Abstract
Purpose:
To assess the effect of preservative-free dorzolamide–timolol on nonvisual symptoms and intraocular pressure (IOP) in newly diagnosed and untreated patients with open-angle glaucoma or ocular hypertension.
Methods:
This was a prospective, 8-week, open-label, Canadian multicenter study. All patients were treated with preservative-free dorzolamide–timolol formulation. The primary outcome was the change in the nonvisual symptom score of the Glaucoma Symptom Scale (GSS-SYMP-6) from baseline to 8 weeks. Secondary effectiveness outcome measures were absolute and percent changes in IOP from baseline to 4 and 8 weeks.
Results:
One hundred and seventy-eight patients were enrolled. Mean (SD) age was 65.6 (12.1) years and 90 (50.6%) were females. There were 92 patients diagnosed with open-angle glaucoma, 62 with ocular hypertension, and 23 with both diseases (diagnosis was missing for one patient). The mean (SD) GSS-SYMP-6 score increased from 73.6 (21.8) at baseline to 76.1 (20.7) at 8 weeks (P = 0.097). Mean (SD) IOP significantly decreased by 11.7 (5.1) mmHg at 4 weeks (P < 0.001) and by 11.5 (5.3) mmHg at 8 weeks (P < 0.001), representing reductions of −38.5% (P < 0.001) and −38.0% (P < 0.001), respectively.
Conclusion:
Preservative-free dorzolamide–timolol does not increase eye discomfort while significantly reducing IOP in patients with open-angle glaucoma or ocular-hypertension.
Keywords: open-angle glaucoma, ocular hypertension, GSS-SYMP-6, intraocular pressure, dorzolamide–timolol, preservative-free
Introduction
Open-angle glaucoma is a chronic progressive disease characterized by asymptomatic elevation of intraocular pressure (IOP), progressive optic nerve damage, and visual field loss that can lead to blindness.1 Glaucoma is the second leading cause of blindness worldwide2 and in Canada,3 and its incidence increases with advancing age.4,5
Currently, the goal of glaucoma therapy is to reduce the rate of retinal ganglion cell loss by decreasing IOP.6 Since open angle-glaucoma and ocular hypertension are progressive chronic conditions, their management requires long-term and even lifelong treatment. As with all chronic conditions, treatment benefits must be balanced against possible risk for side effects. In addition, tolerability of long-term treatment becomes an important factor contributing to therapeutic effectiveness given its impact on compliance. Therefore, the aim of glaucoma therapy is the reduction of IOP and preservation of visual field and vision while reducing the impact of chronic therapy and potential side effects on the patients’ quality of life.
Dorzolamide, a topical carbonic anhydrase inhibitor, and timolol, a nonselective beta receptor blocking agent, are both effective in the management of elevated IOP, and are well established as ocular hypotensive treatments. Further, they have an additive IOP-lowering effect when administered concomitantly.7 Several clinical trials have demonstrated the efficacy of dorzolamide–timolol fixed combination in the treatment of open-angle glaucoma and ocular hypertension.8–19 However, the preservative agents included in the formulation of these eye-drop therapies have been shown to decrease the stability of the precorneal tear film and to have a detergent effect on the lipid layer; resulting in increased evaporation, dry-eyes,20 and irritation.21 Preservative-free eye drops may therefore be useful in the efforts to protect and maintain ocular surface integrity, especially as over 50% of patients treated for glaucoma have concurrent ocular surface disorders.20,22,23 Indeed, preservative-free medications could provide an effective alternative for long-term treatment of glaucoma and ocular hypertension for patients who are sensitive to a preservative and those with a history of dry or irritated eyes.
The principal aim of this study was to describe the change in nonvisual ocular symptoms in newly diagnosed patients with open-angle glaucoma or ocular hypertension treated with a preservative-free dorzolamide-timolol formulation.
Materials and methods
Study design
This was an 8-week prospective, multicenter, open-label study performed in 18 Canadian ophthalmologists’ clinics between May 2007 and October 2008. Assessments were performed at baseline (week 0), and at 4 and 8 weeks of treatment at the treating ophthalmologists’ clinics. At each study visit, patients completed the six-item nonvisual symptom scale (GSS-SYMP-6) extracted from the 10-item glaucoma symptom scale (GSS).24 The six nonvisual symptoms assessed were: i. burning, smarting and stinging, ii. tearing, iii. dryness, iv. itching, v. soreness and tiredness, and vi. feeling of something in the eye. Patients were asked to rate each of these six nonvisual symptoms using a five-point Likert Scale with 0 being very bothersome and 4 representing the absence of the symptom. The score of this scale was transformed between 0 and 100 with lower scores indicating higher severity of the nonvisual symptoms. A score of 100 was the best possible score and positive changes indicate improvement in the patient’s condition. In addition, at each study visit, the treating ophthalmologists measured the IOP in patients’ both eyes using a calibrated Goldmann’s applanation tonometer. This was calculated as the mean of two consecutive and independent measures in the same eye. Patients were assessed at any time during the day. However, each patient was assessed at approximately the same time of the day at all visits.
