Abstract
Following unilateral vestibular damage (UVD), vestibular compensation restores both static and dynamic vestibular reflexes. The cerebellar cortex provides powerful GABAergic inhibitory input to the vestibular nuclei which is necessary for compensation. Metabotropic GABA type B (GABAB) receptors in the vestibular nuclei are thought to be involved. However, the contribution of GABAB receptors may differ between static and dynamic compensation. We tested static and dynamic postural reflexes and gait in young mice, while they compensated for unilateral vestibular damage (UVD) caused by injection of air into the vestibular labyrinth. The effects of an agonist (baclofen), an antagonist (CGP56433A) and a positive allosteric modulator (CGP7930) of the GABAB receptor were evaluated during compensation. Static postural reflexes recovered very rapidly in our model, and baclofen slightly accelerated recovery. However, CGP56433A significantly impaired static compensation. Dynamic reflexes were evaluated by balance-beam performance and by gait; both showed significant decrements following UVD and performance improved over the next 2 days. Both CGP56433A and baclofen temporarily impaired the ability to walk on a balance beam after UVD. Two days later, there were no longer any significant effects of drug treatments on balance-beam performance. Baclofen slightly accelerated the recovery of stride length on a flat surface, but CGP7930 worsened the gait impairment following UVD. Using immunohistochemistry, we confirmed that GABAB receptors are abundantly expressed on the vestibulospinal neurons of Deiters in mice. Our results suggest that GABAB receptors contribute to the compensation of static vestibular reflexes following unilateral peripheral damage. We also conclude that impairment of the first stage of compensation, static recovery, does not necessarily result in an impairment of dynamic recovery in the long term.
Keywords: Posture, vestibular reflexes, gait, Deiters’ nucleus, cerebellum, locomotion
INTRODUCTION
After the vestibular labyrinth is damaged on one side, postural reflexes, gaze stability, and normal locomotion are largely restored by vestibular compensation (Schaefer and Meyer, 1974, Curthoys and Halmagyi, 1995). Despite a long history of investigation, compensation is poorly understood, particularly for movements of the limbs. It is useful to consider two main processes in compensation: First, tonic limb muscle tone and gaze stability (with the subject stationary) are restored (“static compensation”). Second, the dynamic reflexes that operate during locomotion and during head movements must be recalibrated (“dynamic compensation”). Although dramatic, vestibular compensation is not perfect. In fact, some 30% of patients with unilateral vestibular damage (UVD) fail to recover even approximately normal function (Curthoys and Halmagyi, 1999).
Compensation is thought to involve plasticity within the vestibular nuclei of the brainstem (Fetter and Zee, 1988, Darlington et al., 2001, Broussard and Hong, 2003, Bergquist et al., 2008). The vestibular nuclei are the targets of a powerful, tonic GABAergic input from Purkinje cells (Ito, 1984) as well as from the inhibitory vestibular commissure. Following UVD, GABA levels become asymmetric (Bergquist et al., 2008). Metabotropic GABA type B (GABAB) receptors are present in all subdivisions of the vestibular nuclei (Holstein et al., 1992, Eleore et al., 2005) and have been proposed to contribute to compensation (Johnston et al., 2001). Presynaptic GABAB receptors can inhibit the release of both excitatory and inhibitory neurotransmitters (Mouginot and Gahwiler, 1996, Aroniadou-Anderjaska et al., 2000, Yamada et al., 2000, Wang et al., 2003). Postsynaptically, GABAB-receptor activation has multiple effects; these include hyperpolarization (Seabrook et al., 1990, Luscher et al., 1997), which can trigger intrinsic plasticity (Nelson et al., 2003). The varied intracellular targets of GABAB receptors position them to participate in multiple plasticity mechanisms via second-messenger systems in both pre-and postsynaptic terminals.
In the vestibular nuclei and in the cerebellum, resting discharge rates are high, suggesting that pharmacological agents that depend on tonic activation of neurotransmitter receptors (such as modulators) may have more pronounced effects in cerebellar and vestibular networks than in other brain regions. If so, therapeutic intervention at GABAB receptors could improve the rate and/or the final outcome of compensation. The antagonist CGP56433A has been reported to exacerbate both static and dynamic vestibular symptoms during compensation for hemilabyrinthectomy in rats, while the agonist baclofen can cause transient improvement (Magnusson et al., 2002). In brain slices, suppression of firing due to GABAB activation is reduced on the damaged side, a few hours following hemilabyrinthectomy (Yamanaka et al., 2000), and this reduction is believed to contribute to static compensation. Other brainstem mechanisms involving GABAB receptors have also been proposed (Peterson et al., 1996). The cerebellar cortex, including the flocculus, contains a high density of GABAB receptors (Bowery et al., 1987). The flocculus is thought to contribute to compensation, possibly by modifying vestibular neuronal excitability (Johnston et al., 2002).
Static and dynamic compensation have different mechanistic requirements. Static compensation likely requires a tonic increase in the activity of secondary vestibular neurons on the damaged side (Smith and Curthoys, 1988). Dynamic compensation requires not only the restoration of tonic activity, but also recalibration of the transmission of vestibular sensory signals that change during movement, i.e., dynamic signals. Signal transmission by the inhibitory commissural pathways is thought to be important in dynamic compensation (Precht et al., 1966, Galiana et al., 1984). Signal transmission by excitatory brainstem pathways is also modified during dynamic compensation (Broussard and Hong, 2003, Farrow and Broussard, 2003). Cerebellar motor learning may participate in the restoration of dynamic reflex function (Broussard et al., 1999, Murai et al., 2004, Faulstich et al., 2006, Beraneck et al., 2008). Finally, recent evidence suggests that GABAB receptors may contribute to cerebellar motor learning (Titley et al., 2009). Except for one study describing circling (Gacek and Khetarpal, 1998), there has been little investigation of static or dynamic compensation of postural reflexes or gait in the mouse model.
We tested both static and dynamic vestibular function in young mice that had mild unilateral damage caused by injection of air into the vestibular labyrinth. We measured the effects of an agonist (R-baclofen) and an antagonist (CGP56433A) on compensation. We also tested a positive allosteric modulator (CGP7930) which in the presence of GABA, enhances the activity of the GABAB receptor (Urwyler et al., 2001).
No evidence has yet been obtained for any long-term effects of GABAB drugs on vestibular compensation. One hypothesis suggests that if compensation fails to occur during an early, critical period it will fail to occur in the long term (Newlands et al., 2005, Lacour, 2006). If this is the case, then interference with compensation at an early stage should prevent complete recovery at later stages. We have made an attempt to test this prediction.
EXPERIMENTAL PROCEDURES
Animals
A total of 68 male C57/Bl6 mice, either bred in our facility or purchased from Taconic Farms (New York), were used in this study (Table 1). Mice were on a normal light cycle with continuous access to food and water. All experiments were initiated between 7:30 and 10:30 AM. All procedures followed the guidelines of the Canadian Council for Animal Care and were approved by the Animal Care Committee at the University Health Network.
Table 1. Experimental groups and protocols.
The use of each mouse is indicated, including treatment groups, preliminary dosage-determination groups, training-effect and activity-measurement groups (see text for details). The dosage determinations and activity measurements were made following isoflurane anesthesia. During measurement of training effects, no drug was administered.
