport and proteolytic activation of SREBPs, which are membrane-bound transcription factors governing the synthesis of cholesterol and other lipids [1,2,23]; and Patched, a membrane receptor for the cholesterol-linked signaling peptide Hedgehog [24]. The functional significance of the SSD in these proteins remains elusive.
Biochemical evidence suggests that the SSD may be involved in sterol binding. For example, cholesterol was shown to directly bind to the purified SSD-containing membrane region of SCAP through receptor-ligand interaction; thus, SCAP was defined as a receptor for free cholesterol in the endoplasmic reticulum [25]. The requirement of a functional SSD for NPC1 binding to a photo-activatable cholesterol analog was reported years ago [26], although a sterol-binding pocket was recently localized to its cysteine-rich N-terminal domain [20].
The subcellular localization of SSD-containing proteins is often regulated by cellular cholesterol content [22,27]. The SSD may regulate sterol-dependent trafficking of these proteins. In animals, NPC1L1 mainly resides at the apical membrane of enterocytes and hepatocytes [7,28–31], a membrane region that is exposed to unesterified free cholesterol. In cultured hepatoma cells, we showed that NPC1L1 can localize to both the plasma membrane and endocytic recycling compartment (ERC), mirroring the distribution of free cholesterol in the cell, and its subcellular location is regulated by cellular cholesterol availability [29]. Under normal culture conditions, NPC1L1 is enriched in the ERC, which has been shown to be a cholesterol-rich region of cells [32]. When cholesterol is depleted in cultured cells with cyclodextran, NPC1L1 is translocated to the apical subdomain of the plasma membrane from the ERC. Conversely, when cholesterol is replenished, NPC1L1 is internalized, which is coupled with cellular cholesterol uptake [29]. These findings are further supported by subsequent cell biology studies using the same cell line [33]. Future studies are required to examine the role of NPC1L1's SSD in the sterol-regulated trafficking of NPC1L1.
Molecular mechanisms for NPC1L1-dependent cholesterol uptake
Several pieces of evidence suggest that the molecular mechanism for NPC1L1-dependent cholesterol uptake may be cholesterol-regulated clathrin-mediated endocytosis [29,33–35]. First, NPC1L1 protein cycles between the cell surface and intracellular compartments in a cholesterol-dependent manner [29]. Second, this cholesterol-regulated NPC1L1 translocation is coupled to cholesterol uptake [29], which can be blocked by potassium depletion [34], a treatment that inhibits clathrin-mediated endocytosis [36]. Third, caveolin-1, a structural protein of caveolae, is dispensable for intestinal cholesterol absorption [37], demonstrating that caveolae-mediated endocytosis is not the cellular basis for NPC1L1-dependent cholesterol uptake. Fourth, NPC1L1 co-immunoprecipitates with the μ2 (mu2) subunit of the adaptor protein (AP) complex AP2, and with clathrin heavy chain [33], two proteins necessary for clathrin-dependent endocytosis.
A cytosolic tyrosine-based sorting signal YXXØ (tetrapeptide) has been shown to facilitate the clathrin-mediated endocytosis of many plasma membrane proteins via interaction with the μ subunit of AP2 [38]. In YXXØ motif, the tyrosine (Y) residue is functionally indispensable. The Ø represents a residue with a bulky hydrophobic side chain. The X residues vary highly. The endocytic YXXØ signals are most often 10–40 residues away from the membrane spanning domains, but not at the carboxy-termini of proteins [38]. Intriguingly, NPC1L1 has two potential YXXØ motifs (Y721QRL and Y836APF) [21] (Figures 1 and 2). The Y721QRL sequence is within the SSD region and the two motifs are conserved in NPC1L1 proteins from drosophila to mammals, implying their functional significance. It is tempting to speculate that one of these motifs regulates NPC1L1-dependent cholesterol uptake through interaction with proteins of the clathrin-mediated endocytic pathway in a cholesterol-dependent manner (Figure 2). In this case, NPC1L1 appears to functions as a free cholesterol receptor in the plasma membrane, resembling SCAP which is a receptor for free cholesterol in the endoplasmic reticulum [25].
Figure 2. Proposed model of NPC1L1-dependent cholesterol uptake.
