Abstract
The authors have previously documented decreased epithelial basement membrane (BM) components and α3β1 epithelial integrin, and increased expression of matrix metalloproteinase (MMP)-10 in corneas of patients with diabetic retinopathy (DR) compared to normal corneas. The purpose of this study was to examine if organ-cultured DR corneas exhibited the same alterations in wound healing and diabetic marker distribution as the autopsy DR corneas. Twenty normal and 17 DR corneas were organ-cultured in serum-free medium over agar–collagen gel at the air–liquid interface for up to 45 days. Circular 5 mm central epithelial wounds were made with n-heptanol, the procedure that will preserve fragile diabetic corneal BM. Wound healing was monitored microscopically every 12 hr. Distribution of diabetic corneal epithelial markers including laminin-10 α5 chain, nidogen-1/entactin, integrin α3β1, and MMP-10, was examined by immunofluorescence. Normal corneas healed the central epithelial defect within 3 days (mean=2.3 days), whereas DR corneas on average healed about two times slower (mean=4.5 days). In wounded and completely healed organ-cultured corneas, the patterns of studied markers were the same as in the unwounded organ-cultured corneas. This concerned both normal and DR corneas. As in vivo, normal organ-cultured corneas had continuous staining for laminin-10 and nidogen-1/entactin in the epithelial BM, strong and homogeneous staining for both chains of α3β1 integrin in epithelial cells, and little if any staining for MMP-10. Organ-cultured DR corneas also had marker patterns specific for in vivo DR corneas: interrupted to no staining for laminin-10 and nidogen-1/entactin in the epithelial BM, areas of weak or disorganized α3β1 integrin in epithelial cells, and significant MMP-10 staining in the epithelium and keratocytes. Fibrotic extracellular matrix and myofibroblast markers were largely absent. Thus, epithelial wound healing was much slower in organ-cultured DR corneas than in normal corneas, in complete accordance with clinical data in diabetic patients. DR corneas in organ culture preserved the same marker abnormalities as in vivo. The marker distribution was unchanged in wounded and healed organ-cultured corneas, compared to unwounded corneas. The established corneal organ culture provides an adequate system for elucidating mechanisms of epithelial alterations in human DR corneas.
Keywords: diabetic retinopathy, cornea, organ culture, basement membrane, integrin, laminin, nidogen, stromelysin, matrix metalloproteinase, MMP-10, tenascin-C, fibrillin-1, α-enolase, keratin 3
1. Introduction
Diabetic retinopathy (DR) is the leading cause of legal blindness in elderly people in the Western world (Aiello et al., 1998). It is a severe vision-impairing diabetic complication mainly affecting retinal vasculature. However, diabetes mellitus, both insulin-dependent (IDDM) and noninsulin-dependent (NIDDM), damages not only retina and lens, but also cornea, eyelids, iris, ciliary body and cranial nerves (Herse, 1988; Lim and Murphy, 1991; Pickup and Williams, 1994). Corneal abnormalities are found in more than 70% of diabetic patients (Didenko et al., 1999). It was recommended that routine eye examination in diabetic patients should include assessment of cornea (Aiello et al., 1998). Corneal epithelial alterations are very frequent in diabetes and are referred to as diabetic keratopathy (Herse, 1988; Ohashi, 1997; Aiello et al., 1998; Sánchez-Thorin, 1998; Didenko et al., 1999). Clinically observed corneal diabetic alterations include epithelial defects, fragility and recurrent erosions, ulcers, edema, decreased sensitivity, abnormal wound repair, increased autofluorescence and susceptibility to injury (Herse, 1988; Cavallerano, 1992; Chang et al., 1995; Saini and Khandalavla, 1995; Saini and Mittal, 1996; Ohashi, 1997; Sánchez-Thorin, 1998; Van Schaik et al., 1998–1999; Didenko et al., 1999; Zagon et al., 2002). Experimental studies have detected abnormal epithelial basement membrane (BM), decreased number of hemidesmosomes, functional impairment of the endothelium (Tabatabay et al., 1988; Azar and Gipson, 1989; Azar et al., 1989; 1992; Ljubimov et al., 1996; Meller et al., 1996; Saini and Mittal, 1996; Sato et al., 1999) in diabetic corneas. Increased autofluorescence and epithelial fragility are augmented in DR patients (Chang et al., 1995; Saini and Mittal, 1996; Van Schaik et al., 1998–1999). Diabetic patients account for more than 80% of cases with corneal complications after vitrectomy for vitreous hemorrhage (Sánchez-Thorin, 1998). Diabetes also presents a contra-indication to refractive surgery (Sánchez-Thorin, 1998). Diabetic corneal neuropathy accompanies epitheliopathy/keratopathy and is more advanced in DR patients (Saini and Mittal, 1996). Treatment of diabetic corneal problems remains symptomatic (Cavallerano, 1992). In preliminary studies, aldose reductase inhibitors had a positive effect but they are still at the clinical trial stage (Cavallerano, 1992; Sánchez-Thorin, 1998). In a rat model of diabetes, impaired corneal wound healing could be significantly improved by treatment with naltrexone, an opioid antagonist (Zagon et al., 2002). Overall, diabetic corneal disease is a significant clinical problem. Its efficient treatment is hampered by lack of information about molecular changes in diabetic corneas and underlying mechanisms.
