Abstract
Gonadotropin-releasing hormone (GnRH) is the primary hypothalamic factor responsible for the control of gonadotropin secretion in vertebrates. However, within the last decade, two other hypothalamic neuropeptides have been found to play key roles in the control of reproductive functions: gonadotropin-inhibitory hormone (GnIH) and kisspeptin. In 2000, we discovered GnIH in the quail hypothalamus. GnIH inhibits gonadotropin synthesis and release in birds through actions on GnRH neurons and gonadotropes, mediated via GPR147. Subsequently, GnIH orthologs were identified in other vertebrate species from fish to humans. As in birds, mammalian and fish GnIH orthologs inhibit gonadotropin release, indicating a conserved role for this neuropeptide in the control of the hypothalamo-pituitary-gonadal (HPG) axis across species. Following the discovery of GnIH, kisspeptin, encoded by the KiSS-1 gene, was discovered in mammals. In contrast to GnIH, kisspeptin has a direct stimulatory effect on GnRH neurons via GPR54. GPR54 is also expressed in pituitary cells, but whether gonadotropes are targets for kisspeptin remains unresolved. The KiSS-1 gene is also highly conserved and has been identified in mammals, amphibians and fish. We have recently found a second isoform of KiSS-1, designated KiSS-2, in several vertebrates, but not birds, rodents or primates. In this review, we highlight the discovery, mechanisms of action, and functional significance of these two chief regulators of the reproductive axis.
Keywords: gonadotropins, gonadotropin-inhibitory hormone (GnIH), gonadotropin-releasing hormone (GnRH), kisspeptin, hypothalamus, reproduction
Introduction
Gonadotropin-releasing hormone (GnRH) primarily regulates secretion of both of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and is a crucial neuropeptidergic component of the vertebrate reproductive system. Since the discovery of the hypothalamic decapeptide, GnRH, in the brain of mammals at the beginning of 1970s (1, 2), several other GnRHs have been identified in the brain of non-mammalian vertebrates (3–7).
Based on extensive studies in vertebrates, it was generally believed that GnRH is the only hypothalamic regulator of pituitary gonadotropin synthesis and release. In 2000, Tsutsui and co-workers discovered a novel hypothalamic neuropeptide that, in contrast to GnRH, actively inhibits gonadotropin release in quail and termed it gonadotropin-inhibitory hormone (GnIH) (8). From the past 10 years of research, we now know that GnIH exists in several avian species, including quail, chickens, sparrows and starlings, and regulates avian reproduction by decreasing gonadotropin release and synthesis via action on the GnRH system and the anterior pituitary gland, mediated via GPR147 (8–23).
To place our findings in a broader perspective, we have further identified GnIH orthologs in a number of other vertebrates from fish to humans [for reviews, see (24–27)]. Importantly, as in birds, mammalian GnIH orthologs [RFamide-related peptides (RFRPs)] act to inhibit gonadotropin release across mammalian species, including rats, hamsters, and sheep (28–32). RFRP-3 has been shown to inhibit GnRH-stimulated gonadotropin synthesis in sheep pituitary gonadotropes (33). Very recently, an inhibitory action of a fish GnIH ortholog was also reported in goldfish (34). In general, GnIH and its orthologs seem to act similarly across vertebrate species to regulate reproduction. To clarify the evolutionary origin of GnIH and its orthologs, we further sought to identify novel hypothalamic neuropeptides from the brain of hagfish, an extant group of the oldest lineage of vertebrates, Agnatha (Osugi et al., unpublished results).
Following the discovery of GnIH, kisspeptin, encoded by the KiSS-1 gene, was discovered in mammals. In contrast to GnIH, kisspeptin has a stimulatory effect on GnRH neurons via its receptor, GPR54, causing up-regulation of the hypothalamo-pituitary-gonadal (HPG) axis [for reviews, see (24–27)]. Kisspeptin and GPR54 are considered to be essential for puberty and subsequent fertility in mammals. At present, the KiSS-1 gene has been identified in most vertebrates, including mammals, amphibians and fish [for reviews, see (24–27)]. Most recently, we found a second isoform of KiSS-1, designated KiSS-2, in several vertebrate groups, but not in birds, rodents or primates.
Thus, the discovery of GnIH and kisspeptin has changed our understanding of the vertebrate reproductive axis in the last 10 years. Based on these findings over the past decade, this paper summarizes the discovery and evolutionary history of GnIH and kisspeptin, new key neuropeptides controlling reproduction in vertebrates.
GnIH
Discovery of GnIH
GnIH possesses the RFamide (Arg-Phe-NH2) motif at its C-terminus and thus belongs to the RFamide peptide family. The first identified RFamide peptide, Phe-Met-Arg-Phe-NH2 (FMRFamide), was a cardioexcitatory molecule isolated from the ganglia of the venus clam Macrocallista nimbosa (35). Since this initial discovery almost 30 years ago, numerous RFamide peptides that act as neurotransmitters, neuromodulators and peripheral hormones have been identified in various invertebrate phyla (36).
The presence of uncharacterized RFamide peptides in the vertebrate nervous system was suggested by immunohistochemical studies (37, 38). Likewise in birds, FMRFamide-like immunoreactive neurons project to the hypothalamic region close to the pituitary gland, suggesting the presence of a novel RFamide peptide that regulates anterior pituitary function. As a result, in 2000, an RFamide peptide was isolated from the brain of the Japanese quail using high-performance liquid chromatography (HPLC) and a competitive enzyme-linked immunosorbent assay for the dipeptide Arg-Phe-NH2 (8). The isolated peptide was a previously-unreported dodecapeptide (SIKPSAYLPLRFamide) [see (8) and Table 1], the C-terminus of which was identical to the first isolated vertebrate RFamide peptide (39), which may be a degraded fragment of the dodecapeptide, as suggested by Dockray and Dimaline (40). Interestingly, the novel dodecapeptide was shown to be located in the quail hypothalamo-hypophysial system and to decrease gonadotropin, but not prolactin (PRL), release from cultured quail anterior pituitaries (8) (Table 1). Tsutsui and co-workers therefore designated this novel RFamide peptide gonadotropin-inhibitory hormone (GnIH) (8).
Table 1.
Amino acid sequences of GnlH and its orthologs in vertebrates.