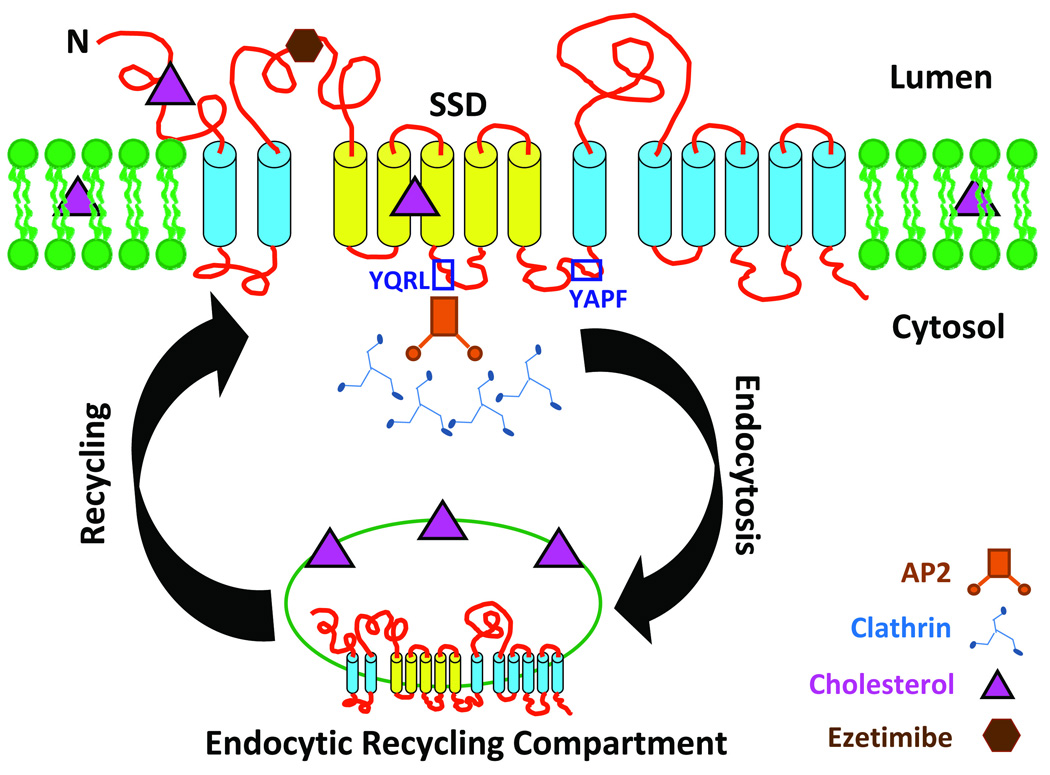
NPC1L1 is enriched at the plasma membrane when cellular cholesterol levels are low. Extracellular cholesterol is recruited to the NPC1L1-containing plasma membrane microdomain by binding to NPC1L1 or other mechanisms. When cholesterol content increases to a threshold in the microdomain, it is sensed by NPC1L1's SSD, which then triggers NPC1L1 protein conformational changes and subsequent internalization of the NPC1L1/cholesterol-containing membrane microdomain via clathrin-mediated endocytosis. Two potential YXXØ motifs known to facilitate the clathrin-mediated endocytosis of many plasma membrane proteins via interaction with the μ subunit of AP2 are highlighted in blue squares. NPC1L1 and its sterol cargo are dissociated in the sorting endosome and/or the endocytic recycling compartment, freeing NPC1L1 to be recycled back to the plasma membrane to take up additional cholesterol, particularly during cholesterol deprivation. Ezetimibe interacts with the second extracellular loop of NPC1L1, causing conformational changes of NPC1L1 protein, thereby inhibiting cholesterol uptake.
How does NPC1L1 efficiently transport cholesterol into a cell? One plausible possibility is that NPC1L1 recruits extracellular free cholesterol through its N-terminus to its cell membrane location, which may create a RAFT-like plasma membrane microdomain. The cholesterol content in this microdomain is sensed by NPC1L1's SSD. When cholesterol is enriched to a threshold in this membrane region, the entire microdomain is endocytosed to facilitate cholesterol uptake.
