Abstract
Background
In Tanzania, the International Working Formulation [WF] rather than the WHO Classification is still being used in diagnosing malignant lymphomas (ML) and the biological characterization including the HIV/EBV association is sketchy, thus restraining comparison, prognostication and application of established therapeutic protocols.
Methods
Archival, diagnostic ML biopsies (N = 336), available sera (N = 35) screened by ELISA for HIV antibodies and corresponding clinical/histological reports at Muhimbili National Hospital (MNH) in Tanzania between 1996 and 2006 were retrieved and evaluated. A fraction (N = 174) were analyzed by histopathology and immunohistochemistry (IHC). Selected biopsies were characterized by flow-cytometry (FC) for DNA ploidy (N = 60) and some by in-situ hybridization (ISH) for EBV-encoded RNA (EBER, N = 37).
Results
A third (38.8%, 109/281) of the ML patients with available clinical information had extranodal disease presentation. A total of 158 out of 174 biopsies selected for immunophenotyping were confirmed to be ML which were mostly (84. 8%, 134/158) non-Hodgkin lymphoma (NHL). Most (83.6%, 112/134) of NHL were B-cell lymphomas (BCL) (CD20+), of which 50.9%, (57/112) were diffuse large B-cell (DLBCL). Out of the 158 confirmed MLs, 22 (13.9%) were T-cell [CD3+] lymphomas (TCL) and 24 (15.2%) were Hodgkin lymphomas (HL) [CD30+]. Furthermore, out of the 60 FC analyzed ML cases, 27 (M:F ratio 2:1) were DLBCL, a slight majority (55.6%, 15/27) with activated B-cell like (ABC) and 45% (12/27) with germinal center B-cell like (GCB) immunophenotype. Overall, 40% (24/60) ML were aneuploid mostly (63.0%, 17/27) the DLBCL and TCL (54.5%, 6/11). DNA index (DI) of FC-analyzed ML ranged from 1.103-2.407 (median = 1.51) and most (75.0%) aneuploid cases showed high (>40%) cell proliferation by Ki-67 reactivity. The majority (51.4%, 19/37) of EBER ISH analyzed lymphoma biopsies were positive. Of the serologically tested MLs, 40.0% (14/35) were HIV positive, mostly with high (≥40.0%) Ki-67 reactivity.
Conclusions
According to the 2001 WHO Classification, most subtypes are represented in Tanzanian ML. Extranodal presentation was common among MNH lymphoma patients who also showed high aneuploidy, tumor proliferation (KI-67) and EBER positivity. DLBCL was frequent and phenotype heterogeneity appeared similar to observations in Western countries suggesting applicability of established intervention approaches. HIV was apparently associated with high ML cell proliferation but extended studies are needed to clarify this.
Background
Malignant lymphomas (ML) represent a spectrum of lymphoid neoplasms with varying prognosis [1] including non-Hodgkin lymphoma (NHL), Burkitt lymphoma (BL) and Hodgkin lymphoma (HL). MLs occur worldwide with an increasing incidence both in industrialized countries and Africa [1,2]. Lymphomas represent today an important cause of morbidity and mortality in sub-Saharan Africa, including Tanzania partly due to the HIV and AIDS epidemic [3-5].
The classification of NHL has changed over time through the Rappaport Classification developed before lymphoid cells were divided into B-cells and T-cells [6], the International Working Formulation [WF] based on clinical aggressiveness [6], the Kiel Classification (based on histological grade) [7], the Lukes and Collins Classification which separated B-cell and T-cell lymphomas by immunologic techniques [8], the Revised European-American classification of Lymphoid neoplasms [REAL] [4] and most recently, the World Health Organization [WHO] classification [9,10]. The later two classifications recognize three major categories, B-cell neoplasms, T/NK-cell neoplasms and HL [9]. For HL the Rye Classification has been used for many years although, now slightly modified in the WHO/REAL Classification system [11,12]. Various countries have tested the applicability and adopted the WHO classification [11,13]. In Tanzania the WF is still used and attempts to apply the WHO classification have so far not been documented. Geographic and racial-ethnic differences in relative frequency of various ML have been reported [5,9]. Such geographic and racial-ethnic differences may influence the adaptation of prognostication as well as therapeutic protocols/algorithms shown to be effective elsewhere. An update and re-appraisal of Tanzanian ML diagnoses and characteristics possibly peculiar to this country is therefore needed.
It is now well documented that ML in HIV and AIDS patients also called AIDS-related lymphomas (ARL) have distinct clinical features including frequent extranodal presentation, which has not been evaluated in Tanzanian ML patients before our current study [4,14].
New insights into the pathogenesis of ML are continuously gained with the development in cytogenetics, molecular biology and immunological techniques [9]. Thus, similar to other cancers, ML may present with genomic instability including specific translocations and numerical chromosomal abnormalities (aneuploidy) [15-17]. Furthermore, the ML association with different viral infections is now well established including the Epstein-Barr virus (EBV) [4,17,18], the human immunodeficiency virus (HIV) [4,17] and recently, Kaposi's sarcoma associated herpes virus/human herpes virus type 8 (KSHV/HHV-8) in primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) y. However, the prevalence of such viral associations is still sketchy in sub-Saharan Africa particularly Tanzania and is elucidated in our current study [3-5].
Likewise, the diffuse large B-cell lymphoma (DLBCL) group is now no longer one entity but rather a spectrum of NHL with heterogeneous histopathology, genotype, phenotype and prognosis including germinal center B-cell like (GCB) and activated B-cell-like (ABC) lymphomas [23,24]. Thus, the expression of proteins related to germinal centre B (GCB) cell or activated B-cells (ABC) and that of apoptosis-regulating proteins have been found to be associated with clinical outcome [25] and in particular Bcl-2 expression is strongly related to poor prognosis [25].
Ki-67 expression by proliferating tumor cells is a well established prognostic marker of malignancies including ML [26,27]. Such prognostic markers are poorly documented in Africa including Tanzania [14] impeding the development of comparable prognostication and adaptation of corresponding therapeutic protocols emphasized by our current studies.
Furthermore, data on clinical presentation and demographic (sex and age) characteristics for Tanzanian ML are scanty [14]. Our recent report on ML tumor proliferation included only mean values for major ML subtypes [14]. Those data are extended by our current long-term study
Methods
Study area
The study was conducted in the Histopathology Unit of the Department of Laboratory Services at the Muhimbili National Hospital (MNH) in Dar es Salaam, which is also the teaching hospital for the Muhimbili University of Health and Allied Sciences (MUHAS). MNH is the national referral health care facility in Tanzanian with a bed capacity of over 1000 and receives biopsies from most of the country except the lake zone which has its own manned pathology department.
Clinical presentation
This was retrieved from clinical records and was determined by physical examination, chest radiography and abdominal ultrasonography during the study period.
Biopsies and HIV serology
Archival diagnostic biopsy material collected at MNH between 1996 and 2006 (10 years) was reviewed for histopathology and analyzed by immunohistochemistry (IHC), flow-cytometry (FC) and in-situ hybridization (ISH) at the Immunopathology Lab (Karolinska University Hospital Solna, Stockholm). The fixation and tissue processing protocols remained unchanged during the study period. Biopsies were selected for IHC or FC depending on the basis of representative tissue material per block. Clinical notes and histological reports for all ML biopsies submitted for diagnosis during the same period were also evaluated. The clinical evaluation protocol did not change during the study period. HIV-1 antibody serology (ELISA) was performed at the Microbiology/Immunology department at Muhimbili University of Health and Allied Sciences (MUHAS) as previously described [14,28].
Histology
Primary histological diagnosis on hematoxylin and eosin (H & E) stained formalin-fixed paraffin embedded (FFPE) sections was done as previously described, at MNH according to the International Working Formulation [4,28].