The study was approved by an Independent Ethics Review Board (Insitutional Review Board Services, Aurora, Ontario) and was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) “Good Clinical Practice guidelines”, the World Medical Association Declaration of Helsinki and all applicable local regulations. A written informed consent was obtained from every patient prior to conducting any study related procedures including the evaluation of study eligibility and enrolment.
Patients
Eligible patients (≥18 years) were recently diagnosed with open-angle glaucoma or ocular hypertension according to the Canadian Ophthalmological Society evidence-based clinical practice guidelines.25 Open-angle glaucoma was diagnosed when there was evidence of glaucomatous optic neuropathy (GON), with or without elevated IOP, while ocular hypertension was diagnosed when IOP was elevated, but without evidence of GON or visual field damage. Eligible patients also had an IOP of ≥27 mmHg in at least one eye and a baseline GSS-SYMP-6 total score of 75 or less. All were treatment-naive. In addition, patients may have been either sensitive to a preservative or had “less than perfect” ocular surface issues, as per the clinical judgment of the treating ophthalmologist.
Patients were excluded from the study if they had any fundus pathology likely to change during the study or to influence IOP, hypersensitivity to any component of preservative-free dorzolamide–timolol, severe renal impairment (serum creatinine >150 μmol/L or creatinine clearance <30 mL/min), or any contraindication to the use of preservative-free dorzolamide–timolol including bronchospasm (eg, bronchial asthma or any history of bronchial asthma and chronic obstructive pulmonary disease) or sinus bradycardia, or second or third degree AV block, or overt cardiac failure or cardiogenic shock). Patients with a history of diabetic retinopathy were eligible for inclusion in the study. Prohibited concomitant medications were carbonic anhydrase inhibitors (systemic and topical), systemic or dermatological medications known to affect the IOP (eg, clonidine, corticosteroids or oral beta-blocking agents), and any medications containing one of the following preservative agents: benzalkonium chloride, benzododecinium bromide, or stabilized oxychloro complex.
Treatment
All patients were treated with the preservative-free formulation of dorzolamide–timolol (COSOPT® without benzalkonium chloride as preservative, Merck Frosst Canada Ltd, Kirkland, Canada) for eight weeks on a self-administered regimen of one drop, twice daily in the morning and bedtime, in each affected eye.
Outcome measures
The primary outcome measure was the change in GSS-SYMP-6 score from baseline to 8 weeks of treatment. Secondary outcome measures were the mean absolute change in GSS-SYMP-6 score from baseline to 4 weeks of treatment. The presence and severity of the nonvisual symptoms in patients’ worse eye, defined as the eye with the highest IOP measure at baseline, were also described at each visit. Therapeutic effectiveness outcomes were the absolute and percent changes in IOP measured in the worse eye from baseline to 4 and 8 weeks of treatment. Patient and ophthalmologist satisfaction with treatment after 8 weeks were assessed with a five-point Likert scale question ranging from 0 (very dissatisfied) to 4 (very satisfied). Compliance with study treatment was also assessed by self-reporting at 4 and 8 weeks during clinic visits. Patients who reported missing more than 20% of scheduled doses were considered non-compliant. Safety was assessed by the incidence of adverse events that occurred during treatment and up to 14 days after study drug discontinuation.
Statistical methods
A difference of seven points was observed in the SYMP-6 validation study,24 in which patients with glaucoma had a mean (SD) score of 78 (23) compared to 85 (21) for the reference group. The present study sought to detect this clinically significant difference of seven points. With an 80% power and allowing for 10% drop out rate, a minimum of 170 patients were to be enrolled.
Descriptive statistics including the mean and standard deviation for continuous variables and frequency distributions for discrete variables were reported. The statistical significance of the change in GSS-SYMP-6 score from baseline to 4 and 8 weeks was assessed with the Student’s t-test for paired observations. The 95% confidence intervals of the mean change were also computed as measures of precision and to allow inference to the target population. χ2 statistics were used to test the difference in the presence of nonvisual symptoms and to assess the change in the distribution of the symptom severity from baseline to 4 and 8 weeks of treatment.
The statistical significance of the mean absolute and percent change in IOP was assessed with the Student’s t-test for paired observations. For this outcome, 95% confidence intervals were also computed. The observed mean absolute change in IOP from baseline to 4 and 8 weeks was compared to the conventional value of zero (Ho: δ = 0 mmHg) and to the a priori established value of −4.0 mmHg (Ho: δ ≤ −4.0 mmHg). The test value for the comparison of the observed mean percent change in IOP was of −20% (Ho: δ ≤ −20.0%). An absolute change of −4.0 mmHg or percent change of −20.0% were considered as the minimum for clinical significance.
All analyses were based on observed cases and no imputation methods were used for replacing missing data. As per the intention-to-treat (ITT) principle, all patients with outcome measurements at baseline and one of the follow-up visits were included in the analyses regardless of protocol violations and compliance with treatment. The minimum level of statistical significance was a priori defined at 5%. All statistical analyses were performed using STATA (version 10.0; College Station, TX) and SPSS (version 12.0 for Windows; SPSS Inc, Chicago, IL).