| Animal | Name | Sequence | Reference |
|---|---|---|---|
| Quail | GnIH | SIKPSAYLPLRFa | Tsutsui et al. (8) |
| GnIH-RP-1* | SLNFEEMKDWGSKNFMKVNTPTVNKVPNSVANLPLRFa | Satake et al. (13) | |
| GnIH-RP-2 | SSIQSLLNLPQRFa | Satake et al. (13) | |
| Chicken | GnIH* | SIRPSAYLPLRFa | Ikemoto et al. (111) |
| GnIH-RP-1* | SLNFEEMKDWGSKNFLKVNTPTVNKVPNSVANLPLRFa | Ikemoto et al. (111) | |
| GnIH-RP-2* | SSIQSLLNLPQRFa | Ikemoto et al. (111) | |
| Sparrow | GnIH* | SIKPFSNLPLRFa | Osugi et al. (12) |
| GnIH-RP-1* | SLNFEEMEDWGSKDIIKMNPFT ASKMPNSVANLPLRFa | Osugi et al. (12) | |
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Osugi et al. (12) | |
| Starling | GnIH | SIKPFANLPLRFa | Ubuka et al. (21) |
| GnIH-RP-1* | SLNFDEMEDWGSKDIIKMNPFTVSKMPNSVANLPLRFa | Ubuka et al. (21) | |
| GnIH-RP-2* | GSSQSLLNLPQRFa | Ubuka et al. (21) | |
| Human | RFRP-1 | MPHSFANLPLRFa | Ubuka et al. (53) |
| RFRP-3 | VPNLPQRFa | Ubuka et al. (53) | |
| Monkey | RFRP-3 | SGRNMEVSLVRQVLNLPQRFa | Ubuka et al. (52) |
| Rat | RFRP-1* | SVTFQELKDWGAKKDIKMSPAPANKVPHSAANLPLRFa | Hinuma et al. (62) |
| RFRP-3 | ANMEAGTMSHFPSLPQRFa | Ukena et al. (54) | |
| Hamster | RFRP-1* | SPAPANKVPHSAANLPLRFa | Kriegsfeld et al. (31) |
| RFRP-3* | TLSRVPSLPQRFa | Kriegsfeld et al. (31) | |
| Bovine | RFRP-1 | SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRFa | Fukusumi et al. (51) |
| RFRP-3 | AMAHLPLRLGKNREDSLSRWVPNLPQRFa | Yoshida et al. (55) | |
| Bullfrog | fGRP | SLKPAANLPLRFa | Koda et al. (56) |
| fGRP-RP-1 | SIPNLPQRFa | Ukena et al. (58) | |
| fGRP-RP-2 | YLSGKTKVQSMANLPQRFa | Ukena et al. (58) | |
| fGRP-RP-3 | AQYTNHFVHSLDTLPLRFa | Ukena et al. (58) | |
| Goldfish | goldfish LPXRFa-1* | PTHLHANLPLRFa | Sawada et al. (59) |
| goldfish LPXRFa-2* | AKSNINLPQRFa | Sawada et al. (59) | |
| goldfish LPXRFa-3 | SGTGLSATLPQRFa | Sawada et al. (59) | |
Putative peptides
Following the isolation of GnIH from birds, its precursor polypeptide was examined (12, 13, 21). Following 3' and 5' rapid amplification of cDNA ends (3'/5' RACE), the resulting cDNA allowed deduction of the GnIH precursor, which consisted of 173 amino acid residues encoding one GnIH and two putative GnIH-related peptides (GnIH-RP-1 and GnIH-RP-2) possessing a -LPXRFamide (X = L or Q) sequence at their C-termini (Table 1). Subsequently, Satake et al. (13) identified GnIH-RP-2 as a mature peptide. Osugi et al. (12) also cloned a GnIH cDNA from Gambel's white-crowned sparrow brain. As in quail, the deduced sparrow GnIH precursor consisted of 173 amino acid residues, encoding one sparrow GnIH and two sparrow GnIH-related peptides (sparrow GnIH-RP-1 and GnIH-RP-2) that included -LPXRFamide (X = L or Q) at their C-termini (Table 1). A cDNA encoding GnIH and GnIH-RPs was also reported in the chicken from a gene database. Recently, Ubuka et al. (21) further cloned a cDNA that encoded GnIH in the brain of European starlings. In agreement with other bird species, starling GnIH precursor mRNA encoded three peptides that possess characteristic LPXRFamide (X = L or Q) motifs at their C-termini (Table 1). The structures of these GnIH precursor polypeptides in different orders of birds indicate that the chicken LPLRFamide discovered as a first RFamide peptide in vertebrates (39) is highly likely to be a fragment of GnIH and GnIH-RP-1 (8, 12, 13, 21).
Function and mode of action of GnIH
Because the localization of GnIH in the brain is essential to understand its function, several studies investigated the precise localization of GnIH in the avian brain by immunohistochemistry (8, 9, 12, 18, 21, 22). Clusters of distinct GnIH-immunoreactive (-ir) neurons were found mostly in the paraventricular nucleus (PVN) in the hypothalamus. Importantly, GnIH-ir neurons were clearly distinct from vasotocin- or mesotocin-expressing neurons (22). In contrast to the discretely-localized clusters of cell bodies, GnIH-ir nerve fibers were widely distributed in multiple diencephalic and mesencephalic regions, implying multiple regulatory roles for GnIH. In all the avian species studied so far except for rufous-winged sparrows (Aimophila carpalis) sampled at one time of year (41), there are extensive networks of branching, beaded fibers extending to terminals in the median eminence (ME), consistent with a role for GnIH in pituitary gonadotropin regulation [for reviews, see (14–17)]. Rufous-winged sparrows express GnIH receptor in the pituitary, however (N.L. McGuire and G.E.Bentley, unpublished results). Importantly, GnIH fibers are further observed in extremely close proximity to GnRH neurons in the preoptic area (POA) in birds (9, 21). Contact also occurs between GnIH and GnRH fibers in the ME (9). Therefore, GnIH likely acts at both the hypothalamic and pituitary levels to regulate gonadotropin release (21).
In view of the initial immunohistochemical findings in quail, Tsutsui and co-workers analyzed the effect of the isolated SIKPSAYLPLRFamide, GnIH, on the release of LH, FSH and PRL from cultured quail anterior pituitaries (8). GnIH significantly inhibited LH release in a dose-dependent manner in vitro (8) (Table 2). A similar inhibitory effect of GnIH on FSH release but not on PRL release was also detected (8). GnIH was also effective in inhibiting circulating LH in vivo (Table 2). When administered intraperitoneally (i.p.) to quail via osmotic pumps, GnIH significantly reduced plasma LH (20) (Table 1). GnIH injected simultaneously with GnRH acutely inhibited the surge of plasma LH above the baseline in song sparrows (12) (Table 2). Furthermore, GnIH injections also acutely decreased LH levels in breeding free-living Gambel's white-crowned sparrows (12). Thus, acute GnIH administration can rapidly reduce circulating LH (12). The same acute effect was not seen in a similar study on a different species, rufous-winged sparrow (42), although the reason for this disparity is not clear, given that this species expresses GnIH receptor mRNA in its pituitary gland (McGuire and Bentley, unplublished results). In addition to reducing gonadotropin release, GnIH inhibits gonadotropin synthesis (11, 20). Addition of GnIH to diced pituitary glands from chickens suppressed gonadotropin common α and FSHβ subunit mRNAs (11) (Table 2). When administered i.p. to quail in vivo via osmotic pumps, GnIH significantly reduced gonadotropin common α and LHβ subunit mRNAs, as well as reducing plasma LH (20) (Table 2). Thus it is clear that, in birds, GnIH not only reduces the release of the gonadotropins from the pituitary, but also reduces gonadotropin subunit synthesis.
Table 2.
Hypophysiotropic actions of GnIH and its orthologs in vertebrates.