Histopathological re-evaluation
(H & E and immunoperoxidase assay) of available tissue blocks was done at Karolinska University Hospital Solna independently and by three pathologists (ARM, PB and AP) according to the 2001 WHO classification of lymphoid neoplasms [9]. Inter-observer discrepancies were resolved by all three pathologists sitting together. Out of the H & E-evaluated cases biopsies selected based on the histological ML group, tumor:stroma ratio and absent/minimal necrosis, were further evaluated by immunohistology, cytomorphometry, flow-cytometry and in-situ hybridization.
Immunohistochemistry (IHC)
Immunostaining was done (Immunopathology Lab) by the avidin-biotin complex immunoperoxidase technique as previously described [4,14,28]. Briefly, tissue sections were mounted on SuperFrost® slides (Menzel GmbH & Co KG, Braunschweigh, Germany) deparaffinized, rehydrated and boiled for antigen retrieval at 750W by microwave (6 min) in citrate buffer pH 6. Endogenous peroxidase activity was quenched by incubating the sections in 30% hydrogen peroxide in distilled water (30 min) at room temperature (RT) then followed by washing in phosphate-buffered saline (PBS) and incubation with 1:20 normal serum from the species of the secondary antibody and washing (PBS). The sections were incubated overnight at 4°C with primary antibody for human antigens including pan-leucocyte CD45 (clone LCA), B-cell CD20 (clone L26), Reed-Sternberg (RS)/Hodgkin (HC) cells CD30 (clone Ber-H2), the Ki-67 (MIB-1) proliferation marker as well as the polyclonal rabbit CD3 T-cell marker. For DLBCL sub-typing, the immunophenotype of the tumors classified as germinal-center B-cell like (GCB) or activated B-cell like (ABC) lymphomas was determined using mouse anti-human CD10 (SS2/36), MUM1p (clone MUM1p), BCL-6 (clone PG-B6p) and BCL-2 (clone 124) all obtained from DakoCytomation, (Glostrup, Denmark) were used. The BCL-6/CD10/MuM1p markers were used for phenotypic and the BCL-2/CD10/MuM1p antibodies for prognostic sub-grouping. Thus DLBCLs could be categorized into activated B-cell like (ABC) if they were CD10-/BCL6+/MUM1p+, CD10-/BCL6- or CD10-/MUM1p+ and germinal center B-cell like (GCB) if they showed CD10+, CD10-/BCL6+/MUM1p- or CD10-/MUM1p- immunophenotypes. Furthermore, DLBCLs were categorized into prognostic group 1 (favourable prognosis) including all BCL-2- cases, BCL-2+/CD10+ and BCL-2+/CD10-/MUM1p- and group 2 (poor prognosis) which included cases with BCL-2+/CD10-/MUM1p+ reactivity [25]. For primary antibody detection, the sections were incubated (30 min, RT) with anti-species (secondary) antibody and the avidin-biotin complex respectively, and later developed (visualized) with 3,3'-diaminobenzidine (DAB) chromogen (Sigma-Aldrich, St. Louis MO, USA) as previously described [4,14,28]. After PBS washing, the slides were lightly counter-stained with H & E, blued in running tap water (30 min), dehydrated in ascending grades of ethanol, cleared in two runs of xylene and mounted with coverslips using Mountex (Histolab Products AB, Göteborg, Sweden).
Negative controls included sections from tissues not expressing the respective antigen as well as substitution of the primary antibody by buffer. Positive controls included tissue sections (lymph nodes and tonsils) with known expression of the antigen under investigation. These controls were included in each experiment.
Microscopic evaluation
A fluorescence microscope (Olympus BX60, Tokyo, Japan) with a digital camera (Sony DKC-5000, Tokyo, Japan) and various filter cubes was used to document bright field and fluorescence microphotography. Cells were scored on color micrographs in eight adjacent fields (256 × 190 μm each) of characteristic lesions and the mean count of DAB-positive cells for each primary antibody (marker) was calculated. Strength of CD20 reactivity was subjectively scaled from 1+ to 4+ depending on staining intensity, thus intensity ≤2+ was regarded as weak and intensity>2+ was regarded as strong). Picture processing and printing was done using Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, USA) and Microsoft-Power Point, 2003 (Microsoft Corporation, Redmond, WA, USA).
Tumor sample selection, enucleation and flow-cytometric analysis (FC)
FC on extracted lymphoma and tonsillar nuclei was performed as previously described [29]. Selected, non-necrotic tumour regions containing ≥70% neoplastic tissue in 90 μm thick sections were dissected, deparaffinized, rehydrated and digested for 1 hour at 40°C with 0.1% w/v Sigma protease XXIV (Sigma P8038) [Sigma-Aldrich, St. Louis, MO, USA] in Tris buffer [0.1 M Tris, 0.07 M NaCl (pH 7.2) [Merck, Darmstadt, Germany]. The obtained free nuclei in suspension were stained for 30' with 6-diamidino-2-phenylindole (DAPI) solution (10 μM DAPI in 800 mM disodiumhydrogenphosphate) [Sigma D9542, Sigma-Aldrich, St. Louis, MO, USA] and evaluated for DNA content by flow cytometry (≥2 × 104 nuclei per histogram). For the FC analysis, a PAS II (Particle Analysing System)-cytometer (Partec, Münster, Germany) and a LSRII Flow Cytometer (BD Biosciences, San Jose, CA) were used. The ModFit Program (Verity Software House; Topsham, ME, USA) was used for cell cycle analysis. Ploidy [DNA index (DI)] of diploid and aneuploid ML cell populations was compared to normal tonsil cells as previously described [30,31].
Cytomorphometric evaluation
Extracted ML and control tonsillar nuclei were stained with DAPI and mounted with Vectashield [Vector Laboratories, Inc. Burlingame, CA, USA] mounting medium for fluorescence microscopy on SuperFrost® slides. The mean size (S) and pleomorphism (P) of nuclei extracted from the tonsils were used as unity and were termed S0 and P0. Thus relative nuclear size (RNS) was the percentage size in excess of S0 evaluated as S0 + size increase ≤25% = S1, S0 + increase of 26 - 50% = S2, S0 + increase of 51 - 75% = S3 and S0 + increase >75% = S4. Evaluation of nuclear pleomorphism was P1 = mild, P2 = moderate and P3 = high. Mitotic figure counts (MFC) were evaluated on routine H & E sections. High power field (HPF) refers to ×400 microscopic magnification.
In-situ hybridization (ISH)
Epstein-Barr Virus (EBV) infection detection was done by automated ISH as previously described [32] (Pathology Cell analysis Lab, Cancer Center Karolinska) using a fluorescein (FITC)-conjugated oligonucleotide probe to EBV-encoded (EBER) transcripts on FFPE tissue sections optimized for use with Bond Polymer Refine Detection (DS9800) and Anti-Fluorescein Antibody (AR0833) on the Bond-max system (Leica Biosystems Nussloch GmbH, Nussloch, Germany) according to the manufacturer's instructions.
Statistical analysis
Data was analyzed using Statistical Package for the Social Sciences (SPSS) [SPSS Inc., Chicago Ill]. The Fisher exact test was used for smaller sample sizes. P-values of ≤ 0.05 were considered statistically significant.
Ethical considerations and MNH guidelines
These studies were approved by the MUHAS Research Ethics Committee and the Ethical Committee, Karolinska University Hospital Solna (Dnr 01-096).
Upon obtaining individual informed consent and upholding safety, confidentiality and privacy, patients and material at MNH are also available for education and research since it was established as a University Teaching Hospital by an Act of Parliament. A strictly confidential and coded specimen processing and evaluation was conducted. HIV screening was performed by clinicians upon informed consent in the respective in-/out-patient units.
