Abstract
The surround suppression of the receptive field is important for basic visual information processing, such as orientation specificity. To date, the effects of aging on the strength of surround suppression are not clear. To address this issue, we carried out extracellular single-unit studies of the receptive field properties of cells in the primary visual cortex (area V1) in young and old rhesus (Macaca mulatta) monkeys. When presented with the oriented central stimulus, we found that cells in old animals showed reduced orientation and direction selectivity compared with those in young animals. When presented with the oriented central stimulus together with the optimal surround stimulus, more selective cells {orientation bias (OB) ≥ 0.1; a bias of 0.1 is significant at the P < 0.005 level} in animals of both ages showed reduced orientation selectivity compared with the experiment that presented only the oriented central stimulus. When presented with the optimal central stimulus together with the oriented surround stimulus, cells in old animals showed reduced orientation and direction selectivity compared with young animals. Moreover, broadly tuned cells (OB<0.1) in old animals exhibited significantly reduced suppression indices that quantified the strength of the surround suppression of the receptive field, when compared with those in young animals. These results suggest that aging may seriously affect the surround suppression of the receptive field of V1 cells. Thus, the decreased strength of surround suppression of the receptive field may be one possible reason for the decreased stimulus selectivity of V1 cells previously found in the senescent brain. This work will contribute to an understanding of the physiological mechanisms mediating surround suppression of the receptive field.
Keywords: primary visual cortex, orientation selectivity, receptive field, surround inhibition, rhesus monkey
INTRODUCTION
Human visual function degrades with increasing age (Spear, 1993). Animal studies have indicated some declines in the response properties of visual cortical cells, such as delayed information processing (Yu et al., 2005; Wang et al., 2006; Wang et al., 2005) as well as decreased orientation and direction selectivity (Hua et al., 2006; Yu et al., 2006; Schmolesky et al., 2000). Moreover, these changes were accompanied by increased spontaneous activity and visual responsiveness and a decreased ability to signal visual stimuli above background activity (signal-to-noise ratio) (Yu et al., 2006; Schmolesky et al., 2000; Hua et al., 2006; Wang et al., 2006). It has been suggested that many of these declines in cells during old age are linked to a reduced gamma-aminobutyric acid (GABA)-mediated inhibition (Schmolesky et al., 2000; Leventhal et al 2003).
The response of a primary visual cortex (V1) neuron to visual stimulation of its classical receptive field (CRF) is profoundly modulated by a second stimulus concurrently presented beyond the CRF, which elicits no response by itself (Jones et al., 2001). Many previous studies have indicated that the effect of surround stimulation is inhibition (Walker et al., 2000; Walker et al., 1999; Sceniak et al., 1999; Sengpiel et al., 1997; Knierim and van, 1992; Akasaki et al., 2002; Nelson and Frost, 1978; DeAngelis et al., 1994; Blakemore and Tobin, 1972) rather than facilitation (Sillito et al., 1995; Maffei and Fiorentini, 1976; Polat et al., 1998; Li and Li, 1994; Levitt and Lund, 1997), which is tuned for orientation and direction in V1 cells (Shapley et al., 2003). Furthermore, the preferred orientation and direction of this inhibitory receptive field (IRF) was suggested to be the same as the CRF (Anderson et al., 2000; Allman et al., 1985; Nelson and Frost, 1978; Fries et al., 1977; DeAngelis et al., 1994; Blakemore and Tobin, 1972). Based on these data, we were interested in determining whether aging has effects on the suppressive strength of the inhibitory receptive field of V1 cells.
In the present study, we tried to assess the effects of aging on the strength of surround suppression of the receptive field of V1 cells using extracellular single-unit recording techniques. The orientation and direction biases of cells were compared with and without the optimal surround suppression in young and old monkeys. In addition, the stimulus selectivity of the surround of the receptive field of cells to induce suppression and the suppression index of the surround were measured and compared between the two age groups.
EXPERIMENTAL PROCEDURES
Animals
Four young and three old male rhesus monkeys (Macaca mulatta) were used in this study. Young monkeys were 2.1–6.1 kg with a mean age of 5.5 years, and old monkeys were 5.6–9.5 kg with a mean age of 28 years. An initial ophthalmological examination was performed under ketamine (100 mg/kg i.m., Ketalar, Parke-Davis, Morris Plains, NJ, USA) anesthesia to screen for monkeys with ocular pathology. None of the monkeys used in this study exhibited signs of glaucoma or had retinal pathology such as hemorrhage, macular degeneration, edema, neovascularization or scarring. In addition, the intraocular pressure was also within the normal range for rhesus monkeys. The experiments were conducted in accordance with the guidelines for the National Care and Use of Animals approved by the National Animal Research Authority.