| Animal | Name | Function | Reference |
|---|---|---|---|
| Quail | GnIH | Inhibition of GTH release | Tsutsui et al. (8) |
| Inhibition of GTH synthesis and release | Ubuka et al. (20) | ||
| Chicken | GnIH | Inhibition of GTH synthesis and release | Ciccone et al. (11) |
| Sparrow | GnIH | Inhibition of GnRH-elicited GTH release | Osugi et al. (12) |
| Inhibition of GTH release | Osugi et al. (12) | ||
| Human | RFRP-1* | Stimulation of PRL release | Hinuma et al. (62) |
| Ovine | RFRP-3* | Inhibition of GnRH-elicited GTH release | Clarke et al. (28) |
| Inhibition of GnRH-elicited GTH synthesis | Sari et al. (33) | ||
| Rat | RFRP-3 | Inhibition of GTH release | Johson et al. (30) |
| Inhibition of GnRH-elicited GTH release | Murakami et al. (32) | ||
| Hamster | GnIH | Inhibition of GTH release | Kriegsfeld et al. (31) |
| Bullfrog | fGRP | Stimulation of GH release | Koda et al. (56) |
| fGRP-RP-2 | Stimulation of GH/PRL release | Ukena et al. (58) | |
| Goldfish | goldfish LPXRFa-1* | Stimulation of GTH and GH release | Amano et al. (68) |
| goldfish LPXRFa-2* | Stimulation of GTH and GH release | Amano et al. (68) | |
| goldfish LPXRFa-3 | Stimulation of GTH and GH release | Amano et al. (68) | |
| zebrafish LPXRFa-3* | Inhibition of GTH release | Zhang et al. (34) | |
Putative peptides; GTH, gonadotropin; GH, growth hormone; PRL, prolactin
Once GnIH had been discovered in birds, identification of the GnIH receptor (GnIH-R) was essential. Yin et al. (23) therefore identified and cloned a novel G protein-coupled receptor (GPCR) (GPR147) cDNA encoding a putative GnIH-R in quail. Crude membrane fractions of COS-7 cells transfected with the putative GnIH-R cDNA specifically bound to GnIH and GnIH-RPs, but not to neuropeptides lacking the C-terminal LPXRFamide (X = L or Q) motif (23). Scatchard plot analysis of the binding also showed that this putative GnIH-R possesses a single class of high affinity binding sites (Kd = 0.752 nM) for GnIH and GnIH-RPs (23). These data indicate that the identified GnIH-R is a functional receptor in the quail. Subsequently, GnIH-R has also been identified in chicken (43, 44) and European starling (21).
To clarify the mode of action of GnIH on gonadotropin release and synthesis, the expression of GnIH-R mRNA was further characterized in birds (23). Southern blotting analysis of reverse-transcriptase-mediated PCR products revealed the expression of GnIH-R mRNA in the pituitary and several brain regions, including the hypothalamus, in the quail (23). Because GnIH-R-ir cells were colocalized with LHβ mRNA- and FSHβ mRNA-containing cells in the quail pituitary (unpublished observation) and chickens (44), GnIH can act directly on gonadotropes in the pituitary via its specific receptor to inhibit gonadotropin release and synthesis. Although GnIH fibers project to the ME and GnIH-R is present on gonadotropes, further studies are needed to determine whether GnIH is effectively released into the hypophysial portal system to control gonadotrope function in a physiologically relevant manner.
The expression of GnIH-R mRNA was significantly higher in the pituitaries of sexually immature chickens relative to sexually mature chickens (44). Administration of estradiol (E2) or E2 plus progesterone (P4) to chickens caused a significant decrease in the amount of pituitary GnIH-R mRNA relative to vehicle controls (44). Thus, the expression of GnIH-R in birds may be down-regulated by increased circulating E2 and P4 levels during sexual maturation. As already described, in vivo treatment with GnIH inhibits GnRH-elicited LH release in sparrow species (9, 12). Bentley et al. (9) and Ubuka et al. (21) found that GnIH neurons project to GnRH neurons as well as to the ME in birds. Furthermore, GnIH-R was expressed in GnRH-I neurons as well as in the pituitary gland (21). Thus, GnIH appears to act on GnRH-I neurons to inhibit gonadotropin release and synthesis, in addition to acting on the pituitary gland.
GnIH-R was shown to inhibit adenylyl cyclase (AC) activity and alter GnRH-induced stimulation of cAMP responsive elements (CRE) on target genes (45). To understand the mode of action of GnIH in more detail, the analysis of the secondary messenger system downstream of GnRH-R is now in progress.
To demonstrate the functional significance of GnIH, we investigated the effects of GnIH on gonadal development and maintenance in male quail (20). Chronic treatment with GnIH via osmotic pumps decreased plasma testosterone concentrations as well as gonadotropin synthesis and release (20). Further, administration of GnIH to mature birds induced testicular apoptosis and decreased spermatogenic activity in the testis (20). In immature birds, chronic treatment with GnIH suppressed normal testicular growth and plasma testosterone concentrations (20). These results show that GnIH inhibits gonadal development and maintenance by inhibiting gonadotropin synthesis and release. Thus, GnIH may act as an important regulatory factor in reproductive maturity and competence [for reviews, see (15, 17, 24, 27)]. It is presumed that the antigonadal effects of GnIH in these experiments are mediate via GnIH action on pituitary gonadotropin synthesis and release. However, these results do not preclude a direct effect of GnIH on the gonads (see refs 46, 47).
The mechanisms regulating GnIH biosynthesis have been investigated in birds. The pineal gland and eyes are the major sources of melatonin in quail (48). We found that pinealectomy combined with orbital enucleation (Px + Ex) decreased the expression of GnIH precursor mRNA and the mature GnIH peptide in the diencephalon (19). Melatonin administration to Px + Ex birds caused an increase in the expression of GnIH precursor mRNA and production of mature peptide (19). In addition, the expression of GnIH increased under short day (SD) photoperiods (19), when the nocturnal duration of melatonin secretion increases (49). Mel1c, a melatonin receptor subtype, was expressed by GnIH-ir neurons in the PVN, suggesting a direct action of melatonin on GnIH neurons (19). These data indicate that melatonin acts directly on GnIH neurons via its receptor to induce GnIH expression and that GnIH is capable of transducing photoperiodic information to the avian reproductive axis via changes in the melatonin signal. To further explore this possibility, we investigated the role of melatonin in the regulation of GnIH release and the correlation of GnIH release with LH release in quail. Melatonin administration increased GnIH release from hypothalamic explants in vitro (50). Furthermore, GnIH release was photoperiodically controlled in quail with diurnal changes negatively correlated with plasma LH concentrations; GnIH release increased at night and LH decreased during the same time period (50). Based on these results, we suggest that melatonin plays a role in stimulating not only GnIH expression but also GnIH release, thus inhibiting plasma LH concentrations in quail.
Evolutionary history of GnIH structure and function
To begin to understand the evolutionary history of GnIH, several studies have been conducted to identify GnIH orthologs in other vertebrates. As summarized in Table 1, GnIH orthologs have now been documented in a variety of vertebrates from fish to mammals, including humans: RFamide-related peptides (RFRPs) in mammals (51–55), frog growth hormone-releasing peptide (fGRP) and fGRP-RPs in amphibian (56–58) and goldfish LPXRFamide peptide (gfLPXRFa) in teleosts (59).
In amphibians, a GnIH ortholog which consisted of 12 amino acids (SLKPAANLPLRFamide) with a C-terminal LPLRFamide motif was identified in the bullfrog hypothalamus (56) (Table 1). This frog neuropeptide, named fGRP, has a high sequence homology (75%) with quail GnIH (8, 56). Note that GnIH and fGRP possess the same C-terminal motif, LPLRFamide (8, 56). The fGRP precursor also encoded one fGRP and three fGRP-related peptides [fGRP-RP-1, -2, and -3 (57)], later identified as mature LPXRFamide peptides (58) (Table 1). At the same time, fGRP and fGRP-RP-1 were independently purified from the European green frog [R-RFa (60)] and bullfrog [fNRP (61)], respectively. Subsequently, from the goldfish brain, a cDNA that encoded three fish GnIH orthologs [goldfish LPXRFamide peptide (gfLPXRFa)-1, -2, and -3)] was characterized and gfLPXRFa-3 was identified as a mature peptide (59) (Table 1). In the mammalian brain, cDNAs that encode GnIH orthologs have been uncovered from a gene database search (62). The cDNAs of human, monkey and cow (bovine) also encoded three GnIH orthologs, which were termed RFRP-1, -2 and -3 (62). However, in contrast to other vertebrates, RFRP-2 is not predicted to be an RFamide peptide, although this mature peptide has not yet been isolated so it is unknown if it is actually cleaved from the precursor. Subsequently, RFRP-1 and -3 were purified from bovine hypothalami (51, 55) (Table 1). Ukena et al. (54) also identified RFRP-3 in the rat hypothalamus (Table 1). Recently, Ubuka et al. (52, 53) further identified RFRP-3 in the monkey hypothalamus and RFRP-1 and -3 in the human hypothalamus (Table 1). Mammalian and primate RFRP-1 and -3 also contain a C-terminal LPXRFamide (X = L or Q) sequence (51–55) as does avian GnIH (Table 1). Because all of the identified neuropeptides possess a LPXRFamide (X = L or Q) motif at their C-termini (Table 1), we designated GnIH and these GnIH orthologs as LPXRFamide peptides which form new members of the RFamide peptide family [for reviews, see (24–27) and Fig 1]. Thus, the presence of GnIH and its orthologs is a conserved property in vertebrates.