Results
ML frequency and general demography
During the period of study, the histopathology unit at MNH received approximately 50,000 biopsies including about 7,000 tumors out of which a total of 336 histologically diagnosed (H & E) lymphoma cases consecutively collected were evaluated. In 311 of 336 cases the information on patient sex (males 63.3%, n = 197/311 and females 36.7%, n = 114/311) was available. In 281 of 336 cases the age of patients was known, including 107 (38.1%) children (≤18 years), 131 (46.6%) adults (19-54 years) and 43 (15.3%) elderly (≥55 years) (Table 1). The overall age ranged from 4 to 91 years with a mean 31 and median of 30 years respectively.
Table 1.
General demographic characteristics of confirmed malignant lymphomas (ML) at MNH between 1996 and 2006
| Category | Number of ML cases | Percent |
|---|---|---|
| Available sex data | 311/330 | 94.2 |
| Female/Male | 114/197 | 36.7/63.3 |
| Available age data (years) | 265/330 | 80.3 |
| Juveniles (≤18y) | 107 | 32.4 |
| Adults (19-54y) | 131 | 39.7 |
| Elderly (≥55y) | 43 | 13.0 |
| Mean age | 31.03 | na* |
*na = not applicable
Clinical presentation
Data on clinical presentation (based on physical examination, radiography, abdominal ultrasonography and endoscopy) was available in 265 patients excluding cases later ruled out as ML after histological and immunohistological review. Majority 64.9%, (172/265) of these ML had nodal and 35.1% (109) extranodal disease at diagnosis. Extranodal presentation was reported in just over half (50.9%, 54/106) of childhood ML cases and in 20.9% (9/43) of the elderly, which difference was statistically highly significant (P = 0.00635, Chi-square Test) [Table 2]. Anatomical sites of extranodal presentation included visceral (12.5%), bone (10.0%) and soft tissues (6.4%) while cutaneous, oral cavity, nasal and ocular in descending order were rare. Furthermore, evaluation of ML anatomical distribution showed that majority (57.7% (153/265) had supra-diaphragmatic, 27.5% (73/265) sub-diaphragmatic and 26.8% (71/265) disseminated lymphoma. However, no primary effusion lymphoma (PEL) or primary central nervous system lymphoma (PCNSL), were found in this cohort [Table 3]. Data on clinical presentation was available in 115 cases morphologically reviewed at the Immunopathology Lab [Table 4]. Differences in distribution of anatomical sites of disease presentation by sex were not statistically significant (p-value 0.4695, Chi-square Test) [Table 2].
Table 2.
Anatomical presentation of ML by age-group at MNH from 1996-2006
| Age (Years) | Anatomical presentation [No. (%)] | Total [No. (%)] |
P-value | |
|---|---|---|---|---|
| Nodal | Extra-nodal | |||
| Young (≤18) | 52 (33.1) | 54 (50.0) | 106 (40.0) | 0.00635 |
| Adults (19-54) | 71 (45.2) | 45 (41.7) | 116 (43.8) | |
| Elders (≥55) | 34 (21.7) | 9 (8.3) | 43 (16.2) | |
| Total | 157 (59.2) | 108 (40.8) | 265 (100.0) | |
| Female | 58 (36.9) | 45 (41.7) | 103 (38.9) | 0.4695 |
| Male | 99 (63.1) | 63 (58.3) | 162(61.1) | |
| Total | 157 (100.0) | 108 (100.0) | 265 (100.0) | |
NB: 265 is the number of confirmed ML whose clinical data was available
Table 3.
Anatomical site of disease presentation at diagnosis among TZ ML patients at MNH from 1996-2006
| Anatomical site | Number of ML cases | Percent |
|---|---|---|
| Lymph node | 157 | 59.2 |
| Soft tissues | 17 | 6.4 |
| Oral cavity | 8 | 3.0 |
| Skin | 9 | 3.4 |
| Bone | 28 | 10.6 |
| Viscera | 35 | 13.2 |
| Ocular | 4 | 1.5 |
| Nasal | 7 | 2.6 |
| Total | 265 | 100.0 |
NB: 265 is the number of confirmed ML whose clinical data was available
Table 4.
Anatomical presentation of ML at diagnosis (MNH) by their reviewed histological subtypes (1996 -2006)
| Reviewed histological diagnosis | Anatomical Site [No. (%)] | Reviewed cases with available clinical data | Clinical data unrecorded | Sub-totals | Total reviewed ML | |
|---|---|---|---|---|---|---|
| Nodal | Extranodal | [No. (%)] | [No. (%)] | [No. (%)] | [No. (%)] | |
| DLBCL | 35 (64.8) | 7 (25.0) | 42 | 15 | 57 (50.9) | |
| BL | 2 (3.7) | 15 (53.6) | 17 | 6 | 23 (20.5) | |
| Other BCL* | 17 (31.5) | 6 (21.4) | 23 | 9 | 32 (28.6) | |
| Total BCL | 54 (83.1) | 28 (80.0) | 82 | 30 | 112 (83.6) | 112 (83.6) |
| TCL | 11 (16.9) | 7 (20.0) | 18 | 4 | 22 (13.7) | |
| TOTAL NHL | 65 (65.0) | 35 (35.0) | 100 | 34 | 134 (84.8) | |
| HL | 10 (66.7) | 5 (33.3) | 15 | 9 | 24 (15.2) | |
| TOTAL REVIEWED ML [No. (%)] | 75 (65.2) | 40 (34.8) | 115 (72.8) | 43 (27.2) | 158 (100.0) | |
*BCL = B-cell lymphomas
Histology and immunohistochemistry (IHC)
A total of 174 biopsies out of the 336 cases above, were selected and stained by H & E and immunoperoxidase assay (IHC) based on availability of clinical notes, tissue blocks as well as quantity of material per block. After histopathological review and immunophenotyping using a panel LCA, CD20, CD3 and CD30 cell markers, a total of 158/174 were confirmed to be ML and 16 cases (9.2%) were excluded from further ML sub-classification, DNA ploidy and EBER studies but retained in the general study cohort to account for misdiagnosis. These represented mostly poorly differentiated metastatic carcinomas and sarcomas in lymph nodes as well as neuroendocrine tumors (carcinoids) but also a few cases of chronic inflammation including follicular hyperplasia and tuberculosis. Furthermore, the evaluation revealed 134 (84.8%) NHL, including 112 (83.6%) B-cell lymphomas (BCL) [CD20+, CD3-] and 22 (13.7%) mature T-cell lymphomas (TCL) [CD3+, CD20-] (Table 4). Most of the reviewed BCLs, (50.9%, 57/112) were DLBCL [Figure 1(a-d)], 20.5% (n = 23) were BL [Figure 2(a-d)] and 28.6% (n = 32) were other B-cell lymphomas [Table 4], which included small cell (SCL), lymphoplasmacytic (LP), follicular (FL) and marginal zone B-cell lymphomas (MZBCL) as well as plasmacytoma and oral plasmablastic lymphoma (OPBL). However, primary mediastinal large B-cell lymphoma was not found. TCL included peripheral T-cell lymphoma, extranodal NK/T-cell lymphoma (nasal type), angioimmunoblastic T-cell lymphoma as well as anaplastic large-cell lymphoma. Furthermore, 15.2% (n = 24) cases were HL (CD30+) [Table 4]. As expected, majority (53.6%, n = 15/28) B-cell lymphomas (BCL) with extranodal presentation were BL cases and most (80.0%, 28/35) NHL with extranodal presentation were TCL [Table 4]. Of the reviewed HL cases (Table 5), most showed a classical HL (CHL) histopathology) often of the mixed cellularity (MC) subtype [Figure 3(a)]. Furthermore, 6 (25.0%) cases were CHL nodular sclerosis (NS), 3 (12.5%) lymphocyte rich (LR) and 2 (8.3%) were lymphocyte depleted (LD). The non-classical nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) subtype appeared rare (8.3%, 2/24) [Table 5] in this cohort.