Results
Patient disposition
A total of 178 patients formed the ITT population and were included in the study. Of these, 169 (94.9%) and 176 (98.9%) were assessed at 4 and 8 weeks, respectively. There were 9 (5.1%) patients who were discontinued from the study: 2 (1.1%) patients withdrew consent, 3 (1.7%) were lost to follow-up, 1 (0.6%) experienced a serious adverse event, and 3 (1.7%) were discontinued for other reasons. Seven of the discontinued patients were retrieved drop-outs that were included in the 8-week assessment based on their visit date.
Patient demographics
The patient demographics are summarized in Table 1. The mean (SD) age of the 178 patients included in the study was 65.6 (12.1) years; 21 (11.8%) were ≤52 years of age and 58 (32.6%) were >72 years. The majority were Caucasian (n = 168; 94.4%) and 90 (50.6%) were females. A total of 92 patients were diagnosed (worse eye) with open-angle glaucoma, 62 with ocular hypertension, and 23 with both diseases; diagnosis was missing for 1 patient. There were 125 patients (70.2%) who had both eyes affected by either open-angle glaucoma or ocular hypertension.
Table 1.
Demographics of the 178 patients included in the study
| Characteristics | n | % |
|---|---|---|
| Age (years)a | ||
| ≤52 | 21 | 11.8 |
| >52 to ≤72 | 97 | 54.5 |
| >72 | 58 | 32.6 |
| Gender | ||
| Male | 88 | 49.4 |
| Female | 90 | 50.6 |
| Race | ||
| Caucasian | 168 | 94.4 |
| Black | 3 | 1.7 |
| Hispanic | 2 | 1.1 |
| Asian | 4 | 2.3 |
| Other | 1 | 0.6 |
| Worse eyeb | ||
| Left eye | 97 | 54.5 |
| Right eye | 81 | 45.5 |
| Presence of open-angle glaucoma | ||
| Left eye | 91 | 51.1 |
| Right eye | 102 | 57.3 |
| Presence of ocular hypertension | ||
| Left eye | 76 | 42.7 |
| Right eye | 82 | 46.1 |
| Presence of glaucoma or ocular hypertension in both eyes | 125 | 70.2 |
| Medical history | ||
| Family history of open-angle glaucoma or ocular hypertension | 42 | 23.6 |
| Type I diabetes | 4 | 2.3 |
| Type II diabetes | 37 | 20.8 |
| Hypertension | 53 | 29.8 |
| Myopia | 35 | 19.7 |
| Migraine/headache | 18 | 10.1 |
Notes:
The age of 2 (1.1%) patients was unknown.
The worse eye was defined as the eye with the highest intraocular pressure measure at baseline.
Effectiveness
The GSS-SYMP-6 scores for the worse eye were available for 122 patients at baseline, 115 at 4 weeks, and 113 at 8 weeks. The mean (SD) GSS-SYMP-6 score increased from 73.6 (21.8) at baseline to 74.5 (19.3) at 4 weeks and 76.1 (20.7) at 8 weeks of treatment (Table 2). The mean (SD) absolute changes in GSS-SYMP-6 scores from baseline to 4 and 8 weeks of treatment were 1.8 (16.9) and 3.2 (20.2), respectively (Table 2).
Table 2.
Glaucoma nonvisual symptoms scale (GSS-SYMP-6) measurements
| Visits | N | Mean (SD) |
95% CI |
P-values | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Baseline (week 0) | 122a | 73.6 (21.8) | 69.5 | 77.1 | – |
| Week 4 | 115b | 74.5 (19.3) | 71.1 | 78.1 | – |
| Absolute change from baseline | 114c | 1.8 (16.9) | −1.3 | 4.9 | 0.260 |
| Week 8 | 113d | 76.1 (20.7) | 72.3 | 79.7 | – |
| Absolute change from baseline | 111e | 3.2 (20.2) | −0.6 | 7.0 | 0.097 |
Notes:
The GSS-SYMP-6 was not completed for both eyes at baseline by 56 of the 178 patients.
The GSS-SYMP-6 was not completed for both eyes at week 4 by 54 of the 169 patients.
The GSS-SYMP-6 was not completed for both eyes at baseline and week 4 by 55 of the 169 patients.
The GSS-SYMP-6 was not completed for both eyes at week 8 by 63 of the 176 patients.
The GSS-SYMP-6 was not completed for both eyes at baseline and week 8 by 65 of the 176 patients.
Abbreviation: CI, confidence interval.
Table 3 describes the presence and severity of nonvisual symptoms in the worse eye at baseline, 4, and 8 weeks of treatment. While the presence of burning, smarting, and stinging symptoms significantly increased from 28.0% at baseline to 63.0% at 4 weeks (P < 0.001), and 61.9% at 8 weeks (P < 0.001), there was no statistical difference in the presence of these symptoms between 4 and 8 weeks of treatment (P = 0.859). The proportion of patients with itching was reduced from 49.2% at baseline to 35.7% at 4 weeks (P = 0.028) and 35.2% at 8 weeks (P = 0.023). From baseline to 8 weeks of treatment, decreases in the presence of the following symptoms were observed: dryness (39.4% to 28.8%; P = 0.074), and soreness and tiredness (49.2% to 36.0%; P = 0.032). The severity profile of these symptoms did not change during the course of the study.
Table 3.