Since many of the diabetic corneal abnormalities are apparently related to changes in cell adhesion and tissue repair, they are likely to be due to alterations of adhesive molecules of the extracellular matrix (ECM) and BM. Our previous data (Ljubimov et al., 1996; 1998a) showed that DR corneas had a significant decrease in immunostaining for major epithelial BM components, nidogen-1/entactin, laminin-10 (α5β1γ1), and of their binding integrin, α3β1. These alterations, especially of α3β1 integrin, may be specific for diabetic corneas since they were not pronounced in corneas from patients with a common corneal disease, bullous keratopathy (Ljubimov et al., 1998a). Most recently, we demonstrated a specific upregulation of matrix metallo-proteinase (MMP)-10/stromelysin-2 in the epithelium and stroma of DR corneas (Saghizadeh et al., 2001). We have proposed that MMP-10 overexpressed in diabetic corneas might cause degradation of specific corneal BM and cell surface components, which could be the mechanism underlying diabetic corneal epithelial abnormalities.
To further test this hypothesis, a dynamic system was needed to study corneal diabetic alterations in time. Since animal models do not reproduce human proliferative DR (Kern and Engerman, 1996; Kern et al., 2000), our attention was turned to the tissue culture. Recently, a new organ culture system has been developed that allowed to easily and reproducibly culture corneas at the air–liquid interface on top of the collagen–agar layer (Foreman et al., 1996; Xu et al., 2000; Zieske et al., 2000). This system was chosen here because (1) it reproduced well the process of normal wound healing, and (2) corneas could be successfully transplanted to patients after long-term culture with this technique (Harper et al., 1998). It is shown here that normal and DR organ-cultured corneas preserve their in vivo differences in respect to rates of wound healing and diabetic corneal markers distribution.
2. Methods
2.1. Tissue
Age-matched autopsy corneas from 20 individuals without eye disease and diabetes (referred to as normal-healthy; mean age 65.9 ± 3.7 years) and from 17 patients with clinically diagnosed DR (mean age 66.4 ± 2.7 years; 15 with IDDM and two with NIDDM) were obtained in chilled Optisol solution within 48 hr after death from the National Disease Research Interchange (NDRI, Philadelphia, PA, USA). NDRI has a human tissue collection protocol approved by the managerial committee and subject to National Institutes of Health oversight. Upon arrival, corneas were immediately processed for organ culture.
2.2. Organ culture
Corneas were organ-cultured over agar–collagen gel essentially as described (Foreman et al., 1996; Xu et al., 2000; Zieske et al., 2000). Corneas were first washed in antibiotic–antimycotic mixture (ABAM, Invitrogen Life Technologies, Carlsbad, CA, USA), and then placed epithelial side down in sterile Chiron’s corneal transportation vials with a small volume of medium to prevent drying. Corneal concavity was filled with serum-free minimum essential medium (MEM, Invitrogen Life Technologies) containing ABAM, 1 mg ml−1 calf skin collagen (made from stock solution of 10 mg ml−1 in 0.1N acetic acid), and 1% agar (both from Sigma Chemical Co. St Louis, MO, USA). This mixture was microwaved to boiling to sterilize it and to dissolve agar and then cooled down to 37–39°C. After the addition to corneas, it solidified within 2–3 min. Corneas were then placed agar side down on a sterile 60 mm dish. Serum-free MEM containing ABAM and insulin–transferrin–sodium selenite (Sigma) was then added dropwise to the central corneal part until it reached the limbus. Corneas were kept in a humidified CO2 incubator at 35°C and 100 μl medium was added one to two times a day to moisten the epithelium. Corneal morphology and cell viability were monitored microscopically using Olympus BH-2 microscope (Olympus USA, Inc. Melville, NY, USA) with a 4 × objective. Cultures were successfully kept for up to 45 days but presented results concern corneas cultured for 10–20 days.