| Number of mice |
Treatment or protocol | Test substance | Dosage | Test volume (ml) | Vehicle | Administration times† (hours) |
Measurement times or activity monitoring (hours) † |
|---|---|---|---|---|---|---|---|
| Immunolabeling | |||||||
| 4 | no treatment | None | -- | -- | -- | -- | |
| CGP56433A | |||||||
| 6 | treatment group | CGP56433A | 5 mg/kg | 0.34 ± 0.05 | saline | −3.5, 1, 3, 7, 19, 31 | −3.5, −1.5, 4, 19, 44 |
| 1 | died during surgery | CGP56433A | 5 mg/kg | 0.37 | saline | −3.5 | −3.5, −1.5 |
| R-baclofen | |||||||
| 6 | treatment group | R-baclofen | 1 mg/kg | 0.24 ± 0.03 | saline | −3.5, 1, 3, 7, 19, 31 | −3.5, −1.5, 4, 19, 44 |
| 3 | died during surgery | R-baclofen | 1 mg/kg | 0.27 ± 0.01 | saline | −3.5 | −3.5, −1.5 |
| 2 | air injection ineffective | R-baclofen | 1 mg/kg | 0.23 | saline | −3.5 | −3.5, −1.5 |
| CGP7930 | |||||||
| 6 | treatment group | CGP7930 | 25 mg/kg | 0.27 ± 0.02 | methylcellulose | −3.5, 1, 3, 7, 19, 31 | −3.5, −1.5, 4, 19, 44 |
| 1 | died during surgery | CGP7930 | 25 mg/kg | 0.26 | methylcellulose | −3.5 | −3.5, −1.5 |
| 1 | air injection ineffective | CGP7930 | 25 mg/kg | 0.29 | methylcellulose | −3.5 | −3.5, −1.5 |
| Saline | |||||||
| 6 | treatment group | Saline | -- | 0.28 ± 0.04 | -- | −3.5, 1, 3, 7, 19, 31 | −3.5, −1.5, 4, 19, 44 |
| 2 | died during surgery | Saline | -- | 0.28 ± 0.00 | -- | −3.5 | −3.5, −1.5 |
| Methylcellulose | |||||||
| 12* | treatment group | methylcellulose | -- | 0.29 ± 0.01 | -- | −3.5, 1, 3, 7, 19, 31 | −3.5, −1.5, 4, 19, 44 |
| 4 | died during surgery | methylcellulose | -- | 0.26 ± 0.03 | -- | −3.5 | −3.5, −1.5 |
| Preliminary tests | |||||||
| 4** | practice effects on beam- crossing | None | -- | -- | -- | -- | 0, 24, 48, 72, 96 |
| 4 | dosage determination | R-baclofen | 0.5–1.5 mg/kg | 0.08–0.2 | saline | 1.75 | 1.75–5.25 |
| 1 | dosage determination | CGP56433A | 5 mg/kg | 0.35 | saline | 1.75 | 1.75–5.25 |
| 2 | dosage determination | CGP7930 | 25–30 mg/kg | 0.25–0.3 | methylcellulose | 1.75 | 1.75–5.25 |
| 1 | control for dosage determinations | None | -- | -- | -- | -- | 1.75–5.25 |
| 1 | activity measurements | R-baclofen | 1 mg/kg | 0.26 | saline | −2, 2.25, 4.25 | 1.75–5.25 |
| 2 | activity measurements | CGP56433A | 5 mg/kg | 0.34 ± 0.01 | saline | −2, 2.25, 4.25 | 1.75–5.25 |
| 1 | activity measurements | saline | -- | 0.23 | -- | −2, 2.25, 4.25 | 1.75–5.25 |
| 1 | activity measurements | methylcellulose | -- | 0.29 | -- | −2, 2.25, 4.25 | 1.75–5.25 |
This group originally consisted of two experimental groups that were later combined.
Three of these mice were also used for dosage determination.
Drug-administration and reflex-measurement times are with reference to the air injection in the drug-treatment groups, with reference to the start of anesthesia for the preliminary tests (which did not include an air injection), and with reference to the first measurement for practice effects. In cases where the measurement and administration times are the same, the measurement was made immediately before the drug was administered.
Immunohistochemistry
GABAB receptors were labeled using immunohistochemistry in 4 mice, 42–48 days old. Mice were anesthetized with 0.1 ml of sodium pentobarbital (24 mg/ml, ip) and perfused through the heart, at a flow rate of 6 ml/min, with 30 ml of 0.1 M phosphate-buffered saline (PBS) followed by 30 ml of 4% paraformaldehyde in PBS. Both solutions were at room temperature. The brain was removed and stored at 4°C in 4% paraformaldehyde/PBS for 24 hours, then cryoprotected in 15% sucrose/PBS followed by 30% sucrose/PBS. Coronal sections through the brainstem, 40 μm thick, were cut on a sliding freezing microtome (American Optical). For immunohistochemistry, sections were rinsed in PBS (all rinses were 3 × 10 min at room temperature with gentle agitation). Non-specific binding was blocked by incubating in a solution of 0.1M PBS with 1% bovine serum albumin, 1.5% normal goat serum (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame CA) and 0.5% Triton X-100 (Sigma Chemical Company, St. Louis, MO, USA) for 30 minutes. Sections were incubated overnight at 4°C in that same solution with the addition of a rabbit antiserum to the GABAB receptor (Santa Cruz Biotechnology, GABAB R2 (H-300): sc-28792, 1:400), rinsed, and incubated with the Vector anti-rabbit IgG secondary antibody (0.5%, following manufacturer’s instructions) in PBS with T-X and NGS, and then in the Vector ABC reagent according to manufacturer’s instructions. Immunoreactivity was visualized with the DAB/glucose oxidase method (Shu et al., 1988). Sections were mounted on slides, air-dried, dehydrated in graded ethanol solutions, cleared with Histosol (National Diagnostics) and coverslipped. Digital images of selected sections were captured with a SPOT camera mounted on a Leitz Dialux 20 microscope. Control sections were processed identically, except that the primary antibody was omitted from the first incubation solution. No immunostaining was seen on the control sections.
UVD test groups and protocols
For the UVD study, mice were 1 to 3 months old and weighed 15–23 grams; this size range was chosen because small individuals recovered rapidly from anesthesia. Thirty-six mice were included in the experimental groups, and 14 additional mice died or were excluded following surgery (see Table 1). The remaining mice were used for preliminary tests, as described below.
Drug dosages and activity levels
Recovery from vestibular damage is thought to be affected by the amount of locomotor activity experienced post-lesion (Mathog and Peppard, 1982). In addition, baclofen has been shown to interact with isoflurane (Sugimura et al., 2002) and CGP56433A can increase locomotor activity in the rat (Slattery et al., 2005). Appropriate drug doses for this study were chosen based on previously published results and on preliminary testing. Our goal was to select the maximum dose that would not alter activity levels following isoflurane anesthesia. We anesthetized a separate group of 5 mice for 1.75 hours and then immediately injected one dose of the drug to be tested. The mouse was then observed for the next 3.5 hours, noting any obvious differences in activity levels. These mice are included in Table 1, along with the range that was tested for each drug. For CGP56433A, we tested only 5 mg/kg, because that dose was higher than the dose that is known to exacerbate vestibular signs following compensation (Magnusson et al., 2002).
After a dose was chosen for each drug, we verified that activity was not affected by the chosen dose, in another group of 8 mice (see Table 1). In this group, mice were given repeated doses of either CGP56433A (5 mg/kg, sc in saline), R-baclofen (1 mg/kg, sc in saline) or CGP7930 (25 mg/kg, sc, suspended in 0.5% methylcellulose), or no injection, 2 hrs before and 0.5 and 2.5 hrs following the end of a 1.75-hour period of isoflurane anesthesia at surgical levels (the injection times with reference to the start of anesthesia are given in Table 1). The total duration of active walking during each 5-minute interval was then averaged over the entire 3.5-hour period after anesthesia was discontinued. None of the test substances had any measurable effects on activity levels.
Experimental protocol
For our study of compensation, mice were divided into four groups of 6 and one group of 12. The 5 experimental groups were tested in random order. H. K.T. prepared all drug solutions and administered all injections. R. H.-S., who was blind to the experimental group, carried out all surgery and all vestibular testing. Each of the five groups received repeated doses of one of the following: the GABAB receptor antagonist CGP56433A (5 mg/kg, sc in saline), the GABAB receptor agonist R-baclofen (1 mg/kg, sc in saline), the GABAB receptor positive allosteric modulator CGP7930 (25 mg/kg, sc, suspended in 0.5% methylcellulose), methylcellulose alone, or saline alone. Six doses were given at 3.5 hrs pre-UVD and 1, 3, 7, 19, and 31 hrs post-UVD. The first three injection times corresponded roughly to the times at which injections were given during the preliminary measurements of activity levels. Those times were measured with respect to the start of anesthesia, since there was no air injection.
The largest group (n=12) received the 0.5% methylcellulose vehicle alone (14 ml/kg sc); for half of this group the initial three doses of methylcellulose were stored at 4°C. We found that refrigeration had no significant effect on any of our results, so the 4°C and room-temperature groups were pooled for post-hoc analysis. Other drug solutions were prepared on the day of use and refrigerated until needed. All drugs were obtained from Tocris.
Surgical procedure
Vestibular damage was generated using the air-injection method (Faulstich et al., 2006). Although we did not confirm the lesion histologically, the behavioral deficit was clear and consistent in 40 of the 42 air-injected mice (see Table 1). Others have found damage to sensory epithelia following this type of lesion (Dr. Michael Faulstich, The Salk Institute, pers. comm.).
Before surgery, mice were given meloxicam (2 mg/kg, sc) and sterile lactated Ringer’s solution (1 ml, sc) and anesthetized using isoflurane. To stabilize the head, we attached a nail to the skull using cyanoacrylate. The head was positioned under a surgical microscope (Zeiss, OPMI 1 FC), and a second incision was made behind the right pinna. We exposed the right horizontal semicircular canal and opened the it using a surgical drill (Foredom TXH Flex Shaft Kit, Gesswein) with a 0.3-mm bur. A blunt 30 ga. needle was inserted into the opening and three ml of air were injected repeatedly until no more endolymph was expelled. A #15 dental paper point (Patterson Dental) was then inserted into the opening, trimmed and sealed in with bone wax. The end of this procedure was recorded as the time of UVD for each mouse. Lactated Ringer’s was administered again at the end of the surgery and meloxicam (1 mg/kg, sc) was administered again 24 hours later.