Presence and severity of the nonvisual symptoms in the worse eye
| Nonvisual symptoms (GSS-SYMP-6)* |
Baseline (N = 132)a |
Week 4 (N = 127)b,c |
Week 4 vs BaselineP-value |
Week 8 (N = 125)d,e |
Week 8 vs BaselineP-value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Burning, smarting, stinging | ||||||||
| Absence of symptom | 95 | 72.0 | 47 | 37.0 | <0.001 | 48 | 38.1 | <0.001 |
| Presence of symptom | 37 | 28.0 | 80 | 63.0 | 78 | 61.9 | ||
| Very bothersome | 5 | 13.5 | 6 | 7.5 | 0.356 | 6 | 7.7 | 0.219 |
| Somewhat bothersome | 11 | 29.7 | 21 | 26.3 | 18 | 23.1 | ||
| A little bothersome | 19 | 51.4 | 50 | 62.5 | 50 | 64.1 | ||
| Not at all bothersome | 2 | 5.4 | 3 | 3.8 | 4 | 5.1 | ||
| Tearing | ||||||||
| Absence of the symptom | 75 | 56.8 | 61 | 48.0 | 0.158 | 64 | 51.2 | 0.367 |
| Presence of the symptom | 57 | 43.2 | 66 | 52.0 | 61 | 48.8 | ||
| Very bothersome | 7 | 12.3 | 4 | 6.1 | 0.129 | 7 | 11.5 | 0.233 |
| Somewhat bothersome | 18 | 31.6 | 18 | 27.3 | 14 | 23.0 | ||
| A little bothersome | 29 | 50.9 | 37 | 56.1 | 33 | 54.1 | ||
| Not at all bothersome | 3 | 5.3 | 7 | 10.6 | 7 | 11.5 | ||
| Dryness | ||||||||
| Absence of the symptom | 80 | 60.6 | 87 | 68.5 | 0.185 | 89 | 71.2 | 0.074 |
| Presence of the symptom | 52 | 39.4 | 40 | 31.5 | 36 | 28.8 | ||
| Very bothersome | 13 | 25.0 | 6 | 15.0 | 0.106 | 4 | 11.1 | 0.057 |
| Somewhat bothersome | 19 | 36.5 | 11 | 27.5 | 9 | 25.0 | ||
| A little bothersome | 15 | 28.8 | 19 | 47.5 | 22 | 61.1 | ||
| Not at all bothersome | 5 | 9.6 | 4 | 10.0 | 1 | 2.8 | ||
| Itching | ||||||||
| Absence of the symptom | 67 | 50.8 | 81 | 64.3 | 0.028 | 81 | 64.8 | 0.023 |
| Presence of the symptom | 65 | 49.2 | 45 | 35.7 | 44 | 35.2 | ||
| Very bothersome | 5 | 7.7 | 1 | 2.2 | 0.220 | 3 | 6.8 | 0.274 |
| Somewhat bothersome | 15 | 23.1 | 8 | 17.8 | 5 | 11.4 | ||
| A little bothersome | 40 | 61.5 | 32 | 71.1 | 33 | 75.0 | ||
| Not at all bothersome | 5 | 7.7 | 4 | 8.9 | 3 | 6.8 | ||
| Soreness, tiredness | ||||||||
| Absence of the symptom | 67 | 50.8 | 81 | 63.8 | 0.035 | 80 | 64.0 | 0.032 |
| Presence of the symptom | 65 | 49.2 | 46 | 36.2 | 45 | 36.0 | ||
| Very bothersome | 6 | 9.2 | 1 | 2.2 | 0.217 | 2 | 4.4 | 0.091 |
| Somewhat bothersome | 24 | 36.9 | 16 | 34.8 | 11 | 24.4 | ||
| A little bothersome | 30 | 46.2 | 24 | 52.2 | 28 | 62.2 | ||
| Not at all bothersome | 5 | 7.7 | 5 | 10.9 | 4 | 8.9 | ||
| Feeling of something in the eye | ||||||||
| Absence of the symptom | 84 | 63.6 | 89 | 70.1 | 0.272 | 83 | 66.4 | 0.643 |
| Presence of the symptom | 48 | 36.4 | 38 | 29.9 | 42 | 33.6 | ||
| Very bothersome | 6 | 12.5 | 4 | 10.5 | 0.472 | 6 | 14.3 | 0.544 |
| Somewhat bothersome | 11 | 22.9 | 8 | 21.1 | 7 | 16.7 | ||
| A little bothersome | 29 | 60.4 | 22 | 57.9 | 25 | 59.5 | ||
| Not at all bothersome | 2 | 4.2 | 4 | 10.5 | 4 | 9.5 | ||
Notes:
Glaucoma symptom scale.
At baseline, the GSS-SYMP-6 was not completed for the worse eye by 46 of the 178 patients.
At week 4, the GSS-SYMP-6 was not completed for the worse eye by 42 of the 169 patients.
N = 126 for itching nonvisual symptom; at week 4, the itching nonvisual symptom was not completed for the worse eye by 43 of the 169 patients.
At week 8, the GSS-SYMP-6 was not completed for the worse eye by 51 of the 176 patients.
N = 126 for burning, smarting and stinging symptoms; at week 8, burning, smarting and stinging symptoms were not completed for the worse eye by 50 of the 176 patients.
