Abstract
Fever can provoke “febrile” seizures (FS). Because complex FS may promote development of temporal lobe epilepsy, understanding their mechanisms is clinically important. Using an immature rodent model and transgenic technology, we examined the role of interleukin-1β, (IL-1β), a pyrogenic, proinflammatory cytokine, in FS. IL-1β receptor–deficient mice were resistant to experimental FS. This resistance appeared independent of genetic background and was attributed to lack of IL-1β signaling, because exogenous cytokine reduced seizure threshold in wild-type but not receptor-deficient mice independent of strain. In addition, high IL-1β doses induced seizures only in IL-1β receptor–expressing mice. These data indicate that IL-1β signaling contributes critically to fever-induced hyperexcitability underlying FS, constituting a potential target for their prevention.
Fever is a systemic response to infection, inflammation, or stress.1 Fever evokes convulsions (febrile seizures [FSs]) in 3 to 5% of infants and children in the Western world, and up to 14% in Japan, which is consistent with genetic variability in the mechanisms for fever-evoked seizures.2 Long or repetitive FSs have been closely linked to the development of temporal lobe epilepsy, a commonly intractable disorder with enormous health consequences.3,4 Therefore, understanding the basis of FSs, thus enabling their potential prevention, is important. However, the mechanisms by which fever induces these seizures have not been elucidated.5
Several proinflammatory cytokines, including interleukin-1β (IL-1β), act as pyrogens,1 causing fever when administered into the lateral cerebral ventricles (icv) or peripherally.5 In addition, fever of any cause, as well as brain hyperthermia, induce IL-1β synthesis in brain microglia.6 When released, IL-1β binds to the type 1 receptor (IL-1RI) that is expressed by neurons in hippocampus and other regions that are sensitive to seizures,7 leading to enhanced neuronal excitability and decreased seizure threshold.5,8 The presence of neuro-immune mediators and their contribution to neuro-pathological processes has been established for neonatal brain.9 These observations have raised the possibility that IL-1β contributes to the generation of FS by two independent mechanisms: in addition to pyrogenesis, hyperthermia- or fever-induced synthesis and secretion of hippocampal IL-1β10 might directly evoke seizures. In this study, we tested the hypothesis that endogenous IL-1β contributes to the mechanisms of fever-induced seizures, and that these IL-1β actions are independent of genetic background.
Materials and Methods
Animals
Mice deficient in IL-1R1 (IL-1R1−/−) were generated on a 129/Sv background.11 Briefly, genomic clones of the IL-1R1 gene were isolated from a λ-129/Sv genomic library, and a targeting vector was constructed and transfected (by electroplation) into embryonic stem cells (ES) cells. Chimeric mice were originally mated to 129/Sv and C57BL female mice,11 but the mice used in this experiment were backcrossed to 129/Sv for more than six generations. IL-1R1−/− mice, as well as wild-type 129/Sv and C57BL mice (Charles River, Wilmington, MA), were kept in quiet facilities under controlled temperature and light schedule. The day of birth was considered postnatal day 0. All experimental procedures were approved by Institutional Animal Care Committees and conformed to National Institutes of Health guidelines.
Generation of Experimental Febrile Seizures
The experimental FS paradigm was modified from methods published for the rat.12 In brief, on postnatal days 14 and 15, mice were placed in a glass container, and hyperthermia (increased core and brain temperatures) was induced to (as during high fever) by using a regulated stream of heated air. Core temperatures were noted at baseline, at experimental FS onset, and every 2 minutes thereafter. Behavioral and electrographical seizures were correlated in a separate animal group (see later). The behavioral manifestations of FS in mouse are stereotyped: After hyperthermia-induced hyperactivity, sudden immobility signifies the onset of electroencephalograms seizures. The immobility is associated with reduced response to stimulation (“altered consciousness”) and often facial automatisms.