Static vestibular reflexes
Static signs were monitored continuously over the first four hours following the lesion. Beginning immediately upon recovery from anesthesia, the tail was pulled at 5-minute intervals to maintain a consistent level of arousal. Signs were scored as follows: 1 point for each barrel roll, fall, or circle toward the side of the lesion; 3 points for 5 minutes of continuous head tilt; 6 points for 5 minutes of body tilt (leaning). Head tilt and leaning were toward the side of the lesion in all cases. A total score was calculated every 20 minutes for each subject. Two mice that did not exhibit a minimum score of 6 after UVD were excluded from the study (Table 1; “air injection ineffective”).
Beam-crossing test
Dynamic vestibular function was assessed using a beam-crossing test and a footprint-based gait analysis. These tests were carried out before the first dose, 2 hrs after the first dose but before UVD, and at 4 hours, 19 hrs and 44 hrs after UVD. The 19-hour measurement was made just before the 19-hour dose.
The “beam” was a 30 cm × 2 cm wooden dowel, 15 cm above a padded surface. In each trial, the mouse was placed at the start of the beam and permitted to explore the apparatus for one minute. At the opposite end was an opening (3cm × 3cm) which led into an enclosed box (8.5 × 11 × 8 cm) and a dab of peanut butter. When the minute was up, the mouse was returned to the start, and the time required either to reach the opening or to fall from the beam was recorded. Performance on each trial was assigned a score as follows: 0–5 s, 1 point; 6–10 s, 2 points; 11–15 s, 3 points; 16–20 s, 4 points; 21–25 s, 5 points; >25 s, 6 points; fall, 10 points. Crossing times greater than 25 s were rare. We looked for possible practice effects of repeated trials on the beam-crossing times in a separate group of normal mice (n=4; see Table 1). These mice were tested once a day for 5 consecutive days. No differences were observed between any of these trials (p>0.05, paired Student’s t-test).
Gait analysis
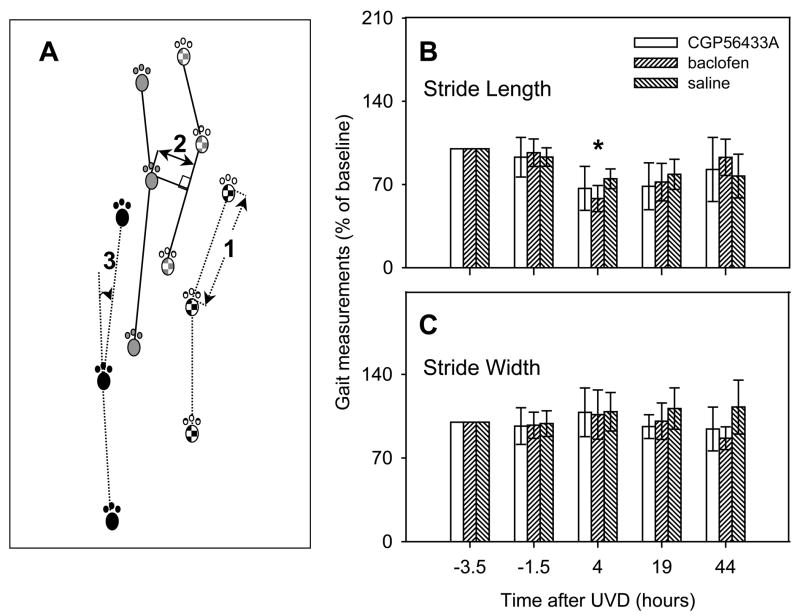
We used the footprint method of gait analysis (Clarke and Still, 1999). Front and hind paws of each mouse were painted with different colors of ink before the mice walked freely on a sheet of white paper. From each set of footprints, we measured right and left front-paw stride length, right and left hind-paw stride length, and front- and hind-paw stride width (Zhao et al., 2008). Fig. 5A illustrates the measurement method. Stride length (1) was measured along a line connecting two successive prints from the same paw, in this case the right rear paw. Front and hind stride widths (2) were then measured, using the connecting lines. If the angle (3) between two successive stride lengths was greater than 45 degrees, the stride width was not used. Measurements within a single trial (n = 3.97+−2.38 per trial) were averaged, and the means were used in the data analysis. Very wide or narrow stride-widths were produced by changes in direction. All stride-lengths that were above 50% of the maximum within-trial value and all turns greater than 45° were excluded from further analysis.
Figure 5.
Stride length, but not stride width, was significantly affected by UVD (see text for details). Baclofen temporarily impaired the recovery of stride length at 4 hours following UVD. A: Methods for measuring gait parameters. All four paw prints are illustrated for two strides. Front paw prints are shown in gray; hind paw prints are black. The right paw prints are textured. Stride length (1) was measured along a line connecting two successive prints from the same paw, in this case the right rear paw. Front and hind stride widths (2) were then measured, using the connecting lines. If the angle (3) between two successive stride lengths was greater than 45 degrees, the stride width was not used. See methods for details. B: Stride lengths and C: stride widths, expressed as percentages of baseline values, before and after UVD. Error bars are standard deviations. * indicates a significant difference between the baclofen and saline groups (P<0.05).
There were no significant differences between the right and left stride lengths, front and hind stride lengths, or front and hind stride widths at any time point in the UVD experiment (p>0.05, 1-way ANOVA). All stride lengths were therefore pooled into one sample, and stride widths into another sample. These data were expressed as percentages of the sample mean initial values.
Grip Test
In conjunction with the dynamic tests, the ability to grip a wire mesh and hang upside down was measured. For each mouse, this was the amount of time it could hold on, up to a maximum of 1 minute. This test was done to ensure that no damage was done to any of the muscles involved in locomotion during surgery. We observed no failures on the grip test at any time in our protocol.
Termination
Mice that completed the UVD experiment were euthanized 48 hours following surgery, using a gradually increased concentration of CO2. The temporal bone was inspected postmortem to ensure that the bone-wax seal was intact.
RESULTS
GABAB receptor expression in the lateral vestibular nucleus
We visualized the distribution of GABAB receptors in the mouse brainstem and cerebellum, using an antibody to the R2 subunit. Fig. 1 shows an exmaple of a labeled section including the vestibular nuclei and cerebellar cortex. All subdivisions of the vestibular nuclei contained labeled somata. The molecular layer of the cerebellar cortex was also heavily labeled. For this study of postural reflexes and locomotion, the giant neurons of Deiters which project to the spinal cord were of particular interest, and these neurons showed dark staining (Fig. 1B and C). Punctate staining was visible at higher magnification, and presynaptic terminals were clearly labelled (Fig. 1C). The intense immunostaining in Deiters’ nucleus raised the possibility that drugs acting at GABAB receptors may affect postural reflexes, by means of their direct effects on vestibulospinal neurons and/or by presynaptic effects.
Figure 1.
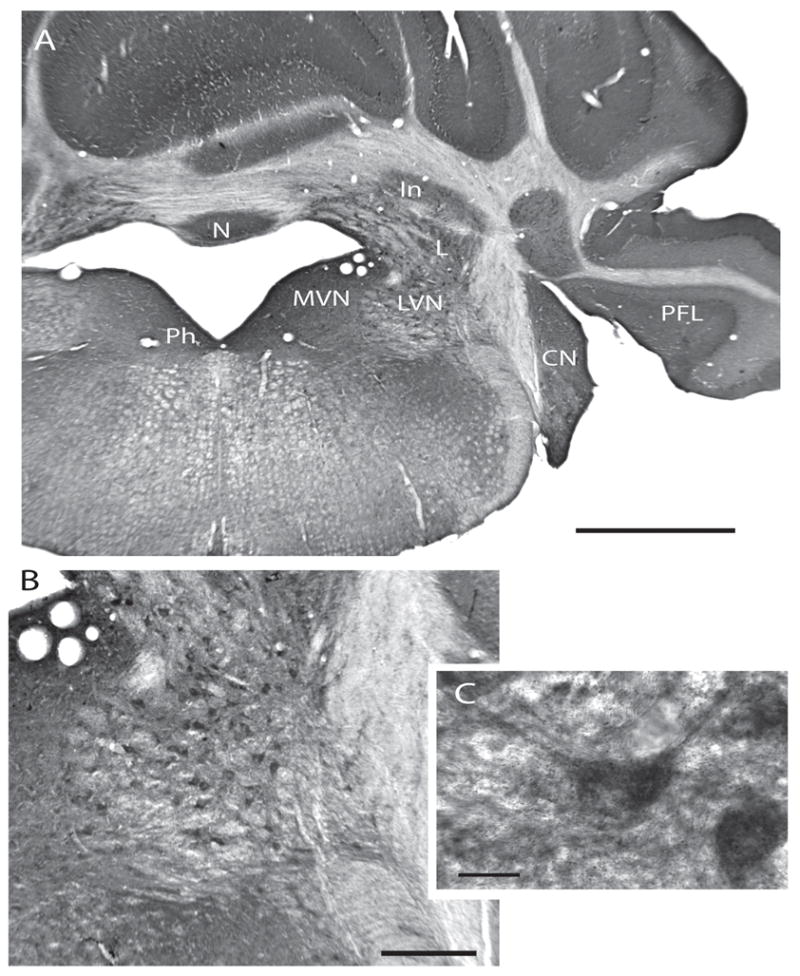
Expression of GABAB receptor protein in the rostral vestibular nuclei and cerebellum. A: CN: Cochlear nucleus. In: Nucleus interpositus. L: Lateral cerebellar nucleus. LVN: Lateral vestibular nucleus. MVN: Medial vestibular nucleus. N: Nodulus. PFL: Paraflocculus. Ph: Nucleus prepositus hypoglossi. Scale bar: 1 mm. B: Higher magnification view of the LVN, showing the heavily stained somata of the large neurons of Deiters. Scale bar: 250 μM. C: A higher-magnification view of a labelled neuron in the LVN. Note punctate labelling. Scale bar: 20 μM.