The IOP measurements at each study visit and the mean absolute changes in IOP from baseline to 4 and 8 weeks of treatment are presented in Table 4. The mean (SD) IOP decreased from 29.6 (4.2) mmHg at baseline to 18.1 (3.7) mmHg at 4 weeks and 18.1 (3.9) mmHg at 8 weeks of treatment. The mean (SD) absolute reduction in IOP from baseline to 4 weeks was −11.7 (5.1) mmHg (P < 0.001) and −11.5 (5.3) mmHg (P < 0.001) at 8 weeks. These changes were significantly higher than the test value of −4.0 mmHg (P < 0.001). At 4 and 8 weeks, a total of 156 (92.3%) and 158 (89.8%) patients achieved a clinically significant reduction in IOP of at least 4.0 mmHg, respectively.
Table 4.
Intraocular pressure (IOP) measurements (mmHg) in the worse eye
| Visits | N | Mean (SD) |
95% CI |
P-values |
||
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | H0: δ = 0 | H0: δ = −4 | |||
| Baseline (Week 0) | 178 | 29.6 (4.2) | 29.0 | 30.2 | – | – |
| Week 4 | 165a | 18.1 (3.7) | 17.5 | 18.7 | – | – |
| Absolute change from baseline | 165b | −11.7 (5.1) | −12.5 | −10.9 | <0.001 | <0.001 |
| Week 8 | 167c | 18.1 (3.9) | 17.5 | 18.8 | – | – |
| Absolute change from baseline | 166d | −11.5 (5.3) | −12.3 | −10.7 | <0.001 | <0.001 |
Notes:
IOP measurement at week 4 was not available for 4 of the 169 patients.
IOP measurements at baseline and week 4 were not available for 4 of the 169 patients.
IOP measurement at week 8 was not available for 9 of the 176 patients.
IOP measurements at baseline and week 8 were not available for 10 of the 176 patients.
Abbreviations: SD, standard deviation; CI, confidence interval;δ, difference.
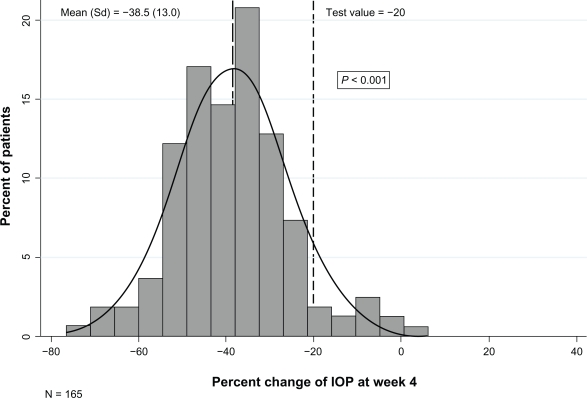
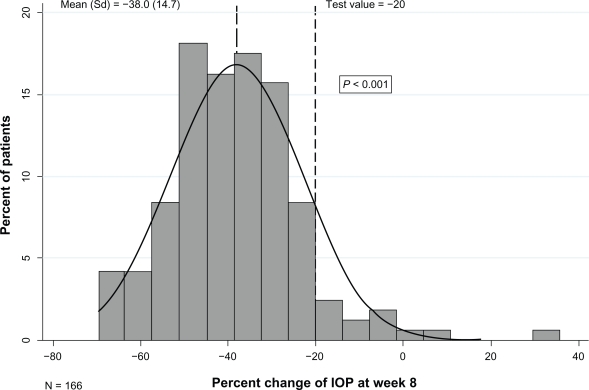
Figures 1 and 2 depict the distribution of the mean percent change in IOP from baseline to 4 and 8 weeks of treatment. The mean (SD) percent reduction in IOP was 38.5% (13.0) at 4 weeks (P < 0.001) and 38.0% (14.73) at 8 weeks (P < 0.001). A clinically significant IOP reduction of at least 20% at 4 and 8 weeks of treatment was observed for 153 (90.5%) and 154 (87.5%) patients, respectively.
Figure 1.
Percent change in intraocular pressure (IOP) of the worse eye between week 4 and baseline.
Figure 2.
Percent change in intraocular pressure (IOP) of the worse eye between week 8 and baseline.
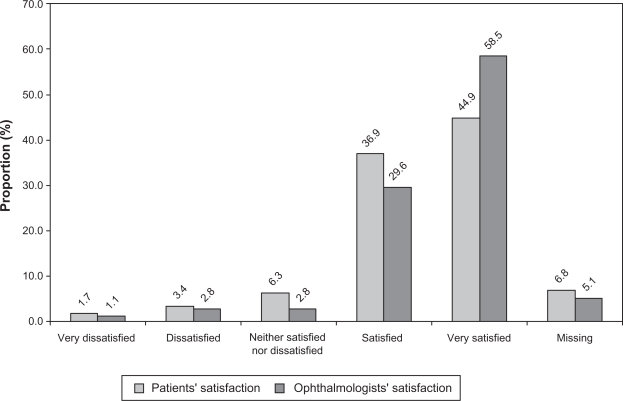
Figure 3 summarizes the results of the patient and ophthalmologist global satisfaction with treatment rating after 8 weeks of therapy. After 8 weeks of treatment, 144 (81.8%) patients were either satisfied or very satisfied with the preservative-free dorzolamide-timolol formulation, while 9 (5.1%) patients were either dissatisfied or very dissatisfied with treatment. After 8 weeks of treatment, the ophthalmologists were either satisfied or very satisfied with the preservative-free dorzolamide-timolol formulation for 155 (88.1%) of the patients, while they were either dissatisfied or very dissatisfied for 7 (3.9%) of them.