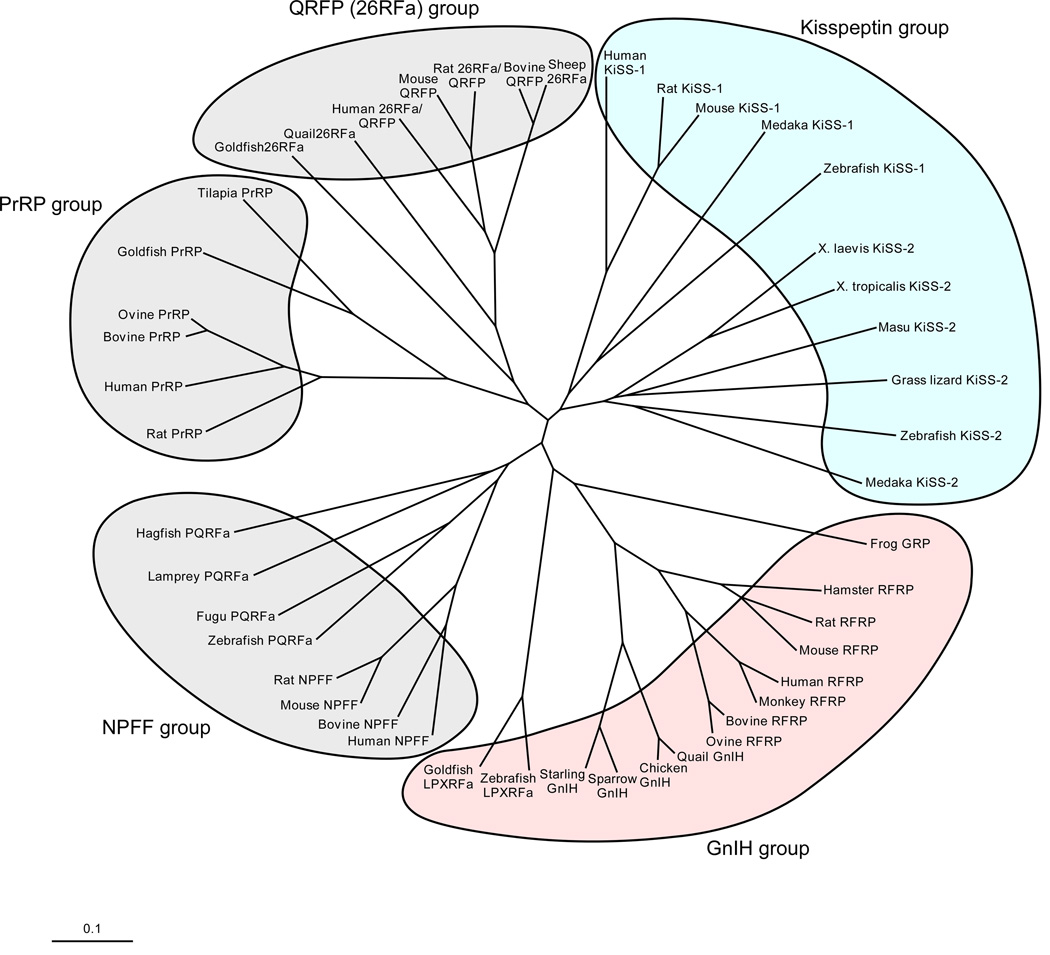
Fig. 1.
Phylogenetic tree of the RFamide peptide family. Extensive studies over the past decade have demonstrated that vertebrate brains produce a variety of RFamide peptides. To date, five groups of the RFamide peptide family have been documented as follows: (1) NPFF group, (2) PRL-releasing peptide (PrRP) group, (3) GnIH group, (4) kisspeptin group, (5) pyroglutamylated RFamide peptide (QRFP)/26RFamide group. GnIH was discovered in the quail hypothalamus. GnIH inhibits gonadotropin synthesis and release in birds. GnIH orthologs sharing a common C-terminal LPXRFamide (X = L or Q) motif have been identified in other vertebrates from fish to humans. As in birds, mammalian and fish GnIH orthologs inhibit gonadotropin secretion. By contrast, the newly identified neuropeptide kisspeptin, encoded by the KiSS-1 gene, acts to stimulate the reproductive axis. Mammalian kisspeptin possesses a C-terminal RFamide or RYamide motif. The KiSS-1 gene has been identified in mammals, amphibians and fish. A second isoform of KiSS-1, designated KiSS-2, has also been identified in various vertebrates, but not birds, rodents and primate.
The identified GnIH orthologs also regulate pituitary hormone release [for reviews, see (24–27) and Table 2]. Mammalian cDNAs encode the two biologically active GnIH orthologs, i.e., RFRP-1 and RFRP-3. To date, mammalian GnIH orthologs, RFRP-1 and RFRP-3, have been identified in cow (both RFRP-1 and RFRP-3), rat (RFRP-3 only), monkey (RFRP-3 only) and human (both RFRP-1 and RFRP-3) brain (51–55, 63, 64). When injected via intracerebroventricular (i.c.v.) cannulae or i.p. GnIH reduces plasma LH levels in ovariectomized Syrian hamsters (31). Likewise, i.c.v. injections of RFRP-3 reduce plasma levels of LH in one study on rats (30) although in a separate study RFRP-3 was found to reduce plasma LH only after intravenous (i.v.) administration, and not after i.c.v. administration (32). The reasons for these disparate data are not clear, but GnIH and RFRP-3 act as functional orthologs in terms of reducing circulating gonadotropin concentrations.
In all mammalian species studied to date (30, 31, 52, 53, 65), RFRP terminal fibers create putative contacts with GnRH cells, suggesting important functional interactions. In mice, direct application of RFRP to GnRH cells in cultured brain slices decreases firing rate in a subpopulation of cells, further suggesting a direct action of RFRP on GnRH neurons (66). In addition to the evidence for RFRP action on GnRH neurons, RFRP may impact gonadotrope function as seen in birds. Suggestive evidence for this comes from studies showing that the RFRP receptor (RFRP-R; GPR147) is localized to rat, Syrian hamster and human pituitary (29, 53, 62) and RFRP-ir fibers have been reported to extend into the external layer of the ME in hamsters, sheep, monkeys and humans (28, 29, 52, 53). Additionally, recent studies in rats suggest that RFRP inhibits GnRH-elicited LH release at the level of the pituitary (32). However, in another study, RFRP-ir fibers were not observed in the ME and i.p. injections of the retrograde tracer Fluorogold labeled very few RFRP cell bodies in rats (67), suggesting that these cells do not reach the hypophysial portal system. Despite this, in the same study peripheral (i.v.) injections of RFRP-3 rapidly (within 3 min) inhibited GnRH-induced LH secretion in ovariectomized rats, indicating action of RFRP-3 on pituitary gonadotropin secretion. Notwithstanding such a difference in the projections of RFRP-ir terminals to the neurosecretory zone of the ME in mammals, Clarke et al. (28) also found that peripheral administration of the deduced ovine GnIH ortholog, RFRP-3, reduces the amplitude of LH pulses in sheep, and reduces LH and FSH release in vitro. Likewise, in culture, RFRP-3 inhibits GnRH-stimulated LH and FSH secretion associated with a reduction in LHβ and FSHβ subunit expression (33). Thus, it is considered that GnIH and its mammalian ortholog RFRP-3 act to inhibit gonadotropin release and synthesis in both birds and mammals (see Table 2). However, more detailed studies, analyzing peptide release into the hypophysial portal blood, peptide projection by retrograde labeling, etc., are needed to elucidate the hypophysiotropic actions of GnIH and its mammalian ortholog RFRP-3. More detailed information in mammals has been described by Kriegsfeld et al. in this volume.