Specificity of Interleukin-1β and the Role of Genetic Background
To determine whether endogenous IL-1β contributed to the generation of FSs, we measured threshold temperatures in 26 IL-1R1−/− mice, 21 129/Sv wild-type mice, and 17 C57BL wild-type mice. To investigate the specificity of the increased resistance to FSs in IL-1R1−/− mice with the null mutation, we administered human recombinant IL-1β icv (5ng/μl, in sterile water)13 to wild-type and IL-1R1−/− mice (n = 14 and n = 21, respectively) 6 minutes before seizure induction.8 To eliminate potential confounders resulting from the infusion procedure, we infused vehicle (sterile water) to wild-type (n = 12) and IL-1R1−/− mice (n = 19). To study further whether IL-1β promoted seizures, we injected mouse recombinant IL-1β (R&D, Minneapolis MN; 116ng/2μl, icv) into wild-type (n = 5) and IL-1R1−/− (n = 5) mice, compared with vehicle-infused controls for each genotype (n = 3 each).
Electrophysiological Recording In Vivo
To study the concordance of behavioral and electrophysiological seizures provoked by hyperthermia and IL-1β, we implanted bipolar electrodes unilaterally in the dorsal hippocampus.12,13 Baseline hippocampal electroencephalograms were recorded 24 hours later, followed by seizure induction through hyperthermia or cytokine infusion. Hippocampal electroencephalograms were obtained in freely moving mice through long, flexible wires, and electrode and cannula placement was verified in all animals.
Statistical Considerations
Differences among groups were analyzed using Student's t test or one-way analysis of variance, as appropriate, followed by Bonferroni post hoc test. Significance = 0.05. Values are expressed as means ± standard error of the mean.
Results
Threshold Temperature for the Generation of Experimental Febrile Seizures Is Increased in the Absence of the Interleukin-1β Receptor
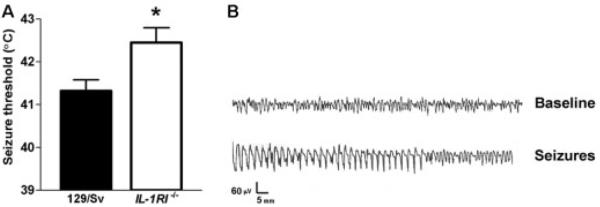
We reasoned that if the actions of IL-1β were required for the generation of FSs, then mice deficient in the IL-1R1 receptor (IL-1R1−/− mice) will be more resistant to the development of experimental FSs. Indeed, threshold temperatures for the onset of these seizures in IL-1R1–deficient mice were significantly greater than those in wild-type control mice of a similar genetic background (129/Sv; Fig 1A). This difference in threshold temperature (~0.9°C) is significant, because anticonvulsants effective for treatment of FSs in children increase seizure threshold to a similar degree (Dubé and Baram, unpublished data). The experimental FSs involved the hippocampal formation, as shown by recordings from bipolar electrodes positioned in the hippocampi of a separate group of freely moving mice (see Fig 1B). Behaviorally, the seizures consisted of sudden immobility and reduced response to tactile stimulation, sometimes associated with limbic automatisms.
Fig 1.
(A) Mice deficient in the interleukin-1 receptor (IL-1R1−/−) are more resistant to the generation of experimental febrile seizures (FSs) compared with wild-type control mice of a similar genetic background (129/Sv). Threshold temperatures for the onset of these seizures, a reliable measure of susceptibility,12 were significantly greater in IL-1R1−/− mice: 42.4 ± 0.3°C versus 41.3 ± 0.2°C in wild-type mice. (See Materials and Methods for details of IL-1R1−/− mice generation and background.) (B) Hippocampal electroencephalogram recordings from unanesthetized wild-type mouse. Normal θ rhythm is evident, which is attenuated by progressive hyperthermia and is replaced by epileptiform discharges. The behavioral correlates of the electroencephalogram seizure consist of sudden immobility associated with reduced response to stimulation (“altered consciousness”), which is typically associated with limbic automatisms. * indicates p < 0.05
The Role of Interleukin-1β Signaling Is Independent of Genetic Background
The increased seizure threshold in IL-1R1−/− mice was consistent with a role for the IL-1 signaling cascade in fever-evoked seizures. However, several studies have found that genetic alterations of transgenic mouse strains, including effects of flanking genes or genetic compensation, may result in effects that are independent from those of the mutated gene.14,15 To examine this possibility, we also measured threshold temperatures for the onset of FSs in wild-type C57BL mice; these temperatures were found to be significantly less than those in wild-type 129/Sv (Fig 2A). These differences in seizure threshold between the two mouse strains suggested that 129/Sv and C57BL mice carry gene repertoires that intrinsically confer differences in susceptibility to the induction of experimental FSs and highlighted the importance of considering the specific mouse strain. Therefore, it was crucial to determine whether the resistance to experimental FSs found in IL-1R1−/− mice was specific to the deletion of this receptor; this was determined by infusing both wild-type and IL-1R1–deficient (129/Sv) mice with IL-1β.