Figure 1.
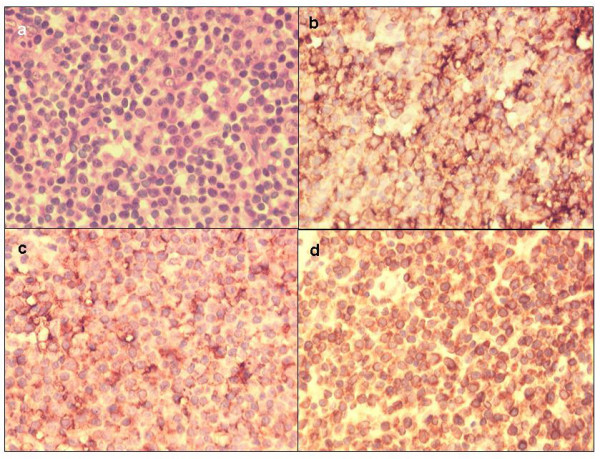
(a, b, c, d)- Sections of an activated B-cell like (ABC) diffuse large B-cell lymphoma (DLBCL) case showing (a) H & E staining, and granular membranous and cytoplasmic immunoperoxidase (brown) reactivity in all tumor cells for (b) CD20 antigen, (c) BCL-6 antigen and (d) BCL-2 antigen (all ×400).
Figure 2.
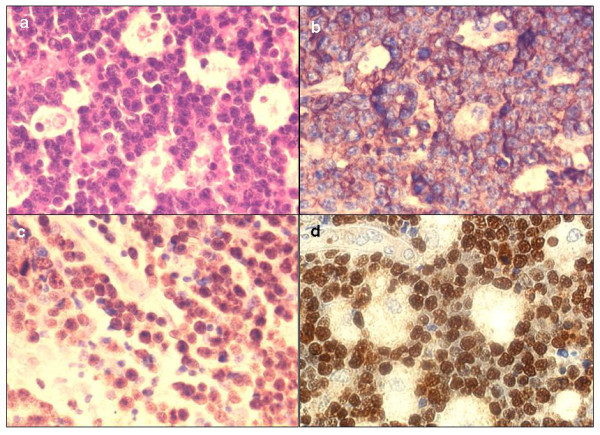
(a, b, c, d)- H & E and immunoperoxidase staining (brown) of a BL case showing (a) H & E section with "starry sky pattern," (b) granular membranous and cytoplasmic reactivity in all tumor cells for CD20 antigen, (c) a high proportion of nuclear Ki-67 immunoreactivity (brown) and (d) EBER probe (dark brown) in situ hybridization (ISH) nuclear reactivity in all tumor cells, (all ×400).
Table 5.
Histological subtypes of reviewed Hodgkin lymphoma (HL) cases from MNH between 1996 and 2006
| Histological diagnosis | Number | Percent |
|---|---|---|
| Classical HL (CHL) | ||
| Mixed cellularity (MC) | 11 | 45.8 |
| Nodular sclerosing (NS) | 6 | 25.0 |
| Lymphocyte rich (LR) | 3 | 12.5 |
| Lymphocyte depleted (LD) | 2 | 8.3 |
| Non-classical HL | ||
| Nodular lymphocyte predominant (NLPHL) | 2 | 8.3 |
| Total HL | 24 | 100.0 |
Figure 3.
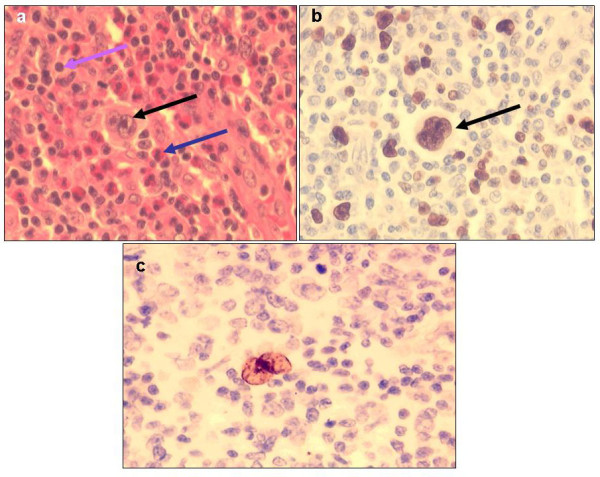
(a, b, c,)- HL histologic sections: (a) H & E staining of a classical HL mixed cellularity (MC) case showing Reed-Sternberg (RS) cells (black arrow), eosinophils (blue arrow) and lymphocytes (purple arrow), (b) nuclear Ki-67 immunoperoxidase reactivity (brown) in an RS cell, and (c) an EBER positive (brown) RS cell (all ×400).
DLBCL sub-typing
Of the 27 subtyped DLBCL (M:F ratio 1.7:1) cases, 3 (11.1%) were diagnosed in children and 24 (88.9%) in adults (Table 6). When sub-grouped for histological presence/absence of follicular structures most (74.1%) cases (n = 20) showed completely diffuse architecture and 25.9% (n = 7) had follicular remnants (Table 6). Using CD10, MUM1p, and BCL-6 markers together, we found that slightly more DLCBL in our series showed an ABC immunophenotype (55.6%, n = 15), by comparison to GCB immunophenotype 44.4% (12) although this was not statistically significant (P = 0.547, Chi2 test) [Table 6]. As expected, all DLBCL with follicular remnants were GCB and most (75.0%) of those completely diffuse were ABC. The DLBCL immunophenotypes were associated with varying strength of CD20 reactivity and majority (57.1%, 12/21) of cases with strong reactivity were GCB (p = 0.02). Thus most (70.4%, 19/27) DLBCL examined were group 1 and 29.6% (8/27) were group 2 according to BCL-2/CD10/MuM1p sub-grouping (Table 6). In the present cohort, DLBCL subtype was not significantly correlated to age-group, HIV serostatus, tumor proliferation (Ki-67+) or DNA index/ploidy status which however, could depend on the small sample size.
Table 6.