2.3. Epithelial wound healing
To perform central epithelial debridement (Chung et al., 1998), a 5 mm filter paper disc soaked in n-heptanol (Sigma) was placed on the central corneal anterior surface for 60–90 sec before applying agar–collagen mixture. The filter was removed, cornea washed in complete serum-free medium and prepared for organ culture as above. This procedure removes the epithelium but leaves behind an intact BM both in normal and DR corneas. Mechanical debridement was not used because it will disrupt fragile diabetic epithelial BM (Hatchell et al., 1983). After n-heptanol debridement, residual epithelial cells usually died and sloughed off the next day leaving behind microscopically intact and homogeneous BM, so there was no need for additional mechanical cell removal. Live healing and fellow unwounded (control) corneas were photographed every 12 hr until the epithelial defect was completely healed. After 10–45 days in culture, corneas were cut in half, embedded in OCT compound (Ted Pella, Inc. Redding, CA, USA) and processed for indirect immunofluorescence on cryostat sections (Ljubimov et al., 1995).
2.4. Immunofluorescence
This was done as we have described previously (Ljubimov et al., 1995, 1998a; Saghizadeh et al., 2001). Monoclonal antibodies to α5 laminin chain (clone 4C7), fibrillin-1 (clone 11C1.3), α3 integrin subunit (clone P1B5), and β1 integrin subunit (clone HB1.1) were from Chemicon International (Temecula, CA, USA). A monoclonal antibody to MMP-10/stromelysin 2 (clone 117239) was from R&D Systems (Minneapolis, MN, USA). A monoclonal antibody to α-smooth muscle actin (clone 1A4) was from Sigma Chemical Co. Monoclonal antibodies to tenascin-C (clone BC-8, a gift from Dr L. Zardi, Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy), nidogen-1/entactin (clone A9, a gift from Dr R.J. Butkowski, INCSTAR Corporation, Stillwater, MN, USA), keratin 3 (clone AE5, a gift from Dr T.-T. Sun, New York University Medical School, New York, NY, USA), α-enolase (clone 4G10), and a polyclonal antibody to MMP-10 have been described previously (Zieske et al., 1992; Ljubimov et al., 1995, 1998b,c; Saghizadeh et al., 2001). A monoclonal and a polyclonal antibody to MMP-10 reacted similarly. Another monoclonal antibody to MMP-10 (clone 110304, R&D Systems) gave a strong nonspecific staining of keratocytes in preliminary experiments and was not further used.
Each antibody was analyzed at least twice on most cases, with the same results. Routine specificity controls were negative. Cryostat sections of normal and diseased corneas were exposed to the same dilutions of antibodies simultaneously. Monoclonal antibodies were used as straight hybridoma supernatants or at 20–50 μg/ml when purified, and polyclonal antibodies were diluted according to supplier’s recommendations.
2.5. Statistical analysis
Patient age and wound healing data were analyzed by the unpaired two-tailed Student t-test using InStat software program (GraphPad Software, San Diego, CA, USA). Data are expressed as mean ± standard error of mean (s.e.m.). Immunostaining results were analyzed using a double-sided Fisher’s exact test. P-value less than 0.05 was considered significant for both tests.
3. Results
3.1. Wound healing
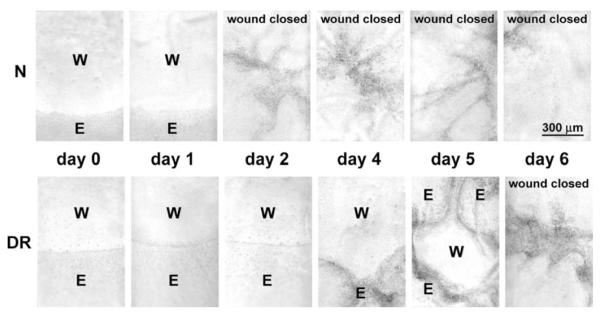
Wounded normal/healthy corneas in organ culture (n = 13) completely closed n-heptanol-induced epithelial defects on average in 2.3 ± 0.2 days (Fig. 1), in accordance with earlier data (Foreman et al., 1996). Wounded DR corneas in organ culture (n = 12) healed significantly slower on average (Fig. 1), with a mean healing time of 4.5 ± 0.7 days (p < 0.005 vs. normal group). There was more heterogeneity in healing times between individual corneas in the DR group compared to normal group, possibly related to differences in the severity and/or duration of disease. These data were consistent with previous findings on impaired diabetic corneal wound healing (Hallberg et al., 1996; Rosenberg et al., 2000). Control normal and DR corneas retained intact epithelial layer in culture; the longest follow-up so far was for 45 days.
Fig. 1.
Representative wound healing dynamics of normal and DR corneas in organ culture. An organ-cultured normal cornea shown (N) completely closed 5 mm circular epithelial defect within 2 days, whereas a DR cornea shown healed completely only between 5 and 6 days after wounding. Photographs of live unstained corneas are shown. W, wound area; E, epithelium.