Fig 2.
The resistance to experimental febrile seizures (FSs) in IL-1R1−/− mice appears independent of potential confounders related to their genetic background. (A) Experimental FS threshold in (wild-type) C57BL mice is less than in (wild-type) 129/Sv mice (39.7 ± 0.2°C vs 41.3 ± 0.2°C). A difference of gene repertoires between these two strains could underlie changes in susceptibility to the induction of experimental FSs. However, the absence of interleukin-1 type 1 receptor (IL-1R1) leads to significant increase of seizure threshold in both genetic strains. The asterisk indicates different from threshold of wild-type 129/Sv and C57BL. Minus sign indicates different from C57BL. (B) Infusion of 5ng IL-1β, but not of vehicle (water) into the cerebral ventricles (icv), does not affect seizure threshold of the IL-1R1−/− mice, but significantly (asterisk) decreases seizure threshold of wild-type mice of a similar genetic background, suggesting that the resistance to experimental FSs found in IL-1R1−/− mice is specific to the null mutation in the receptor gene.
High Doses of Interleukin-Iβ Are Convulsant in Immature Mice and Require the Interleukin-1β Type 1 Receptor
As expected, infusion of 5ng IL-1β (icv) decreased the threshold for experimental FSs in wild-type 129/Sv mice (see Fig 2B), but not in IL-1R1−/− mice, supporting the notion that their resistance to these seizures stemmed from their deficiency in the IL-1β signaling cascade. In addition, infusion of a high dose (116ng) of IL-1β to normothermic mice resulted in prolonged limbic seizures in wild-type 129/Sv mice (Fig 3), whereas the IL-1R1–deficient mice were resistant.
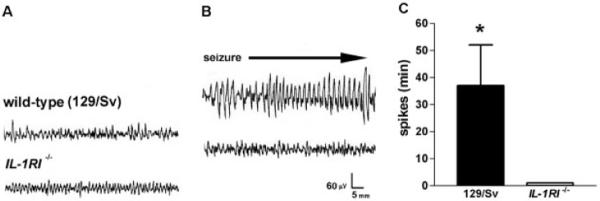
Fig 3.
High doses of interleukin (IL)-1β (116ng) result in limbic behavioral and electrical seizures only in wild-type mice. (A, B) Hippocampal electroencephalographic (EEG) recordings from mice before (A) and 3 hours after IL-1β administration (B). (A) Normal EEGs in wild-type (129/Sv) and IL-1R1−/− mice. (B) Differential effects of IL-1β in these two groups include: prolonged spike trains develop in wild-type 129/Sv mice, whereas the EEG remains normal in IL-1R1−/− mice. (C) Quantitative analysis of IL-1β–induced spike duration in the 6 hours after administration. IL-1β induces limbic seizures, with a mean duration of 37.0 ± 15.2 minutes (n = 5), in all wild-type mice, whereas no IL-1R1−/− mice (n = 5) experienced development of seizures.
Discussion
There are three principal findings of these experiments. First, absence of IL-1R1 provides resistance to the generation of experimental FSs. Second, the IL-1β signaling cascade is involved in FSs generated on a variety of genetic backgrounds, although the susceptibility to these seizures varies with mouse strain (as also found for different human populations). Third, high doses (~100ng) of this cytokine are sufficient to generate seizures even without increased brain temperature. Taken together, these data implicate IL-1β among the mechanisms by which fever provokes seizures in the developing brain.
The experiments described in this article suggest that IL-1β may contribute to the hyperexcitability and seizures generated by fever and hyperthermia. A likely mechanism for this effect is through increased N-methyl-D-aspartate function through IL-1R1–mediated activation of the Src family of kinases and subsequent NR2A/B subunit phosphorylation, resulting in increased intracellular calcium.16 IL-1R1 and N-methyl-D-aspartate receptors are colocalized on dendrites of hippocampal neurons, promoting cross talk between proinflammatory and excitatory pathways.
