Abstract
A major challenge for tuberculosis control is mycobacterial detection in paucibacillary disease, particularly in pediatric, extrapulmonary and smear-negative pulmonary infections. We developed a simple and efficient DNA extraction and real-time quantitative PCR (qPCR) protocol for mycobacterial detection and quantification in paucibacillary specimens. The method was refined using an in vitro model mimicking blood specimens which are characterized by the presence of numerous qPCR inhibitors. Mycobacterial DNA detection in blood is of interest given the high sensitivity we previously reported using conventional PCR in blood of patients with tuberculosis lymphadenitis. Mechanical lysis of mycobacteria in the presence of an organic solvent provided the highest sensitivity. Mycobacterial DNA amplification was compromised when the human:bacterial genome ratio was at least 190:1. Separation of the specimen into bacterial- and host-rich fractions prior to DNA extraction improved mycobacterial DNA detection by 30%. Preliminary testing of our protocol in smear-negative, culture-positive specimens (gastric and lymph node aspirates, pleural and cerebrospinal fluid, blood) confirmed the applicability of our technique to a range of paucibacillary specimens for the detection, quantification and speciation (M. tuberculosis versus M. avium) of mycobacteria, several weeks before culture results were available. Our protocol provides a novel efficient and simple strategy to improve the performance of qPCR in paucibacillary specimens, including those with excess human DNA background. This tool is useful to study the pathophysiology of early pulmonary or occult tuberculosis, and for more rapid and accurate diagnosis in difficult to diagnose infections.
Keywords: Mycobacterium, tuberculosis, real-time polymerase chain reaction, DNA extraction, paucibacillary, pediatric, mycobacteremia
1. Introduction
Tuberculosis is a serious public health concern, affecting 2-3 million new individuals annually. A major challenge is lack of tools to detect and study paucibacillary disease, that is, where the number of bacteria in clinical specimens is below the level of detection using direct smears. This is a classic problem in smear-negative pulmonary, pediatric and extrapulmonary tuberculosis (American Thoracic Society, 2000; Starke, 2000). Early detection of M. tuberculosis is key to disease control, by preventing progression to the more contagious, smear-positive disease (Murray et al., 1990). Prompt identification of children with tuberculosis has high impact, particularly in prevention of meningitis-related deaths or neurological sequelae. Pediatric tuberculosis is important evidence of recent transmission and thus an indicator of the need for immediate contact tracing (Loeffler, 2003). Finally, distinction between mycobacterial species is essential for appropriate clinical management (Myers, 2005; Wagner and Young, 2004).
PCR offers a highly sensitive and rapid option for detection of few mycobacteria directly from specimens (Richeldi et al., 1995), when compared with the poorly-sensitive direct smear, or the inherent delay using mycobacterial cultures (American Thoracic Society, 2000). The main limitation of conventional, primer-based PCR is false negatives in about 20% of (sputum) specimens due to the presence of Taq DNA polymerase inhibitors (Amicosante et al., 1995), or false-positives due to contamination of the specimen with mycobacterial DNA or PCR amplicons. The new generation of PCR, real-time quantitative PCR (qPCR), is superior to conventional PCR because it is more sensitive, very specific (based on the requirement for exact matches for two PCR primers plus the fluorescent hybridization probe), and post-PCR analysis is done without further manipulation of the amplified product. Not only is assay setup and post-run analysis simplified, but the risk of amplicon contamination in the laboratory is greatly reduced (Uhl et al., 2002). Furthermore, qPCR provides precise information on the number of mycobacterial genomes in the specimen, opening the possibilities for further understanding on the pathogenesis of paucibacillary tuberculosis, about which so little is know.
An alternative or complementary approach to detection of mycobacterial DNA in sputum or specimens from the site of infection, is using peripheral blood mononuclear cells (PBMCs)(De Francesco et al., 1996). The hypothesis is that patients with active infection harbor M. tuberculosis DNA in peripheral scavenging cells (De Francesco et al., 1996; Schluger et al., 1994). This strategy has been useful for diagnosis of mycobacteremia in HIV-positive patients with M. tuberculosis or M. avium infection, with sensitivities comparable to blood cultures (De Francesco et al., 1996; Kulski and Pryce, 1996; Schluger et al., 1994). In our hands, detection of mycobacterial DNA present in PBMCs of 48 non-HIV infected patients with a clinical diagnosis of extrapulmonary disease (tuberculous lymphadenitis) provided an improved sensitivity (65%) and 100% specificity, when compared with microbiological methods such as direct smear and culture (42 and 27% sensitivity, respectively). Cytology had a higher sensitivity (77%), but 35% of these cases were not confirmed by visualization of bacteria(Mirza et al., 2003). More recently, conventional PCR on DNA extracted from PBMCs detected M. avium subspecies paratuberculosis DNA in 13 of 28 patients with Crohn's disease (Naser et al., 2004). Despite these promising results the appropriate design for effective detection of mycobacterial DNA in blood by PCR remains a challenge. For example, blood-based PCR assays have yielded variable sensitivity (33-95%) in studies of non-HIV infected patients with M. tuberculosis infection, depending on the patient population, DNA extraction method and PCR assay used (Aguado et al., 1996; Ahmed et al., 1998; Condos et al., 1996).