Figure 3.
Patients’ and ophthalmologists’ global satisfaction with 8 weeks preservative-free dorzolamide–timolol treatment.
Adherence to treatment was high. There were 162 (95.8%) and 159 (90.3%) patients who were ≥80% compliant with treatment at 4 and 8 weeks, respectively. At 4 weeks, 107 (63.3%) patients reported perfect adherence (taking 100% of their medication) while another 55 (32.5%) reported adherence (taking 80%–99.9% of their study medication). Similar results were obtained at week 8, with 106 (60.2%) patients reporting perfect adherence and another 53 (30.1%) reporting adherence. The median missed dose at each visit was 0.0 drops.
Safety
During the course of this 8-week study, three serious adverse events were experienced by three patients. A 67-year-old female experienced a retinal vein occlusion that was considered by the treating ophthalmologist to be probably not related to study medication. A 95-year-old male had nausea and another 61-year-old male experienced bradycardia. These two events were considered to be definitely related to the study medication; however, both patients recovered without any sequelae or permanent disability. The patient who had nausea discontinued study medication after 2 weeks of treatment.
Discussion
This was the first Canadian observational study aimed at assessing nonvisual ocular symptoms and the effectiveness of preservative-free dorzolamide–timolol formulation in a real-life setting. The results of this study showed that treatment with preservative-free dorzolamide–timolol does not increase discomfort related to nonvisual ocular symptoms, while maintaining therapeutic effectiveness in reducing IOP.
It is postulated that discomfort with eye-drop therapy can lead to patient discontinuation of treatment.26–28 While a small increase in GSS-SYMP-6 score (indicating improvement) was observed in the current study, this increase was neither statistically nor clinically significant. The use of the preservative-free formulation therefore did not increase eye discomfort, which may have been an important factor contributing to high compliance with therapy. This could result in optimization of long-term treatment effectiveness.
In this study, over 80% of patients and ophthalmologists were satisfied with the preservative-free dorzolamide–timolol formulation. From the perspective of the patient, the high level of satisfaction can be mainly explained by the reduction in dryness, itching, soreness, and tiredness in the eyes. This could contribute to improved quality of life during the course of treatment. From the perspective of the physician, the high level of satisfaction reported by the ophthalmologists may be due to the observed therapeutic effectiveness of the preservative-free dorzolamide–timolol formulation in reducing IOP. The magnitude of IOP reduction observed in these patients is likely associated with their treatment-naïve drug status since more robust IOP reduction is known to occur in treatment-naïve patients.
In the current study, self-administration of preservative-free dorzolamide–timolol during eight weeks produced an IOP reduction of approximately 40%, which exceeds the treatment targets established by the American Academy of Ophthalmology and the European Glaucoma Society. In fact, the American Academy of Ophthalmology currently recommends IOP lowering of at least 20% from baseline IOP29 and the European Glaucoma Society recommends lowering of at least 30% from baseline IOP.30
The observed change in IOP is comparable but higher than that generally reported by randomized clinical trials of dorzolamide–timolol,7,8,15,18,31–34 further supporting the efficacy of dorzolamide–timolol. Importantly, the present study indicates that the absence of preservative did not seem to thwart the efficacy. It is conceivable that by disrupting the corneal epithelium, preservatives partially contribute to ocular penetration and hence, therapeutic effectiveness. The results in this study suggest that preservative-induced effects on the ocular surface are not necessary for the drug efficacy. Since there is no difference in efficacy between preserved and preservative-free formulations,26,35,36 the current study further suggests that the dorzolamide–timolol preservative-free formulation may constitute an advantageous treatment alternative that provides a better tolerability for patients sensitive to preservative or for whom the utilization of preservative-free formulation is otherwise advisable.
Limitations of the current study relate to the open-label, single cohort design that did not include a comparative group. The study design was thus not amenable to answer some potentially interesting questions. For instance, recent observations from daily practice indicate that while most patients are satisfied with their medication, 9% of new users had their medication stopped by their ophthalmologist due to side effect.28 Comparing the nonvisual symptoms between various treatments or between preserved and unpreserved formulations of dorzolamide–timolol would have been informative. However, the principal objective of the present study was to measure changes in eye comfort from baseline to 8 weeks of treatment with preservative-free dorzolamide–timolol formulation and not to perform between treatment group comparisons. By conducting within- instead of between-group comparison, all possible confounding bias related to disease and lifestyle factors that may affect IOP changes were avoided since each patient provided both control (pretreatment) and on treatment data.37 Further, a blinded treatment regimen would not have been compatible with a clinical practice setting. The current single-cohort, open-label, prospective design was thus implemented in order to achieve study objectives and to more accurately reflect real-life clinical settings.