DLBCL (ABC/GCB) subtypes by clinical presentation, viral (HIV/EBV) status, IHC (Ki-67 and BCL-2) and DNA ploidy at MNH (1996-2006)
| Subtypes [No. (%)] |
Sex | Age-group | Clinical Presentation in relation to the diaphragm |
HIV status | Ki-67 reactivity | EBER ISH reactivity | DNA Ploidy | BCL2/Mum1p subtype |
Total [No. (%)] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | ≤18 | >18 | Supra- | Sub- | Gen | Pos | Neg | Low | High | Pos | Neg | nd | Diploid | Aneuploid | Grp. 1 | Grp. 2 | No.(%) | |
| ABC | 9 (50.0) | 6 (66.7) | 0 | 15 (65.2) | 9 (66.7) | 4 (80.0) | 4 (50.0) | 2 (40.0) | 1 (33.3) | 11 (61.1) | 4 (44.4) | 5 (50.0) | 8 (53.3) | 2 | 6 (66.7) | 9 (50.0) | 7 (36.8) | 8 | 15 (55.6) |
| GCB | 9 (50.0) | 3 (33.3) | 3 | 8 (34.8) | 3 (33.3) | 1 (20.0) | 4 (50.0) | 3 (60.0) | 2 (66.7) | 7 (38.9) | 5 (55.6) | 5 (50.0) | 7 (46.7) | 0 | 3 (33.3) | 9 (50.0) | 12 (63.2) | 0 | 12 (44.4) |
| Total | 18 (66.7) | 9 (33.3) | 3 (11.1) | 23 (88.9) | 12 (44.4) | 5 (18.5) | 8 (29.6) | 5 (62.5) | 3 (37.5) | 18 (66.7) | 9 (33.3) | 10 (37.0) | 15 (55.5) | 2 (7.4) | 9 (33.3) | 18 (66.7) | 19 (70.0) | 8 (30.0) | 27* (100.0) |
*This is the total number of DLBCLs analyzed by FACS for DNA Ploidy
nd = not determined
ABC = activated B-cell like DLBCL, GCB = germinal center B-cell like DLBCL
Cell proliferation by Ki-67 immunohistochemistry and flow-cytometry (FC)
As expected, the highest (mean = 91.5%, range 75-98%) reactivity was found among BL cases [Figure 2(c)] followed by DLBCL (mean 42.4%, median 40.0%, range 10-90%, SD 23.5) and closely by TCL (mean 42.3%, median 50.0%, range 10-80%, SD 24.2) and lowest for HL [Figure 3(b)]. Furthermore, possible associations between Ki-67 index, sex, age or clinical presentation of ML cases as well as with that of different DLBCL subtypes, were not statistically significant. However, as expected, most (88.9%, 8/9) aneuploid ML cases showed high tumor proliferation rates (>40% Ki-67 reactivity) while only 11.1% of diploid ML had high tumor proliferation which difference appeared to be statistically significant (p-value 0.04, Fisher's Exact Test). FC S-phase Fraction (SPF) showed a mean for all ML of 19.6% (median = 16.5%) ranging from 3.8-39.7%. Comparison between Ki-67 index and SPF showed a significant correlation (R2 = 0.55) between the mean SPF and Ki-67 reactivity.
Epstein-Barr virus (EBV)
EBER in-situ hybridization (ISH) [Figures 2(d) &3(c)] was evaluated on 37 ML cases of which the majority (51.4%) were positive (19/37). Most (68.4%) of the EBER+ cases were found in the adult age-group (13/19) followed by juveniles (26.3%, 5/19) and rare in the group of elderly patients, which difference was statistically significant (p = 0.048). EBER reactivity was not correlated with either of the DLBCL immunohistological subtypes, p = 0.87 (not statistically significant, Chi-square test). Furthermore, EBER positivity correlation with sex, clinical presentation, tumor proliferation (Ki-67) and DNA index (DI) was also not statistically significant.
Cytomorphometry results
DAPI-stained nuclei from 60 selected out of the 158 evaluated ML as well as from 3 tonsils were examined microscopically for changes in nuclear size, shape pleomorphism and cellularity (Figure 4) and as expected the highest nuclear size (S4) was most frequently seen in HL cases while a difference of nuclear pleomorphism (NP) and for DI between different ML groups did not appear to be statistically significant. Obviously, cellularity (number of nuclei/hpf) appeared significantly higher in BL followed by DLBCL, TCL and least in HL and appeared to correlate well with the median Ki-67 immunoreactivity.
Figure 4.
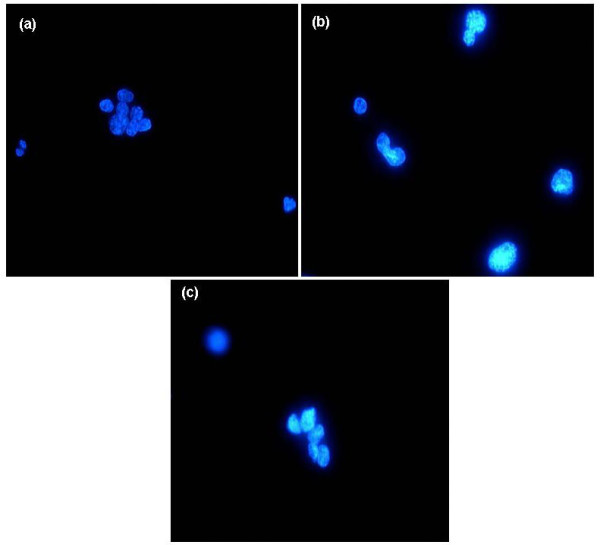
(a, b, c)- DAPI stained nuclei from a (a) normal paraffinized tonsil showing relative monomorphism and from DLBCLs showing pleomorphism (b & c) and mitoses (b).
Flow cytometric (FC) results
The nuclei extracted from the 60 selected cases evaluated by cytomorphometry above [including 27 DLBCL, 7 BL, 4 other B-cell lymphomas (BCL), 11 TCL and 11 HL] as well as those from three normal tonsils were analyzed for DNA index (DI) and tumor proliferation [S-Phase fraction (SPF)]. Microdissection and/or laser-capture microscopy (LCM) could not be done for HL cases in the current study due to logistical limitations. Thus, the HL ploidy results presented hereinafter are expected to serve as a proxy for HL cases at MNH. All the tonsillar controls were euploid or diploid (DI = 1) [Figure 5 (a)] but also some DLBCL [Figure 5(b)] and other BCL (including BL) as well as some TCL and most HL. Overall, 40% (24/60) of the selected ML cases showed DNA aneuploidy and the highest (63.0%, 17/27) frequency was seen in the DLBCL group followed by TCL (54.5%, 6/11) while aneuploidy was rare (14.3%, 1/7) in BL cases respectively, which differences were not statistically significant (p-value 0.062, Chi2 test). Aneuploid DNA indices (DI) for tested ML biopsies ranged from 1.103 to 2.407 (mean = 1.65, median = 1.51, SD = 0.445). About 37.5% (n = 8) aneuploid cases were hyperdiploid (1 > DI < 1.3) mostly DLBCL (77.7%, 7/8) [Figure 5(c)] but also one TCL (12.5%) [Figure 5(d)] (p = 0.369, not statistically significant). Furthermore, only 12.5% (n = 3/24) of aneuploid ML cases showed triploidy (1.3 ≥ DI < 1.7) including two DLBCL [Figure 6(a)] and one BL whereas 50.0% were tetraploid/multiploid (DI ≥ 1.7) [12/24] including DLBCL (58.3%, n = 7) [Figure 6(b)] and TCL (41.7%, n = 5) [Figure 6(c)]. Thus tetraploidy appeared to be more (83.3%, 5/6) frequent among TCL cases compared to DLBCL (47.1%, 7/17) which difference was not statistically significant (p = 0.161, Fisher Exact Test).
Figure 5.
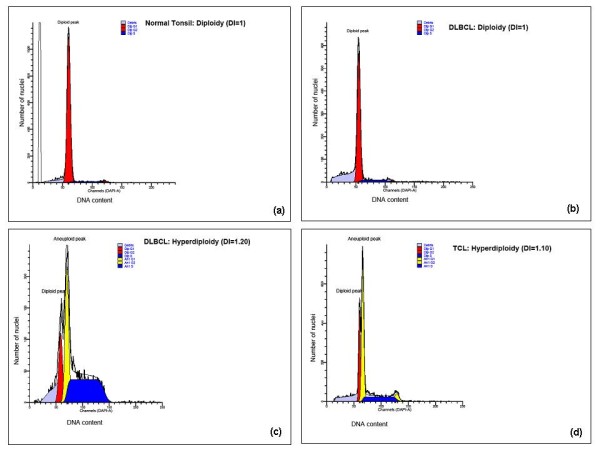
(a, b, c, d)- DNA histograms of a diploid normal tonsil control (a), diploid (b) and hyperdiploid (c) DLBCLs as well as a hyperdiploid TCL (d).
Figure 6.