These findings may also provide important clues for the natural variability in the prevalence of FSs in diverse human populations worldwide: increased expression and release of IL-1β may confer susceptibility to these seizures. Indeed, polymorphisms in the IL-1β gene have been associated with FSs. Specifically, increased frequency of the IL-1β-511*2 allele, a variant that promotes enhanced expression of the cytokine, has been found in children with FSs compared with the appropriate ethnic cohort.17 In this context, it should be noted that increased IL-1β levels have been reported in cerebrospinal fluid of children with FSs18 (see also Lahat and colleagues19). Interestingly, homozygosity for the same gene variant, promoting enhanced cytokine production, the IL-1β-511*2 allele was overrepresented in temporal lobe epilepsy patients with hippocampal sclerosis compared with both control subjects and temporal lobe epilepsy patients without hippocampal sclerosis.20 This suggests that this cytokine also contributes to seizure-related hippocampal lesions.
In summary, the data presented in this article suggest that IL-1β contributes to the generation of human FSs, and it potentially contributes to long-lasting hyperexcitability and excitotoxicity associated with hippocampal epilepsy. Better understanding of this relation should lead to therapeutic strategies targeting the IL-1β signaling cascade.
Acknowledgments
This study was supported by the NIH (National Institute of Mental Health, T.B.; National Institute of Neurological Disorders and Stroke, NS28912 and NS35439, T.Z.B.; R01 NS043501, M.B., T.B.), the Ellison Foundation (T.B.), and an Epilepsy Foundation of America postdoctoral research fellowship (EP-36421, C.D.).
We thank M. Hinojosa for expert editorial help and R. Gonzalez-Vega for technical assistance.
References
- 1.Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179:S294–S304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35:S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 3.Cendes F, Andermann F. Do febrile seizures promote temporal lobe epilepsy? Retrospective studies. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego: 2002. pp. 77–86. [Google Scholar]
- 4.Shinnar S. Do febrile seizures lead to temporal lobe epilepsy? Prospective and epidemiological studies. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego: 2002. pp. 87–101. [Google Scholar]
- 5.Gatti S, Vezzani A, Bartfai T. Mechanisms of fever and febrile seizures: putative role of interleukin-1 system. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego: 2002. pp. 169–188. [Google Scholar]
- 6.Haveman J, Geerdink AG, Rodermond HM. Cytokine production after whole body and localized hyperthermia. Int J Hyperthermia. 1996;12:791–800. doi: 10.3109/02656739609027685. [DOI] [PubMed] [Google Scholar]
- 7.Takao T, Tracey DE, Mitchell WM, De Souza EB. Interleukin-1 receptors in mouse brain: characterization and neuronal localization. Endocrinology. 1990;127:3070–3078. doi: 10.1210/endo-127-6-3070. [DOI] [PubMed] [Google Scholar]
- 8.Vezzani A, Conti M, De Luigi A, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barks JD, Silverstein FS. Inflammation and neonatal brain injury. In: Donn SM, Sinha SK, Chiswick ML, editors. Birth asphyxia and the brain. Futura; Armonk, NY: 2002. pp. 71–87. [Google Scholar]
- 10.Cartmell T, Southgate T, Rees GS, et al. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci. 1999;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labow M, Shuster D, Zetterstrom M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 12.Dubé C, Chen K, Eghbal-Ahmadi M, et al. Prolonged febrile seizures in immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- 13.Baram TZ, Hirsch E, Snead OC, 3rd, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- 16.Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1 beta (−511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192–195. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- 18.Ichiyama T, Nishikawa M, Yoshitomi T, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50:407–411. doi: 10.1212/wnl.50.2.407. [DOI] [PubMed] [Google Scholar]
- 19.Lahat E, Livne M, Barr J, Katz Y. Interleukin-1 beta levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr Neurol. 1997;17:34–36. doi: 10.1016/s0887-8994(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 20.Kanemoto K, Kawasaki J, Miyamoto T, et al. Interleukin (IL)-1 beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571–574. [PubMed] [Google Scholar]