3.2. Distribution of DR markers in organ-cultured corneas
The next step was to determine whether the markers (Ljubimov et al., 1996, 1998a; Saghizadeh et al., 2001) altered in the in vivo DR corneas (laminin-10, nidogen-1/entactin, integrin α3β1, and MMP-10) retained their expression patterns in organ-cultured normal and DR corneas. After 10–20 days in organ culture, control and wounded normal and DR corneas were stained by immunofluorescence for α5 chain of laminin-10, α3 and β1 subunits of integrin α3β1, nidogen-1/entactin, and MMP-10. As shown in Fig. 2 top, normal patterns of all markers were typical for organ-cultured normal corneas: (1) strong continuous staining for laminin-10 (and nidogen-1/entactin, not shown here) in the epithelial BM, (2) strong staining for α3β1 integrin in epithelial cells, mostly in the basal cells (shown for α3 subunit, with similar results for the β1 subunit), and (3) little or no staining for MMP-10. In organ-cultured DR corneas (Fig. 2 bottom), the marker patterns specific for intact DR corneas were also evident: (1) discontinuous or little to no staining for laminin-10 and nidogen-1/entactin in the epithelial BM, (2) areas of weak or disorganized staining for α3β1 integrin chains in epithelial cells, and (3) pronounced staining for MMP-10 in the epithelium and some keratocytes. Differences in marker distribution between normal and DR organ-cultured corneas were statistically significant, with p < 0.04 for laminin α5 chain, nidogen-1/entactin, α3 and β1 integrin subunits, and p < 0.005 for MMP-10.
Fig. 2.
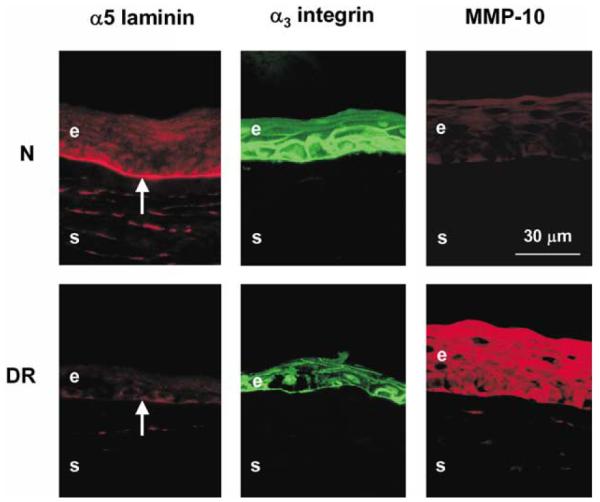
Distribution of DR markers in control (unwounded) normal and DR organ-cultured corneas. Note continuous BM staining for laminin-10 α5 chain, strong epithelial staining for α3 integrin and lack of staining for MMP-10 in normal (N) corneas. In DR corneas, little staining is seen for laminin-10, weak and disorganized staining for α3 integrin, and strong staining for MMP-10. Thus, all markers in organ-cultured corneas have the same patterns as in the respective intact normal or DR corneas. All presented corneas were kept in organ culture for 10 days. Central parts of all corneas are shown. E, epithelium; S, stroma; arrows point to the epithelial BM. Indirect immunofluorescence.
To find out whether organ-cultured corneas would show nonspecific alterations in the epithelium or stroma compared to the in vivo corneas, stainings were performed for keratin 3 (corneal epithelial marker), α-enolase (limbal epithelial marker), α-smooth muscle actin (myofibroblast marker), fibrillin-1, and tenascin-C (stromal fibrotic components). Both central normal and DR corneal epithelium was usually positive in all layers for keratin 3 (Fig. 3). Only about 25% of cases showed pronounced staining exclusively in the suprabasal cells as in limbus. All these corneas were also usually negative for α-enolase (Fig. 3), same as normal central epithelium in vivo (again, only 25% cases had significant staining of basal epithelial cells). As in the in vivo corneas, tenascin-C (Fig. 3) and fibrillin-1 (not shown here) were generally absent from both normal and DR organ-cultured corneas. α-smooth muscle actin was not seen in any of the corneas (not shown here) indicating the absence of myofibroblasts and, therefore, no active tissue remodeling in organ-cultured corneas.
Fig. 3.