The aim of this study was to develop a highly-sensitive DNA extraction method followed by qPCR to detect and study M. tuberculosis and M. avium in paucibacillary specimens. In order to mimic the most challenging conditions for extraction and selective amplification of mycobacterial DNA, we chose blood as our model. Blood usually has very few bacteria and high levels of human DNA “background” which may be potentially inhibitory for mycobacterial DNA amplification. Furthermore, it contains PCR inhibitors (Al-Soud and Radstrom, 2001). Overcoming these limitations would improve the sensitivity of our current PBMC-PCR assay for further studies on tuberculosis lymphadenitis, and contribute to the development of a protocol useful for paucibacillary specimens other than blood (American Thoracic Society, 2000). We tested our final protocol prospectively in specimens from six children with clinical suspicion of tuberculosis and negative direct smear. qPCR results were compared to mycobacterial culture and final clinical diagnosis.
2. Methods
2.1. M. avium identification and quantification
A clinical isolate of M. avium identified at the Texas Department of State and Health Services laboratory in Harlingen, Texas was used for all experiments. Bacteria were grown in Middlebrook 7H9 broth (Becton Dickinson, Franklin Lakes, NJ) for one week at 37°C and harvested by washing and resuspending with PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.3)-0.05% v/v Tween 80. Clumping was reduced by sonicating trice for three-second pulses at level 8 (Sonic dismembrator model 100, Fisher Scientific, Pittsburg, PA) and bacteria were stored in frozen aliquots at -70°C until required. For quantification, an aliquot was thawed, submitted to serial 10-fold dilutions in Middlebrook broth and plated by spreading into Middlebrook 7H10 agar plates with a glass rod and a turntable (Fisher). Plates were incubated at 37°C, colonies were counted by day 14, and CFU per ml of stock were calculated.
2.2. In vitro model for extraction of mycobacterial DNA from blood
An in vitro simulation of mycobacterium-containing blood was used to develop our DNA extraction and qPCR protocols. Four serial 10-fold dilutions of M. avium estimated to range from 2.7 × 102 to 2.7 × 105 viable counts based on CFUs were prepared. In a separate tube PBMCs were isolated from EDTA-anticoagulated blood from a healthy volunteer using Lymphocyte Separation Medium (MP Biomedicals, Solon, Ohio). PBMCs were washed once with Hanks Buffered salt solution (Sigma-Aldrich, St. Louis, MO) containing 1 mM of EDTA (HBSS-EDTA), counted in a hemacytometer chamber (Housser Sci., Horsham, PA), and either used immediately or stored in aliquots at -70°C with 10% glycerol in HBSS-EDTA. For each experiment, between 2.7 × 102 and 2.7 × 105 M. avium were dispensed either alone or in combination with 1.2 × 107 PBMCs isolated from 3 mls of human blood. These preparations were immediately used for DNA extraction and qPCR amplification.
2.3. Specimen collection and initial processing
Processing of paucibacillary specimens from pediatric patients with suspicion of tuberculosis was as follows: PBMCs were isolated from blood as described above, but following washing with HBSS-EDTA, the cells were resuspended in 1.5 ml of 10% glycerol in HBSS-EDTA. Gastric aspirates were centrifuged at 1,100 RCF at 4°C for 30 min and the pellets were treated with 1.5 ml of 10X TE (100 mM Tris-HCl, 10 mM EDTA, pH 8.0) to increase the pH of the specimen. The cerebrospinal fluids, lymph node aspirate and the pleural fluid were centrifuged at 1,100 RCF at 4°C for 30 min and the pellets resuspended in 1.5 ml of 10% glycerol in TE. All specimens were aliquoted and stored frozen at -70°C. The protocol was approved by the Committee for the Protection of Human subject from the University of Texas, Houston Medical Center.
2.4. DNA extraction
M. avium DNA was extracted by proteinase K-organic lysis (Mirza et al., 2003), heat lysis (Afghani and Stutman, 1996) or alkaline heat lysis (Kulski and Pryce, 1996) as described previously, or by the organic bead-beater lysis (OBL) method described in this study. The latter was developed by modifying the manufacturer's instructions for TRI Reagent (Sigma-Aldrich Corp., St. Louis, MO) and a previously reported protocol used with M. tuberculosis (Shi et al., 2003). Essentially, a pre-established number of CFU from M. avium (with or without PBMCs) were taken to a final volume of 0.5 ml in TE, and mixed with an equal volume of TRI Reagent and 0.5 mls of zirconia beads (0.1 mm) (BioSpec Prods, Bartlesville, OK). The suspension was placed on a BeadBeater-8 (BioSpec Prods, Bartlesville, OK) at maximum speed for one min followed by cooling at -20°C for one minute. After centrifugation, the DNA was transferred from the organic to the aqueous phase by back-extraction. This was achieved by adding one volume of TESS solution (100 mM Tris/HCl, 1 mM EDTA, 50 mM NaCl, 1% SDS), incubating at 65°C for 15 min and centrifugating at 18,300 RCF. The aqueous supernatant containing DNA was collected, and back-extraction of the DNA remaining in the organic phase was performed as described above. Both aqueous supernatants containing DNA were combined. DNA extracted by all methods (proteinase K-organic lysis, heat lysis, alkaline heat lysis and OBL) was cleaned from salts and concentrated by addition of 1/10 volume of 3M sodium acetate pH 5.2 followed by isopropanol precipitation (Eickbush and Moudrianakis, 1978). The DNA pellet was resuspended in 0.06 ml of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0).