Animal preparation and recording
Subjects were sedated with ketamine HCl (10–15 mg/kg i.m.) and then anesthetized with 3–5% halothane (Halocarton Laboratories, River Edge, NJ, USA) in a 70:30 mixture of N2O:O2. Intravenous and tracheal cannulae were inserted. Animals were placed in a stereotaxic frame, and all pressure points and incisions were infiltrated with a long-acting local anesthetic (2% lidocaine HCl; Copley Pharmaceuticals, Canton, MA, USA). A mixture of d-tubocurarine (0.4 mg/kg/h; Sigma, St. Louis, MO, USA) and gallamine triethiodide (7 mg/kg/h; Sigma, St. Louis, MO, USA) was infused intravenously to induce and maintain paralysis. Animals were ventilated, and anesthesia was maintained with a mixture of N2O (70%), O2 (30%) and halothane (0.25–1.0% as needed). Heart rate was monitored continuously to assess the level of anesthesia. The dose of halothane was increased if the heart rate changed in response to nociceptive stimulation (paw or tail pinch) and was decreased if the heart rate showed continuous slowing. Comparable levels of anesthesia were induced in old and young animals. End-tidal CO2 partial pressure was monitored and maintained at approximately 4%. Body temperature was maintained at 38°C by an automatically regulated heating pad.
The nictitating membrane was prepared with neosynephrine (0.5%; Bayer, Morristown, NJ, USA), the pupils were dilated with atropine (Sigma, St. Louis, MO, USA), and the eyes were covered with contact lenses for protection from desiccation. Spectacle lenses and artificial pupils were used when needed. The optics and retinal vasculature were monitored throughout the experiment. No visible deterioration in the optics occurred during the experimental period in any of the animals. A craniotomy was performed above the area in V1 that corresponded to central vision. Extracellular action potentials of isolated neurons were recorded by glass or glass-coated tungsten microelectrodes with 1–3 MΩ impedance, which were advanced by a hydraulic microdrive (David Kopf Instruments, Tujunga, CA, USA).
The recording sites in V1 were determined as described in our previous studies (Schmolesky et al. 1998; Yang et al. 2009). Briefly, at the end of each experiment, small lesions were made at the end of each penetration by passing DC current through the tip of the electrode (2 μA for 5 s, negative). The monkeys were perfused transcardially, and brains were removed. Blocks of tissue containing V1 were dissected and post-fixed in 4% paraformaldehyde (Tianjin Chemical Reagents Research Institute, PR China) in 0.1 M phosphate-buffered saline for later verification of the recording sites.
Visual stimulation
When a neuron was encountered, the eye affiliation was determined, and all stimuli were presented monocularly to the dominant eye. The receptive field of each cell was manually plotted using an ophthalmoscope. A Sony Multiscan 17se color monitor (85-Hz frame rate; Sony, Tokyo, Japan) was placed 57 cm in front of the animal’s eye and centered on the receptive field of the cell. The program used to generate the stimulus was written in MATLAB (Math Works, Natick, MA, USA) using the extensions provided by the high-level Psychophysics Toolbox (Brainard, 1997) and low-level Video Toolbox (Pelli, 1997).
The optimal orientation and direction were assessed for each neuron with drifting bars that were oriented from 0 to 345° in 15° steps (24 trials). Based on the optimal orientation and direction, the optimal spatial frequency and temporal frequency were determined using drifting sinusoidal gratings (also see Zhang et al., 2008). With these optimal parameters, the size-tuning curve was obtained for each neuron at a fixed contrast (0.8). For the size-tuning curve data, a difference of Gaussian (DOG) function, as described by Sceniak et al. (2001), was used to fit the data. From the fitted function, we determined the smallest stimulus diameter at which the response of the neuron peaked and asymptoted. We took the smallest stimulus diameter at peak response as a measure of CRF size and the smallest stimulus diameter at the asymptoted response as a measure of IRF size. Only cells that showed surround suppression were included in this analysis (Angelucci et al., 2002). Next, three block stimuli were carried out as follows (Fig. 1):
Figure 1.
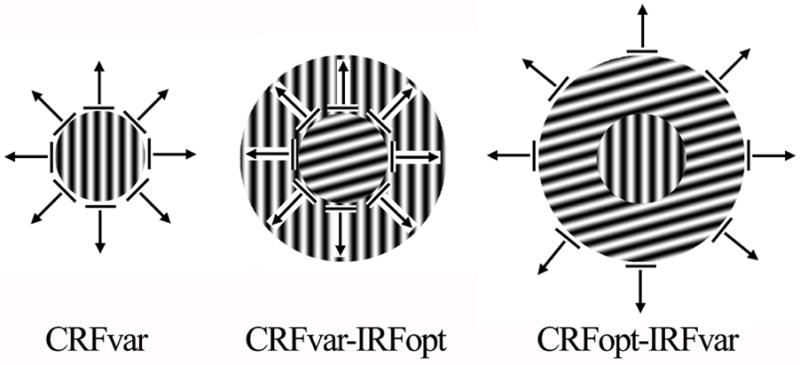
Patterns of the CRFvar (left), CRFvar-IRFopt (middle) and CRFopt-IRFvar (right) stimuli. The gratings in the CRF (center) and the IRF (annulus) either drifted in the optimal orientation and direction or were oriented in each of the 24 randomly generated orientations or directions from 0° to 360° in 15° steps. The drifting grating that was oriented was confined to the CRF (left). The drifting grating was oriented in the CRF and kept optimal in the IRF (middle). The drifting grating in the CRF was in the optimal orientation and direction, and the grating in the IRF was oriented (right). Eight of the 24 directions from 0° to 360° in 15° steps are represented with black arrows.