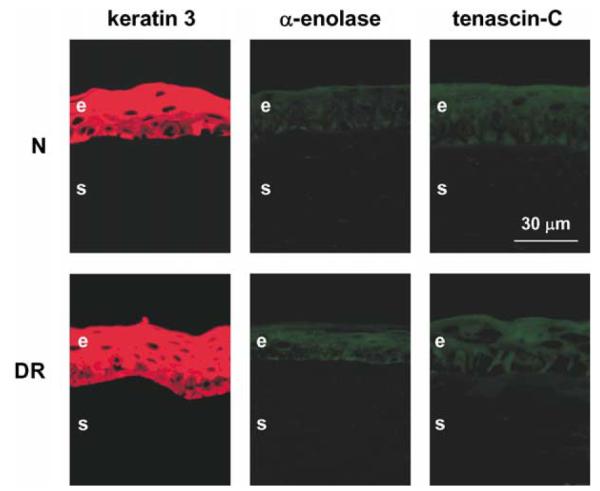
Distribution of keratin 3, α-enolase, and tenascin-C in control organ-cultured corneas. In both normal (N) and DR corneas, all markers have patterns very similar to the in vivo central corneas. Keratin 3 is present in all epithelial layers, limbal marker, α-enolase, is scarce to absent, and fibrosis marker, tenascin-C, is absent. These patterns did not change in wounded and healed corneas (not shown here). All corneas were kept in organ culture for 13 days. Central parts of all corneas are shown. E, epithelium; S, stroma. Indirect immunofluorescence.
In normal or DR wounded corneas after 10–20 days in organ culture when the healing of the epithelial defect was complete, all markers had the same patterns as in the respective unwounded corneas (Fig. 4). Moreover, keratin 3, α-enolase, α-smooth muscle actin, tenascin-C, and fibrillin-1 also did not change their distribution in wounded and healed corneas compared to the unwounded corneas (not shown here).
Fig. 4.
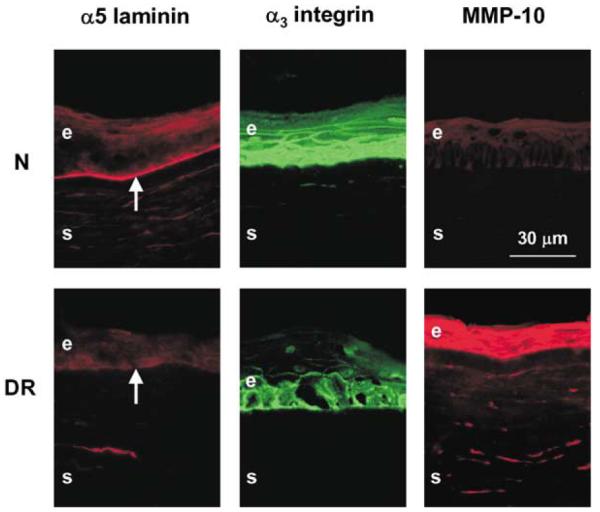
Distribution of DR markers in wounded and healed normal and DR organ-cultured corneas. Note that in both normal (N) and DR corneas, marker distribution is the same as in the respective unwounded corneas (Fig. 2). Normal corneas presented were cultured for 10 days (8 days after healing was complete). DR corneas presented were cultured for 13 days (9–10 days after healing was complete). Central parts of all corneas are shown. E, epithelium; S, stroma; arrows point to the epithelial BM. Indirect immunofluorescence.
4. Discussion
Corneal organ cultures have been used to study wound healing, cell proliferation, and the expression of MMPs, growth factors and integrins (Fini and Girard, 1990; Stepp et al., 1993; Moller-Pedersen and Moller, 1996; Gan et al., 1998; Redbrake et al., 1999; Messent et al., 2000; Shi et al., 2000; Zagon et al., 2000; Zieske et al., 2000). Most studies concluded that different corneal organ culture systems adequately represented the processes going on in vivo. However, certain organ cultures were shown to change protein expression patterns from what was observed in the in vivo corneas. One such example is MMP-9 that is not expressed in intact rabbit cornea but can be readily detected either in monolayer cultures of corneal cells or in organ-cultured corneas (Fini and Girard, 1990). Potentially, this could occur only with certain culture systems, since most recent papers reported good preservation of in vivo parameters in corneal organ culture including the system used here (Redbrake et al., 1999; Liminga and Oliw, 2000; Ma and Bazan, 2000; Messent et al., 2000; Zagon et al., 2000; Zieske et al., 2000; Crewe and Armitage, 2001). In general, however, the usage of organ cultures to study the mechanisms of corneal disease requires a prior accurate comparison of cultured corneas with in vivo corneas for the expression patterns of proteins of interest.