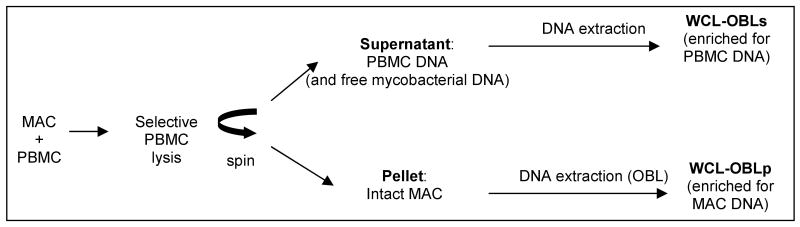
In some experiments (specified in Results) where M. avium was mixed with PBMCs, the OBL protocol was preceded with a PBMC lysis step to obtain separate fractions enriched for mycobacterial and human DNA (white cell lysis-OBL or WCL-OBL DNA extraction protocol; Figure 1). Aliquots containing M. avium plus PBMCs, or M. avium alone (control with no human DNA) were mixed with white blood cell lysis buffer (WCL buffer: 0.06M NaCl, 0.02M EDTA, 0.22% SDS, 1.03M sodium perchlorate) and vortexed for 10 seconds. After centrifugation at 5300 RCF, the white blood cell DNA released in the supernatant, as well as any free mycobacterial DNA originally present in the specimen were isolated by a modified ‘salting-out’ protocol (Miller et al., 1988). The pellet containing intact mycobacteria was submitted to the OBL method as described above.
Figure 1.
Flow diagram of WCL-OBL protocol. Abbreviations: MAC, M. avium; PBMC, peripheral blood mononuclear cells; OBL, organic bead-beater lysis; WCL, white blood cell lysis; WCL-OBLs, supernatant fraction; WCL-OBLp, pellet fraction
2.5. qPCR assays
Taqman assays were developed using Primer Express assay design software version 2.0 for Windows (Applied Biosystems, Foster City, CA). Probes were labeled with a fluorescent dye (6-FAM) on the 5′ end and a non-fluorescent quencher dye (Black Hole Quencher 1-BHQ1) on the 3′ end. The system for the M. tuberculosis complex-specific IS6110 element and heat shock protein of 65 kD (Hsp65), the 16S rRNA gene of M. avium, and the apolipoprotein B (ApoB) assay for quantification of human genomes are presented in Table 1. The latter was developed by the Quantitative Genomics Core Laboratory of the University of Texas Health Science Center Houston. All qPCR assays were run on a 7900HT Sequence detector (Applied Biosystems, Foster City, CA) as 25 μl reactions containing 10 mM Tris/HCl pH 8.0, 50 mM KCl, 5 mM MgCl2, 200 μM dNTP mix, 400 nM each primer, 100 nM probe and 0.75 units of hot start JumpStart Taq DNA polymerase (Sigma-Aldrich Corp., St. Louis, MO, USA). Conditions were 1 cycle of 94°C for 2 min for activation of Taq and initial DNA denaturation, followed by 40 cycles at 95°C 15 seconds for denaturation, and 60°C 1 min for primer annealing, probe degradation and primer extension. Each run included experimental samples, a reagent control (reagents used to extract DNA to rule out experimental contamination during DNA extraction), a no-template negative control (water; to exclude contamination in the qPCR reagents) and a positive control (standard curve with known amounts of each gene target). The templates for each standard curve were in vitro synthesized ssDNA oligonucleotide targets (BioSource, Camarillo, CA) quantified by OD260 and diluted in 100 ng/μl of yeast tRNA (Invitrogen, Carlsbad, CA) to known numbers/well. Sample quantification was determined by extrapolating the measured number of cycles required for detection (Ct) from a standard curve with known amounts of each mycobacterial target in triplicates, using SDS 2.1 software (Applied Biosystems)(Heid et al., 1996).
Table 1.
Primers and Probes for Taqman assays
| Gene name | Accession No | Primers and Probes |
|---|---|---|
| M. tuberculosis IS6110a | AF390039 | 1253 (+) GGGTCCAGATGGCTTGC |
| 1315 (-) GGGTCGCTTCCACGATG | ||
| 1275 (+) FAM-CGCGTCGAGGACCATGGAGGT-BHQ1 | ||
| M. tuberculosis Hsp65a | M15467 | 429 (+) GAGCTGGAGGATCCGTACG |
| 496 (-) TCATCGGTCTTCTTGGCTACC | ||
| 472 (-) FAM-TTGACCAGCTCGGCGCCG-BHQ1 | ||
| M. avium 16S rRNAa | M61672.1 | 64 (+) GAACGGAAAGGCCTCTTCG |
| 138 (-) GAAGTGCAGGGCAGATTGC | ||
| 88 (+) FAM-ACTCGAGTGGCGAACGGGTGAGTA-BHQ1 | ||
| Human Apolipoprotein Ba | NM_000384 | 4253 (+) TGGCAACACCAGCACAGAC |
| 4326 (-) GCAGGTCAACCACAGAGTCAG | ||
| 4280 (+) FAM-CCTTCGGGCTCGTTACCACATGAA-BHQ1 |
Lowest limit of quantification of each assay is ten targets
2.6. Calculation of DNA extraction sensitivity
The DNA extraction efficiency was evaluated based on the number of M. avium 16S rRNA targets detected by qPCR. The total number of targets detected in 5 μls of the M. avium DNA sample was extrapolated from a standard curve as described above. This number was then multiplied by 12, providing the total number of 16S rRNA targets that should be present in the original 60 μl DNA sample. The sensitivity of DNA detection was then estimated, assuming one 16S rRNA target per bacterial genome(Cocito et al., 1994), or one bacterial CFU, as follows:
2.6. Gel electrophoresis
After PCR, the amplicon was submitted to 12% polyacrylamide-gel electrophoresis (Chory and Pollard, 2005). DNA was visualized by staining with 2 μM SYBR Green I (Molecular Probes, Carlsbad, CA), and band size was estimated using a 50 bp ladder as reference (New England Biolabs Inc., Ipswich, MA).