CRFvar block: A drifting grating, whose orientation and direction were randomly oriented from 0 to 345° in 15° steps, was confined to the CRF. In this case, the optimal orientation and direction were confirmed again for this neuron, which would be used in the following 2 blocks (Fig. 1 left).
CRFvar-IRFopt block: While displaying a stimulus as CRFvar, the IRF of this neuron was simultaneously stimulated by an annular drifting grating with the optimal orientation and direction obtained in the above block (Fig. 1 middle).
CRFopt-IRFvar block: This stimulus was just the opposite of the CRFvar-IRFopt. The CRF of this neuron was stimulated by a drifting grating in the optimal orientation and direction, while the IRF was stimulated by an annular drifting grating in a randomly oriented orientation and direction from 0 to 345° in 15° steps (Fig. 1 right).
There was 5-min interval between each block. Each block included 2 cycles, and each cycle consisted of 5 presentations of the drifting grating at each of the 24 trials (from 0 to 345° in 15° steps) to compile the orientation tuning curve for the neuron studied.
Data collection and analysis
Signals from the microelectrode were amplified (×1000), bandpass filtered (300–3000 Hz), and digitized (sampling frequency of 10,000 Hz) using an acquisition board (National Instruments, Austin, TX, USA) controlled by IGOR software (WaveMetrics, Portland, OR, USA). The original waves were stored in the computer for off-line analysis.
Orientation and direction selectivity were calculated for each cell using the statistical methods described in detail elsewhere (Leventhal et al., 1995). Briefly, the responses of each cell to the different stimulus orientations and directions were represented as vectors. The sum of the vectors was divided by the sum of their absolute values. The angle of the resulting vector indicated the preferred orientation and direction of the cell. The length of the resulting vector (taking values between 0 and 1), termed the orientation bias (OB) or direction bias (DB), provided a quantitative measure of the orientation or direction sensitivity of the cell. Zero indicated that the neuron responded equally to all orientations (directions), and one meant that the neuron only responded to one stimulus orientation (direction). Previous studies in our laboratory have indicated that a bias of 0.1 is significant at the P < 0.005 level (Rayleigh test), and orientation biases of 0.1, 0.3 and 0.5 correspond to maximum to minimum response ratios of 1.5:1, 3.7:1 and 10.8:1, respectively (Leventhal et al., 1995).
The suppression index of each cell was calculated as the ratio of the maximal response in the CRFvar block to the minimal response in the CRFopt-IRFvar block. For the CRFvar block, the maximal response of the cell could be obtained from the orientation and direction tuning curve. For the CRFopt-IRFvar block, the response of the cell changed with the strength of surround suppression of the receptive field, which varied with the oriented IRF grating. Thus, the minimal response of the cell appeared when the strength of surround suppression was maximal.
Comparisons were statistically analyzed by chi-square tests and Mann-Whitney U tests where appropriate. A level of P < 0.05 was considered as significant and P < 0.01 as highly significant.
RESULTS
We studied a total of 81 neurons in young monkeys and 46 neurons in old monkeys. In most monkeys, the data were collected from three to five penetrations in each animal. Neurons studied in both age groups were recorded from the same range of cortical depths to avoid laminar bias. They also had similar eccentricities of the receptive fields (less than 8°). The lengths of penetration did not differ between the two groups, and there was no difference in the thickness of V1 in coronal sections.
The responses of cells in the CRFvar and the CRFvar-IRFopt blocks
Previously, we reported that the orientation and direction selectivity of V1 cells significantly decreased in old monkeys (Schmolesky et al., 2000, Leventhal et al 2003), and the current results confirmed this finding. When the CRFvar stimulus was presented, the percentages of V1 cells that were biased for orientation and direction (≥ 0.1 for both) were smaller for old monkeys (OB: 24%, 11 of 46; DB: 11%, 5 of 46) than for young monkeys (OB: 60%, 49 of 81, χ2 = 15.75, P < 0.001; DB: 72%, 58 of 81, χ2 = 43.29, P < 0.001; Chi-square tests) (Figs. 2 and 3).
