Abstract
Background
Stroke thrombolysis-related intracerebral hemorrhage may occur remotely from the anatomical site of ischemia. One postulated mechanism for this is simultaneous multiple embolization with hemorrhage into a "silent" area of ischemia.
Results
A patient suffered a disabling stroke affecting the right cerebral hemisphere. He was treated with intravenous alteplase and underwent extensive early imaging with multimodal MRI. Several hours after treatment he developed a brainstem hemorrhage despite having no evidence of ischemia on DWI MRI in the brainstem.
Conclusion
Not all occurrences of remote ICH after stroke thrombolysis are secondary to multiple emboli with silent ischemia.
Case
A 66-year-old right-handed man awoke from sleep feeling well. At 06:55, while having breakfast, he developed sudden onset of painless weakness of his left arm associated with left facial droop and a subtle change in sensation over the left face and arm. He went directly to his local Emergency room where a diagnosis of stroke was made. He was transferred immediately to our centre for consideration of thrombolytic therapy, arriving at 08:28 (1 h 33 min from onset). He had a past history of a minor stroke with full recovery 3 years previously and longstanding hypertension. He was taking no medication having elected to discontinue his anti-hypertensive therapy two years earlier.
On examination, he was alert with no hemi-neglect or aphasia. He had a left facial droop and slight dysarthria. He was proximally weak but able to hold his left arm up against gravity, but had completely absent left distal hand function. He had reduced left leg power but was able to walk unsteadily. There was a reduction in pinprick sensation over the left arm and face. He was in normal sinus rhythm with a blood pressure of 200/115. A brain CT scan was performed, which demonstrated a small old right anterior parietal infarct which was interpreted as consistent with his previous minor stroke. There were no other acute changes. His National Institutes of Health Stroke Scale Score was 3.
Based on his clinical syndrome of severe distal weakness, it was postulated that his stroke involved the primary motor cortex or underlying fibres. After careful discussion with the patient including explanation of the risks of intracerebral hemorrhage, he was offered intravenous alteplase therapy. His deficits were considered disabling because of his retirement hobby of carpentry. His blood pressure fell to 170/95 with 10 mg of intravenous labetolol, after which an intravenous alteplase infusion was started at 09:28, 2 h 33 min after stroke onset. His blood pressure remained controlled (<185/110) throughout the infusion and subsequently with no change in his neurological status.
Immediately post-infusion (3 h 30 min from onset) he underwent an acute brain magnetic resonance scan (Figure 1), which demonstrated a new stroke in the right primary motor cortex with a parallel perfusion deficit (not shown). There was no perfusion-diffusion mismatch. No additional lesions were observed in the brain (Figure 2). All proximal intracranial arteries were patent on MR angiography.
Figure 1.
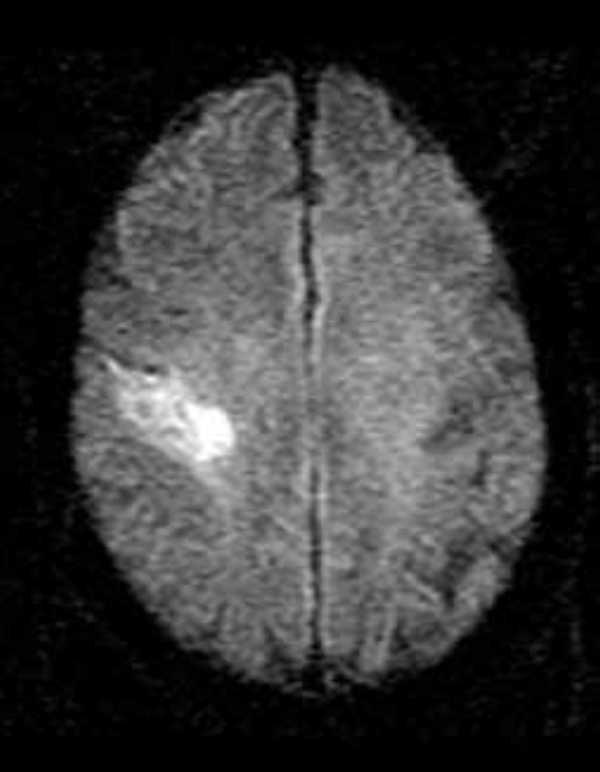
Post-alteplase infusion (~ 230 min after stroke onset) diffusion weighted brain magnetic resonance image showing cortical infarct in the motor strip
Figure 2.