3. Results
3.1. Selection of the most efficient DNA extraction method for mycobacterial DNA
Our previous study indicated a proteinase K-organic lysis method was suitable for detection of M. tuberculosis DNA in blood of patients with tuberculous lymphadenitis (Mirza et al., 2003). We examined if there was a simpler DNA isolation protocol that would offer higher sensitivity for a variety of paucibacillary specimens. Aliquots containing 2.7 × 104 M. avium CFU were submitted to DNA extraction by the proteinase-K-organic, heat lysis, alkaline heat lysis or the OBL methods. Results from qPCR amplification of the 16S rRNA gene for M. avium, present at one copy per genome (genome GenBank #NC002944), showed optimal recovery of DNA using the OBL method (Table 2). If we assume there is one genome per bacterium, the number of genomes detectable by qPCR was 169% of the viable count. This apparent discrepancy can be accounted for by clumping and non-viable bacteria. With much reduced sensitivity the heat lysis method was next with 73% of the viable count, followed by proteinase K-organic lysis with 59% and alkaline-heat lysis with a similar sensitivity of 57%. The greatly enhanced sensitivity of the OBL method (2.8-fold over the proteinase K-organic lysis) prompted us to focus further on this technique.
Table 2.
DNA extraction efficiency of different protocols when starting with 2.7 × 104 M. avium CFU
| DNA extraction method | Number of M. avium genomes detected by qPCRa (Average±SEM) | Sensitivity (%) based on qPCR/CFUb | Fold-improvement over PKOc |
|---|---|---|---|
| PKO | 15,832±507 | 59 | Reference |
| Heat Lysis | 19,645±426 | 73 | 1.2 |
| Alkaline Heat Lysis | 15,421±268 | 57 | 1.0 |
| OBL | 45,556±848 | 169 | 2.9 |
Based on number of 16S rRNA targets detected by qPCR in 5 μls of specimen, present at one copy/genome, and then extrapolated to the total amount present in the 60 μl of total volume of the specimen
Total number of M. avium genomes detectable by 16S rRNA qPCR as described in footnote “a”. Assuming there is one genome/bacteria, then total number of genomes was divided by total number of CFUs (2.7 × 104) placed in each reaction
Recovery obtained by each method divided by recovery obtained by reference Proteinase K-Organic DNA extraction method
PKO, Proteinase K-Organic; OBL, organic beat-beater lysis
3.2. Amplification of mycobacterial DNA in the presence of surplus human DNA
Successful amplification of few mycobacterial DNA targets is a challenge when biological specimens contain several logs of excess human DNA (Morata et al., 1998). Internal Taq inhibitors naturally present in specimens may further block or substantially reduce amplification (Al-Soud and Radstrom, 2001). To determine if excess human DNA compromises amplification of mycobacterial DNA, serial 10-fold dilutions of M. avium containing 2.7 × 102 to 2.7 × 105 cells were mixed with (experimental) or without (control) human PBMCs isolated from three mls of blood. This volume was arbitrarily chosen as the maximum amount of blood that would be sufficient to detect mycobacterial genomes, without having to handle excess human DNA. DNA was extracted from these preparations by the OBL method and the efficiency of the protocol was measured by amplification of the M. avium 16S rRNA gene. Results are shown in Table 3. Amplification of M. avium without human DNA yielded sensitivities ranging from 76-199% with respect to the original viable count. Using as a reference the number of 16S rRNA targets amplified in the absence of human DNA (control), the addition of human DNA background (experimental) significantly lowered the sensitivity of M. avium amplification when the ratio of human to mycobacterial DNA exceeded 190:1. This inhibitory effect escalated as the ratio of human to mycobacterial DNA increased (Table 3). Visualization of the amplicons by agarose gel electrophoresis suggested that the excess human DNA competed with the mycobacterial genome for binding and amplification of the mycobacterium-specific primers, leading to amplification of non-specific products (Fig. 2).
Table 3.
Amplification of M. avium DNA in the presence or absence of 2.4 × 107 genomes of human DNA background following the OBL extraction method
| M. avium | ||||
|---|---|---|---|---|
| CFU submitted to DNA extraction and qPCR | Average number ± SEM of 16S rRNA targets amplified in presence (+) or absence (-) of human DNAa (sensitivity based on CFU) | Amplification efficiency in presence of human DNA (%)b | Human:MAC genome ratioc,d | |
| - | + | |||
| 270 | 206±61 (76%) | 131±77 (49%) | 64% | 31,100 |
| 2,700 | 2,105±128 (78%) | 1,453±112 (54%) | 69% | 3,500 |
| 27,000 | 53,600±330 (199%) | 39,430±377 (146%) | 74% | 190 |
| 270,000 | 441,264±16,910 (163%) | 472,860±9,259 (175%) | 107% | 30 |
Data shown for number of M. avium targets in the 60 μl final volume, estimated from results obtained from a 5 μl specimen submitted to qPCR
Percentage of M. avium targets amplified when human DNA is in background, compared to no human DNA background as reference
Calculations based on estimate that there is one genome for every 16S rRNA for M. avium, or ApoB gene for human
Data rounded to the nearest 10th
Figure 2.