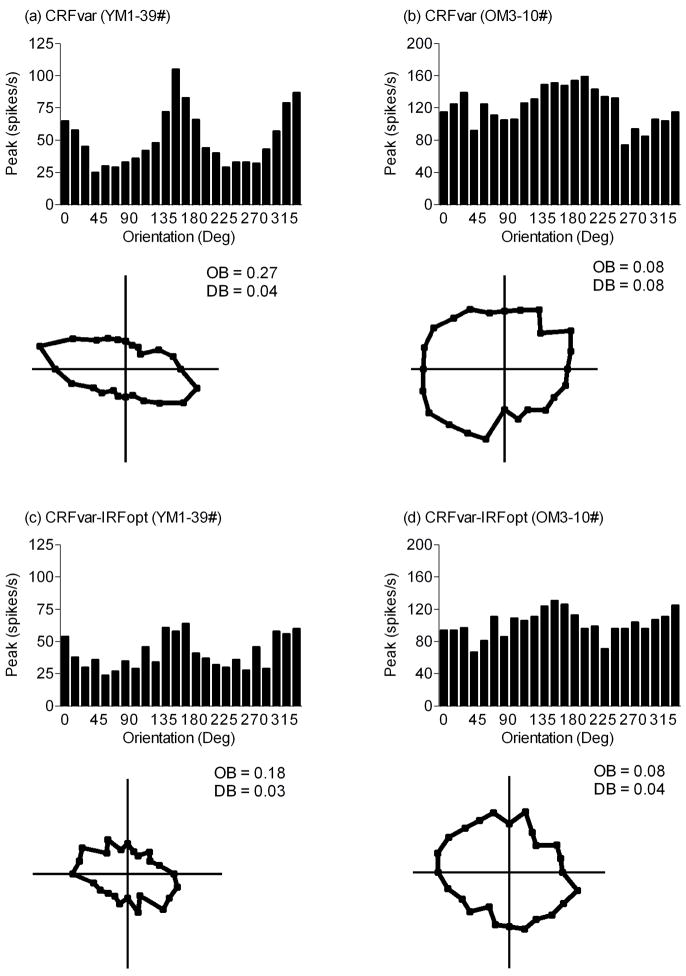
Figure 2.
Typical orientation tuning curves and corresponding polar plots of young (a, c) and old (b, d) monkey V1 cells. These responses were to the CRFvar stimulus (a, b) and the CRFvar-IRFopt stimulus (c, d). Each point in the polar plots represents the response to the stimulus moving in each of the 24 directions. The OB and DB of cells are shown.
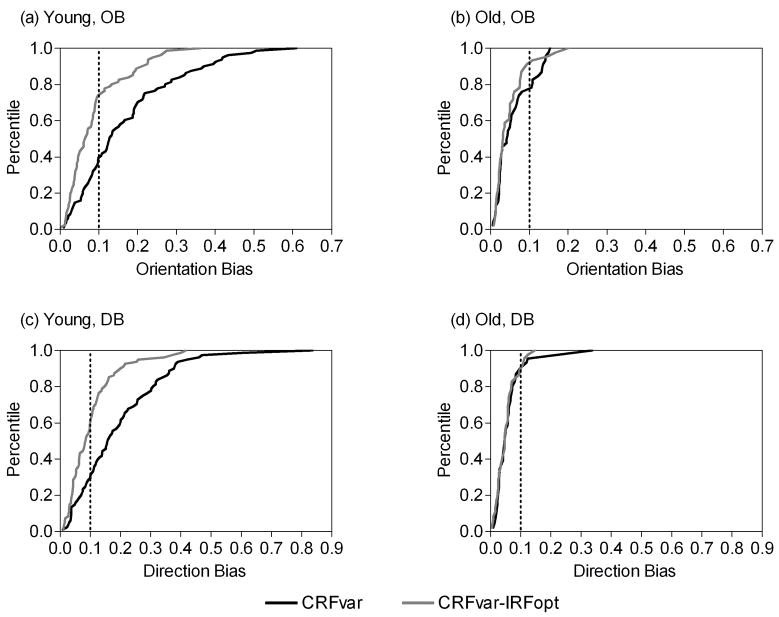
Figure 3.
Cumulative distribution plots showing OB and DB in response to the CRFvar and the CRFvar-IRFopt stimuli in young (a, c) and old (b, d) monkey V1 cells. The percentage of cells with any given OB or DB value is shown. The dotted line indicates an orientation bias or a direction bias of 0.1.
Compared with the CRFvar stimulus, presentation of the CRFvar-IRFopt stimulus significantly decreased the percentages of V1 cells that were biased for orientation and direction in young monkeys (OB: 26%, 21 of 81, χ = 19.72, P < 0.001; DB: 41%, 33 of 81, χ2 = 15.67, P < 0.001); however, only those cells that were biased for orientation decreased in old monkeys (OB: 9%, 4 of 46, χ2 = 3.90, P < 0.05) (Figs. 2 and 3). It is notable that there may be little significance in the statistics of these changes in DB in cells from old monkeys because of the low number of cells that were biased for direction in this study (n = 5).
Accordingly, the OB of more selective cells (cells with OB ≥ 0.1) were compared between the CRFvar and the CRFvar-IRFopt stimuli in either age group. In both groups, the OB was lower for the CRFvar-IRFopt stimulus than for the CRFvar stimulus (P < 0.001 and 0.01 for young and old groups, respectively; Mann-Whitney U test) (Table 1).
Table 1.