For intestinal cholesterol absorption, free cholesterol has to be sorted to the endoplasmic reticulum for incorporation into cholesterol ester, which will then be packaged into chylomicrons. How is NPC1L1-derived free cholesterol sorted to the endoplasmic reticulum? Is it sorted out directly from the early endosome or from the endocytic recycling compartment? What will carry it to its destination? Answers to these questions would greatly enhance the molecular understanding of intestinal cholesterol absorption. Based on cell culture studies [29,33–35], NPC1L1 sorted to the endocytic recycling compartment can be recycled back to the cell surface, a process that appears to involve an endocytic recycling triple complex consisting of the microfilament-interacting motor myosin Vb, the small GTPase Rab11a, and the adaptor Rab11-FIP2 [39]. Thus, it is likely that free cholesterol is dissociated from NPC1L1 in the endocytic recycling compartment or earlier endocytic stages.
Substrate specificity of NPC1L1
Evidence indicates that NPC1L1 can absorb both animal and plant sterols because both sterol classes are dramatically reduced in NPC1L1 knockout mice [7,9,40,41] and in ezetimibe-treated sitosterolemic patients [42] and mice [43]. But NPC1L1 does not seem to absorb all sterols equally. Uptake of sitosterol versus cholesterol from various donors is significantly lower in cells over-expressing NPC1L1 [33,34,44], suggesting that NPC1L1 has a greater affinity for cholesterol than sitosterol, a major plant-derived sterol in the diet. It was thought that the heterodimer ABCG5/G8 was the major discriminator of animal-derived sterols from plant-derived phytosterols because mutations in these genes cause a genetic disorder, sitosterolemia that is characterized by massive accumulation of phytosterols in the body [3,4]. However, the rank order of intestinal sterol absorption rates (cholesterol > cholestanol > campesterol > sitosterol) is maintained in mice lacking ABCG5/G8 [5,45]. This finding implies that the sterol efflux transporter ABCG5/G8 does not discriminate sterols, but rather, the sterol influx transporter NPC1L1’s affinity for different sterols may largely contribute to the rank order of the individual sterols’ intestinal absorption rates.
Whether NPC1L1 transports other substrates remains controversial. NPC1L1, like its homolog NPC1, shares similarity with the resistance-nodulation-division (RND) family of prokaryotic permeases that are involved in the efflux of lipophilic drugs, detergents, fatty acids, bile acids, metal ions, and dyes from the cytosol of bacteria [16,46], suggesting a potential role of NPC1L1 in the transport of other substrates. In one study using Caco-2 cells, NPC1L1 over-expression appears to facilitate α-tocopherol (vitamin E) uptake in an ezetimibe-sensitive manner, but not the uptake of retinol (vitamin A) or cyclosporin A [47]. In this same study, rats treated with ezetimibe showed reduced absorption of radiolabeled α-tocopherol. But the serum concentrations of vitamin E do not differ significantly in steady state in ezetimibe-treated patients [48]. The physiological significance of NPC1L1 in vitamin E absorption remains elusive. An earlier study showed that ezetimibe does not inhibit intestinal absorption of triglyceride and vitamins A and D in rodents [49]. Using a very sensitive method to measure intestinal fat absorption, a group recently demonstrated a modest, but significant reduction in intestinal absorption of long chain saturated fatty acids in NPC1L1 knockout mice or ezetimibe-treated mice on a high fat diet [50]. How an intestinal cholesterol transporter influences fatty acid absorption, and whether this phenomenon can be recapitulated in animals fed other diets, is uncertain.
NPC1L1 as the molecular target of ezetimibe
The fact that NPC1L1 is involved in an ezetimibe-sensitive pathway for cholesterol uptake is clearly documented [7,9,18,28,29,33,34,44,51]. However, whether NPC1L1 is the direct molecular target of the drug has been controversial [52–55]. NPC1L1 overexpression in cultured cells promotes cholesterol uptake and this NPC1L1-dependent cholesterol uptake can be blocked by ezetimibe [29,33,34,44]. Garcia-Calvo and colleagues [28] found that isotope-labeled ezetimibe binds the enterocyte brush border membrane vesicles of wild-type mice, but not NPC1L1 knockout mice. In addition, the degree of ezetimibe inhibition correlates with NPC1L1 binding affinity to ezetimibe across many species [18]. In humans, NPC1L1 sequence variations are associated with sterol absorption rates, LDL-C levels [56] and LDL-C response to ezetimibe therapy [57–59]. Importantly, ezetimibe was recently shown to directly bind NPC1L1, and the location of ezetimibe binding to NPC1L1 was mapped to the second extracellular loop of NPC1L1 [60] (Figure 1). Taken together, these data support the notion that NPC1L1 is the molecular target of ezetimibe. Ezetimibe binding to NPC1L1 may result in NPC1L1 protein conformational changes, thereby disturbing NPC1L1 and free cholesterol interactions and ultimately inhibiting cholesterol-induced NPC1L1 endocytosis [29,33].