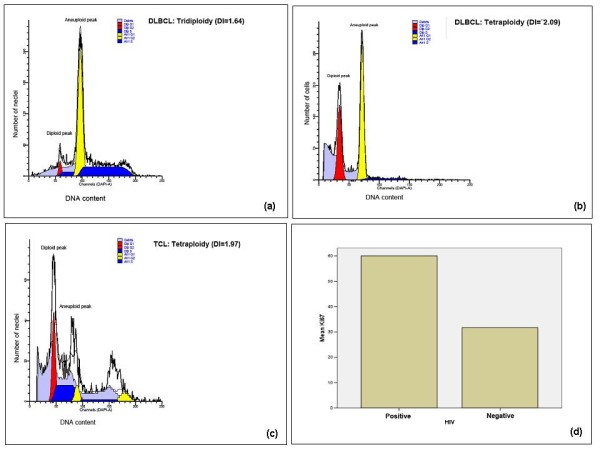
(a, b, c, d)- DNA histograms of triploid (a) and tetraploid (b) Tanzanian DLBCLs, a tetraploid TCL (c), as well as a histogram of median Ki-67 reactivity (proliferation) and HIV serostatus in Tanzanian ML patients (d).
ML association with HIV infection (serology)
HIV screening was possible in only 22.2% (35/158) of the histologically reviewed ML cases of which 40.0% (14/35) were seropositive. Apparently, most (85.7%, 12/14) of the positive cases were seen in the younger adults (age 18-54). Furthermore, most (77.8%, 7/9) of the HIV seroreactive ML stained for Ki-67 showed high (≥40.0%) reactivity, which difference was statistically significant (p = 0.021, Fisher's Exact Test) [Figure 6(d)]. HIV association with sex, clinical presentation (disease extent and anatomic location), EBV infection (EBER+ ISH), and DNA index (ploidy) of ML cases appeared not statistically significant which could be due to small samples.
Discussion
The ML demographics found in the studied, extended 10 year cohort are generally consistent with age and sex distribution of Tanzanian lymphomas discussed in our previous reports [4,14]. The finding that about one-third of the ML had extranodal presentation at diagnosis is of therapeutic importance and remarkably concordant with the 30-40% extranodal presentation observed among DLBCLs in Wurzburg, Germany [33] but not previously documented in Tanzania and in contrast to the frequency reported in Japan where extra-nodal presentation was higher (83.3%) in the young than (60.0%) in the older age-group [34]. Concordant with our findings in juvenile ML cases [34], nodal versus extranodal presentation is reportedly an important factor for determination of ML prognosis and pathogenicity related to genotypic and phenotypic differences in the two clinical groups which however is not well established [35]. Thus it seems that nodal and extranodal DLBCL, as well as DLBCL from different primary sites, are heterogeneous with regard to different biologic characteristics and prognostic implications [35]. Corresponding to other studies [36,37] we also found that TCL were more often associated with extranodal presentation particularly cutaneous and nasal compared to other ML subtypes [36,37].
Lymphoma classification remains a challenge particularly to resource-limited countries in Africa, where methods complementary to routine histology such as IHC, FACS, PCR and cytogenetics are mostly lacking. This is also the reason why the WHO classification has been difficult to implement in Tanzania. As realized from the present study combined immunophenotyping and H & E staining, clearly improved diagnostic specificity and should be implemented routinely. Thus the diagnosis of TCL by IHC in the current ML cohort to our knowledge is novel in Tanzania. Furthermore, the dominance of B-cell lymphomas, mostly DLBCL also reported in our previous study [14], and the proportions of BL, TCL and HL at MNH appear similar to other studies [9] suggesting the applicability of the WHO classification for Tanzanian ML.
The sub-phenotyping of African DLBCL by IHC using CD10, MUM1p and BCL-6 as well as BCL-2 cell markers in the present study has not our knowledge, been reported before in Tanzania and Africa. Thus, our findings indicate that previously established DLBCL sub-phenotypes also exist in Tanzania/Africa, which should allow the application of the same prognostication criteria and therapeutic protocols as those in developed countries. The apparently higher frequency of the ABC than GCB lymphomas, in the current MNH cohort appear to be concordant with several DLBCL studies from North America and Western Europe [23,25] although our sample size was small. However, this concordance may support the notion of pathogenic and biological similarity between Caucasian and African DLBCL heterogeneity. Our finding of architectural (histological) differences between diffuse DLBCL and those with follicular remnants suggests also the existence of DLBCL which may develop from nodal follicular lymphoma (FL) [38].
Furthermore, the application of the WHO classification of Tanzanian HL in the current study is novel including the reporting of classical (CHL) and the non-classical nodular lymphocyte predominant (NLPHL) cases. However, our HL findings of more frequent mixed cellularity (MC) than nodular sclerosing (NS) among cases with CHL, were not in agreement with reports from other countries were NS is reportedly more common [39,40] although other reports seem to concur with our current findings [11,41,42]. The reason for this discrepancy are not clear but the may possibly include the role of HIV infection in altering the epidemiology, pathogenesis and natural history of HL [41,42] considering that Tanzania is within the epicenter of the HIV and AIDS pandemic. However, these results could also depend on the sample size.
The 40% rate of aneuploidy found among the Tanzanian ML patients indicates a relatively high prevalence of genomic instability (chromosomal aberrations) and is in general in agreement with previous reports from Western countries [43,44] but higher than that observed among NHL in a Swedish report [16]. High DNA indices (triploidy, tetraploidy and multiploidy) were also found in our current study as well as a high mean DI comparable to other reports [16,45], which appears to correlate with biological aggressiveness and poor prognosis. The high DI (tetra-/multi-ploidy) and proportion of aneuploidy found most frequently among the current DLBCL cases is concordant with previous reports [45]. However, the lack of aneuploidy among our HL cases is in contrast to other reports [46] and could probably be due to the fact that microdissection/LCM for the neoplastic Reed-Sternberg cells (RSC) and/or Hodgkin cells (HC) was not done in our current study due to resource limitations as well as difficulty in suspending intact RSC known to be aneuploid. The strong correlation between aneuploidy and tumor proliferation (Ki-67) found in our study was expected as previously reported by others [47] and is indicative of a biological high tumor grade. There was no association between aneuploidy and HIV infection in our cohort but previous reports suggested higher proliferation and lower DI among HIV-associated lymphomas [44]. However, these reports are scanty, and further documentation is needed [48]. Furthermore, the finding that EBER positivity in our cohort, did not appear to be clearly associated with high tumor proliferation (Ki-67) could partly be due to the small sample size. Furthermore, the high frequency of EBER+ ML in our ISH-tested biopsies indicates a significant association of lymphomas with EBER positivity at MNH particularly in the adult age-group (68.4%) and is consistent with our previous report [4], in which we also showed co-expression (31.8%) of tumoral EBV-encoded RNA (EBER) and HHV-8 DNA (PCR) but not ML cell HHV-8 latency-associated nuclear antigen (LANA) association [4].
The finding that increased tumor proliferation in our current cohort appeared correlated to HIV infection, is concordant with previous reports [44] and supports the notion of viral-associated/driven tumor proliferation as a biological role in the oncogenesis of HIV-related malignancies similar to that reported for HHV-8 and Kaposi's sarcoma [28].
Conclusions
The WHO Classification can apparently be applied for the diagnosis of lymphomas at MNH in Tanzania. Extranodal presentation of ML was frequent particularly for TCL. DLBCL subtype phenotype heterogeneity and frequency was similar to that observed in Western countries suggesting applicability of similar, diagnostic, prognostic and therapeutic approaches. Lymphomas at MNH appear to have frequent aneuploidy and EBER positivity as well as high DNA indices and tumor proliferation (Ki-67). HIV is apparently associated with increased ML cell proliferation but extended studies are needed to confirm this.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ARM, PB, EK and AP designed research. ARM collected data. ARM, TH, JC, GW and FP performed research. ARM, PB, AP, TH, JC, GW, EK analyzed and interpreted data. ARM performed statistical analysis and wrote the manuscript. PB, AP, TH and EK corrected manuscript. PB contributed vital reagents and analytical tools. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Amos R Mwakigonja, Email: amwakigonja@muhas.ac.tz.