There are only a few reports about diabetic organ-cultured corneas. In animal diabetic organ-cultured corneas, wound healing rates and metabolism were similar to the in vivo corneas (Shimazaki et al., 1995; Hallberg et al., 1996). The only report to date on cultured human diabetic corneas described metabolic recovery of corneas during culture (Redbrake et al., 1997). There was no information available on the wound healing rates of human diabetic corneas compared to normal in organ culture, or on the influence of organ culture on specific diabetic corneal protein markers. This paper thus provides the first description in organ-cultured human normal and DR corneas of the patterns of specific markers altered in the in vivo DR corneas and of the epithelial wound healing.
It is reported here that epithelial wound healing was impaired in organ-cultured human DR corneas. This result is in agreement with previous clinical observations of abnormal corneal wound healing in diabetic patients (Azar et al., 1989, 1992; Sánchez-Thorin, 1998; Didenko et al., 1999; Rosenberg et al., 2000) and animals (Takahashi et al., 2000b; Zagon et al., 2002). Our current hypothesis is that the corneal epithelial BM, which is altered in diabetes by proteolysis (Takahashi et al., 2000a; Saghizadeh et al., 2001) and/or accumulation of advanced glycation end products (Kaji et al., 2000), does not support the migration of epithelial cells that is essential (Zieske, 2001; Suzuki et al., 2003) for wound healing. This might result in slow closing of the epithelial defects and recurrent erosions observed clinically (Sánchez-Thorin, 1998; Didenko et al., 1999).
We have shown previously that diabetic, especially DR corneas have altered distribution of epithelial BM components and α3β1 epithelial integrin, and increased levels of MMP-10 (Ljubimov et al., 1996, 1998a; Saghizadeh et al., 2001). In organ-cultured corneas, the expression patterns of all these DR corneal markers were identical to those seen in respective corneas in vivo (Fig. 2). Moreover, organ-cultured corneas did not show significant nonspecific epithelial alterations as judged by the staining for corneal epithelial differentiation markers, keratin 3 and α-enolase. Fibrotic markers, tenascin-C and fibrillin-1 (Ljubimov et al., 1998b,c), were not observed in normal or DR organ-cultured corneas. Moreover, α-smooth muscle actin-positive myofibroblasts were also absent indicating no active tissue remodeling. These results show that organ culture does not significantly change specific protein expression patterns in normal or DR corneas.
The differences in marker expression between normal and DR corneas were also maintained in wounded corneas after the healing was complete (Fig. 4). This finding may be important for future studies of the efficacy and toxicity of novel therapeutics aimed at restoring normal wound healing to diabetic corneas.
Previous thorough work in mice by Stepp’s group has shown that BM components did not change their distribution during healing of small epithelial wounds (up to 1.5 mm), nor was epithelial BM fragmented as judged by electron microscopy (Sta. Iglesia and Stepp, 2000). In a limbus to limbus debridement wounds, however, discontinuous staining was seen for laminin-5, nidogen-1/entactin and perlecan, which reverted back to normal within 3 days. The 5 mm human corneal epithelial wounds studied here are more similar to the small wounds than to large wounds in Stepp’s work in terms of their surface area compared to total epithelial surface area. We, therefore, assume that no major changes in BM structure and component distribution occurred during healing of these wounds in human corneal organ culture. A comparison of BM changes in normal and DR organ-cultured corneas during healing of larger wounds would present interest for further studies.
Thus, the established corneal organ culture provides an adequate system for the studies of normal and diabetic human corneas. Organ cultures retain in vivo-like parameters of normal and diabetic intact corneas (wound healing dynamics and distribution of markers). It remains to be established whether the mechanisms of the observed diabetes-related alterations are the same in vitro and in vivo. Overall, this system would help unravel mechanisms of impaired wound healing and altered BM marker expression in DR corneas and develop means to prevent or slow down diabetic keratopathy.
Acknowledgements
We are grateful to Drs L. Zardi (Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy), R.J. Butkowski (INCSTAR Corporation, Stillwater, MN), and T.-T. Sun (New York University Medical School, New York, NY) for their generous gift of antibodies. We thank Dr F.X. Yu and A.E.K. Hutcheon, BS, for helpful suggestions concerning corneal organ culture. Supported by NIH grants EY12605 and EY13431 to A.V.L.