An important strength of this study is the generalizability of its results to the Canadian target population. This study was conducted in real-life clinical settings where physicians treated patients as per their clinical judgment within the constraints of their clinical practice. These characteristics thus better emulate the routine clinical practice and permit the assessment of real-life effectiveness and safety. In addition, the use of a standardized and validated questionnaire (the GSS)24 to assess the ocular symptoms experienced by the patients enhances study validity.24 Based on their baseline scores, patients enrolled in this study likely represent the patient population who would benefit from the preservative-free medication.
In conclusion, the results of this study conducted in a real-life setting demonstrated that preservative-free dorzolamide–timolol formulation does not increase eye discomfort while significantly reducing intra–ocular pressure in treatment-naïve, newly diagnosed patients with open-angle glaucoma or ocular hypertension. Future real-life studies assessing the relative difference in nonvisual symptoms between various glaucoma treatments and between preserved and unpreserved formulations of dorzolamide–timolol, would further contribute to the management of glaucoma.
Acknowledgments
This study was supported by Merck Frosst Canada Ltd. The authors would like to acknowledge the study investigators: Jeffrey Chambers, David Neima, Carl Peters, Roberto LG Piemontesi, Lindsay Ong-Tone, Adian Long, George Colev, Cindy Hutnik, Fahim Ibrahim, Thomas Klein, Derek P-K Lui, Girair Basmadijian, Rama R Behki, Serge Boucher, François Demay, Stephen H Fichman, Clovis Eid, Robert Scott.
Trial registration number: This study is registered at clintrial.gov: NCT # 000545064.
Footnotes
Disclosures
C Hutnik, D Neima, F Ibrahim, and R Scott have no competing financial interests to declare. N Bastien and S Foucart are employees of Merck Frosst Canada Ltd; J Vaillancourt, D Haine, and J Sampalis are employees of JSS Medical Research Inc (contract research organization); all study investigators received grants related to the conduct of the study.
References
- 1.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull WHO. 2004;82(11):844–852. [PMC free article] [PubMed] [Google Scholar]
- 3.Adatia FA, Damji KF. Chronic open-angle glaucoma. Review for primary care physicians. Can Fam Physician. 2005;51:1229–1237. [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AG, Beaver HA. Visual loss in the elderly – Part I: Chronic visual loss: what to recognize and when to refer. Clin Geriatr. 2003;11(6):46–53. [Google Scholar]
- 5.Rouland JF, Berdeaux G, Lafuma A.The economic burden of glaucoma and ocular hypertension: implications for patient management: a review Drugs Aging 2005224315–321.15839720 [Google Scholar]
- 6.Damji KF, Behki R, Wang L. Canadian perspectives in glaucoma management: setting target intraocular pressure range. Target IOP workshop participants. Can J Ophthalmol. 2003;38(3):189–197. doi: 10.1016/s0008-4182(03)80060-1. [DOI] [PubMed] [Google Scholar]
- 7.Frampton JE, Perry CM. Topical dorzolamide 2%/timolol 0.5% ophthalmic solution: a review of its use in the treatment of glaucoma and ocular hypertension. Drug Aging. 2006;23(12):977–995. doi: 10.2165/00002512-200623120-00005. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JE, Ghosh K, Gieser DK, Adamsons IA. A randomized trial comparing the dorzolamide-timolol combination given twice daily to monotherapy with timolol and dorzolamide. Dorzolamide-Timolol Study Group. Ophthalmology. 1998;105(10):1945–1951. doi: 10.1016/s0161-6420(98)91046-6. [DOI] [PubMed] [Google Scholar]
- 9.Choudhri S, Wand M, Shields MB. A comparison of dorzolamide-timolol combination versus the concomitant drugs. Am J Ophthalmol. 2000;130(6):832–833. doi: 10.1016/s0002-9394(00)00717-0. [DOI] [PubMed] [Google Scholar]
- 10.Clineschmidt CM, Williams RD, Snyder E, Adamsons IA. A randomized trial in patients inadequately controlled with timolol alone comparing the dorzolamide-timolol combination to monotherapy with timolol or dorzolamide. Dorzolamide-Timolol Combination Study Group. Ophthalmology. 1998;105(10):1952–1959. doi: 10.1016/s0161-6420(98)91047-8. [DOI] [PubMed] [Google Scholar]
- 11.Fechtner RD, Airaksinen PJ, Getson AJ, et al. Efficacy and tolerability of the dorzolamide 2% timolol 0.5% combination (COSOPT) versus 0.005% (XALATAN) in the treatment of ocular hypertension or glaucoma: results from two randomized clinical trials. Acta Ophthalmol Scand. 2004;82(1):42–48. doi: 10.1046/j.1600-0420.2004.0205.x. [DOI] [PubMed] [Google Scholar]
- 12.Fechtner RD, McCarroll KA, Lines CR, Adamsons IA. Efficacy of the dorzolamide/timolol fixed combination versus latanoprost in the treatment of ocular hypertension or glaucoma: combined analysis of pooled data from two large randomized observer and patient-masked studies. J Ocul Pharmacol Ther. 2005;21(3):242–249. doi: 10.1089/jop.2005.21.242. [DOI] [PubMed] [Google Scholar]