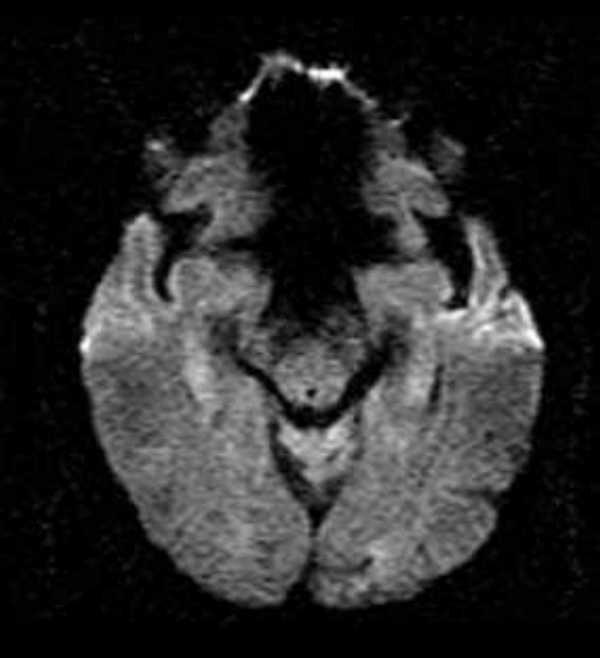
Post-alteplase infusion (~ 230 min after stroke onset) diffusion weighted brain magnetic resonance image showing absence of any acute lesion in the brainstem.
Approximately 3 hours post-alteplase infusion (5 h 30 min from onset), he developed sudden vomiting and hypertension followed by bradycardia. His level of consciousness declined, he developed vertical strabismus and he became hemiplegic on the left. He was intubated and an urgent CT scan was performed (Figure 3) which demonstrated a pontomesencephalic hemorrhage. He died several weeks later from bronchopneumonia. An autopsy was not performed.
Figure 3.
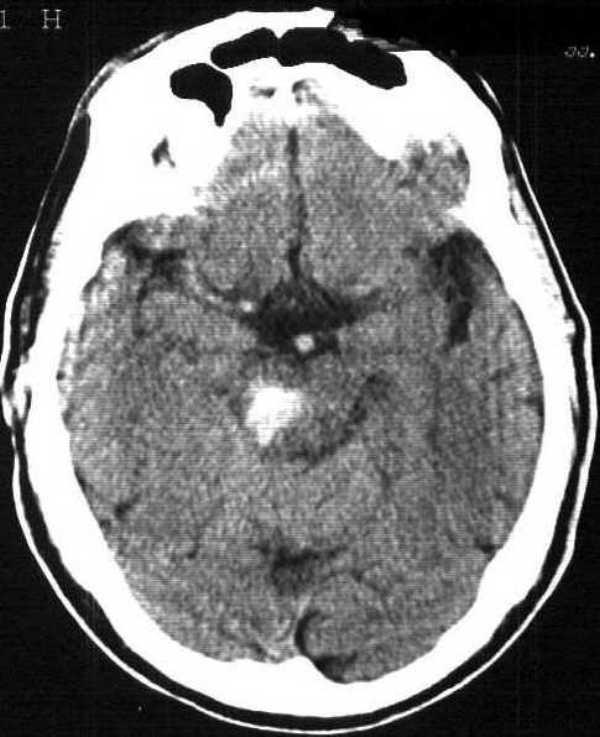
6 h CT scan showing new pontomesencephalic hemorrhage.
Discussion
Although most thrombolysis related intracerebral hemorrhages (ICH) occur at the site of stroke, up to 20% occur at sites away from the area of ischemia [1]. Despite this, no brainstem hemorrhages occurred in the NINDS tPA Stroke Trial and only 3% of ICH in the GUSTO-1 trial were in the brainstem [2]. One postulated explanation for the phenomenon of hemorrhage distant from the site of ischemia has been that strokes from proximal embolic sources, may be multiple such that co-existing small silent strokes are the source areas of hemorrhage. Multiple areas of DWI abnormality have been detected in a significant minority of patients with acute stroke [3].
This case concisely demonstrates that other mechanisms are at play. By both clinical examination and early diffusion-weighted imaging, there was no evidence of any area of concomitant brainstem ischemia. It remains possible that he had an initial DWI abnormality in the pons that was reversible with alteplase treatment, or that a small DWI lesion was missed for technical reasons such as slice thickness. DWI lesions associated with a brief duration of ischemia have been clinically shown to be reversible [4]. We consider this possibility to be remote because of his lack of clinical signs suggesting brainstem ischemia. In addition, DWI lesions in both anterior and posterior circulation concurrently are very uncommon [5]. Finally, no perfusion deficit (mean transit time or cerebral blood volume) was demonstrable in the pons.