Descriptive statistics of OB and DB either in more selective cells (OB ≥ 0.1) or in all cells in response to the CRFvar and CRFvar-IRFopt stimuli in young and old monkeys.
| CRFvar |
CRFvar-IRFopt |
Mann-Whitney U test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SEM | n | Median | Mean | SEM | n | |||
| OB ≥ 0.1 | Young | 0.20 | 0.24 | 0.02 | 49 | 0.08 | 0.11 | 0.01 | 49 | P < 0.001 |
| Old | 0.13 | 0.13 | 0.01 | 11 | 0.06 | 0.07 | 0.02 | 11 | P < 0.01 | |
| OB (Total) | Young | 0.13 | 0.17 | 0.01 | 81 | 0.06 | 0.09 | 0.01 | 81 | P < 0.001 |
| Old | 0.04 | 0.06 | 0.01 | 46 | 0.03 | 0.05 | 0.01 | 46 | P = 0.36 | |
| DB (Total) | Young | 0.16 | 0.19 | 0.02 | 81 | 0.09 | 0.10 | 0.01 | 81 | P < 0.001 |
| Old | 0.05 | 0.06 | 0.01 | 46 | 0.05 | 0.05 | 0.00 | 46 | P = 0.65 | |
Moreover, the OB and DB of all cells were also compared between the two stimuli in the two groups. Both OB and DB were lower for the CRFvar-IRFopt stimulus than for the CRFvar stimulus in young monkeys (P < 0.001 for both) but not in old monkeys (P = 0.36 and 0.65 for OB and DB, respectively) (Table 1).
The responses of cells in the CRFvar and the CRFopt-IRFvar blocks
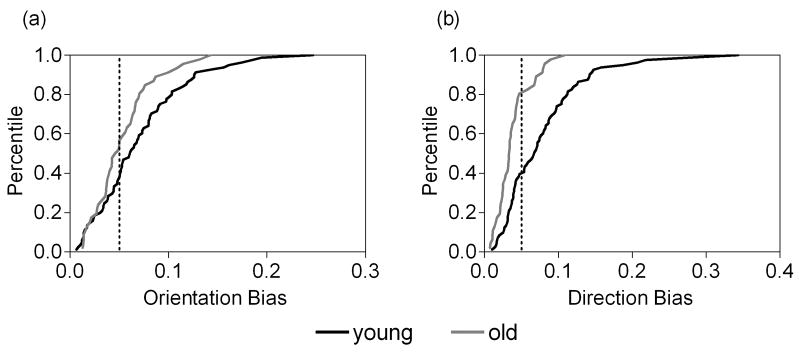
When the CRFopt-IRFvar stimulus was presented, the OB and DB of all cells were compared between the two groups. The OB was lower (P = 0.06), and the DB was significantly lower (P < 0.001) for old monkeys compared with young monkeys (Table 2). Meanwhile, when the OB and DB were greater than “0.05”, the percentages of cells in the old monkeys (OB: 43%, 20 of 46; DB: 20%, 9 of 46) were significantly lower than those in the young monkeys (OB: 63%, 51 of 81, χ2 = 4.52, P < 0.05; DB: 60%, 49 of 81, χ2 = 19.81, P < 0.001) (Figs. 4 and 5).
Table 2.
Descriptive statistics of OB and DB in response to the CRFopt-IRFvar stimulus in young and old monkeys.
| Young | Old | Mann-Whitney U test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SEM | n | Median | Mean | SEM | n | ||
| OB | 0.06 | 0.07 | 0.01 | 81 | 0.05 | 0.05 | 0.00 | 46 | P = 0.06 |
| DB | 0.07 | 0.08 | 0.01 | 81 | 0.03 | 0.04 | 0.00 | 46 | P < 0.001 |
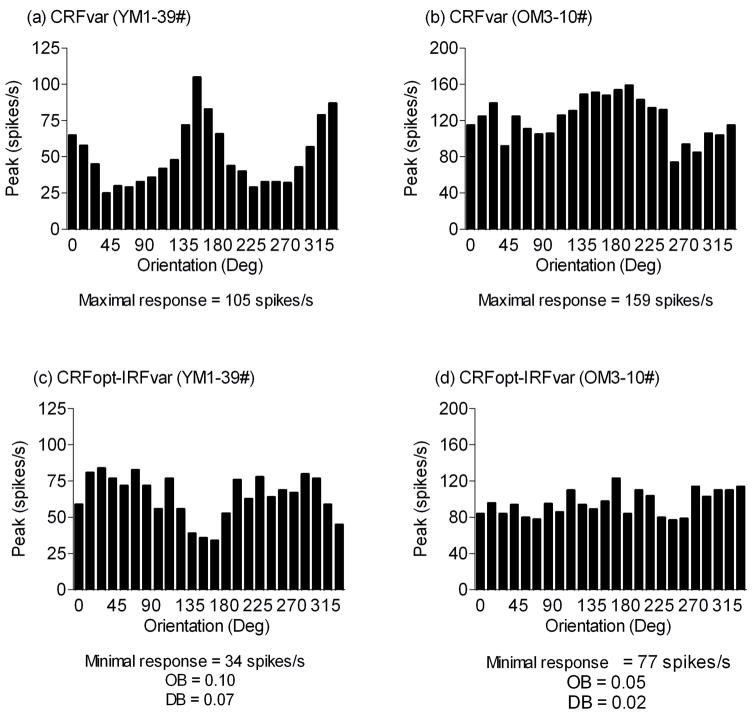
Figure 4.