- 13.Francis BA, Du LT, Berke S, Ehrenhaus M, Minckler DS. Comparing the fixed combination dorzolamide-timolol (COSOPT) to concomitant administration of 2% dorzolamide (trusopt) and 0.5% timolol – a randomized controlled trial and a replacement study. COSOPT Study Group. J Clin Pharm Ther. 2004;29(4):375–380. doi: 10.1111/j.1365-2710.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 14.Gandolfi SA, Rossetti L, Cimino L, Mora P, Tardini M, Orzalesi N. Replacing maximum-tolerated medications with latanoprost versus adding latanoprost to maximum-tolerated medications: a two-center randomized prospective trial. J Glaucoma. 2003;12(4):347–353. doi: 10.1097/00061198-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Henderer JD, Wilson RP, Moster MR, et al. Timolol/dorzolamide combination therapy as initial treatment for intraocular pressure over 30 mmHg. J Glaucoma. 2005;14(4):267–270. doi: 10.1097/01.ijg.0000169389.09804.33. [DOI] [PubMed] [Google Scholar]
- 16.Hutzelmann J, Owens S, Shedden A, Adamson I, Vargas E. Comparison of the safety and efficacy of the fixed combination of dorzolamide/timolol and the concomitant administration of dorzolamide and timolol: a clinical equivalence study. International Clinical Equivalence Study Group. Br J Ophthalmol. 1998;82(11):1249–1253. doi: 10.1136/bjo.82.11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konstas AG, Kozobolis VP, Tsironi S, Makridaki I, Efremova R, Stewart WC. Comparison of the 24-hour intraocular pressure-lowering effects of latanoprost and dorzolamide/timolol fixed combination after 2 and 6 months of treatment. Ophthalmology. 2008;115(1):99–103. doi: 10.1016/j.ophtha.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Rolle T, Tofani F, Brogliatti B, Grignolo FM. The effects of dorzolamide 2% and dorzolamide/timolol fixed combination on retinal and optic nerve head blood flow in primary open-angle glaucoma patients. Eye. 2008;22(9):1172–1179. doi: 10.1038/sj.eye.6703071. [DOI] [PubMed] [Google Scholar]
- 19.Whitson JT, Henry C, Hughes B, Lee DA, Terry S, Fechtner RD. Comparison of the safety and efficacy of dorzolamide 2% and brimonidine 0.2% in patients with glaucoma or ocular hypertension. J Glaucoma. 2004;13(2):168–173. doi: 10.1097/00061198-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 22.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 23.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 24.Lee BL, Gutierrez P, Gordon M, et al. The glaucoma symptom scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol. 1998;116(7):861–866. doi: 10.1001/archopht.116.7.861. [DOI] [PubMed] [Google Scholar]
- 25.Canadian Ophthalmological Society Evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44(Suppl 1):S7–S93. doi: 10.3129/cjo44s1. [DOI] [PubMed] [Google Scholar]
- 26.Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi: 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 27.Chawla A, McGalliard JN, Batterbury M. Use of eyedrops in glaucoma: how can we help to reduce non-compliance? Acta Ophthalmol Scand. 2007;85(4):464. doi: 10.1111/j.1600-0420.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 28.Beckers HJ, Schouten JS, Webers CA, van der Valk R, Hendrikse F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008;246(10):1485–1490. doi: 10.1007/s00417-008-0875-7. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Ophthalmology . Preferred practice pattern: primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2005. Available from: http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=a5a59e02-450b-4d50-8091-b2dd21ef1ff2 (Accessed October 07, 2009). [Google Scholar]
- 30.European Glaucoma Society . Terminology and Guidelines for Glaucoma. 2nd ed. Savona, Italy: The Society; 2003. Available from: http://www.eugs.org (accessed October 07, 2009) [Google Scholar]
- 31.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 32.Martinez A, Sánchez M. Effects of dorzolamide 2% added to timolol maleate 0.5% on intraocular pressure, retrobulbar blood flow, and the progression of visual field damage in patients with primary open-angle glaucoma: a single-center, 4-year, open-label study. Clin Ther. 2008;30(6):1120–1134. doi: 10.1016/j.clinthera.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Stewart WC, Konstas AG, Nelson LA, Kruft B. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117–1122. doi: 10.1016/j.ophtha.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Webers CA, van der Valk R, Schouten JS, Zeegers MP, Prins MH, Hendrikse F. Intraocular pressure-lowering effect of adding dorzolamide or latanoprost to timolol: a meta-analysis of randomized clinical trials. Ophthalmology. 2007;114(1):40–46. doi: 10.1016/j.ophtha.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr Eye Res. 2001;22(1):8–18. doi: 10.1076/ceyr.22.1.8.6977. [DOI] [PubMed] [Google Scholar]
- 36.Liesegang TJ. Conjunctival changes associated with glaucoma therapy: implications for the external disease consultant and the treatment of glaucoma. Cornea. 1998;17(6):574–583. doi: 10.1097/00003226-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma. 2009;18(6):423–428. doi: 10.1097/IJG.0b013e31818d3899. [DOI] [PMC free article] [PubMed] [Google Scholar]