Hypertensive small vessel disease was likely this patient's major risk factor for ICH. In phase 2 studies of alteplase for ischemic stroke, hypertension (diastolic BP > 100 mmHg immediately pre-treatment) was a clear risk factor for ICH [6]. Although treating patients "off-protocol" has been shown in post-marketing studies to be associated with higher hemorrhage rates and poorer outcomes [7], the NINDS protocol included both the treatment of hypertension prior to administration of thrombolytic therapy and the treatment of patients with low NIHSS scores, but with disabling deficits. Recent experimental evidence suggests that chronically hypertensive rats have higher rates of intracerebral hemorrhage after alteplase therapy for stroke [8]. Nevertheless, the NINDS investigators suggested that the treatment of hypertension (after randomisation) in the alteplase treated group was associated with poorer outcome but could not draw definitive conclusions because of the trial's design [9].
Conclusion
Thrombolysis-related ICH may occur unexpectedly and even DWI MRI may not be predictive. The decision to treat acute stroke patients with alteplase in the setting of hypertension is still one based on clinical judgement rather than solid evidence.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/content/backmatter/1471-2377-1-1-b1.pdf
Acknowledgments
Acknowledgements
Drs. Hill and Buchan were funded in part by grants from the Medical Research Council of Canada, the Heart & Stroke Foundation of Canada and the Alberta Heritage Foundation for Medical Research
Dr. Frayne was funded in part by grants from the Heart & Stroke Foundation of Canada and the Alberta Heritage Foundation for Medical Research
Dr. Demchuk was funded in part by a grant from the Medical Research Council of Canada
Contributor Information
Michael D Hill, Email: michael.hill@crha-health.ab.ca.
Philip A Barber, Email: phil.barber@crha-health.ab.ca.
Andrew M Demchuk, Email: ademchuk@dcns.ucalgary.ca.
Robert J Sevick, Email: rob.sevick@crha-health.ab.ca.
Richard Frayne, Email: rfrayne@ucalgary.ca.
Alastair M Buchan, Email: buchan@ucalgary.ca.
References
- Intracerebral hemorrhage after intravenous rtPA therapy for ischemic stroke. Stroke NINDS rtPA Stroke Study Group. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Gebel JM, Sila CA, Sloan MA, Granger CB, Mahaffey KW, Weisenberger J, Green CL, White HD, Gore JM, Weaver D, Califf RM, Topol EJ. Thrombolysis-related intracranial hemorrhage. Stroke . 1998;29:563–569. doi: 10.1161/01.str.29.3.563. [DOI] [PubMed] [Google Scholar]
- Albers GW, Lansberg MG, Norbash AM, Tong DC, O'Brien MW, Woolfenden AR, Marks MP, Moseley ME. Yield of diffusion-weighted MRI for detection of potentially relevant findings in stroke patients. Neurology . 2000;54:1562–1567. doi: 10.1212/wnl.54.8.1562. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, Saver JL. Diffusion MRI in Patients With Transient Ischemic Attacks. Stroke. 1999;30:1174–1180. doi: 10.1161/01.str.30.6.1174. [DOI] [PubMed] [Google Scholar]
- Roh JK, Kang DW, Lee SH, Yoon BW, Chang KH. Significance of Acute Multiple Brain Infarction on Diffusion-Weighted Imaging. Stroke . 2000;31:688–694. doi: 10.1161/01.str.31.3.688. [DOI] [PubMed] [Google Scholar]
- Levy DE, Brott TG, Haley C, Jr., Marler JR, Sheppard GL, Barsan W, Broderick JP. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke. 1994;25:291–297. doi: 10.1161/01.str.25.2.291. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Barber PA, Newcommon N, Karbalai HG, Demchuk AM, Hoyte KM, Klein GM, Feasby TE. Effectiveness of rtPA in acute ischemic stroke: outcome relates to appropriateness. Neurology. 2000;54:679–684. doi: 10.1212/wnl.54.3.679. [DOI] [PubMed] [Google Scholar]
- Brinker G, Pillekamp F, Hossmann KA. Brain hemorrhages after rt-PA treatment of embolic stroke in spontaneously hypertensive rats. Neuroreport. 1999;10(9):1943–6. doi: 10.1097/00001756-199906230-00027. [DOI] [PubMed] [Google Scholar]
- Brott T, Lu M, Kothari R, Fagan SC, Frankel M, Grotta JC, Broderick J, Kwiatkowski T, Lewandowski C, Haley C, Jr., Marler JR, Tilley BC. Hypertension and Its Treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29:1504–1509. doi: 10.1161/01.str.29.8.1504. [DOI] [PubMed] [Google Scholar]