Hepatic NPC1L1 and canalicular re-uptake of cholesterol
NPC1L1 is highly expressed in the small intestine across mammalian species [7,40]. The regulation of NPC1L1 expression is largely unknown and data on this topic are inconsistent [61–78]. Readers interested in regulation of NPC1L1 expression are referred to references listed above and recent reviews [79,80].
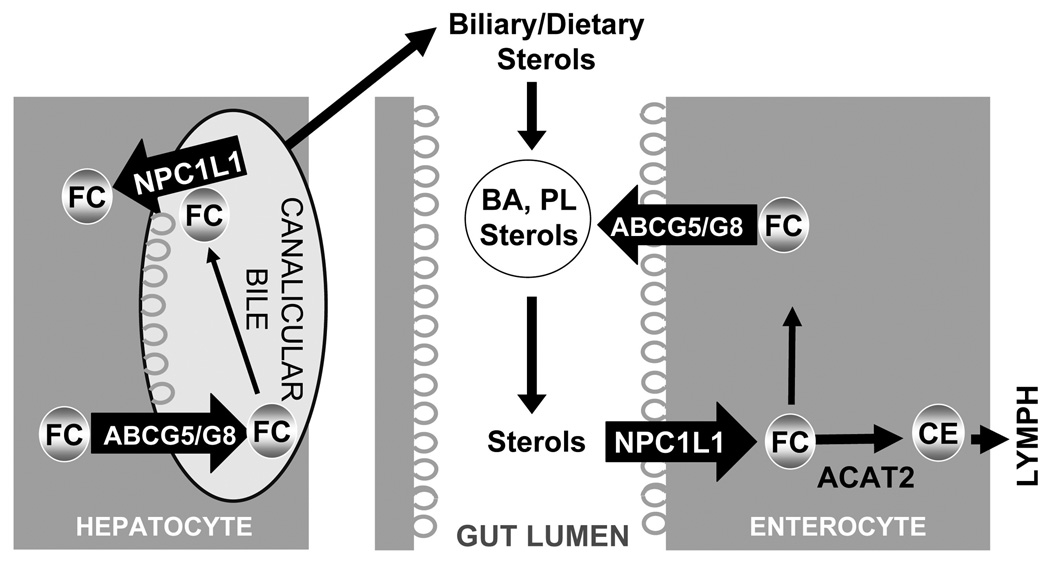
The tissue pattern of NPC1L1 expression differs among species. Although rodent livers express only trivial amounts of NPC1L1 [65], NPC1L1 is readily detectable in human livers [7,30,40]. Why human livers express NPC1L1 remains unclear. In the small intestine, NPC1L1 transports biliary and dietary cholesterol from the intestinal lumen into the enterocyte to facilitate cholesterol absorption. In the liver, NPC1L1 localizes to the bile canalicular membrane [29,30]. We found that human NPC1L1, when transgenically over-expressed in the mouse liver, is localized to the bile canalicular membrane [30]. In these transgenic animals, cholesterol levels in the bile are dramatically reduced with no effects on the hepatic expression of the canalicular cholesterol efflux transporter ABCG5/G8 [30]. This finding supports the idea that hepatic NPC1L1 may counterbalance ABCG5/G8-mediated biliary secretion of sterols by transporting sterols in the bile canaliculus back to hepatocytes (Figure 3). This mechanism may have evolved to protect against excessive biliary loss of cholesterol, a crucial structural component of the cell membrane. The fact that the two opposing sterol transporters, NPC1L1 and ABCG5/G8, evolved in the same subcellular location of both enterocytes and hepatocytes is intriguing, highlighting the importance of sterol balance in life.