Ephata E Kaaya, Email: ekaaya@muhas.ac.tz.
Thomas Heiden, Email: Thomas.Heiden@charite.de.
German Wannhoff, Email: wannhoff@gmail.com.
Juan Castro, Email: Juan.Castro@ki.se.
Fatemeh Pak, Email: parisa_amin@yahoo.com.
Anna Porwit, Email: anna.porwit@karolinska.se.
Peter Biberfeld, Email: Peter.Biberfeld@ki.se.
Acknowledgements
We are greatly indebted to the MNH patients who provided their biopsies and sera as well as to the department of surgery for submitting them, and to Onjection Byobangamba and Jafari Sufi (MUHAS) for technical assistance. Constructive criticism from Dr. Esmeralda Castanõz-Velèz and Prof. Birger Christensson is also appreciated. Financial Support was provided by the Swedish International Development Agency (Sida), Department of Research Cooperation (SAREC), Cancer Fonden and KI Fonden.
References
- Omoti CE, Halim NK. Adult lymphomas in Edo state, Niger Delta region of Nigeria--clinicopathological profile of 205 cases. Clin Lab Haematol. 2005;27(5):302–306. doi: 10.1111/j.1365-2257.2005.00711.x. [DOI] [PubMed] [Google Scholar]
- Baris D, Zahm SH. Epidemiology of lymphomas. Current opinion in oncology. 2000;12(5):383–394. doi: 10.1097/00001622-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Chokunonga E, Levy LM, Bassett MT, Borok MZ, Mauchaza BG, Chirenje MZ, Parkin DM. Aids and cancer in Africa: the evolving epidemic in Zimbabwe. AIDS (London, England) 1999;13(18):2583–2588. doi: 10.1097/00002030-199912240-00012. [DOI] [PubMed] [Google Scholar]
- Kaaya E, Castanos-Velez E, Ekman M, Mwakigonja A, Carneiro P, Lema L, Kitinya J, Linde A, Biberfeld P. AIDS and non AIDS-related malignant lymphoma in Tanzania. African health sciences. 2006;6(2):68–74. doi: 10.5555/afhs.2006.6.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA: a cancer journal for clinicians. 1999;49(1):33–64. doi: 10.3322/canjclin.49.1.33. 31. [DOI] [PubMed] [Google Scholar]
- Christensson B, Tribukait B, Linder IL, Ullman B, Biberfeld P. Cell proliferation and DNA content in non-Hodgkin's lymphoma. Flow cytometry in relation to lymphoma classification. Cancer. 1986;58(6):1295–1304. doi: 10.1002/1097-0142(19860915)58:6<1295::AID-CNCR2820580620>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Lindemalm C, Biberfeld P, Bjorkholm M, Holm G, Johansson B, Mellstedt H, Nilsson B, Ost A. Immunodeficiency and prognosis in patients with non-Hodgkin's lymphomas. Acta radiologica. 1985;24(2):159–165. doi: 10.3109/02841868509134380. [DOI] [PubMed] [Google Scholar]
- Higgins RA, Blankenship JE, Kinney MC. Application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphoma. Archives of pathology & laboratory medicine. 2008;132(3):441–461. doi: 10.5858/2008-132-441-AOIITD. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Suzumiya J, Kikuchi M. The World Health Organization classification of malignant lymphoma: incidence and clinical prognosis in HTLV-1-endemic area of Fukuoka. Pathol Int. 2002;52(1):1–12. doi: 10.1046/j.1440-1827.2002.01308.x. [DOI] [PubMed] [Google Scholar]
- Hasserjian RP, Ott G, Elenitoba-Johnson KS, Balague-Ponz O, de Jong D, de Leval L. Commentary on the WHO classification of tumors of lymphoid tissues (2008): "Gray zone" lymphomas overlapping with Burkitt lymphoma or classical Hodgkin lymphoma. Journal of hematopathology. 2009. in press . [DOI] [PMC free article] [PubMed]
- Chan JK. The new World Health Organization classification of lymphomas: the past, the present and the future. Hematological oncology. 2001;19(4):129–150. doi: 10.1002/hon.660. [DOI] [PubMed] [Google Scholar]
- Lukes RJ, Butler JJ. The pathology and nomenclature of Hodgkin's disease. Cancer research. 1966;26(6):1063–1083. [PubMed] [Google Scholar]
- Xiao C, Su ZL, Wu QL, Gao HY, Fang JC, Xia ZJ, Guan ZZ. Clinical and pathological reassessment of 493 cases of non-Hodgkin's lymphomas according to current WHO classification of lymphoid neoplasms. Zhonghua bing li xue za zhi Chinese journal of pathology. 2005;34(1):22–27. [PubMed] [Google Scholar]
- Mwakigonja AR, Kaaya EE, Mgaya EM. Malignant lymphomas (ML) and HIV infection in Tanzania. J Exp Clin Cancer Res. 2008;27(1):9. doi: 10.1186/1756-9966-27-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin ZD, Barista I, Canpinar H, Sungur A, Tekuzman G. Gastric lymphomas in Turkey. Analysis of prognostic factors with special emphasis on flow cytometric DNA content. Cancer. 2000;89(1):12–20. doi: 10.1002/1097-0142(20000701)89:1<12::AID-CNCR3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Czader M, Porwit A, Tani E, Ost A, Mazur J, Auer G. DNA image cytometry and the expression of proliferative markers (proliferating cell nuclear antigen and Ki67) in non-Hodgkin's lymphomas. Mod Pathol. 1995;8(1):51–58. [PubMed] [Google Scholar]
- Castanos-Velez E, Heiden T, Lindvall C, Capello D, Sandlund A, Imreh S, Blennow E, Andersson LC, Gaidano G, Nordenskjold M. Simian AIDS-related lymphoma growth in severe combined immunodeficiency mice is independent of karyotypic abnormalities or Bcl-6 mutations. AIDS research and human retroviruses. 2002;18(5):383–390. doi: 10.1089/088922202753519160. [DOI] [PubMed] [Google Scholar]
- Biberfeld P, Christensson B, Andersson-Anvret M, Ernberg I, Lewensohn R, Ekman M, Johansson B, Tribukait B. An EBV-associated, Swedish case of Burkitt-type malignant lymphoma. Acta pathologica et microbiologica Scandinavica. 1981;89(6):417–424. doi: 10.1111/j.1699-0463.1981.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Pak F, Pyakural P, Kokhaei P, Kaaya E, Pourfathollah AA, Selivanova G, Biberfeld P. HHV-8/KSHV during the development of Kaposi's sarcoma: evaluation by polymerase chain reaction and immunohistochemistry. J Cutan Pathol. 2005;32(1):21–27. doi: 10.1111/j.0303-6987.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Katano H, Suda T, Morishita Y, Yamamoto K, Hoshino Y, Nakamura K, Tachikawa N, Sata T, Hamaguchi H, Iwamoto A. Human herpesvirus 8-associated solid lymphomas that occur in AIDS patients take anaplastic large cell morphology. Mod Pathol. 2000;13(1):77–85. doi: 10.1038/modpathol.3880012. [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Vaccher E, Cerri M, Gaidano G, Dalla-Favera R, Tirelli U. Kaposi's sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7(1):17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R, Carpenter L, Casabonne D, Beral V, Babiker A, Darbyshire J, Weller I, Weiss R, Kwan A, Bourboulia D. A prospective study of Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus in adults with human immunodeficiency virus-1. British journal of cancer. 2006;94(10):1504–1509. doi: 10.1038/sj.bjc.6603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Berglund M, Thunberg U, Amini RM, Book M, Roos G, Erlanson M, Linderoth J, Dictor M, Jerkeman M, Cavallin-Stahl E. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005;18(8):1113–1120. doi: 10.1038/modpathol.3800396. [DOI] [PubMed] [Google Scholar]