References
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- Azar DT, Gipson IK. Repair of the corneal epithelial adhesion structures following keratectomy wounds in diabetic rabbits. Acta Ophthalmol. Suppl. 1989;192:72–79. doi: 10.1111/j.1755-3768.1989.tb07097.x. [DOI] [PubMed] [Google Scholar]
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Decreased penetration of anchoring fibrils into the diabetic stroma. A morphometric analysis. Arch. Ophthalmol. 1989;107:1520–1523. doi: 10.1001/archopht.1989.01070020594047. [DOI] [PubMed] [Google Scholar]
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch. Ophthalmol. 1992;110:537–540. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- Cavallerano J. Ocular manifestations of diabetes mellitus. Optom. Clin. 1992;2:93–116. [PubMed] [Google Scholar]
- Chang SW, Hsu HC, Hu FR, Chen MS. Corneal autofluorescence and epithelial barrier function in diabetic patients. Ophthal. Res. 1995;27:74–79. doi: 10.1159/000267600. [DOI] [PubMed] [Google Scholar]
- Chung JH, Kim WK, Lee JS, Pae YS, Kim HJ. Effect of topical Na-hyaluronan on hemidesmosome formation in n-heptanol-induced corneal injury. Ophthal. Res. 1998;30:96–100. doi: 10.1159/000055460. [DOI] [PubMed] [Google Scholar]
- Crewe JM, Armitage WJ. Integrity of epithelium and endothelium in organ-cultured human corneas. Invest. Ophthalmol. Vis. Sci. 2001;42:1757–1761. [PubMed] [Google Scholar]
- Didenko TN, Smoliakova GP, Sorokin EL, Egorov VV. Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes. Vestn. Oftalmol. 1999;115:7–11. [PubMed] [Google Scholar]
- Fini ME, Girard MT. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Invest. Ophthalmol. Vis. Sci. 1990;31:1779–1788. [PubMed] [Google Scholar]
- Foreman DM, Pancholi S, Jarvis-Evans J, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp. Eye Res. 1996;62:555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- Gan L, Fagerholm P, Ekenbark S. Expression of proliferating cell nuclear antigen in corneas kept in long term culture. Acta Ophthalmol. Scand. 1998;76:308–313. doi: 10.1034/j.1600-0420.1998.760311.x. [DOI] [PubMed] [Google Scholar]
- Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant trolox. Res. Commun. Mol. Pathol. Pharmacol. 1996;93:3–12. [PubMed] [Google Scholar]
- Harper CL, Boulton ME, Marcyniuk B, Tullo AB, Ridgway AE. Endothelial viability of organ-cultured corneas following penetrating keratoplasty. Eye. 1998;12:834–838. doi: 10.1038/eye.1998.214. [DOI] [PubMed] [Google Scholar]
- Hatchell DL, Magolan JJ, Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch. Ophthalmol. 1983;101:469–471. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am. J. Optom. Physiol. Opt. 1988;65:224–230. doi: 10.1097/00006324-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Kaji Y, Usui T, Oshika T, Matsubara M, Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S, Amano S. Advanced glycation end products in diabetic corneas. Invest. Ophthalmol. Vis. Sci. 2000;41:362–368. [PubMed] [Google Scholar]
- Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch. Ophthalmol. 1996;114:986–990. doi: 10.1001/archopht.1996.01100140194013. [DOI] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru RA, Nagaraj RH, Romeo G, Podesta F, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest. Ophthalmol. Vis. Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- Lim JI, Murphy RP. Review of diabetic retinopathy. Curr. Opin. Ophthalmol. 1991;2:315–323. [Google Scholar]
- Liminga M, Oliw EH. Studies of lipoxygenases in the epithelium of cultured bovine cornea using an air interface model. Exp. Eye Res. 2000;71:57–67. doi: 10.1006/exer.2000.0852. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang Z, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J. Histochem. Cytochem. 1996;44:1469–1479. doi: 10.1177/44.12.8985139. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun T-T, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- Ljubimov AV, Huang Z, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J. Histochem. Cytochem. 1998a;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, Spirin KS, Khin HL, Lewin SL, Zardi L, Bourdon MA, Kenney MC. Expression of tenascin-C splice variants in normal and bullous keratopathy human corneas. Invest. Ophthalmol. Vis. Sci. 1998b;39:1135–1142. [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, Spirin KS, Mecham RP, Sakai LY, Kenney MC. Increased expression of fibrillin-1 in human corneas with bullous keratopathy. Cornea. 1998c;17:309–314. [PubMed] [Google Scholar]
- Ma X, Bazan HE. Increased platelet-activating factor receptor gene expression by corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 2000;41:1696–1702. [PubMed] [Google Scholar]
- Meller D, Augustin AJ, Koch FH. A modified technique of impression cytology to study the fine structure of corneal epithelium. Ophthal. Res. 1996;28:71–79. doi: 10.1159/000267877. [DOI] [PubMed] [Google Scholar]