In a marked departure from the actions of GnIH and its mammalian homolog, RFRP-3, frog GnIH orthologs (fGRP and fGRP-RP-2) stimulate the release of growth hormone (GH) and/or PRL in amphibians both in vitro and in vivo (56, 58) (Table 2). On the other hand, goldfish GnIH orthologs (gfLPXRFa-1, -2 and -3) stimulate the release of gonadotropins and GH but not PRL in vitro (68) (Table 2). However, another recent study (34) found that the zebrafish GnIH ortholog (zfLPXRFa-3) reduces LH release in goldfish in vivo (Table 2). The reason for this discrepant finding in fish might be due to technical variation across studies. Therefore, further studies are necessary to clarify the generality of GnIH function on gonadotropin release in lower vertebrates.
Evolutionary origin of GnIH
To clarify the evolutionary origin of GnIH and its orthologs, Tsutsui and co-workers further investigated novel LPXRFamide peptides from the brain of sea lamprey and hagfish, two extant groups of the oldest lineage of vertebrates, Agnatha. Three PQRFamide peptides, another RFamide group in the RFamide peptide family, were found in the brain of sea lamprey (69). Lamprey PQRFamide peptides have the C-terminal structure PQRFamide similar to LPXRFamide peptides (X = L or Q). However, neither LPXRFamide peptides nor their cDNA could be identified from the brain of sea lamprey (69). On the other hand, three PQRFamide peptides and one LPXRFamide peptide were found in the brain of hagfish. In the hagfish, two cDNAs were identified; one encoded three PQRFamide peptides and the other encoded two PQRFamide peptides and one LPXRFamide peptide (Osugi et al., unpublished results). Hagfish LPXRFamide peptide may be involved in the regulation of pituitary hormones because this peptide was expressed in the hypothalamus (Osugi et al., unpublished results). Further studies, such as genome synteny analysis and functional analysis of hagfish LPXRFamide peptide, are needed to clarify whether the hagfish peptide may be the earliest known evolutionary origin of GnIH and its orthologs.
Kisspeptin
Discovery and function of kisspeptin
Following the discovery of GnIH in 2000, kisspeptin, encoded by the KiSS-1 gene, was discovered in mammals. As with GnIH, kisspeptin is also a member of the RFamide peptide family (Fig 1). In contrast to GnIH, kisspeptin has a stimulatory effect on GnRH neurons via its receptor, GPR54, and an up-regulation of the HPG axis.
A major breakthrough in the initial discovery of kisspeptin came when the ligand of an orphan GPCR, OT7T175, which is nearly identical to AXOR12 and GPR54, was identified. Ohtaki et al. (70) found that an extract from human placenta reacts with OT7T175, and purified a novel 54-amino acid-residue RFamide peptide, the KiSS-1 gene product. Although KiSS-1 was known to be a human metastasis suppressor gene that suppresses metastasis of human melanomas and breast carcinomas without affecting tumorigenicity, until recently the KiSS-1 gene product and its functional mechanism had not been clarified. The KiSS-1 gene product was originally referred to as metastin (71, 72) and inhibited chemotaxis and invasion of OT7T175-transfected Chinese hamster ovary cells in vitro, and attenuated the pulmonary metastasis of the receptor-transfected B16-BL6 melanomas in vivo. Two other research groups reported similar results, and termed the peptide KiSS-1 (73) and kisspeptin (74). Based on this nomenclature history, in this review the gene product of the KiSS-1 gene will be referred to as KiSS-1 mRNA and its peptide product as kisspeptin. Muir et al. (73) showed that KiSS-1 mRNA was mainly present in the placenta and brain. In the brain, KiSS-1 mRNA is highly expressed in the hypothalamus and basal ganglia. A role for kisspeptin in regulating reproductive function was first reported in 2003 when individuals with hypogonadism were found to have a mutation in the GPR54 gene (75, 76). In humans and mice lacking GPR54, the hypothalamus fails to drive adequate secretion of gonadotropins (75, 76). These results indicate that kisspeptin and its receptor may play an important role in the regulation of the HPG axis.
To date, it appears that kisspeptin neurons located within the hypothalamus provide direct excitatory inputs to GnRH neurons. Kisspeptin activates GnRH (77) and LH (78– 80) secretion in vivo, and GnRH neurons are surrounded by kisspeptin fibers (81), express GPR54 mRNA (82), and are activated intensely by kisspeptin (83). Kisspeptin is unable to stimulate LH secretion in GPR54 knockout mice (77), and kisspeptin knockout mice are infertile (84). Thus, kisspeptin and GPR54 are essential for puberty and subsequent fertility in mammals. The majority of research on kisspeptin to date has focused on mammals that maintain relatively continuous reproductive function (e.g., laboratory mice and rats, monkeys and humans). In addition, the kisspeptin system is likely critical for seasonal changes in the reproductive axis of seasonally breeding hamsters (85, 86) and sheep (65, 87–89).
As for a direct action of kisspeptin on gonadotropes, conflicting findings have been reported in mammals (90–95), although GPR54 is expressed both in pituitary cells and GnRH neurons (73, 74, 77, 82). Several reports have indicated that kisspeptin stimulates gonadotropin release in vitro from cultured rat, ovine and bovine primary pituitary cells, suggesting that kisspeptin may act directly on gonadotropes to stimulate gonadotropin secretion (71–73, 75). In contrast, other studies have shown no apparent effect of kisspeptin on in vitro gonadotropin secretion in cultured rat primary pituitary cells or pituitary fragments (70, 74). The reasons for these discrepant findings are unclear and might result from technical variations across studies. It is known that kisspeptin-ir fibers appear to come into close association with GnRH terminals in the ME of mammals (96–98), whereas several reports show the absence of kisspeptin-ir fibers in the external zone of the ME of various mammalian species (81, 98, 99). Thus, whether gonadotropes are targets for kisspeptin action remains unresolved in mammals (for reviews, see 99, 100). To draw a firm conclusion regarding a hypophysiotropic action of kisspeptin, it would be critical to show the release of kisspeptin into the hypophysial portal system as for GnIH.
Evolutionary origin and history of kisspeptin
The human KiSS-1 gene encodes a 145-amino acid precursor that is enzymatically cleaved into peptides of 54, 14, 13, or 10 amino acids that share a common C-terminal sequence with Phe-amidation (70). KiSS-1 genes have been identified in other mammalian species, including rat and mouse (101, 102) (Fig. 1). Kisspeptins in these species show a high conservation of the C-terminal decapeptide sequence with Tyr-amidation (Table 3). Indeed, this C-terminal decapeptide (kisspeptin-10, metastin 45–54) has been shown to be the minimal sequence required for receptor activation (70). Recently, a few KiSS-1 genes have been isolated in fish and amphibian species (103, 104). It is noteworthy that the C-terminal decapeptide of zebrafish and medaka KiSS-1 differs by only one amino acid at position 3 from those of rodents, while the decapeptides of fugu and tetraodon reveal three amino acid substitutions at positions 1, 3, and 5 (103) (Table 3). This relatively high variation within fish species proposed a possibility that KiSS-1s in fugu and tetraodon are isofroms of the KiSS-1s in zebrafish, medaka and mammals. Later, a fugu- and tetraodon-like KiSS-1 was indentified in the African clawed frog (Xenopus laevis) and a synthetic Xenopus KiSS-1 dodecapeptide was found to be more potent than mammalian kisspeptin-10 in activating the bullfrog kisspeptin receptor, bfGPR54, expressed in CV-1 cells (105).