Figure 3. Role of NPC1L1 and ABCG5/G8 in enterohepatic cholesterol recirculation.
Biliary and dietary sterols are mixed in the gut lumen and solubulized by bile acids (BA) and phospholipids (PL) to form mixed micelles. NPC1L1 absorbs sterols, including free cholesterol (FC), from these mixed micelles at the apical surface of the enterocyte. Sterols in enterocytes can be pumped out to the gut lumen by the action of the heterodimeric ATP binding cassette transporters G5 and G8 (ABCG5/G8), or can be esterified by acyl-coenzyme A: cholesterol acyltransferase 2 (ACAT2) to yield cholesteryl ester (CE) for assembly into chylomicrons for secretion into lymph. In the hepatocyte, ABCG5/G8 at the canalicular membrane pumps free cholesterol into the bile canaliculus. This action is opposed by NPC1L1, which reabsorbs the FC back into the hepatocyte. Thus, in both the enterocyte and hepatocyte, sterol efflux by ABCG5/G8 is opposed by NPC1L1-dependent sterol influx.
An alternative explanation for why NPC1L1 resides at the canalicular membrane of human instead of murine hepatocytes may be attributable to the difference in bile acid hydrophobicity between the two species. Bile acids in human bile are more hydrophobic compared to those from rodents. Hydrophobic bile acids are cytotoxic and can cause liver injury and cholestasis. Membrane cholesterol content could be an important factor that renders the canalicular membrane resistant to the cytotoxic effects of concentrated bile acids [81]. Thus, canalicular NPC1L1 protein, by retrieving free cholesterol in the canalicular bile back to the canalicular membrane may protect hepatocytes against cytotoxicity induced by hydrophobic bile acids concentrated in human bile.
Hepatic NPC1L1 as a target of ezetimibe: good or bad?
When liver-specific NPC1L1 transgenic mice are treated with ezetimibe, biliary cholesterol excretion is restored [30]. This finding demonstrated that hepatic NPC1L1 is a target of ezetimibe in mice in addition to intestinal NPC1L1. This action of ezetimibe may permit more biliary cholesterol to be excreted into bile, thus promoting the final step in the reverse cholesterol transport pathway. But excess cholesterol build-up in the bile may negatively impact health by leading to gallstone formation. An increase in gallstone disease, however, was not observed in humans treated with ezetimibe. Conversely, ezetimibe has been shown to prevent cholesterol gallstone formation in mice [82], hamsters [83], and a few human subjects [84]. Inter-individual variation exists in hepatic [30] and perhaps intestinal NPC1L1 expression levels. The relative expression level of NPC1L1 in intestine and liver, and differences in the ezetimibe efficiency in inhibiting intestinal cholesterol absorption may ultimately determine whether ezetimibe increases or decreases biliary cholesterol. In individuals with lower expression of NPC1L1 in liver relative to intestine, if ezetimibe efficiently inhibits intestinal cholesterol absorption, it may actually reduce the amount of cholesterol transported to the liver for hepatobiliary secretion.
NPC1L1 and diseases
Cholesterol is an important biological component of cell membranes, and is a precursor for bile acid and steroid hormone biosynthesis. However, high levels of blood cholesterol are associated with atherosclerotic coronary heart disease. By lowering blood cholesterol levels, NPC1L1 inhibition should have beneficial effects on this disease. Indeed, ezetimibe treatment reduces cholesterol absorption, lowers plasma cholesterol, and inhibits the development and progression of atherosclerosis in apolipoprotein (apo) E knockout mice [85]. Likewise, NPC1L1/apoE double knockout mice have a significant reduction in cholesterol absorption and plasma cholesterol levels, and almost complete protection from the development of atherosclerosis [86]. In humans, ezetimibe monotherapy or coadministration with a statin significantly reduces blood LDL-C in primary hypercholesterolemic subjects [10]. A large clinical trial, IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), is underway to determine whether this reduction is translated to prevention of atherosclerosis and cardiovascular events [10]. An earlier clinical trial ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression) failed to show benefit of Vytorin (ezetimibe plus simvastatin) over simvastatin in improving the carotid artery intima-media thickness (IMT), though the combined therapy versus statin alone resulted in more reduction in plasma LDL-C, in 720 heterozygous familial hypercholesterolemic patients who had been treated with a statin prior to enrollment in the trial [87]. But in this trial, patient selection and methodology may have confounded the outcomes. The IMPROVE-IT trial should provide a definitive answer to whether the combined therapy improves cardiovascular events.