- Messent AJ, Blissett MJ, Smith GL, North AJ, Magee A, Foreman D, Garrod DR, Boulton M. Expression of a single pair of desmosomal glycoproteins renders the corneal epithelium unique amongst stratified epithelia. Invest. Ophthalmol. Vis. Sci. 2000;41:8–15. [PubMed] [Google Scholar]
- Moller-Pedersen T, Moller HJ. Viability of human corneal keratocytes during organ culture. Acta Ophthalmol. Scand. 1996;74:449–455. doi: 10.1111/j.1600-0420.1996.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Ohashi Y. Diabetic keratopathy. Nippon Ganka Gakkai Zasshi. 1997;101:105–110. [PubMed] [Google Scholar]
- Pickup JC, Williams G, editors. Chronic Complications of Diabetes. Blackwell Scientific; Oxford: 1994. p. 313. [Google Scholar]
- Redbrake C, Salla S, Frantz A, Reim M. Metabolic changes of the human donor cornea during organ-culture. Acta Ophthalmol. Scand. 1999;77:266–272. doi: 10.1034/j.1600-0420.1999.770304.x. [DOI] [PubMed] [Google Scholar]
- Redbrake C, Salla S, Vonderhecken M, Sieben P, Reim M. Gewebezustand humaner hornhäute vor und nach organkultur einfluβ der todesursache des spenders. Ophthalmologe. 1997;94:573–577. doi: 10.1007/s003470050161. [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas. A possible mechanism of basement membrane and integrin alterations. Am. J. Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can. J. Ophthalmol. 1995;30:142–146. [PubMed] [Google Scholar]
- Saini JS, Mittal S. Graded corneal sensitivity for screening of diabetic retinopathy. Indian J. Ophthalmol. 1996;44:219–223. [PubMed] [Google Scholar]
- Sánchez-Thorin JC. The cornea in diabetes mellitus. Int. Ophthalmol. Clin. 1998;38:19–36. [PubMed] [Google Scholar]
- Sato N, Nakamura M, Chikama T, Nishida T. Abnormal deposition of laminin and type IV collagen at corneal epithelial basement membrane during wound healing in diabetic rats. Jpn. J. Ophthalmol. 1999;43:343–347. doi: 10.1016/s0021-5155(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tabesh M, Sugrue SP. Role of cell adhesion-associated protein, pinin (DRS/memA), in corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 2000;41:1337–1345. [PubMed] [Google Scholar]
- Shimazaki J, Tsubota K, Yoshida A, Tornheim K, Laing RA. Changes of corneal redox state in diabetic animal models. Cornea. 1995;14:196–201. [PubMed] [Google Scholar]
- Sta. Iglesia DD, Stepp MA. Disruption of the basement membrane after corneal debridement. Invest. Ophthalmol. Vis. Sci. 2000;41:1045–1053. [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest. Ophthalmol. Vis. Sci. 1993;34:1829–1844. [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell–matrix and cell–cell interactions during corneal epithelial wound healing. Prog. Retin. Eye Res. 2003;22:113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM. Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 1988;226:389–392. doi: 10.1007/BF02172973. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Akiba K, Noguchi T, Ohmura T, Takahashi R, Ezure Y, Ohara K, Zieske JD. Matrix metalloproteinase activity is enhanced during corneal wound repair in high glucose condition. Curr. Eye Res. 2000a;21:608–615. [PubMed] [Google Scholar]
- Takahashi H, Ohara K, Ohmura T, Takahashi R, Zieske JD. Glucose transporter 1 expression in corneal wound repair under high serum glucose level. Jpn. J. Ophthalmol. 2000b;44:470–474. doi: 10.1016/s0021-5155(00)00222-7. [DOI] [PubMed] [Google Scholar]
- Van Schaik HJ, del Castillo J.M. Benitez, Caubergh MJ, Gobert A, Leite E, Moldow B, Rosas V, Van Best JA. Evaluation of diabetic retinopathy by fluorophotometry. European concerted action on ocular fluorometry. Int. Ophthalmol. 1998–1999;22:97–104. doi: 10.1023/a:1006132908679. [DOI] [PubMed] [Google Scholar]
- Xu K-P, Li X-F, Yu FX. Corneal organ culture model for assessing epithelial responses to surfactants. Toxicol. Sci. 2000;58:306–314. doi: 10.1093/toxsci/58.2.306. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Jenkins JB, Sassani JW, Wylie JD, Ruth TB, Fry JL, Lang CM, McLaughlin PJ. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes. 2002;51:3055–3062. doi: 10.2337/diabetes.51.10.3055. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Reepithelialization of the human cornea is regulated by endogenous opioids. Invest. Ophthalmol. Vis. Sci. 2000;41:73–81. [PubMed] [Google Scholar]
- Zieske JD. Extracellular matrix and wound healing. Curr. Opin. Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, Yankauckas MA, Wasson ME, Keutmann HT. Alpha-enolase is restricted to basal cells of stratified squamous epithelium. Dev. Biol. 1992;151:18–26. doi: 10.1016/0012-1606(92)90209-y. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AEK, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]