Recovery of postural reflexes following UVD
All mice were able to stand within the first 30 minutes after the anesthetic was turned off following UVD. At that time they also had severe postural deficits, i.e. falling, circling, barrel rolling or head or body tilt. Figs. 2 and 3 illustrate the time course of the static scores over the first four postoperative hours. Some mice scored zero at the earliest postoperative time point because they were not yet able to stand. Means and standard deviations are illustrated in the left-hand panels of each figure, and exponential fits to the entire data sets are shown in the right panels.
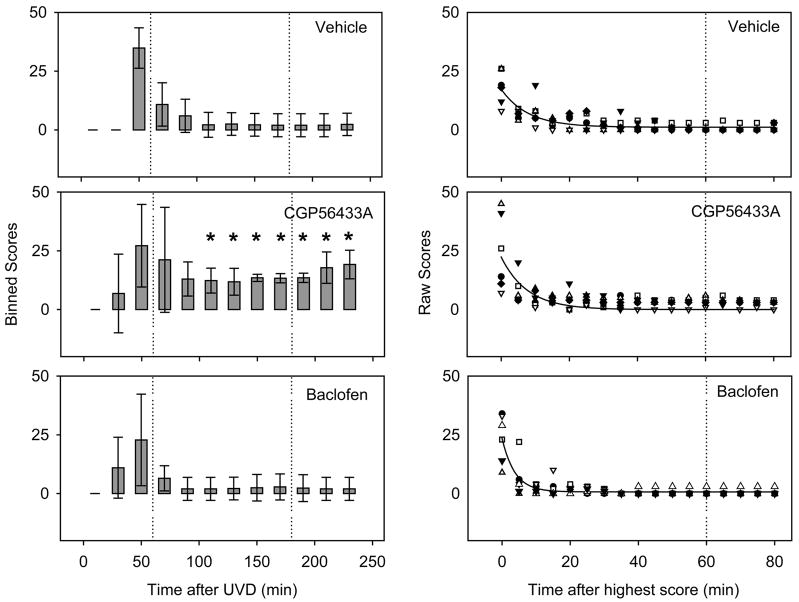
Figure 2.
CGP56433A impaired the compensation of static signs. Each plot shows balance impairment reflected by scoring as a function of time after UVD. Drugs were administered at the times indicated by the vertical dotted lines. Left hand panels: scores (for individual mice) are binned over 20-minute intervals (n=24 scores per bar) and plotted over time after UVD. Error bars are standard deviations. The initial zeroes were before recovery from the anesthetic. Significant differences for CGP56433A compared to the saline vehicle are indicated by * for p<0.05. Right panels: The detailed time course over the first two hours revealed more rapid compensation in the baclofen group. Individual mice in each group are represented by different symbols. Data are aligned on the recovery start time (i.e., the highest score) for each mouse. Curves are best-fit single exponentials.
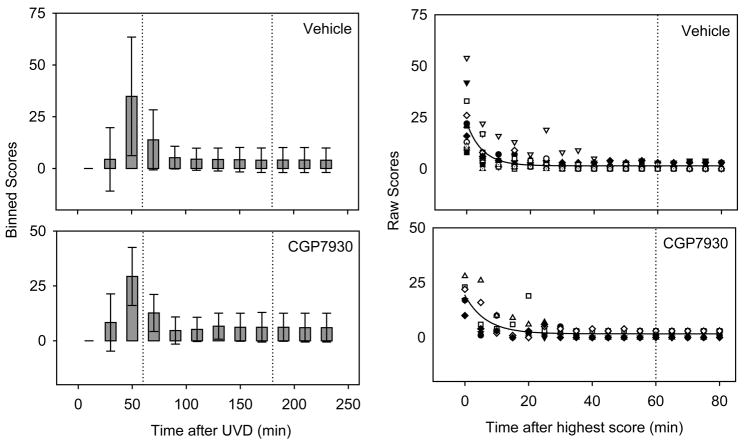
Figure 3.
The CGP7930 group showed no significant differences in static scores, compared with the methylcellulose vehicle. Left panels: Binned static balance scores over 20 minute intervals after UVD (Number of measurements: 24 per bar for CGP7930, 48 per bar for methylcellulose). Times of drug doses are indicated by the vertical dotted lines. Error bars are standard deviations. Right panels: Raw static sign scores over the first 90 minutes after the highest score. Symbols represent individual mice. Curves are best-fit single exponentials. For the raw scores and exponential fits, data have been aligned on the recovery start time (i.e., the highest score) for each mouse.
The signs of vestibular damage were evident immediately on standing after UVD. During the first postoperative hour the scores were variable, possibly due to incomplete recovery from the anesthetic. In general, however, the mice compensated rapidly for the static postural deficit. Circling and barrel rolling subsided within the first hour after waking. In contrast to all other static signs, the head tilt generally failed to recover, resulting in a low residual score (Figs. 2 and 3, vehicle).
During the initial stage of compensation, groups of mice (shown in Table 1) received either the GABAB antagonist, CGP56433A (5 mg/kg); the GABAB agonist, baclofen (1 mg/kg), or an equal volume of physiological saline, at the times indicated by the dotted lines in Fig. 2. Following UVD, the static postural scores of the CGP56433A group were higher than those of the vehicle group, indicating a greater impairment in posture. The difference between groups became significant 110 minutes after UVD (p<0.05, Kruskal-Wallis H test for multiple comparisons; asterisks in Fig. 2). Until the end of the monitoring period, the scores of the group receiving CGP56433A remained significantly higher than the saline controls, consistent with an impairment of compensation. There was also a slight increase in the mean postural score following the administration of the second postoperative dose of CGP56433A (Fig. 2, dotted line). However, the increase was not significant (p>0.05, Mann-Whitney U Test).
Repeated doses of 1 mg/kg baclofen had no significant effect on our measures of postural reflex compensation (Fig. 2), but because the control group recovered essentially to normal levels (p>0.05, Kruskal-Wallis) within the first 90 minutes, any improvement in the baclofen group could not have reached significance. We therefore asked whether the rate of compensation was affected. Data were fitted with single-exponential decay functions (Fig. 2, right hand side; note the different time scale). The best-fit exponential for the baclofen group had a time constant of only 3.7 min, compared with 7.9 min for the saline group. As Fig. 2 shows, the baclofen group had the shortest time constant, while the CGP56433A and saline groups recovered more slowly (τCGP56433A =7.68 min; τsaline = 7.92 min).
In summary, CGP56433A did not affect the time constant of recovery, but it did affect the asymptotic value that was achieved during the first four hours. Baclofen reduced the time constant. Based on these results, it appeared that blocking GABAB receptors was detrimental to compensation, and activating them may have been of some slight benefit in speeding up compensation.
We hypothesized that an allosteric modulator might be more effective than an agonist for targeting GABAB receptors in the postlesion vestibular nuclei. A test group of mice receiving the positive allosteric modulator CGP7930 was compared with a group receiving the methylcellulose vehicle (see Table 1). Fig. 3 shows the time course of recovery for these groups. We found no significant effect of CGP7930 at any time point following UVD (p>0.05, Mann-Whitney U test). The time constant of recovery was slightly longer in the CGP7930 group (6.6 min, vs. 5.0 min for vehicle).