Representative tuning curves obtained from young (a, c) and old (b, d) monkey cells. The presented responses were to the CRFvar (a, b) or CRFopt-IRFvar (c, d) stimulus. The maximal response, the minimal response, the OB or the DB of the cell is shown.
Figure 5.
Cumulative distribution plots showing OB and DB in response to the CRFopt-IRFvar stimulus in young (a) and old (b) monkey V1 cells. The percentage of cells with any given OB or DB value is shown. The dotted line indicates an orientation bias or a direction bias of 0.05.
The suppression index, which was defined as the ratio of the maximal response in the CRFvar block to the minimal response in the CRFopt-IRFvar block, was calculated for each cell and compared between the two groups. The suppression index of broadly tuned cells (cells with OB < 0.1) was significantly lower for the old monkeys compared with the young monkeys (P < 0.01); however, the suppression index was not different between groups when we examined more selective cells (P = 0.57) (Table 3). The percentage of cells was measured in both age groups when the suppression index was greater than “3”. It did not show any significant difference between young (59%, 29 of 49) and old monkeys (64%, 7 of 11, χ2 = 0.07, P = 0.79) when we examined more selective cells. Broadly tuned cells, however, exhibited a significantly lower percentage for old monkeys (20%, 7 of 35) compared with young monkeys (47%, 15 of 32, χ2 = 5.48, P < 0.05) (Fig. 6).
Table 3.
Descriptive statistics of the suppression index of more selective cells (OB ≥ 0.1) or broadly tuned cells (OB < 0.1) in young and old monkeys.
| Young | Old | Mann-Whitney U test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SEM | n | Median | Mean | SEM | n | ||
| OB ≥ 0.1 | 3.43 | 6.79 | 1.46 | 49 | 3.47 | 8.40 | 3.75 | 11 | P = 0.57 |
| OB < 0.1 | 2.87 | 3.60 | 0.32 | 32 | 2.06 | 2.22 | 0.14 | 35 | P < 0.01 |
Figure 6.
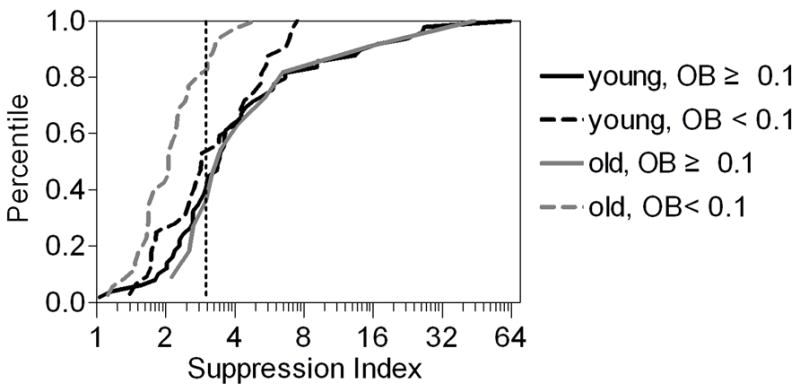
Cumulative distribution plots showing the suppression indices in more selective cells (OB ≥ 0.1) and broadly tuned cells (OB < 0.1) in young and old monkeys. The suppression index was calculated as the ratio of the maximal response of the CRFvar stimulus to the minimal response of the CRFopt-IRFvar stimulus. The x-axis is on a log (base 2) scale, and the dotted line indicates a suppression index of 3.
DISCUSSION
In this study, we investigated the effects of aging on the strength of surround suppression of the receptive field of V1 cells in monkeys. Three kinds of visual stimuli were used: the 1st was the oriented central stimulus; the 2nd was the oriented central stimulus together with the surround stimulus in the optimal orientation and direction; and the 3rd was the central stimulus in the optimal orientation and direction together with the oriented surround stimulus. When the 1st stimulus was used, cells in old monkeys showed significantly lower orientation and direction biases than those in young monkeys. Compared with the 1st stimulus, the 2nd stimulus exhibited significantly reduced orientation and direction biases in the cells of young monkeys, but no reductions were found in old monkeys. However, more selective cells in old monkeys still showed reduced orientation biases, similar to those in the young monkeys. When the 3rd stimulus was used, cells in old animals showed reduced orientation and direction biases compared with young animals. Moreover, the suppression index was calculated as the ratio of the maximal response of the cell when the 1st stimulus was offered to the minimal response of the cell when the 3rd stimulus was offered. In old monkeys, broadly tuned cells exhibited significantly lower suppression indices than those in young monkeys. Our data indicate that the strength of surround inhibition of the receptive field of V1 cells decreases with normal aging.
The primary finding of this study was that broadly tuned cells in old monkeys exhibited significantly reduced suppression indices that quantified the strength of the surround suppression of the receptive field. In previous and current data, the orientation selectivity of V1 cells decreased during senescence, which resulted in an increased percentage of broadly tuned cells (Schmolesky et al., 2000, Leventhal et al 2003). Thus, the age-related degradation of orientation selectivity of V1 cells could be linked to the reduced strength of surround suppression in senescent brain.