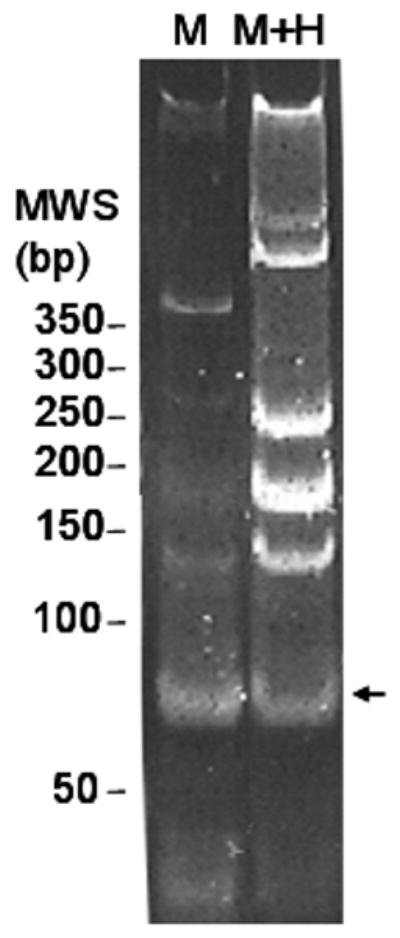
Human DNA background reduces amplification of expected mycobacterial target. SYBR Green I stained 12% polyacrylamide gel illustrates predominance of expected 74 bp amplicon (arrow) when M. avium total DNA is used as substrate to amplify the 16S rRNA gene (M). Additional, fainter, unexpected bands of ∼400, 250, 180 and 130 bp were also detected with mycobacterial DNA alone. An increase in non-specific amplicons and decrease in the intensity of the expected 74 bp product (arrow) is observed when the same reaction is carried out in the presence of 2.4×107 human genomes (M + H). M, M. avium DNA, M+H, M. avium plus human DNA; arrow indicates expected 74 bp amplicon. Molecular weight standards (MWS) are expressed in base pairs (bp).
3.3. Enhanced sensitivity by separation of specimens into bacterial- and leukocyte-rich fractions
We investigated whether the inhibition of mycobacterial DNA amplification in the presence of excess human DNA could be overcome by adding an initial step to the OBL method to selectively lyse PBMCs (WCL-OBL), but not mycobacteria. Using this strategy we expected to free human DNA from the more fragile leukocytes into the supernatant while retaining mycobacterial DNA in intact cells (Fig.1). To do this we added the WBC-lysis buffer, vortexed and centrifuged to bring down all intact M. avium. The cell pellet and supernatant were then separated. The released PBMC DNA in the supernatant (together with any free bacterial DNA) was extracted by salting-out (Miller et al., 1988), and cleaned and concentrated by isopropanol precipitation (WCL-OBL-supernatant fraction; WCL-OBLs, Table 3). The pellet was expected to contain all intact M. avium. This was therefore submitted to the OBL method for extraction of mycobacterial DNA (WCL-OBL-pellet fraction; WCL-OBLp, Table 4). Table 4 also shows the sum of amplicons (16S rRNA) detected in the pellet and supernatant under the fraction denominated WCL-OBL-combined (WCL-OBLc).
Table 4.
| CFU submitted to DNA extraction and qPCR | WCL-OBL fraction (enriched for) | Number ± SEM 16S rRNA targets detected in absence (-) or presence (+) of human DNA (sensitivity based CFU) | % of 16S rRNA targets amplified in pellet or supernatant in absence (-) or presence (+) of human DNAc | % 16S rRNA amplified when human DNA in backgroundd | Ratio ApoB:16S rRNA submitted to DNA extractione | ||
|---|---|---|---|---|---|---|---|
| - | + | - | + | ||||
| 270 | pellet (MAC) | 339±64 (126%) | 222±48 (82%) | 95% | 95% | 65% | |
| supernatant (PBMC) | 18±18 (7%) | 10±10 (4%) | 5% | 5% | |||
| combined (MAC+PBMC) | 357±82 (132%) | 232±58 (86%) | Reference | Reference | 65% | 27,810 | |
| 2,700 | pellet (MAC) | 2,085±145 (77%) | 1,433±130 (53%) | 90% | 87% | 69% | |
| supernatant (PBMC) | 236±34 (9%) | 209±121 (8%) | 10% | 13% | |||
| combined (MAC+PBMC) | 2,320±179 (86%) | 1,642±251 (61%) | Reference | Reference | 71% | 4,590 | |
| 27,000 | pellet (MAC) | 61,904±1,604 (229%) | 48,495±846 (180%) | 96% | 91% | 78% | |
| supernatant (PBMC) | 2,619±182 (10%) | 4,992±2,626 (18%) | 4% | 9% | |||
| combined (MAC+PBMC) | 64,523±1,786 (238%) | 53,486±3,473 (198%) | Reference | Reference | 83% | 220 | |
| 270,000 | pellet (MAC) | 525,141±5,015 (194%) | 444,001±1,527 (164%) | 98% | 95% | 85% | |
| supernatant (PBMC) | 9,163±6,019 (3%) | 25,005±1,950 (9%) | 2% | 5% | |||
| combined (MAC+PBMC) | 534,304±1,004 (198%) | 469,005±3,477 (173%) | Reference | Reference | 88% | 20 | |
Number of M. avium or ApoB targets are extrapolated from results in 5 μls, to number of copies in the 60 μl total specimen volume
Number of ApoB targets in 3 mls of blood, equivalent to 2.4 × 107 genomes
Reference is total number of targets detected in combined fraction
Percentage of M. avium targets amplified when human DNA is in background, compared to no human DNA background as reference
Ratio based on number of ApoB/16S rRNA amplicons
Evaluation of the WCL-OBL was carried out using the simulated blood design described above for the OBL. Table 4 shows that 87-98% of the total amplified M. avium DNA is found in the pellet, and the remaining 2-13% in the supernatant. As anticipated 65-100% of the human DNA was now detected in the supernatant fraction (data not shown). Despite the reduction in human DNA on the mycobacterial fraction, partial inhibition of mycobacterial DNA amplification was still observed. That is, sensitivity ranged from 65-88% for M. avium combined with human cells, when compared to the recovery from M. avium alone. As observed for the OBL, lowest sensitivity (65%) was observed when the initial ratio of human to M. avium was highest (27,810:1 ratio). Data in tables 3 and 4 are from the same experiment, shown separately for simplicity of presentation. Comparison of the two tables leads to the conclusion that the WCL-OBL provided an average 1.3-fold better sensitivity than OBL (Tables 3 and 4, sensitivity based on CFU). This improved sensitivity was observed in three repeated experiments. Thus, WCL-OBL does not abrogate entirely the inhibition of M. avium DNA amplification posed by excess human DNA, but does provide improved sensitivity compared with OBL.