Table 3.
Potent kisspeptin forms in vertebrates.
| Animal | Gene | Putative potent kisspeptin form | Reference |
|---|---|---|---|
| Human | KiSS-1 | YNWNSFGLRFa | Ohtaki et al. (70) |
| Mouse/Rat | KiSS-1 | YNWNSFGLRYa | Stafford et al. (101) |
| Platypus | KiSS-1 | YNWNSFGLRYa | Lee et al. (94), Um et al. (110) |
| KiSS-2 | GQFNFNPFGLRFa | Lee et al. (94), Um et al. (110) | |
| Chicken | Absent | Um et al. (99) | |
| Anole lizard | KiSS-2 | SKFNFNPFGLRFa | Lee et al. (106), Um et al. (99) |
| Western clawed frog | KiSS-1a | pEAQVGYNVNSFGLRYa | Lee et al. (106) |
| KiSS-1b | DLSTYNWNSFGLRYa | Lee et al. (106) | |
| KiSS-2 | SKFNFNPFGLRFa | Lee et al. (106) | |
| African clawed frog | KiSS-1 | DLSTYNWNSFGLRYa | Lee et al. (106) |
| KiSS-2* | SKFNFNPFGLRFa | Lee et al. (106) | |
| Zebrafish | KiSS-1 | pENVAYYNLNSFGLRYa | van Aerle et al. (103), Lee et al. (106), Kitahashi et al. (107) |
| KiSS-2 | SKFNYNPFGLRFa | Lee et al. (106), Kitahashi et al. (107) | |
| Medaka | KiSS-1 | pENVAYYNLNSFGLRYa | van Aerle et al. (103), Kanda et al. (104), Lee et al. (106) |
| KiSS-2 | SKFNYNPFGLRFa | Lee et al. (106) | |
Putative kisspeptin forms are predicted based on the amino acid sequence analysis and/or functional activity at their own receptors (106). For fish KiSS-1 and frog KiSS-1a, N-terminus Gln of the peptide is likely pyroglutamylated (pE).
African clawed frog KiSS-2 was identified as a mature peptide.
Following these reports, cDNAs for the KiSS-1 isoform, KiSS-2, have been further identified in many vertebrate species including lamprey, elephant shark, zebrafish, medaka, see bass, goldfish, western clawed frog (Xenopus (or Silurana) tropicalis), anole lizard, and platypus, a mammalian monotreme species (106–109) (Fig. 1 and Table 3). In addition, another isoform, KiSS-1b, was isolated in X. tropicalis (106). Thus, many vertebrates possess two or three kisspeptin genes (Table 3). The KiSS-1 cDNAs encode kisspeptin decapeptides in which Tyr or Phe residue at the C-terminal end are α-amidated (Table 3). The KiSS-1 decapeptides show variation at position 3 (Leu for fish species, Val for X. tropicalis KiSS-1b, and Trp for other species, including X. tropicalis KiSS-1a) (106, 107). It is of interest to note that fish KiSS-1 and X. tropicalis KiSS-1b precursors have a conserved dibasic site followed by conserved Gln five amino acids upstream of the decapeptide, which allows us to predict production of a mature peptide of 15 amino acids with N-terminal pyroglutamylation (Table 3). Indeed, these pentadecapeptides are more potent than decapeptides in activation of fish and X. tropicalis GPR54-1 (105). The family of the KiSS-2 decapeptide shows difference in three amino acids at positions 1, 3, and 5 compared to those of the KiSS-1 decapeptide (Table 3). Further, the presence of a basic amino acid, Arg, three amino acids upstream of the decapeptide suggests production of a mature peptide with 12 amino acids from the KiSS-2 gene (106) (Table 3). The presence of the KiSS-2 dodecapeptide has been demonstrated in the X. laevis brain using immunoaffinity purification (106).
The KiSS-1 and KiSS-2 genes reside at different chromosomes but chromosomal regions having the KiSS-1 and KiSS-2 genes share some paralogous genes such as GOLT1A/GOLT1B, PLEKHA5/PLEKHA6, PIK3C2B/PIK3CG, and ETNK1/ETNK2, indicating that the KiSS-1 and KiSS-2 genes have been driven by duplication of ancestral chromosome/paralogon (109). Genome synteny analysis data, together with BLAST search results, reveals that some vertebrate species, such as anole lizard (reptilian), chicken (avian), zebra finch (avian), and stickleback (fish) do not contain the KiSS-1 gene, while its neighboring genes such as PLEKHA6, GOLT1A, and REN are present (110). The absence of the KiSS-1 gene in some fish, avian and reptilian species, is likely due to gene loss during evolution. The KiSS-1b gene is only found in X. tropicalis. This gene is located in genome fragments containing ALDH16A1, PIH1D1, PTH2, KRAS and TEAD2. However, it is unable to find any of GOLT1A/GOLT1B, PLEKHA5/PLEKHA6, PIK3C2B/PIK3CG, and ETNK1/ETNK2 near the KiSS-1b gene. Thus, it is difficult to propose the origin of the KiSS-1b gene. The KiSS-2 genes are localized in a genome region containing GOLT1B, C12orf39, GYS2, and LDHB in fish and anole lizards. While these neighboring genes are well conserved for mammalian and avian species, the KiSS-2 gene is not found in this genomic region of these species (110). Thus, in avian species, both the KiSS-1 and the KiSS-2 genes are absent.
Conclusion
The discovery of GnIH in the avian brain has enhanced our understanding of vertebrate reproductive neurobiology. GnIH acts on GPR147 and inhibits gonadal development and maintenance through a decrease in gonadotropin release and synthesis in birds. GnIH orthologs have also been identified in the hypothalamus of a variety of other vertebrate species from fish to mammals, including humans. As in birds, mammalian and fish GnIH orthologs inhibit gonadotropin release, indicating that the inhibitory action of GnIH in the control of the HPG axis has been largely conserved during evolution. Following the discovery of GnIH, kisspeptin, encoded by the KiSS-1 gene, was discovered in mammals. In contrast to GnIH, kisspeptin has a stimulatory effect on GnRH neurons via GPR54 and up-regulates HPG axis activity. Both GnIH and kisspeptin are members of the RFamide peptide family. The distinct opposing roles of these two relatively newly discovered RFamide peptides suggest that GnIH and kisspeptin act as key neuropeptides controlling reproductive activity. Up until now, the KiSS-1 gene has been identified in amphibians and fish as well as in mammals. In addition, a second isoform of KiSS-1, designated KiSS-2, has been found in various vertebrates, but not in birds, rodents and primates. These findings underscore an important role for these novel RFamide peptides in the control of the HPG axis and point to exciting opportunities for further exploration.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (16086206 and 18107002 to K. T.) and by National Institutes of Health Grant HD-050470 (L. J. K.), National Science Foundation Integrative Organismal Systems Grant # 0641188 (to G. E. B.).
Grant support: Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (16086206, and 18107002 to K. T.) and by National Institutes of Health Grant HD-050470 (L. J. K.), National Science Foundation Integrative Organismal Systems Grant # 0641188 (to G.E.B.).