The surround suppression of visual cells is thought to be mediated by inhibitory interneurons (Angelucci and Bullier, 2003; Bair et al., 2003; Lund et al., 1995). Indeed, morphology studies have observed a selective decline in presumptive inhibitory synapses in the sensorimotor cortex of aged rats (Brunso-Bechtold et al., 2000). Moreover, electrophysiological findings by Dustman et al. have suggested that there is weakened inhibition in elderly people (Dustman and Snyder, 1981; Dustman et al., 1981; Dustman et al., 1985; Dustman et al., 1996). For example, evoked potential studies revealed that the inhibitory activity of neurons was negatively affected in the visual cortex as well as some other sensory cortices of humans (Dustman et al., 1996). Thus, our finding of a degenerated strength of surround suppression during senescence is in accord with these previous studies.
Additionally, the age-related degradation of the surround suppression may be due to degeneration and/or dysfunction in the GABAergic system. In a previous study, the ability to produce GABA was suggested to decrease in the visual cortices of old monkeys (Leventhal et al., 2003). Studies on GABAA receptors in aged rats have demonstrated that its subunit mRNA levels are altered (Mhatre and Ticku, 1992) and the combination of its subunits reduced (Gutierrez et al., 1994). Moreover, levels of glutamic acid decarboxylase, an enzyme needed to synthesize GABA, have been shown to decrease with age (Gutierrez et al., 1994). Furthermore, old observers performed better than did younger observers in a motion discrimination task, which prompted the authors to suggest that the reduced GABAergic function in the senescent brain might contribute to the age-related changes in motion discrimination (Betts et al., 2005).
Taken together, the previous results indicate that reduced surround suppression, such as GABA-mediated inhibition in V1, might be a reasonable explanation for the decline of visual functions during normal aging. Nevertheless, according to Webb et al. (2005) and Petrov and McKee (2009), the suppressive signal in V1 is a combination of broadly tuned cortical inhibition and untuned pre-cortical input. Therefore, we could not exclude the possibility that age-related changes in the suppressive signal in V1 may be derived from changes in the pre-feedback mechanism from lateral geniculate nucleus or the post-feedback mechanism from the higher visual cortical regions during normal aging.
Additionally, when the classical receptive field of cells was stimulated by a drifting grating in the optimal orientation and direction while the inhibitory receptive field was stimulated by an annular drifting grating in randomly oriented orientation and direction, the lower OB and DB were observed in the old monkeys compared with the young monkeys. Thus, normal aging may decrease the stimulus selectivity of the surround of the receptive field of cells to induce suppression.
Another finding of this study was that more selective cells in old animals exhibited reduced orientation selectivity, similar to those in young animals, when the optimal surround suppression was presented. In addition, these old cells had a high suppression index comparable to the cells from young monkeys. This may indicate that not all V1 cells display markedly changed properties of the receptive field, such as orientation selectivity, during senescence. As a consequence, we hypothesized that aging is suggestive of a possible cumulative effect on animal cells over time. Conversely, this finding may raise an intriguing question as to why the age difference in the strength of surround suppression is only represented in broadly tuned cells rather than more selective cells. The question may contradict the working hypothesis that GABAergic inhibition is compromised in old animals. However, we think that the ratio of more selective cells to total cells in the old animals in this study was small. Most of the cells (76%, 35/46) still exhibited a reduced suppression index. Thus, the decreased strength of surround suppression may be a possible reason for the reduced stimulus selectivity with age, but the question requires further investigations.
The finding that most of the cells in old monkeys had reduced surround suppression indices could be explained by each cell’s response variability. If the decrease in the response variability of cells was greater in old monkeys compared with young monkeys, the suppression indices based on our calculation might be lower for old monkeys. Our previous data, however, suggest that the increased response variability has already occurred during an early stage of aging (Yang et al., 2009).
In conclusion, our results demonstrate that the strength of surround suppression of the receptive field of V1 cells decreases during normal aging. These findings also contribute to our understanding of the neural mechanisms underlying the declines in orientation and direction sensitivity and perception of aged humans.
Acknowledgments
This study was supported by grants from NIH/NIA R01 AG 17922, National Science Foundation of China (NSFC 30470553, 30530270, 30670669 and 30770700), 973 program (2005CB522803CB522007CB947703), 863 program (O7013810, 2006AA02A116), the Major State Basic Research of China (NO2003CB716600), Chinese-Finnish International Collaboration Project-neuro (30621130076), Program of CASC (KSCX1-YW-R-33, YZ200737, KSCX2-YW-R-261), National Key Technologies R&D Program and Yunnan Science and Technique Program (2006PT08-2), the research foundation of Yunnan University (2008YB007), the Science Foundation of Education Department of Yunnan (09Y0034), and the special fund of the “211” third phase project of Yunnan University (21134018). We thank Shan Yu, Xiangrui Li, Pinglei Bao, Xiang Ye, Yun Yang and Guangxun Li for their assistance with the experiments. We are also thankful for the very helpful comments of the anonymous reviewers.