We finally explored if the reduced amounts of human DNA (and other possible residual blood components) remaining in the WCL-OBLp fraction had an inhibitory effect on amplification. For this, the pellet fraction obtained from extraction of PBMCs from 3 mls of blood were spiked with known amounts (ten-fold increment from 10 to 105 targets) of ssDNA standards for 16S rRNA of M. avium. The amplification efficiency of this standard curve in the presence or absence of the WCL-OBLp fraction was identical, confirming that no residual inhibitors of PCR persisted after DNA extraction (data not shown).
3.4. Evaluation of the DNA extraction and qPCR protocol in pediatric patients with suspected tuberculosis
Our protocols (OBL for CSF, pleural fluid lymph node and gastric aspirates and WCL-OBL for blood) were tested prospectively in specimens from six children with negative direct smears and clinical suspicion of tuberculosis (Table 5). qPCR results for M. tuberculosis IS6110 (multiple copy insertion sequence for improved sensitivity) and Hsp65 (one copy/genome for quantification), and M. avium 16S rRNA (one copy/genome) were available within two days. These data were then compared with the final diagnosis based on culture and clinical criteria available several weeks later. Three children had culture-confirmed tuberculosis. The first had pleural tuberculosis due to M. tuberculosis, with IS6110 and Hsp65 qPCRs positive in pleural fluid, but negative in blood. The second had meningeal tuberculosis due to M. tuberculosis, with IS6110 and Hsp65 qPCRs positive in CSF. The third had tuberculous lymphadenitis due to M. avium intracellulare, with the three specimens (blood, gastric and lymph node aspirates) negative for M. tuberculosis DNA, but positive for the M. avium 16SrRNA gene. In the other three children tuberculosis was excluded by eventual culture and clinical criteria, thus supporting the earlier result of negative qPCR for the three Taqman assays.). We spiked with known amounts of DNA the nucleic acids extracted from two very different types of samples: pleural fluid which is “clean”, and blood which has several potential inhibitors. Amplification of a standard curve for the 16S rRNA of M. avium using as diluent these two specimens (experimental) or water (reference control) showed no evidence of PCR inhibitors. These results confirmed the validity of the quantitative data for positive samples, and ruled out the possibility for false-negatives in the negative specimens.
Table 5.
Summary of qPCR results in pediatric patients with negative smear and suspicion of tuberculosis
| PID | Age (yrs) | Specimen Type | qPCR results interpretation | Number of targets/ml of specimen | Culture | Final Diagnosis | ||
|---|---|---|---|---|---|---|---|---|
| M. tuberculosis Hsp65 | M. tuberculosis IS6110 | M. avium 16S rRNA | ||||||
| 2075 | 18 | Blood | neg | 0 | 0 | ND | ND | Pleural TB |
| Pleural fluid | M. tuberculosis | 800 | 360,000 | ND | M. tuberculosis | |||
| 2101 | 1 | Blood | M. avium | 0 | 0 | 100 | ND | Lymphatic TB |
| Lymph node aspirate | M. avium | 0 | 0 | 400 | M. avium | |||
| Gastric aspirate | M. avium | 0 | 0 | 200,000 | neg | |||
| 2114 | 1 | CSF | M. tuberculosis | 1,400 | 220,000 | ND | M. tuberculosis | Meningeal TB |
| 2116 | 3 | Gastric aspirate | neg | 0 | 0 | ND | neg | no TB |
| CSF | neg | 0 | 0 | ND | neg | |||
| 2117 | 1 | Gastric aspirate | neg | 0 | 0 | ND | neg | no TB |
| 0 | 0 | |||||||
| 2138 | 2 | Gastric aspirate | neg | 0 | 0 | ND | neg | no TB |
qPCR positive for M. tuberculosis by IS6110 and M. avium by species-specific 16S rRNA
Abbreviations: PID, patient identification number; ND, not determined; TB, tuberculosis
4. Discussion
Little is known about the biology of paucibacillary tuberculosis, in part due to the difficulty in detecting the infecting bacteria. We describe a simple and efficient DNA extraction and qPCR amplification protocol for mycobacterial detection, quantification and species identification in challenging specimens. A novel aspect of our protocol is addition of a step to enrich for mycobacterial DNA during extraction, resulting in increased sensitivity in specimens with excess host DNA, such as blood. Pilot testing of our protocol in pediatric specimens suggested our strategy may be: i) at least as sensitive as culture in smear-negative specimens, ii) appropriate for a variety of biological specimens, including blood, and iii) able to distinguish M. tuberculosis from M. avium DNA.