Recovery of dynamic balance
Figure 4 shows the time course of recovery of dynamic postural balance as assessed using a balance beam, in all of the experimental groups. Balance-beam scores were assigned based on whether the mouse stayed on the beam and, if it did, how quickly it crossed. Scoring was done before and after the first injection of the test substance and at 4, 19 and 44 hours postoperatively. The times of all manipulations relative to UVD are illustrated in Fig. 4B and D. The time of UVD is indicated by the solid vertical line at t=0. Six doses of the test substance were given as indicated by the vertical dotted lines. Separate plots in Figure 4B and D represent the baclofen, CGP56433A and CGP7930 groups and their control groups.
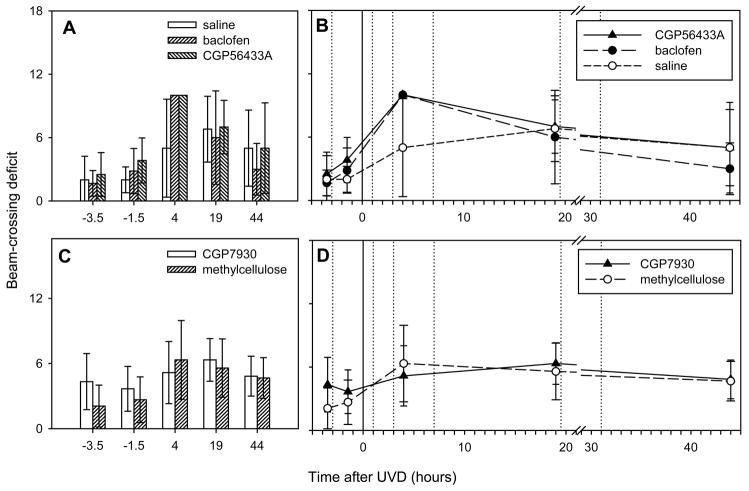
Figure 4.
Mice were less able to cross a balance beam following UVD. Both agonist and antagonist treatments appeared to impair beam crossing compared to the control group. All mice in the baclofen and CGP56433A groups fell from the beam at 4 hours, as did 50% of the control group. These drugs did not affect the scores before surgery. A: Beam-crossing scores of the baclofen and CGP56433A groups and the saline group, before and after UVD at t=0. Note the high scores in both drug groups, relative to controls, at t=4 hours. B: Scores in the methylcellulose and CGP7930 groups. C, D: The time course of performance on the balance beam. The time of UVD is indicated by the vertical solid line and drug doses were administered at the vertical dotted lines. Note the break in the abscissa between 20 and 30 hours.
During the 4-hour period immediately before UVD, we tested the effects of baclofen, CGP56433A and CGP7930 on dynamic postural balance in each mouse (Fig. 4, compare t=−3.5 with t=−1.5 in all plots). Two hours after the prelesion dose, all mice were able to cross the balance beam. No drug had any significant effect on the beam-crossing scores. Following UVD, scores increased in all groups. Next, there was a gradual decrease in the mean scores over the two days following the lesion. There were differences among the groups 4 hours after air injection. All mice in the baclofen and CGP56433A groups fell from the beam at that time (Fig. 4A). However, the group means were not significantly different from the saline vehicle group, three of which also fell (p>0.05, Kruskal-Wallis).
Although drug effects were present during the early phase of compensation (Figs. 2–4), we did not find any lasting effects of the drug treatments. Drug groups and control groups had essentially equal scores by the end of the monitoring period. The lack of any effects on the late phase of compensation could have been due to insufficient drug administration. We also looked for any differences in balance-beam performance in the group of mice that received the positive allosteric modulator CGP7930. Figure 4C and D show the balance-beam assessments for the CGP7930 group and the corresponding vehicle control group. Repeated doses of CGP7930 had no significant effect on beam crossing at any time point (p>0.05, Mann-Whitney).
Since none of the test substances affected beam crossing significantly, we pooled all the experimental groups to evaluate the effect of UVD on beam crossing, which has not been described in mice to our knowledge. In the pooled data, the effect of UVD on beam crossing was highly significant, with scores above the pre-lesion values at both 4 and 19 hours post-lesion (p<0.01, Kruskal-Wallis). The pooled data also showed clear evidence of compensation, with a significant difference between the performances at 4 and 44 hours post-lesion (p<0.05, Kruskal-Wallis).
To summarize the results of the balance-beam tests, UVD impaired the ability of the mice to cross the beam for the two-day period following surgery, and the deficit showed evidence of compensation between 4 and 44 hours postlesion. Both baclofen and CGP56433A appeared to worsen the deficit at 4 hours postlesion. No drug treatment had any significant effect on the scores at 19 or 44 hours postlesion.
Recovery of gait
Gait could be a factor in the beam-crossing deficits if, for example, a broad-based gait led to poor foot placement on the beam. In the same mice, we tested for any effect of UVD on gait and for any effect of the test substances on gait parameters during compensation. We measured stride length and stride width as illustrated in Fig. 5A. The gait measurements were carried out immediately after the balance-beam tests. Stride length was measured for all four paws and the data were combined for a total of up to 24 measurements, per treatment, at each time point. Stride width was measured for both front and rear paws and combined.
The results, normalized with respect to initial values, are illustrated in Figs. 5 and 6. During the 4-hour period immediately before surgery, we tested the effects of baclofen, CGP56433A, and CGP7930 on the gait parameters. None of the test substances had any significant effect on stride width or length during the baseline period (Figs. 5 and 6, compare t=−3.5 with t=−1.5; p>0.05, Student’s t-test).
Figure 6.
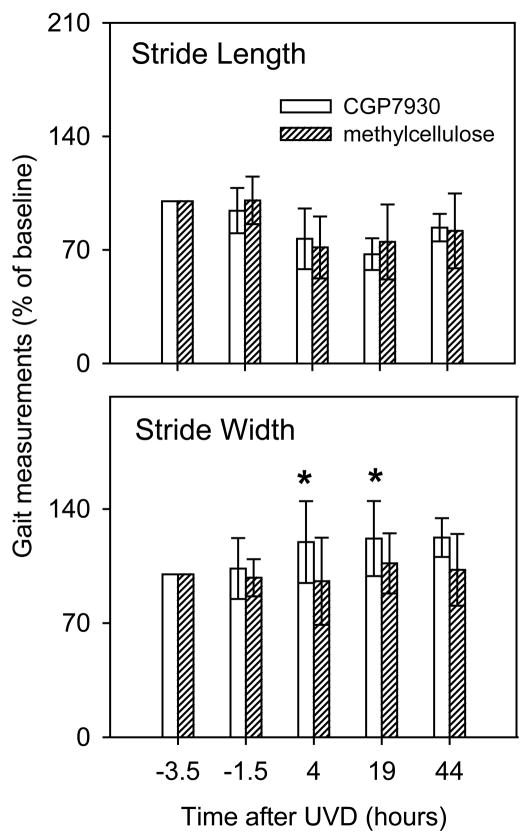
UVD significantly affected stride length (see text). In addition, CGP7930 revealed a significant effect of UVD on stride width but not on stride length. A: stride length and B: stride width are illustrated. Normalization and error bars as in Fig. 5. * at 4 and19 hours, CGP7930 and methylcellulose groups were significantly different (p<0.05, Student’s t-test).
Air injection had a reproducible effect on gait. Stride length was significantly reduced within all groups, compared to the pre-surgical time point, at 4 and 19 hrs (Figs. 5B and 6) (p<0.01; 1-way ANOVA with Tukey-Kramer post-hoc correction). The effect on stride length was similar in all groups. Stride length returned to normal over the first two post-lesion days and by 44 hrs postlesion, only the saline group had stride lengths that remained significantly reduced, compared to baseline (p<0.01; 1-way ANOVA with Tukey-Kramer correction). The baclofen group had a more severe deficit in stride length than the saline group at 4 hrs after UVD (p<0.05; 1-way ANOVA with Tukey-Kramer post-hoc; asterisk in Fig. 5). Neither CGP56433A nor CGP7930 had a significant effect on stride length.
Following UVD there was an increase in the mean stride width. This effect was not significant at any of the time points for any of the test groups with saline vehicle (Fig. 5C), nor was there a measurable effect of baclofen or CGP56433A on stride width during the baseline period. However, the group receiving CGP7930 exhibited an increase in stride width, while the methylcellulose vehicle controls had a slight decrease after UVD (Fig. 6). The difference between the CGP7930 and methylcellulose groups was significant at 4 and 19 hrs (p<0.05, 2-tailed Student’s t-test; asterisks in Fig. 6).
Our observations on gait are discussed below.