- Muris JJ, Meijer CJ, Vos W, van Krieken JH, Jiwa NM, Ossenkoppele GJ, Oudejans JJ. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. The Journal of pathology. 2006;208(5):714–723. doi: 10.1002/path.1924. [DOI] [PubMed] [Google Scholar]
- Dinand V, Malik A, Unni R, Arya LS, Pandey RM, Dawar R. Proliferative index and CD15 expression in pediatric classical Hodgkin lymphoma. Pediatric blood & cancer. 2008;50(2):280–283. doi: 10.1002/pbc.21204. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim BS, Choi CW, Choi J, Kim I, Lee YH, Kim JS. Ki-67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Ann Oncol. 2007;18(8):1382–1387. doi: 10.1093/annonc/mdm183. [DOI] [PubMed] [Google Scholar]
- Mwakigonja AR, Pak F, Pyakurel P, Mosha IJ, Urassa WK, Kaaya EE, Biberfeld P. Oral Kaposi's sarcoma in Tanzania: presentation, immunopathology and human herpesvirus-8 association. Oncology reports. 2007;17(6):1291–1299. [PubMed] [Google Scholar]
- Heiden T, Schumann J, Gohde W. Flow sorting of tumor cells for morphometric analysis, particularly of rare cells. Virchows Archiv. 1991;61(1):29–38. doi: 10.1007/BF02890402. [DOI] [PubMed] [Google Scholar]
- Heiden T, Castro J, Graf BM, Tribukait B. Comparison of routine flow cytometric DNA analysis of fresh tissues in two laboratories: effects of differences in preparation methods and background models of cell cycle calculation. Cytometry. 1998;34(4):187–197. doi: 10.1002/(SICI)1097-0320(19980815)34:4<187::AID-CYTO3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Heiden T, Castanos-Velez E, Andersson LC, Biberfeld P. Combined analysis of DNA ploidy, proliferation, and apoptosis in paraffin-embedded cell material by flow cytometry. Laboratory investigation; a journal of technical methods and pathology. 2000;80(8):1207–1213. doi: 10.1038/labinvest.3780128. [DOI] [PubMed] [Google Scholar]
- Fanaian N, Cohen C, Waldrop S, Wang J, Shehata B. EBER: Automated In Situ Hybridization (ISH) vs. Manual ISH and Immunohistochemistry (IHC) for Detection of EBV in Pediatric Lymphoproliferative Disorders. Pediatr Dev Pathol. 2008;1 doi: 10.2350/07-07-0316.1. [DOI] [PubMed] [Google Scholar]
- Ott G, Rosenwald A. Extranodal diffuse large B-cell lymphoma--an organotypic disease? Der Pathologe. 2007;28(1):29–35. doi: 10.1007/s00292-006-0883-5. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Fujita S, Ikeda J, Nakamichi I, Fukuhara S, Hino M, Kanakura Y, Ogawa H, Sugiyama H, Kanamaru A. Diffuse large B-cell lymphoma in the young in Japan: a study by the Osaka Lymphoma Study Group. American journal of hematology. 2007;82(10):893–897. doi: 10.1002/ajh.20968. [DOI] [PubMed] [Google Scholar]
- Lu JB, Li XQ, Zhang PH, Zhou XY, Zhang TM, Li XM, Zhu XZ. Nodal versus extranodal diffuse large B-cell lymphoma: comparison of clinicopathologic features, immunophenotype and prognosis. Zhonghua bing li xue za zhi Chinese journal of pathology. 2007;36(7):470–473. [PubMed] [Google Scholar]
- Katsaounis P, Alexopoulou A, Dourakis SP, Smyrnidis A, Marinos L, Filiotou A, Archimandritis AJ. An extranodal NK/T cell lymphoma, nasal type: with specific immunophenotypic and genotypic features. International journal of hematology. 2008;88(2):202–205. doi: 10.1007/s12185-008-0137-9. [DOI] [PubMed] [Google Scholar]
- Kim TM, Lee SY, Jeon YK, Ryoo BY, Cho GJ, Hong YS, Kim HJ, Kim SY, Kim CS, Kim S. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19(8):1477–84. doi: 10.1093/annonc/mdn147. [DOI] [PubMed] [Google Scholar]
- Martinez-Climent JA, Alizadeh AA, Segraves R, Blesa D, Rubio-Moscardo F, Albertson DG, Garcia-Conde J, Dyer MJ, Levy R, Pinkel D. Transformation of follicular lymphoma to diffuse large cell lymphoma is associated with a heterogeneous set of DNA copy number and gene expression alterations. Blood. 2003;101(8):3109–3117. doi: 10.1182/blood-2002-07-2119. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Meisner D, Talreja N, Smith R, Swerdlow SH. Classical nodular sclerosis Hodgkin lymphoma presenting with central nervous system disease. Southern medical journal. 2007;100(5):549–550. doi: 10.1097/SMJ.0b013e3180316469. [DOI] [PubMed] [Google Scholar]
- Niitsu N, Nakamine H, Okamoto M, Tamaru JI, Hirano M. A clinicopathological study of nm23-H1 expression in classical Hodgkin's lymphoma. Ann Oncol. 2008;19(11):1941–6. doi: 10.1093/annonc/mdn413. [DOI] [PubMed] [Google Scholar]
- Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killebrew D, Shiramizu B. Pathogenesis of HIV-associated non-Hodgkin lymphoma. Current HIV research. 2004;2(3):215–221. doi: 10.2174/1570162043351237. [DOI] [PubMed] [Google Scholar]
- Christensson B, Lindemalm C, Johansson B, Mellstedt H, Tribukait B, Biberfeld P. Flow cytometric DNA analysis: a prognostic tool in non-Hodgkin's lymphoma. Leuk Res. 1989;13(4):307–314. doi: 10.1016/0145-2126(89)90067-2. [DOI] [PubMed] [Google Scholar]
- McDunn SH, Winter JN, Variakojis D, Rademaker AW, Von Roenn JH, Tallman MS, Gordon LI, Bauer KD. Human immunodeficiency virus-related lymphomas: a possible association between tumor proliferation, lack of ploidy anomalies, and immune deficiency. J Clin Oncol. 1991;9(8):1334–1340. doi: 10.1200/JCO.1991.9.8.1334. [DOI] [PubMed] [Google Scholar]
- Krugmann J, Gschwendtner A, Mairinger T, Fend F. DNA ploidy in gastrointestinal B-cell lymphomas. An image analysis study of 43 cases. Anal Quant Cytol Histol. 2003;25(1):31–38. [PubMed] [Google Scholar]
- Czader M, Porwit A, Ost A, Auer G. DNA content and expression of PCNA and p53 in Hodgkin's disease and Hodgkin's-like B-cell lymphoma. Apmis. 1994;102(11):865–873. doi: 10.1111/j.1699-0463.1994.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Schmitt F, Tani E, Tribukait B, Skoog L. Assessment of cell proliferation by Ki-67 staining and flow cytometry in fine needle aspirates (FNAs) of reactive lymphadenitis and non-Hodgkin's lymphomas. Cytopathology. 1999;10(2):87–96. doi: 10.1046/j.1365-2303.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- Ioakim-Liossi A, Mikou P, Botsoli-Stergiou E, Petrakakou E, Athanassiadou P, Kordosis T, Panagiotakopoulos G, Karakitsos P. Primary effusion lymphoma. A case report. Acta cytologica. 2004;48(2):269–272. doi: 10.1159/000326330. [DOI] [PubMed] [Google Scholar]