References
- 1.Matsuo H, Baba Y, Nair RMG, Arimura A, Schally AV. Structure of the porcine LH-and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 2.Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) Proc Natl Acad Sci USA. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King JA, Millar RP. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem. 1982;257:10722–10728. [PubMed] [Google Scholar]
- 4.Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kangawa K, Matsuo H. Isolation and characterization of chicken hypothalamic luteinizing hormone-releasing hormone. Biochem Biophys Res Commun. 1982;107:820–827. doi: 10.1016/0006-291x(82)90596-4. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. Identification of the second gonadotrophin-releasing hormone in chicken hypothalamus: Evidence that gonadotrophin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci USA. 1984;81:3874–3878. doi: 10.1073/pnas.81.12.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherwood N, Eiden L, Brownstein M, Spiess J, Rivier J, Vale W. Characterization of a teleost gonadotrophin-releasing hormone. Proc Natl Acad Sci USA. 1983;80:2794–2798. doi: 10.1073/pnas.80.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwood NM, Sower SA, Marshak DR, Fraser BA, Brownstein MJ. Primary structure of gonadotrophin-releasing hormone from lamprey brain. J Biol Chem. 1986;261:4812–4819. [PubMed] [Google Scholar]
- 8.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 9.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ. Gonadotrophin inhibitory hormone depresses gonadotropin α and follicle-stimulating hormone β subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 12.Osugi T, Ukena K, Bentley GE, O’Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrows: cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- 13.Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J. 2001;354:379–385. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsui K, Bentley GE, Ciccone N. Structure, action and functional significance of GnIH. In: Dawson A, Sharp PJ, editors. Functional Avian Endocrinology. New Delhi: Narosa Publishing House; 2005. pp. 73–82. [Google Scholar]
- 15.Tsutsui K, Ubuka T, Yin H, Osugi T, Ukena K, Bentley GE, Ciccone N, Inoue K, Chowdhury VS, Sharp PJ, Wingfield JC. Mode of action and functional significance of avian gonadotropin-inhibitory hormone (GnIH): A review. J Exp Zool A Comp Exp Biol. 2006;305:801–806. doi: 10.1002/jez.a.305. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, Sharp PJ, Wingfield JC. Review: The general and comparative biology of gonadotropin-inhibitory hormone (GnIH) Gen Comp Endocrinol. 2007;153:365–370. doi: 10.1016/j.ygcen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui K, Ubuka T, Yin H, Ukena K, Bentley G, Sharp P, Wingfield J. Review: Discovery of gonadotropin-inhibitory hormone in a domesticated bird, and its mode of action and functional significance. J Ornithol. 2007;147:53–54. [Google Scholar]
- 18.Ubuka T, Ueno M, Ukena K, Tsutsui K. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol. 2003;178:311–318. doi: 10.1677/joe.0.1780311. [DOI] [PubMed] [Google Scholar]
- 19.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. Nature Reviews Highlight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- 21.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-Inhibitory Hormone Neurons Interact Directly with Gonadotropin-Releasing Hormone-I and -II Neurons in European Starling Brain. Endocrinology. 2008;149:268–278. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 22.Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003b;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- 23.Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol. 2005;184:257–266. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsui K. Review: A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsui K, Ukena K. Review: Hypothalamic LPXRF-amide peptides in vertebrates: Identification, localization and hypophysiotropic activity. Peptides. 2006;27:1121–1129. doi: 10.1016/j.peptides.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization, and function. Mass Spectrom Rev. 2005;24:469–486. doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Review: Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.03.001. [Epub ahead of print; doi:10.1016/j.yfrne.2010.03.001] [DOI] [PubMed] [Google Scholar]
- 28.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 29.Gibson EM, Humber SA, Jain S, Williams WP3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AA, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brain of mammals. Proc Natl Acad Sci USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 33.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150:5549–5556. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Li S, Liu Y, Lu D, Chen H, Huang X, Liu X, Meng Z, Lin H, Cheng CHK. Structural diversity of the GnIH/GnIH receptor system in teleost: its involvement in early development and the negative control of LH release. Peptides. 2010 doi: 10.1016/j.peptides.2010.03.003. in press. [DOI] [PubMed] [Google Scholar]
- 35.Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg MJ, Price DA. Relationships among the FMRFamide-like peptides. Prog Brain Res. 1992;92:25–37. doi: 10.1016/s0079-6123(08)61162-0. [DOI] [PubMed] [Google Scholar]
- 37.Raffa RB. The action of FMRFamide (Phe-Met-Arg-Phe-NH2) and related peptides on mammals. Peptides. 1988;9:915–922. doi: 10.1016/0196-9781(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi RK, D'Aniello B, Pinelli C, Fiorentino M, Di Fiore MM, Di Meglio M, Iela L. FMRFamide in the amphibian brain: a comprehensive survey. Micros Res Tech. 2001;54:158–172. doi: 10.1002/jemt.1130. [DOI] [PubMed] [Google Scholar]
- 39.Dockray GJ, Reeve JR, Jr, Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305:328–330. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 40.Dockray GJ, Dimaline R. FMRFamide- and gastrin/CCK-like peptides in birds. Peptides. 1985;3:333–337. doi: 10.1016/0196-9781(85)90393-6. [DOI] [PubMed] [Google Scholar]
- 41.Small TW, Sharp PJ, Bentley GE, Millar RP, Tsutsui K, Mura E, Deviche P. Photoperiod-independent hypothalamic regulation of luteinizing hormone secretion in a free-living sonoran desert bird, the Rufous-winged sparrow (Aimophila carpalis) Brain Behav Evol. 2008;71:127–142. doi: 10.1159/000111459. [DOI] [PubMed] [Google Scholar]
- 42.Deviche P, Small T, Sharp P, Tsutsui K. Control of luteinizing hormone and testosterone secretion in a flexibly breeding male passerine, the Rufous-winged Sparrow. Aimophila carpalis. Gen Comp Endocrinol. 2006;149:226–235. doi: 10.1016/j.ygcen.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Maddineni SR, Ocon-Grove OM, Krzysik-Walker SM, Hendricks GL3rd, Ramachandran R. Gonadotropin-inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: potential role of GnIH in follicular maturation. Reproduction. 2008;135:267–274. doi: 10.1530/REP-07-0369. [DOI] [PubMed] [Google Scholar]
- 44.Maddineni S, Ocon-Grove OM, Krzysik-Walker SM, Hendricks GL3rd, Proudman JA, Ramachandran R. Gonadotrophin-inhibitory hormone receptor expression in the chicken pituitary gland: potential influence of sexual maturation and ovarian steroids. J Neuroendocrinol. 2008;20:1078–1088. doi: 10.1111/j.1365-2826.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Bedecarrats GY. Activation of the chicken gonadotropin inhibitory hormone receptor reduces gonadotropin releasing hormone receptor signaling. Gen Comp Endocrinol. 2010 doi: 10.1016/j.ygcen.2010.03.029. in press. [DOI] [PubMed] [Google Scholar]
- 46.Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 47.McGuire NL, Bentley GE. A functional neuropeptide system in vertebrate gonads: Gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus) Gen Comp Endocrinol. 2010 doi: 10.1016/j.ygcen.2010.01.010. [Epub ahead of print: doi:10.1016/j.ygcen.2010.01.010] [DOI] [PubMed] [Google Scholar]
- 48.Underwood H, Binkley S, Siopes T, Mosher K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 1984;56:70–81. doi: 10.1016/0016-6480(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 49.Cockrem JF, Follett BK. Circadian rhythm of melatonin in the pineal gland of the Japanese quail (Coturnix coturnix japonica) J Endocrinol. 1985;107:317–324. doi: 10.1677/joe.0.1070317. [DOI] [PubMed] [Google Scholar]