Unexpectedly, ezetimibe treatment or NPC1L1 deficiency was recently shown to improve many metabolic disorders besides hypercholesterolemia in rodents, although similar effects have yet to be examined in humans. For instance, ezetimibe treatment improved hepatic steatosis and insulin sensitivity in leptin receptor-deficient Zucker obese rats [88,89]. Ezetimibe also reduced hepatic steatosis in mice on the methionine choline-deficient diet [90] or diets containing high amounts of cholesterol [91,92]. In a study focusing on obesity and diabetes, ezetimibe treatment or NPC1L1 deficiency was shown to attenuate weight gain and insulin resistance in mice fed a high fat diet, though it remains to be determined whether these interventions influence hepatic steatosis [50]. In another study focusing on the role of NPC1L1 in protein trafficking and diet-induced hypercholesterolemia, NPC1L1 knockout mice were shown to be resistant to hepatic steatosis induced by the Paigen diet, a lithogenic diet that contains high amounts of bile acids plus cholesterol and fat [40]. Despite these interesting observations, the mechanism by which NPC1L1 deficiency or ezetimibe treatment alleviates hepatic steatosis, insulin resistance, and obesity remain largely unexplored. Ezetimibe was reported to decrease reactive oxygen species production, c-Jun N-terminal kinase (JNK) activation, and endoplasmic reticulum stress in the livers of Zucker obese fatty rats, as well as hepatocytes in vitro [89], but it is unclear if this was a result of, or the mechanisms for, reduced hepatic accumulation of cholesterol and fat in ezetimibe-treated animals and hepatocytes. Nonetheless, the aforementioned findings together suggest that NPC1L1 plays an important role in the pathogenesis of metabolic disorders, and its physiological role extends beyond simple facilitation of intestinal cholesterol absorption. Inhibition of NPC1L1 or NPC1L1-dependent intestinal cholesterol absorption could be a potential preventative and therapeutic approach for metabolic diseases such as nonalcoholic fatty liver disease, insulin resistance, type 2 diabetes, and central obesity.
Conclusions
NPC1L1 protein possesses multiple conserved domains, including a SSD that is found in many other proteins involved in cholesterol metabolism and regulation. NPC1L1 mediates intestinal cholesterol absorption and it may also limit hepatobiliary cholesterol excretion, particularly in humans. Thus, NPC1L1 may have evolved to protect the body against excessive loss of cholesterol, a structural component of the cell membrane. Evidence supports NPC1L1 being the molecular target of ezetimibe. The potency of ezetimibe in lowering blood cholesterol may be attributable to its inhibition of NPC1L1 function in both the small intestine and liver. Cell culture studies suggest that NPC1L1 may function as a plasma membrane receptor for free cholesterol and the cellular basis for NPC1L1-dependent cholesterol uptake appears to be clathrin-mediated endocytosis. Future in vitro studies should focus on whether NPC1L1 directly binds cholesterol and how this interaction may affect NPC1L1/cholesterol endocytosis. Recently, NPC1L1 has been the focus of research beyond cholesterol absorption and atherosclerosis. NPC1L1 inhibition showed beneficial effects in animals on many components of the metabolic syndrome, such as fatty liver, obesity, and insulin resistance. Future studies are needed to dissect how NPC1L1 modulates the pathogenesis of metabolic diseases.