An in vitro model mimicking blood was selected for several reasons: i) our pilot study in patients with tuberculous lymphadenitis indicated a blood-based PCR assay could provide improved sensitivity when compared with routine microbiological studies (Mirza et al., 2003), but further refining to simplify and improve the sensitivity of this protocol was required. ii) There is scanty information on the biology of mycobacteremia (Calmette, 1923; Reimer, 1994), and development of an efficient tool to detect mycobacterial DNA would open avenues for further investigation in this field. iii) In addition to our findings with tuberculous lymphadenitis, studies from diverse research groups suggest that a blood-based PCR assay has potential for detection of mycobacterial DNA in blood of patients with pulmonary tuberculosis (especially in AIDS patients)(Aguado et al., 1996; De Francesco et al., 1996; Schluger et al., 1994), other forms of extrapulmonary tuberculosis (Tzoanopoulos et al., 2001), and M. avium subspecies paratuberculosis DNA in patients with Crohn's disease (Naser et al., 2004). iv) Blood presents a number of challenges for successful PCR amplification, making it a model for overcoming potential pitfalls presented by biological specimens from other sources. Blood is paucibacillary, has internal Taq inhibitors, some natural such as heme (Akane et al., 1994) and plasma IgG (Al-Soud et al., 2000), and others added during collection such as anticoagulants containing EDTA (Mg++ chelator) and heparin (binds to Taq). Finally, there is a surplus of genomic DNA that interferes with amplification (Morata et al., 1998).
In our model the major qPCR inhibitor was excess of background human DNA, which led to non-specific amplification, and hypothetically, consumption of primers, nucleotides and Taq polymerase. Under these circumstances, qPCR assays require selective quantification of the expected amplicons, a feature that can be achieved with Taqman assays, but not with double-stranded DNA-binding dyes such as SYBR Green I or SYTO9 (Monis et al., 2005). Our approach to overcome the challenge posed by excess human DNA was to selectively remove leukocyte DNA leaving a mycobacteria-rich fraction. This WCL-OBL approach did not obliterate the inhibitory effect associated with concomitant presence of human nucleic acids, but for this protocol the reduction in sensitivity was more likely explained by the loss of mycobacterial DNA during the additional steps required for enrichment of white blood cell versus mycobacterial DNA. Nevertheless, the WCL-OBL protocol did improve the sensitivity over OBL by 30%. We also attempted to reduce the non-specific amplification when excess human DNA was present by increasing the annealing temperature during qPCR. However, improvement in specificity was compromised by reduction in amplification efficiency of the expected target (data not shown). Overall, we show that the sensitivity of a PCR assay is affected not only by the absolute number of target DNA molecules, but also by the complex environment of the biological specimen in which they are present.
The sensitivity of the OBL and WCL-OBL DNA extraction protocols was above 100% when compared to viable bacteria based on the CFU technique. Possible, non-exclusive explanations for such an overestimation in efficiency include; i) clumping of mycobacteria despite our efforts to form single-cell suspensions, which would result in one CFU from several cells (genomes); and/or ii) the presence of dead bacteria that have intact DNA targets for amplification but do not form CFUs.
Preliminary evaluation of our protocol in various types of smear-negative pediatric specimens showed excellent concordance between qPCR and mycobacterial culture. Quantification of bacterial genomes confirmed the paucibacillary nature of extrapulmonary, pediatric tuberculosis, clearly below the limit of sensitivity of direct smears (104 bacteria/ml) in four of the five positive specimens. The quantitative data also supported the increased sensitivity provided by the multi-copy insertion sequence IS6110 (Table 5). We ruled out that our DNA extraction protocol did not leave internal PCR inhibitors in two of the negative specimens, by spiking these with known amounts of ssDNA standards (data not shown).
We demonstrate here that it is possible to use the latest molecular technology to tackle some of the most difficult clinical and public health challenges of tuberculosis. Addition of the white blood cell lysis step at the beginning of any DNA extraction may be useful for improving the sensitivity in specimens where excess human DNA may also be a challenge (ie. skin, bone marrow). We anticipate in the not too distant future that continued simplification and streamlining of these techniques may make them available, possibly in miniaturized, microfluidic format, so that they can be used in the poorer populations where they are most needed. In the meantime we propose to continue to use these approaches to address issues not only of detection of genomic material, but up-regulation of host and pathogen genes at various stages of this complex infection in the hope that we can throw new light on this important disease.
Acknowledgments
We thank Ms. Nielsa Robinnette, Jaymie Estrella, Mary Mireles and Leticia Armendariz for technical support, and Dr. Monica Trujillo for inviting the pediatric patients to participate in this study.
This project was supported by grant NIH-EXPORT MD000170 020.