- 50.Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151:271–280. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- 51.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta. 2001;1540:221–232. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 52.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol. 2009;517:841–855. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- 53.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human gonadotropin-inhibitory hormone homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE. 2009;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta. 2003;1593:151–157. doi: 10.1016/s0167-4889(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 56.Koda A, Ukena K, Teranishi H, Ohta S, Yamamoto K, Kikuyama S, Tsutsui K. A novel amphibian hypothalamic neuropeptide: isolation, localization, and biological activity. Endocrinology. 2002;143:411–419. doi: 10.1210/endo.143.2.8630. [DOI] [PubMed] [Google Scholar]
- 57.Sawada K, Ukena K, Kikuyama S, Tsutsui K. Identification of a cDNA encoding a novel amphibian growth hormone-releasing peptide and localization of its transcript. J Endocrinol. 2002;174:395–402. doi: 10.1677/joe.0.1740395. [DOI] [PubMed] [Google Scholar]
- 58.Ukena K, Koda A, Yamamoto K, Kobayashi T, Iwakoshi-Ukena E, Minakata H, Kikuyama S, Tsutsui K. Novel neuropeptides related to frog growth hormone-releasing peptide: isolation, sequence, and functional analysis. Endocrinology. 2003;144:3879–3884. doi: 10.1210/en.2003-0359. [DOI] [PubMed] [Google Scholar]
- 59.Sawada K, Ukena K, Satake H, Iwakoshi E, Minakata H, Tsutsui K. Novel fish hypothalamic neuropeptide: Cloning of a cDNA encoding the precursor polypeptide and identification and localization of the mature peptide. Eur J Biochem. 2002;269:6000–6008. doi: 10.1046/j.1432-1033.2002.03351.x. [DOI] [PubMed] [Google Scholar]
- 60.Chartrel N, Dujardin C, Leprince J, Desrues L, Tonon MC, Cellier E, Cosette P, Jouenne T, Simonnet G, Vaudry H. Isolation, characterization, and distribution of a novel neuropeptide, Rana RFamide (R-RFa), in the brain of the European green frog. Rana esculenta. J Comp Neurol. 2002;448:111–127. doi: 10.1002/cne.10253. [DOI] [PubMed] [Google Scholar]
- 61.Kanetoh T, Sugikawa T, Sasaki I, Muneoka Y, Minakata H, Takabatake I, Fujimoto M. Identification of a novel frog RFamide and its effect on the latency of the tail-flick response of the newt. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:259–266. doi: 10.1016/s1532-0456(02)00277-6. [DOI] [PubMed] [Google Scholar]
- 62.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 63.Quennell JH, Rizwan MZ, Relf HL, Anderson GM. Developmental and steroidogenic effects on the gene expression of RFRP and its receptor in the rat brain and pituitary gland. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.01963.x. [Epub ahead of print, doi: 10.1111/j.1365-2826.2010.01963.x] [DOI] [PubMed] [Google Scholar]
- 64.Smith JT, Clarke IJ. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2009.11.010. [Epub ahead of print, doi: 10.1016/j.tem.2009.11.010] [DOI] [PubMed] [Google Scholar]
- 65.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 67.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150:1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 68.Amano M, Moriyama S, Iigo M, Kitamura S, Amiya N, Yamamori K, Ukena K, Tsutsui K. Novel fish hypothalamic neuropeptides stimulate the release of gonadotrophins and growth hormone from the pituitary of sockeye salmon. J Endocrinol. 2006;188:417–423. doi: 10.1677/joe.1.06494. [DOI] [PubMed] [Google Scholar]
- 69.Osugi T, Ukena K, Sower SA, Kawauchi H, Tsutsui K. Evolutionary origin and divergence of PQRFamide peptides and LPXRFamide peptides in the RFamide peptide family: Insights from novel lamprey RFamide peptides. FEBS J. 2006;273:1731–1743. doi: 10.1111/j.1742-4658.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- 70.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 71.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. [PubMed] [Google Scholar]
- 73.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 74.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 75.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 77.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 79.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 80.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 83.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current Biology. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 86.Greives TJ, Kriegsfeld LJ, Demas GE. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus) Gen Comp Endocrinol. 2008;156:552–558. doi: 10.1016/j.ygcen.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 88.Chalivoix S, Bagnolini A, Caraty A, Cognié J, Malpaux B, Dufourny L. Effects of photoperiod on kisspeptin neuronal populations of the ewe diencephalon in connection with reproductive function. J. Neuroendocrinol. 2009;22:110–118. doi: 10.1111/j.1365-2826.2009.01939.x. [DOI] [PubMed] [Google Scholar]
- 89.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 91.Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci. 2008;103:360–365. doi: 10.1016/j.anireprosci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 94.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 95.Richard N, Corvaisier S, Camacho E, Kottler ML. KiSS-1 and GPR54 at the pituitary level: overview and recent insights. Peptides. 2009;30:123–129. doi: 10.1016/j.peptides.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 98.Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36:131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–5404. [PubMed] [Google Scholar]
- 102.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 103.van Aerle R, Kille P, Lange A, Tyler CR. Evidence for the existence of a functional Kiss1/Kiss1 receptor pathway in fish. Peptides. 2008;29:57–64. doi: 10.1016/j.peptides.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 104.Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, Maeda K, Oka Y. Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes) Endocrinology. 2008;149:2467–2476. doi: 10.1210/en.2007-1503. [DOI] [PubMed] [Google Scholar]
- 105.Moon JS, Lee YR, Oh DY, Hwang JI, Lee JY, Kim JI, Vaudry H, Kwon HB, Seong JY. Molecular cloning of the bullfrog kisspeptin receptor GPR54 with high sensitivity to Xenopus kisspeptin. Peptides. 2009;30:171–179. doi: 10.1016/j.peptides.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 106.Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang J-I, Osugi T, Otaki N, Sunakawa Y, Kim K, Vaudry H, Kwon HB, Seong JY, Tsutsui K. Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology. 2009;150:2837–2846. doi: 10.1210/en.2008-1679. [DOI] [PubMed] [Google Scholar]
- 107.Kitahashi T, Ogawa S, Parhar IS. Cloning and expression of kiss2 in the zebrafish and medaka. Endocrinology. 2009;150:821–831. doi: 10.1210/en.2008-0940. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Zhang Y, Liu Y, Huang X, Huang W, Lu D, Zhu P, Shi Y, Cheng CH, Liu X, Lin H. Structural and functional multiplicity of the kisspeptin/GPR54 system in goldfish (Carassius auratus) J Endocrinol. 2009;201:407–418. doi: 10.1677/JOE-09-0016. [DOI] [PubMed] [Google Scholar]
- 109.Felip A, Zanuy S, Pineda R, Pinilla L, Carrillo M, Tena-Sempere M, Gomez A. Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals. Mol Cell Endocrinol. 2009;312:61–71. doi: 10.1016/j.mce.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 110.Um HN, Han JM, Hwang J-I, Hong SI, Vaudry H, Seong JY. Molecular coevolution of kisspeptins and their receptors from fish to mammals. Ann N Y Acad Sci. 2010 doi: 10.1111/j.1749-6632.2010.05508.x. in press. [DOI] [PubMed] [Google Scholar]
- 111.Ikemoto T, Park MK. Chicken RFamide-related peptide (GnIH) and two distinct receptor subtypes: identification, molecular characterization, and evolutionary considerations. J Reprod Dev. 2005;51:359–377. doi: 10.1262/jrd.16087. [DOI] [PubMed] [Google Scholar]