Acknowledgements
Dr. Jenna L. Betters is supported by a Ruth L. Kirschstein National Research Service Award (NRSA) (#1F32DK084582-01) provided by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Liqing Yu is supported by a Scientist Development Grant (#0635261N) from the American Heart Association, and by intramural funds from Wake Forest University Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 2.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis; Cold Spring Harb Symp Quant Biol; 2002. pp. 491–498. [DOI] [PubMed] [Google Scholar]
- 3.Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 4.Lee MH, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmann SW, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 8.Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40:302–308. [PubMed] [Google Scholar]
- 9.Davis HR, Jr, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 10.Davis HR, Veltri EP. Zetia: Inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to Reduce Intestinal Cholesterol Absorption and Treat Hyperlipidemia. J Atheroscler Thromb. 2007;14:99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 11.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- 12.Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr Opin Lipidol. 2003;14:233–240. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 14.Carstea ED, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 15.Loftus SK, et al. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 16.Davies JP, Ioannou YA. The role of the Niemann-Pick C1-like 1 protein in the subcellular transport of multiple lipids and their homeostasis. Curr Opin Lipidol. 2006;17:221–226. doi: 10.1097/01.mol.0000226112.12684.5e. [DOI] [PubMed] [Google Scholar]
- 17.Iyer SP, Yao X, Crona JH, Hoos LM, Tetzloff G, Davis HR, Jr, Graziano MP, Altmann SW. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim Biophys Acta. 2005;1722:282–292. doi: 10.1016/j.bbagen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Hawes BE, O'Neill KA, Yao X, Crona JH, Davis HR, Jr, Graziano MP, Altmann SW. In vivo responsiveness to ezetimibe correlates with niemann-pick C1 like-1 (NPC1L1) binding affinity: Comparison of multiple species NPC1L1 orthologs. Mol Pharmacol. 2007;71:19–29. doi: 10.1124/mol.106.027896. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Chu BB, Ge L, Li BL, Yan Y, Song BL. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J Lipid Res. 2009;50:1653–1662. doi: 10.1194/jlr.M800669-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betters JL, Yu L. Transporters as drug targets: discovery and development of NPC1L1 inhibitors. Clin Pharmacol Ther. 2010;87:117–121. doi: 10.1038/clpt.2009.209. [DOI] [PubMed] [Google Scholar]
- 22.Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 25.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci U S A. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nohturfft A, DeBose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc Natl Acad Sci U S A. 1999;96:11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Calvo M, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci U S A. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, et al. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem. 2006;281:6616–6624. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 30.Temel RE, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sane AT, Sinnett D, Delvin E, Bendayan M, Marcil V, Menard D, Beaulieu JF, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res. 2006;47:2112–2120. doi: 10.1194/jlr.M600174-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J. 2007;406:273–283. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen NH, Faergeman NJ, Yu L, Wustner D. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. J Lipid Res. 2008;49:2023–2037. doi: 10.1194/jlr.M800145-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 37.Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J Biol Chem. 2005;280:28103–28109. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 38.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 39.Chu BB, Ge L, Xie C, Zhao Y, Miao HH, Wang J, Li BL, Song BL. Requirement of myosin Vb.Rab11a.Rab11-FIP2 complex in cholesterol regulated translocation of NPC1L1 to the cell surface. J Biol Chem. 2009;284:22481–22490. doi: 10.1074/jbc.M109.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J Lipid Res. 2009;50:293–300. doi: 10.1194/jlr.M800439-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Salen G, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J Lipid Res. 2005;46:1739–1744. doi: 10.1194/jlr.M500124-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J Pharmacol Exp Ther. 2007;320:559–564. doi: 10.1124/jpet.106.114181. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res. 2004;45:301–317. doi: 10.1194/jlr.M300377-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- 47.Narushima K, Takada T, Yamanashi Y, Suzuki H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol Pharmacol. 2008;74:42–49. doi: 10.1124/mol.107.043034. [DOI] [PubMed] [Google Scholar]
- 48.Knopp RH, et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24:729–741. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 49.van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labonte ED, et al. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G776–G783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinglass AB, et al. Madin-Darby canine kidney II cells: a pharmacologically validated system for NPC1L1-mediated cholesterol uptake. Mol Pharmacol. 2008;73:1072–1084. doi: 10.1124/mol.107.043844. [DOI] [PubMed] [Google Scholar]