DISCUSSION
The compensation of static postural reflexes
Static compensation is usually attributed to the restoration of symmetry in the resting discharge rates of secondary neurons on the two sides of the brainstem (Precht et al., 1966, Yagi and Markham, 1984, Newlands and Perachio, 1990a, Ris et al., 2001), although other mechanisms may contribute (Ris et al., 1997). The rebalancing of resting discharges in the vestibular nuclei may involve a decrease in the efficacy of both GABAA and GABAB receptors (Johnston et al., 2001) and an increase in neuronal excitability on the damaged side (Him and Dutia, 2001, Guilding and Dutia, 2005, Gittis and du Lac, 2006). Interestingly, the change in intrinsic excitability does not persist (Guilding and Dutia, 2005), suggesting that another mechanism is involved in the long-term maintenance of postural compensation. Synaptic plasticity (Precht et al., 1966, Dieringer, 1995, Goto et al., 2001, Gittis and du Lac, 2006), synaptogenesis (Gacek et al., 1989, Li et al., 2002, Paterson et al., 2006) and reactive neurogenesis (Tighilet et al., 2007) are candidate mechanisms.
The concentration of GABA increases acutely in the MVN on the damaged side following UVD (Bergquist et al., 2008). The increase could activate extrasynaptic as well as synaptic GABAB receptors tonically, triggering compensation mechanisms. We hypothesized that baclofen would improve the outcome of compensation by increasing the tonic activation of GABAB receptors, and the mice receiving baclofen tended to compensate more rapidly, supporting our hypothesis. Baclofen had no permanent effect. However, it should be noted that the control group had fully recovered after only 90 minutes, so that there was little opportunity to measure any improvement. Therefore, the lack of any permanent effect of baclofen has no implication for the role of GABAB receptors during static compensation.
It is possible that the more rapid improvement in posture in the baclofen group was due to a reduction in postoperative pain, via GABAB receptors (Goudet et al., 2009). However, for analgesia, baclofen is administered intrathecally. The dose of baclofen that we used (1 mg/kg, sc) was lower than the effective antinociceptive dose for sc or ip administration (Cutting and Jordan, 1975, Franek et al., 2004). In addition, the postoperative meloxicam used in our study would be expected to obscure the antinociceptive effects of baclofen.
The GABAB antagonist CGP56433A impaired the compensation of static signs. Three doses of CGP56433A around the time of surgery significantly worsened the final outcome of static compensation. This finding indicates that during static compensation, GABAB receptors are tonically active, as others had suggested (Magnusson et al., 2002, Bergquist et al., 2008). Our result suggests that GABAB receptors contribute to compensation during the first 4 hours after UVD.
The lack of any effect of the positive allosteric modulator, CGP7930, was disappointing but can perhaps be discounted. CGP7930 is relatively insoluble in aqueous solutions (Urwyler et al., 2001) and was administered sc as a suspension in methylcellulose. Although a similar suspension has an anxiolytic effect at 100 mg/kg when administered by gavage (Jacobson and Cryan, 2008), its concentration in the brain may have been too low to affect compensation.
GABAB receptors were found to be abundant on Deiters’ cells and presynaptic terminals of the lateral vestibular nucleus (LVN), as has also been reported in the rat (Eleore et al., 2005). The axons of Deiters’ neurons make up the lateral vestibulospinal tract and are largely responsible for extensor tone in the limbs (for a review see (Brodal, 1981), pp. 201–204). Deiters’ neurons show clear responses to tilt (Peterson, 1970) and have high resting rates of activity. Their resting discharge rates ipsilateral to a hemilabyrinthectomy are nearly abolished by labyrinthectomy, and recover during compensation (Ris et al., 1995). The giant neurons of the LVN are, therefore, a plausible site for the participation of GABAB receptors in postural balance compensation.
Comparison with earlier studies
In an earlier study using chemical UVD in the rat model, Magnusson and co-workers reported large, transient effects of GABAB agonists and antagonists administered during vestibular compensation (Magnusson et al., 1998, Magnusson et al., 2000, Magnusson et al., 2002). The present study differs from the earlier one in several ways. First, we monitored static signs using quantitative scoring, which permitted us to identify a significant effect of blocking GABAB receptors on the final outcome of static compensation. In the earlier study, the chemical lesion developed over time and may not have been fully compensated at the time that the drugs were administered; also, Magnusson et al. administered drugs intramuscularly, which could have led to a more sudden change in receptor activation. In the present study we included the positive allosteric modulator CGP7930 which had not previously been tested in the vestibular system.
Static compensation occurred rapidly in our mouse model, and we administered repeated doses of the test treatments during a significant part of the compensation process. Falling, circling, and barrel rolling were abolished within 90 minutes following UVD. Rapid clearing of anesthetic in our study (within 30 minutes in all cases) may have contributed to the speed of compensation. All of our subjects were 1–3 months of age and were selected to weigh less than 23 grams. In this age and weight range, mice have little body fat and are active and agile. Very rapid compensation of circling behavior (< 1 hour) has also been described in gerbils following unilateral labyrinthectomy (Kaufman et al., 1999). However, another group of mice (that were up to 6 months of age) required as long as three days to abolish circling (Gacek and Khetarpal, 1998). In young rats, recovery of static postural reflexes requires more than 24 hours (Bergquist et al., 2008) but mice have been reported to recover their static balance within 24 hours after hemilabyrinthectomy (Aleisa et al., 2007). Considered together, the results suggest that young mice and gerbils can correct static postural deficits relatively rapidly. More investigation is needed to establish the effect of age and body size on compensation.
Dynamic compensation
“Dynamic compensation” refers to the compensation of vestibular reflexes that are activated by movement. Pronounced recovery in the performance of the vestibulo-ocular reflex (VOR) is included in dynamic compensation (Fetter and Zee, 1988, Lasker et al., 1999, Broussard and Hong, 2003). There is a corresponding increase in the sensitivities to rotation of secondary vestibular neurons on the damaged side (Precht et al., 1966, Newlands and Perachio, 1990a, Newlands and Perachio, 1990b). The dynamic vestibulo-spinal reflexes, which function in maintaining balance and posture while moving, and are involved in locomotion, also compensate. Changes in the dynamic responses of vestibulospinal neurons have been described following hemilabyrinthectomy (Xerri et al., 1985). Gait deviation was also found to recover over several weeks (Igarashi et al., 1981). This slow recovery supports the view that normal gait requires normal dynamic vestibular function. Here, we report a quantitative analysis of stride width and length on a flat surface, that shows significant changes in length, but not width, following UVD. Our results also demonstrate recovery in stride length during the subsequent 48 hours. The dynamic reflexes mediated by vestibulospinal pathways may compensate in a way analogous to the VOR.
The effect on gait of the positive allosteric modulator, CGP7930, was unexpected. Unlike the other drugs in this study, CGP7930 had no effect on postural reflexes, balance, or stride length, but did have an effect on stride width at all postlesion time points. In the presence of GABA, CGP7930 can enhance the downstream effects of GABAB receptor activation (Urwyler et al., 2001). The selective effects of CGP7930 on gait suggests that following air injection, there is a functional difference in the distribution or in the action of GABAB receptors in the brain pathways subserving gait, compared with pathways subserving extensor tone. The Deiters’ neurons of the LVN participate in both gait and postural reflexes. (Orlovsky, 1972). Therefore, other pathways should be considered that might differentially participate in gait. The highest density of GABAB receptors in the brain is in the molecular layer of the cerebellum, where parallel fibers terminate on Purkinje neurons (Bowery et al., 1987) (see Figure 1). Increased stride width, or broadbased gait, is a classic sign of cerebellar anterior-lobe dysfunction. Accurate paw placing is a function of paravermal regions and the interpositus nuclei (Ito, 1984). It is possible that GABAergic synaptic drive in the anterior-lobe paravermis is affected by UVD, making it vulnerable to disruption by the allosteric modulator. Although the paravermal regions are not generally considered as a locus for vestibular compensation, they may be considered in future studies.
Dynamic compensation of balance
We found the balance beam to be an even more sensitive indicator of dynamic vestibular function than gait on a flat surface. Balance-beam scores can be affected by (for example) paw placing, static postural reflexes, and/or dynamic vestibular reflexes. After the administration of either baclofen or CGP56433A, all mice fell off the beam at 4 hours postoperatively, at which time postural reflexes had recovered fully. The poor performance by the baclofen group at 4 hours is consistent with findings of de Valck et al. (De Valck et al., 2009), who showed that patients with UVD that were given baclofen performed poorly when they had to walk 20 paces heel-to-toe, creating a situation similar to walking on a narrow beam. At the doses used here, baclofen had no effect on the preoperative balance-beam performance. It appears, therefore, that dynamic vestibular reflexes remain more vulnerable to disruption after UVD. Because GABAB receptors are located both pre- and postsynaptically, both agonists and antagonists can impair the normal function of synapses. Thus both drugs could impair the participation of Deiters’ neurons in the locomotor rhythm, sensitivity to tilt, and extensor tone.