References
- Afghani B, Stutman HR. Polymerase chain reaction for diagnosis of M. tuberculosis: comparison of simple boiling and a conventional method for DNA extraction. Biochem Mol Med. 1996;57:14–18. doi: 10.1006/bmme.1996.0003. [DOI] [PubMed] [Google Scholar]
- Aguado JM, Rebollo MJ, Palenque E, Folgueria L. Blood-based PCR assay to detect pulmonary tuberculosis. Lancet. 1996;347:1836–7. doi: 10.1016/s0140-6736(96)91656-6. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Mohanty AK, Mukhopadhyay U, Batish VK, Grover S. PCR-based rapid detection of Mycobacterium tuberculosis in blood from immunocompetent patients with pulmonary tuberculosis. J Clin Microbiol. 1998;36:3094–3095. doi: 10.1128/jcm.36.10.3094-3095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akane A, Matsubara K, Nakamura H, Takahashi S, Kimura K. Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. J Forensic Sci. 1994;39:362–372. [PubMed] [Google Scholar]
- Al-Soud WA, Jonsson LJ, Radstrom P. Identification and characterization of immunoglobulin G in blood as a major inhibitor of diagnostic PCR. J Clin Microbiol. 2000;38:345–350. doi: 10.1128/jcm.38.1.345-350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Soud WA, Radstrom P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol. 2001;39:485–493. doi: 10.1128/JCM.39.2.485-493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- Amicosante M, Richeldi L, Trenti G, Paone G, Campa M, Bisetti A, Saltini C. Inactivation of polymerase inhibitors for Mycobacterium tuberculosis DNA amplification in sputum by using capture resin. J Clin Microbiol. 1995;33:629–630. doi: 10.1128/jcm.33.3.629-630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmette A. Tubercle bacillus infection and Tuberculosis in man and animals. Baltimore, MD: Williams & Wilkins Company; 1923. [Google Scholar]
- Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Chory J, Pollard JD. Preparation and Analysis of DNA: Separation of Small DNA Fragments by Conventional Gel Electrophoresis. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Cambridge: John Wiley & Sons, Inc.; 2005. pp. 2.7.1–2.7.4. [Google Scholar]
- Cocito C, Gilot P, Coene M, de KM, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condos R, McClune A, Rom WN, Schluger NW. Peripheral-blood-based PCR assay to identify patients with active pulmonary tuberculosis. Lancet. 1996;347:1082–5. doi: 10.1016/s0140-6736(96)90281-0. [DOI] [PubMed] [Google Scholar]
- De Francesco MA, Colombrita D, Pinsi G, Gargiulo F, Caligaris S, Bertelli D, Martinelli F, Gao J, Turano A. Detection and identification of Mycobacterium avium in the blood of AIDS patients by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1996;15:551–555. doi: 10.1007/BF01709362. [DOI] [PubMed] [Google Scholar]
- Eickbush TH, Moudrianakis EN. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978;13:295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Kulski JK, Pryce T. Preparation of mycobacterial DNA from blood culture fluids by simple alkali wash and heat lysis method for PCR detection. J Clin Microbiol. 1996;34:1985–1991. doi: 10.1128/jcm.34.8.1985-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler AM. Pediatric tuberculosis. Semin Respir Infect. 2003;18:272–291. doi: 10.1053/s0882-0546(03)00071-9. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S, Restrepo B, McCormick J, Fisher-Hoch S. Diagnosis of tuberculous lymphadenitis using a polymerase chain reaction on peripheral blood mononuclear cells. Am J Trop Med Hyg. 2003;69:461–465. [PubMed] [Google Scholar]
- Monis PT, Giglio S, Saint CP. Comparison of SYTO9 and SYBR Green I for real-time polymerase chain reaction and investigation of the effect of dye concentration on amplification and DNA melting curve analysis. Anal Biochem. 2005;340:24–34. doi: 10.1016/j.ab.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Morata P, Queipo-Ortuno MI, de Dios CJ. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol. 1998;36:2443–2446. doi: 10.1128/jcm.36.9.2443-2446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- Myers JP. New recommendations for the treatment of tuberculosis. Curr Opin Infect Dis. 2005;18:133–140. doi: 10.1097/01.qco.0000160902.48942.31. [DOI] [PubMed] [Google Scholar]
- Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- Reimer LG. Laboratory detection of mycobacteremia. Clin Lab Med. 1994;14:99–105. [PubMed] [Google Scholar]
- Richeldi L, Barnini S, Saltini C. Molecular diagnosis of tuberculosis. Eur Respir J. 1995;8:689s–700s. [PubMed] [Google Scholar]
- Schluger NW, Condos R, Lewis S, Rom WN. Amplification of DNA of Mycobacterium tuberculosis from peripheral blood of patients with pulmonary tuberculosis. Lancet. 1994;344:232–3. doi: 10.1016/s0140-6736(94)92999-8. [DOI] [PubMed] [Google Scholar]
- Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci U S A. 2003;100:241–246. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke JR. Diagnosis of tuberculosis in children. Pediatr Infect Dis J. 2000;19:1095–1096. doi: 10.1097/00006454-200011000-00015. [DOI] [PubMed] [Google Scholar]
- Tzoanopoulos D, Stakos D, Hatseras D, Ritis K, Kartalis G. Detection of Mycobacterium tuberculosis complex DNA in pericardial fluid, bone marrow and peripheral blood in a patient with pericardial tuberculosis. A case report Neth J Med. 2001;59:177–80. doi: 10.1016/s0300-2977(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Uhl JR, Bell CA, Sloan LM, Espy MJ, Smith TF, Rosenblatt JE, Cockerill FR., III Application of rapid-cycle real-time polymerase chain reaction for the detection of microbial pathogens: the Mayo-Roche Rapid Anthrax Test. Mayo Clin Proc. 2002;77:673–680. doi: 10.4065/77.7.673. [DOI] [PubMed] [Google Scholar]
- Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]