Abbreviations
- V1
primary visual cortex
- OB
orientation bias
- DB
direction bias
- CRF
classical receptive field
- IRF
inhibitory receptive field
- GABA
gamma-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akasaki T, Sato H, Yoshimura Y, Ozeki H, Shimegi S. Suppressive effects of receptive field surround on neuronal activity in the cat primary visual cortex. Neurosci Res. 2002;43:207–220. doi: 10.1016/s0168-0102(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Walton EJS, Hupe JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp Brain Res. 1972;15:439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Linville MC, Sonntag WE. Age-related synaptic changes in sensorimotor cortex of the Brown Norway X fischer 344 rat. Brain Res. 2000;872:125–133. doi: 10.1016/s0006-8993(00)02515-4. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat’s primary visual cortex. J Neurophysiol. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996;30:109–126. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- Dustman RE, LaMarche JA, Cohn NB, Shearer DE, Talone JM. Power spectral analysis and cortical coupling of EEG for young and old normal adults. Neurobiol Aging. 1985;6:193–198. doi: 10.1016/0197-4580(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Snyder EW. Life-span change in visually evoked potentials at central scalp. Neurobiol Aging. 1981;2:303–308. doi: 10.1016/0197-4580(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Snyder EW, Schlehuber CJ. Life-span alterations in visually evoked potentials and inhibitory function. Neurobiol Aging. 1981;2:187–192. doi: 10.1016/0197-4580(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Fries W, Albus K, Creutzfeldt OD. Effects of interacting visual patterns on single cell responses in cats striate cortex. Vision Res. 1977;17:1001–1008. doi: 10.1016/0042-6989(77)90002-5. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, Morris SJ, De Blas AL. Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci. 1994;14:7469–7477. doi: 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27:155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Jones HE, Grieve KL, Wang W, Sillito AM. Surround suppression in primate V1. J Neurophysiol. 2001;86:2011–2028. doi: 10.1152/jn.2001.86.4.2011. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, van E. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J Neurophysiol. 1992;67:961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Thompson KG, Liu D, Zhou Y, Ault SJ. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J Neurosci. 1995;15:1808–1818. doi: 10.1523/JNEUROSCI.15-03-01808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature. 1997;387:73–76. doi: 10.1038/387073a0. [DOI] [PubMed] [Google Scholar]
- Li CY, Li W. Extensive integration field beyond the classical receptive field of cat’s striate cortical neurons--classification and tuning properties. Vision Res. 1994;34:2337–2355. doi: 10.1016/0042-6989(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Lund JS, Wu Q, Hadingham PT, Levitt JB. Cells and circuits contributing to functional properties in area V1 of macaque monkey cerebral cortex: bases for neuroanatomically realistic models. J Anat. 1995;187:563–581. [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res. 1976;16:1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Aging related alterations in GABAA receptor subunit mRNA levels in Fischer rats. Brain Res Mol Brain Res. 1992;14:71–78. doi: 10.1016/0169-328x(92)90012-z. [DOI] [PubMed] [Google Scholar]
- Nelson JI, Frost BJ. Orientation-selective inhibition from beyond the classic visual receptive field. Brain Res. 1978;139:359–365. doi: 10.1016/0006-8993(78)90937-x. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Petrov Y, McKee SP. The time course of contrast masking reveals two distinct mechanisms of human. J Vis. 2009;9:21, 1–11. doi: 10.1167/9.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Hawken MJ, Shapley RM. Visual spatial characterization of macaque V1 neurons. J Neurophysiol. 2001;85:1873–1887. doi: 10.1152/jn.2001.85.5.1873. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Sen A, Blakemore C. Characteristics of surround inhibition in cat area 17. Exp Brain Res. 1997;116:216–228. doi: 10.1007/pl00005751. [DOI] [PubMed] [Google Scholar]
- Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 2003;38:689–699. doi: 10.1016/s0896-6273(03)00332-5. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Grieve KL, Jones HE, Cudeiro J, Davis J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature. 1995;378:492–496. doi: 10.1038/378492a0. [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Res. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Asymmetric suppression outside the classical receptive field of the visual cortex. J Neurosci. 1999;19:10536–10553. doi: 10.1523/JNEUROSCI.19-23-10536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Suppression outside the classical cortical receptive field. Vis Neurosci. 2000;17:369–379. doi: 10.1017/s0952523800173055. [DOI] [PubMed] [Google Scholar]
- Wang H, Xie X, Li X, Chen B, Zhou Y. Functional degradation of visual cortical cells in aged rats. Brain Res. 2006;1122:93–98. doi: 10.1016/j.brainres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cereb Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. J Neurosci. 2005;25:11666–11675. doi: 10.1523/JNEUROSCI.3414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Li G, Wang Y, Zhou Y. Aging affects response variability of V1 and MT neurons in rhesus monkeys. Brain Res. 2009;1274:21–27. doi: 10.1016/j.brainres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang XS, Fu Y, Zhang J, Ma YY, Wang YC, Zhou YF. Effects of age on latency and variability of visual response in monkeys. Chinese Sci Bull. 2005;50:1163–1165. [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Wang Y, Fu Y, Liang Z, Ma Y, Leventhal AG. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys. Eur J Neurosci. 2008;28:201–207. doi: 10.1111/j.1460-9568.2008.06300.x. [DOI] [PubMed] [Google Scholar]