Although there is evidence suggesting that GABAB receptors may participate in dynamic compensation (see below), we found no significant effect of baclofen, CGP7930, or CGP56433A on balance-beam performance later on. However, we did not continue to administer the drugs throughout the two-day monitoring period, and drug levels may have fallen too low to be effective. Because CGP56433A interfered with static compensation but did not affect dynamic compensation in the same group of mice, our results support the view that long-term dynamic and short-term static compensation are mediated by separate and independent processes.
Mechanisms of dynamic compensation
Dynamic vestibular compensation is believed to be due to synaptic plasticity and/or synaptogenesis within the vestibular nuclei (Precht et al., 1966, Dieringer, 1995, Goto et al., 2001, Li et al., 2002, Paterson et al., 2006). Mechanisms for compensation may include changes in the ratio of excitatory to inhibitory synapses in the vestibular nuclei (Gacek et al., 1996), increased glycinergic synaptic drive, (Lim et al., 2010), and reduced commissural excitatory synaptic efficacy (Farrow and Broussard, 2003). Vestibular reflexes can also be modified by cerebellar motor learning (for review see (Broussard and Kassardjian, 2004)), for which cerebellar long-term depression (LTD) is thought to be an important mechanism (Ito, 2001, Boyden et al., 2004, Broussard and Kassardjian, 2004). Cerebellar LTD appears not to be required for VOR compensation (Faulstich et al., 2006), although it could be a contributing factor.
GABAB receptors regulate synaptic plasticity at both excitatory (Davies et al., 1991) and inhibitory synapses (Shew et al., 2000) and are thought to interact cooperatively with other pathways in the induction of long-term plasticity (Patenaude et al., 2003, Kamikubo et al., 2007). In the cerebellar cortex, GABAB receptors can enhance synaptic plasticity via their interactions with pathways that are also affected by metabotropic glutamate receptors (Hirono et al., 2001, Kelly et al., 2009). Cerebellar cortical GABAB receptors are tonically active during, and participate in, VOR motor learning (Titley et al., 2009). The present results do not negate these conclusions, because it is possible that in our protocol, CGP56433A did not remain at an adequate systemic level to interfere with dynamic compensation.
To summarize, we found that CGP56433A interfered with static compensation of posture and baclofen increased its speed. These results suggest that GABAB receptors are tonically active following UVD and contribute to the restoration of static postural reflexes. CGP7930 revealed a selective effect of UVD on stride width, which may be mediated by a separate pathway. There were no lasting effects of any of our treatments on dynamic compensation, failing to support the existence of a critical period for recovery of dynamic reflexes, and supporting the concept of separate mechanisms for static and dynamic compensation.
Acknowledgments
We thank C. Wu and N. Paolone for technical assistance, Dr. M. Faulstich for teaching us the air-injection procedure, and Dr. W. Froestl for the gift of CGP56433A. This work was supported by a catalyst award from the Canadian Institutes of Health Research. R. H.-S. and H. K. T. were supported by fellowships from the Vision Science Research Program, University of Toronto.
ABBREVIATIONS
- ANOVA
Analysis of variance
- CN
Cochlear nucleus
- DAB
Diaminobenzedine
- GABA
γ-amino butyric acid
- GABAB
Metabotropic GABA type B
- IgG
Immunoglobulin G
- In
Nucleus interpositus
- L
Lateral cerebellar nucleus
- LVN
Lateral vestibular nucleus
- LTP
Long-term potentiation
- MVN
Medial vestibular nucleus
- N
Nodulus
- PBS
Phosphate-buffered saline
- PFL
Paraflocculus
- Ph
Nucleus prepositus hypoglossi
- UVD
Unilateral vestibular damage
- VOR
Vestibulo-ocular reflex
References
- Aleisa M, Zeitouni AG, Cullen KE. Vestibular compensation after unilateral labyrinthectomy: Normal versus cerebellar dysfunctional mice. J Otolaryngol. 2007;36:315–321. [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Zhou F-M, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol. 2008;586:4441–4452. doi: 10.1113/jphysiol.2008.155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distributions in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: The role of multiple plasticity mechanisms. Ann Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- Brodal A. Neurological anatomy in relation to clinical medicine. Oxford U. Pr; New York: 1981. [Google Scholar]
- Broussard DM, Bhatia JK, Hong JA. The dynamics of the vestibulo-ocular reflex after peripheral vestibular damage. II. Comparison with dynamics after optically-induced learning. Exp Brain Res. 1999;125:365–374. doi: 10.1007/s002210050692. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Hong JA. The response of vestibulo-ocular reflex pathways to electrical stimulation after canal plugging. Exp Brain Res. 2003;149:237–248. doi: 10.1007/s00221-002-1345-9. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Memory. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation. Adv Otorhinolaryngol. 1999;55:82–110. doi: 10.1159/000059059. [DOI] [PubMed] [Google Scholar]
- Cutting DA, Jordan CC. Alternative approaches to analgesia: Baclofen as a model compound. Br J Pharmacol. 1975;54:171–179. doi: 10.1111/j.1476-5381.1975.tb06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CL, Dutia MB, Smith PF. The contribution of the intrinsic excitability of vestibular nucleus neurons to recovery from vestibular damage. Eur J Neurosci. 2001;15:1719–1727. doi: 10.1046/j.1460-9568.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Davies CH, Starkey SL, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- De Valck CFJ, Vereeck L, Wuyts FL, Van de Heyning PH. Failure of γ-aminobutyrate acid-β agonist baclofen to improve balance, gait, and postural control after vestibular schwannoma resection. Otol Neurotol. 2009;30:350–355. doi: 10.1097/MAO.0b013e31819678a7. [DOI] [PubMed] [Google Scholar]
- Dieringer N. ‘Vestibular compensation’: Neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46:97–129. [PubMed] [Google Scholar]
- Eleore L, Vassias I, Bernat I, Vidal PP, de Waele C. An in situ hybridization and immunofluorescence study of GABAA and GABAB receptors in the vestibular nuclei of the intact and unilaterally labyrinthectomized rat. Exp Brain Res. 2005;160:166–179. doi: 10.1007/s00221-004-1997-8. [DOI] [PubMed] [Google Scholar]
- Farrow K, Broussard DM. Commissural inputs to secondary vestibular neurons in alert cats after canal plugs. J Neurophysiol. 2003;89:3351–3353. doi: 10.1152/jn.01060.2002. [DOI] [PubMed] [Google Scholar]
- Faulstich M, van Alphen AM, Luo C, du Lac S, De Zeeuw C. Oculomotor plasticity during vestibular compensation does not depend on cerebellar LTD. J Neurophysiol. 2006;96:1187–1195. doi: 10.1152/jn.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;59:370–393. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- Franek M, Vaculin S, Rokyta R. GABAB receptor agonist baclofen has non-specific antinociceptive effect in the model of peripheral neuropathy in the rat. Physiol Res. 2004;53:351–355. [PubMed] [Google Scholar]
- Gacek RR, Khetarpal U. Neurotrophin 3, not brain-derived neurotrophic factor or neurotrophin 4, knockout mice have delay in vestibular compensation after unilateral labyrinthectomy. Laryngoscope. 1998;108:671–678. doi: 10.1097/00005537-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Lyon MJ, Schoonmaker JE. Morphologic correlates of vestibular compensation in the cat. Acta Oto-Laryngologica suppl. 1989;462:1–16. doi: 10.3109/00016488909098981. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Schoonmaker J, Lyon M. Morphologic changes in contralateral superior vestibulo-ocular neurons following labyrinthectomy in the cat. Ann Otol Rhinol Laryngol. 1996;105:791–794. doi: 10.1177/000348949610501006. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Flohr H, Melvill Jones G. A reevaluation of intervestibular nuclear coupling: its role in vestibular compensation. J Neurophysiol. 1984;51:242–259. doi: 10.1152/jn.1984.51.2.242. [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol. 2006;16:385–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Postlesional vestibular reorganization in frogs: Evidence for a basic reaction pattern after nerve injury. J Neurophysiol. 2001;85:2643–2646. doi: 10.1152/jn.2001.85.6.2643. [DOI] [PubMed] [Google Scholar]
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, Pin J-P. Metabotropic receptors for glutamate and GABA in pain. Brain Res Reviews. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Guilding C, Dutia MB. Early and late changes in vestibular neuronal excitability after deafferentation. Neuroreport. 2005;16:1415–1418. doi: 10.1097/01.wnr.0000176519.42218.a6. [DOI] [PubMed] [Google Scholar]
- Him A, Dutia MB. Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation. Brain Res. 2001;908:58–66. doi: 10.1016/s0006-8993(01)02600-2. [DOI] [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S. GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nature Neurosci. 2001;4:1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]