- 52.Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:3450–3455. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Kramer W, et al. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- 54.Labonte ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta. 2007;1771:1132–1139. doi: 10.1016/j.bbalip.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta. 2007;1771:1140–1147. doi: 10.1016/j.bbalip.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci U S A. 2006;103:1810–1815. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Williams C, Hegele R. Compound heterozygosity for two non-synonymous polymorphisms in NPC1L1 in a non-responder to ezetimibe. Clin Genet. 2005;67:175–177. doi: 10.1111/j.1399-0004.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 58.Hegele RA, Guy J, Ban MR, Wang J. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 2005;4:16. doi: 10.1186/1476-511X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon JS, et al. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 2005;86:648–656. doi: 10.1016/j.ygeno.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Weinglass AB, et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci U S A. 2008;105:11140–11145. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPAR{alpha}-dependent modulation of NPC1L1 expression in mouse. J Lipid Res. 2007;48:2725–2735. doi: 10.1194/jlr.M700345-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Mathur SN, Watt KR, Field FJ. Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J Lipid Res. 2007;48:395–404. doi: 10.1194/jlr.M600325-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun. 2006;340:1259–1263. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- 64.van der Veen JN, et al. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44:1259–1266. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- 66.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res. 2007;48:699–708. doi: 10.1194/jlr.M600439-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Jesch ED, Seo JM, Carr TP, Lee JY. Sitosterol reduces messenger RNA and protein expression levels of Niemann-Pick C1-like 1 in FHs 74 Int cells. Nutr Res. 2009;29:859–866. doi: 10.1016/j.nutres.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 68.Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol. 2007;292:G369–G376. doi: 10.1152/ajpgi.00306.2006. [DOI] [PubMed] [Google Scholar]
- 69.Iwayanagi Y, Takada T, Suzuki H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res. 2008;25:1134–1141. doi: 10.1007/s11095-007-9496-9. [DOI] [PubMed] [Google Scholar]
- 70.Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, Han TQ, Eriksson M, Parini P. HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res. 2009 doi: 10.1194/jlr.M900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvaro A, Rosales R, Masana L, Vallve JC. Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Ontsouka CE, Burgener IA, Mani O, Albrecht C. Polyunsaturated fatty acid-enriched diets used for the treatment of canine chronic enteropathies decrease the abundance of selected genes of cholesterol homeostasis. Domest Anim Endocrinol. 2010;38:32–37. doi: 10.1016/j.domaniend.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Lalloyer F, et al. The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2006;26:2731–2737. doi: 10.1161/01.ATV.0000248101.93488.84. [DOI] [PubMed] [Google Scholar]
- 75.Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism. 2007;56:430–438. doi: 10.1016/j.metabol.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 76.Lally S, Owens D, Tomkin GH. The different effect of pioglitazone as compared to insulin on expression of hepatic and intestinal genes regulating post-prandial lipoproteins in diabetes. Atherosclerosis. 2007;193:343–351. doi: 10.1016/j.atherosclerosis.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 77.Lally S, Tan CY, Owens D, Tomkin GH. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia. 2006;49:1008–1016. doi: 10.1007/s00125-006-0177-8. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura K, Matsui T, Adachi H, Yamagishi SI. Involvement of angiotensin II in intestinal cholesterol absorption. Pharmacol Res. 2009 doi: 10.1016/j.phrs.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Yu L. The structure and function of Niemann-Pick C1-like 1 protein. Curr Opin Lipidol. 2008;19:263–269. doi: 10.1097/MOL.0b013e3282f9b563. [DOI] [PubMed] [Google Scholar]
- 80.Davis HR, Jr, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim Biophys Acta. 2009;1791:679–683. doi: 10.1016/j.bbalip.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Oude Elferink RP, Paulusma CC, Groen AK. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 2006;130:908–925. doi: 10.1053/j.gastro.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 82.Zuniga S, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 83.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008;295:G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 86.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 Prevents Atherosclerosis in ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2007;27:841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 87.Kastelein JJ, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 88.Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–5670. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 89.Nomura M, Ishii H, Kawakami A, Yoshida M. Inhibition of Hepatic Neiman-Pick C1-Like 1 Improves Hepatic Insulin Resistance. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00343.2009. [DOI] [PubMed] [Google Scholar]
- 90.Assy N, Grozovski M, Bersudsky I, Szvalb S, Hussein O. Effect of insulin-sensitizing agents in combination with ezetimibe, and valsartan in rats with non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:4369–4376. doi: 10.3748/wjg.v12.i27.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng S, Hoos L, Cook J, Tetzloff G, Davis H, Jr, van Heek M, Hwa JJ. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118–124. doi: 10.1016/j.ejphar.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 92.Nozaki Y, et al. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J Hepatol. 2009;51:548–556. doi: 10.1016/j.jhep.2009.05.017. [DOI] [PubMed] [Google Scholar]