- Holstein GR, Martinelli GP, Cohen B. L-Baclofen-sensitive GABAB binding sites in the medial vestibular nucleus localized by immunocytochemistry. Brain Res. 1992;581:175–180. doi: 10.1016/0006-8993(92)90361-c. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Levy JK, Uchi TO, Reschke MF. Further study of physical exercise and locomotor balance compensation after unilateral labyrinthectomy in squirrel monkeys. Acta Oto-Laryngologica. 1981;92:101–105. doi: 10.3109/00016488109133243. [DOI] [PubMed] [Google Scholar]
- Ito M. The cerebellum and neural control. Raven Pr; New York: 1984. [Google Scholar]
- Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001;8:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABAA and GABAB receptors during vestibular compensation. Neuroreport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neurons plasticity during vestibular compensation in the rat. J Physiol. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikubo Y, Tabata T, Kakizawa S, Kawakami D, Watanabe M, Ogura A, Iino M, Kano M. Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J Physiol. 2007;585:549–563. doi: 10.1113/jphysiol.2007.141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GD, Shinder ME, Perachio AA. Correlation of Fos expression and circling asymmetry during gerbil vestibular compensation. Brain Research. 1999;817:246–255. doi: 10.1016/s0006-8993(98)01284-0. [DOI] [PubMed] [Google Scholar]
- Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nature Neurosci. 2009;12:693–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- Lacour M. Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opinion. 2006;22:1651–1659. doi: 10.1185/030079906X115694. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Backous DD, Lysakowski A, Davis GL, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. II. Responses after canal plugging. J Neurophysiol. 1999;82:1271–1285. doi: 10.1152/jn.1999.82.3.1271. [DOI] [PubMed] [Google Scholar]
- Li H, Dokas LA, Godfrey DA, Rubin AM. Remodeling of synaptic connections in the deafferented vestibular nuclear complex. J Vest Res. 2002;12 [PubMed] [Google Scholar]
- Lim R, Callister RJ, Brichta AM. An increase in glycinergic quantal amplitude and frequency during early vestibular compensation in mouse. J Neurophysiol. 2010;103:16–24. doi: 10.1152/jn.91223.2008. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Eriksson B, Tham R. Effects of the GABA agonists baclofen and THIP on long-term compensation in hemilabyrinthectomized rats. Brain Res. 1998;795:307–311. doi: 10.1016/s0006-8993(98)00329-1. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Lindstrom S, Tham R. GABAB receptors contribute to vestibular compensation after unilateral labyrinthectomy in pigmented rats. Exp Brain Res. 2000;134:32–41. doi: 10.1007/s002210000438. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Ulfendahl M, Tham R. Early compensation of vestibulo-oculomotor symptoms after unilateral vestibular loss in rats is related to GABA-B receptor function. Neuroscience. 2002;111:625–634. doi: 10.1016/s0306-4522(01)00618-2. [DOI] [PubMed] [Google Scholar]
- Mathog RH, Peppard SB. Exercise and recovery from vestibular injury. Am J Otolaryngol. 1982;3:397–407. doi: 10.1016/s0196-0709(82)80017-3. [DOI] [PubMed] [Google Scholar]
- Mouginot D, Gahwiler BH. Presynaptic GABAB receptors modulate IPSPs evoked in neurons of deep cerebellar nuclei in vitro. J Neurophysiol. 1996;75:894–901. doi: 10.1152/jn.1996.75.2.894. [DOI] [PubMed] [Google Scholar]
- Murai N, Tsuji J, Ito J, Mishina M, Hirano T. Vestibular compensation in glutamate receptor delta-2 subunit knockout mice: dynamic property of vestibulo-ocular reflex. Eur Arch Otorhinolaryngol. 2004;261:82–86. doi: 10.1007/s00405-003-0644-5. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Dara S, Kaufman GD. Relationship of static and dynamic mechanisms in vestibuloocular reflex compensation. The Laryngoscope. 2005;115:191–204. doi: 10.1097/01.mlg.0000154718.80594.2e. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. I. Type I neurons. Exp Brain Res. 1990a;82:359–372. doi: 10.1007/BF00231255. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. II. Type II neurons. Exp Brain Res. 1990b;82:373–383. doi: 10.1007/BF00231256. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Patenaude C, Chapman CA, Bertrand S, Congar P, Lacaille J-C. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J Physiol. 2003;553:155–167. doi: 10.1113/jphysiol.2003.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JM, Short D, Flatman PW, Seckl JR, Aitken A, Dutia MB. Changes in protein expression in the rat medial vestibular nuclei during vestibular compensation. J Physiol. 2006;575:777–788. doi: 10.1113/jphysiol.2006.112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW. Distribution of neural responses to tilting within vestibular nuclei of the cat. J Neurophysiol. 1970;33:750–767. doi: 10.1152/jn.1970.33.6.750. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Kinney GA, Quinn KJ, Slater NT. Potential mechanisms of plastic adaptive changes in the vestibulo-ocular reflex. Ann N Y Acad Sci. 1996;781:499–512. doi: 10.1111/j.1749-6632.1996.tb15723.x. [DOI] [PubMed] [Google Scholar]
- Precht W, Shimazu H, Markham CH. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol. 1966;29:996–1010. doi: 10.1152/jn.1966.29.6.996. [DOI] [PubMed] [Google Scholar]
- Ris L, Capron B, de Waele C, Vidal P-P, Godaux E. Dissociations between behavioural recovery and restoration of vestibular activity in the unilabyrinthectomized guinea-pig. J Physiol. 1997;500:509–522. doi: 10.1113/jphysiol.1997.sp022037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, Capron B, Vibert N, Vidal P-P, Godaux E. Modification of the pacemaker activity of vestibular neurons in brainstem slices during vestibular compensation in the guinea pig. Eur J Neurosci. 2001;13:2234–2240. doi: 10.1046/j.0953-816x.2001.01603.x. [DOI] [PubMed] [Google Scholar]
- Ris L, de Waele C, Serafin M, Vidal P-P, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1995;74:2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Schaefer KP, Meyer DL. Compensation of vestibular lesions. In: Kornhuber HH, editor. Handbook of Sensory Physiology. part 2. VI. Springer; New York: 1974. pp. 463–490. [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slice. Br J Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew T, Yip S, Sastry BR. Mechanisms involved in tetanus-induced potentiation of fast IPSCs in rat hippocampal CA1 neurons. J Neurophysiol. 2000;83:3388–3401. doi: 10.1152/jn.2000.83.6.3388. [DOI] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exptl Ther. 2005;312:290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Res. 1988;444:308–319. doi: 10.1016/0006-8993(88)90939-0. [DOI] [PubMed] [Google Scholar]
- Sugimura M, Kitayama S, Morita K, Imai K, Irifune M, Takarada T, Kawahara M, Dohi T. Effects of GABAergic agents on anesthesia induced by halothane, isoflurane, and thiamylal in mice. Pharmacol, Biochem Behav. 2002:111–116. doi: 10.1016/s0091-3057(01)00728-6. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Brezun JM, Sylvie GDD, Gaubert C, Lacour M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci. 2007;2007:47–58. doi: 10.1111/j.1460-9568.2006.05267.x. [DOI] [PubMed] [Google Scholar]
- Titley HK, Heskin-Sweezie R, Broussard DM. Learning of gain increases in the vestibulo-ocular reflex requires mGluR1 and GABAB receptors in the cerebellar cortex) Society for Neuroscience; Chicago: 2009. [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid beta receptors by 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Molecular Pharmacology. 2001;60:963–971. [PubMed] [Google Scholar]
- Wang D, Cui L-N, Renaud LP. Pre-and postsynaptic GABAB receptors modulate rapid neurotransmission from suprachiasmatic nucleus to parvocellular hypothalamic paraventricular nucleus neurons. Neuroscience. 2003;118:49–58. doi: 10.1016/s0306-4522(02)00906-5. [DOI] [PubMed] [Google Scholar]
- Xerri C, Gianni S, Manzoni D, Pompeiano O. Central compensation of vestibular deficits. IV. Responses of lateral vestibular neurons to neck rotation after labyrinth deafferentation. J Neurophysiol. 1985;54:1006–1025. doi: 10.1152/jn.1985.54.4.1006. [DOI] [PubMed] [Google Scholar]
- Yagi T, Markham CH. Neural correlates of compensation after hemilabyrinthectomy. Exp Neurol. 1984;84:98–108. doi: 10.1016/0014-4886(84)90008-6. [DOI] [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABAB receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 2000;38:1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol. 2000;523:413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Jones SM, Yamoah EN, Lundberg YW. Otoconin-90 deletion leads to imbalance but normal hearing: A comparison with other otoconia mutants. Neuroscience. 2008;153:289–299. doi: 10.1016/j.neuroscience.2008.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]