Abstract
This review deals with individual components regulating the neural control of the urinary bladder. This article will focus on factors and processes involved in the two modes of operation of the bladder: storage and elimination. Topics included in this review include: (1) The urothelium and its roles in sensor and transducer functions including interactions with other cell types within the bladder wall (“sensory web”), (2) The location and properties of bladder afferents including factors involved in regulating afferent sensitization, (3) The neural control of the pelvic floor muscle and pharmacology of urethral and anal sphincters (focusing on monoamine pathways), (4) Efferent pathways to the urinary bladder, and (5) Abnormalities in bladder function including mechanisms underlying comorbid disorders associated with bladder pain syndrome and incontinence.
Keywords: pelvic floor, sensory afferents, urothelium
THE UROTHELIUM
There is evidence that a number of functional pain syndromes are associated with changes in the epithelial layer. Alterations of bladder urothelium at the molecular and structural levels have been reported in both patients and animals modeled for various bladder disorders. Many therapies currently used in the treatment of bladder disease may actually target urothelial receptors and/or their release mechanisms.
Anatomy and Barrier Function of the Urothelium and Response to Injury
The urothelium is the epithelial lining of the lower urinary tract between the renal pelvis and the urinary bladder. Urothelium is composed of at least three layers: a basal cell layer attached to a basement membrane, an intermediate layer, and a superficial or apical layer composed of large hexagonal cells (diameters of 25–250 μm) known as “umbrella cells.”1,2 The umbrella cells are interconnected by tight junctions (which are composed of multiple proteins such as the claudins) and are covered on their apical surface (nearly 70–80%) by crystalline proteins called uroplakins that assemble into hexagonal plaques.3-6 Uroplakins and other urothelial cellular differentiation markers, such as cytokeratin 20, are not expressed in the stratified epithelium of the urethra. In some species, the umbrella cells and perhaps also the intermediate cells have projections to the basement membrane.1
The ability of the bladder to maintain the barrier function, despite large alterations in urine volume and pressure during bladder filling and emptying, is dependent on several features of the umbrella cell layer. These features include tight junction complexes that reduce the movement of ions and solutes between cells and specialized lipid molecules and uroplakin proteins in the apical membrane, which reduce the permeability of the cells to small molecules (water, urea, and protons).1,7 The apical surface of the urothelium is also covered with a sulfated polysaccharide glycosaminoglycan (GAG) or mucin layer that is thought to act as a nonspecific anti-adherence factor and as a defense mechanism against infection.8-10 In addition, during bladder filling the umbrella cells become flat and squamous and this shape change is accompanied by vesicular trafficking (i.e., exocytosis/endocytosis) that adds membrane to the apical surface thereby increasing overall urinary bladder surface area.3,11,12 There is evidence that this stretch-induced exocytosis is dependent on activation of epidermal growth factor receptor (EGFR).13,14 These processes allow the bladder to accommodate increasing volumes of urine during filling without compromising the barrier function. Exocytosis/endocytosis (vesicular recycling) may also play an important role in modulating the release of a number of neurotransmitters/mediators as well as regulation of the function of many receptors and ion channels in urothelial cells.15,16
Though the urothelium maintains a tight barrier to ion and solute flux, a number of local factors such as tissue pH, mechanical or chemical trauma, or bacterial infection can modulate the barrier function of the urothelium.3,17 When the barrier is compromised, water, urea, and toxic substances can pass into the underlying tissue (neural/muscle layers) resulting in urgency, frequency, and pain during bladder filling and voiding. Disruption of urothelial barrier integrity has also been linked to the expression of substances such as antiproliferative factor (APF), which also slows urothelial cell growth.18-20 APF, a frizzled 8 protein detected in the urine of patients with bladder pain syndrome/interstitial cystitis (BPS/IC), is secreted by bladder epithelial cells obtained from these patients. Treatment of urothelial cells from normal patients with purified APF decreases the expression of adhesion and tight junction proteins. Other types of urothelial–neural interactions are also likely, based on the recent reports that various stimuli induce urothelial cells to release chemical mediators that can in turn modulate the activity of afferent nerves.1,16 This has raised the possibility that the urothelium may have a role in sensory mechanisms in the urinary tract.
Roles for Urothelial Cells in Visceral Sensation
While urothelial cells are often viewed as bystanders in the process of visceral sensation, recent evidence has supported the view that these cells function as primary transducers of some physical and chemical stimuli and are able to communicate with underlying cells including bladder nerves, smooth muscle, myofibroblasts, and inflammatory cells (Fig. 1).
Fig. 1.
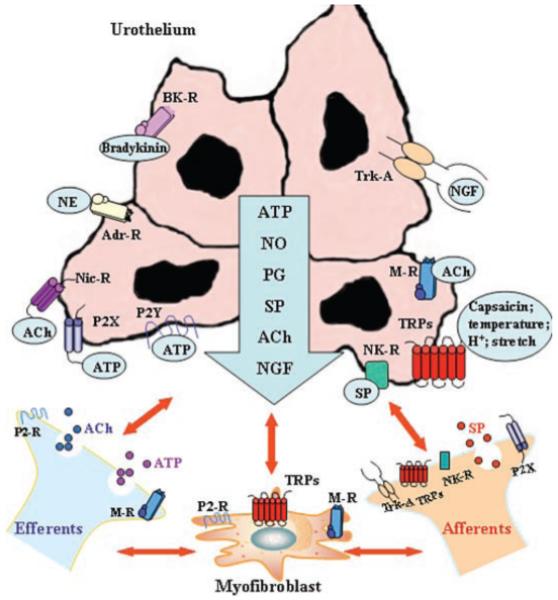
Hypothetical model depicting possible interactions between bladder afferent and efferent nerves, urothelial cells, smooth muscle, and myofibroblasts. Stimulation of urothelial receptors and channels can release mediators that target bladder nerves and other cell types; urothelial cells can also be targets for neurotransmitters released from nerves or other cell types. Urothelial cells can be activated by either autocrine (i.e., autoregulation) or paracrine (release from nearby nerves or other cells) mechanisms. Abbreviations: ACh, acetylcholine; AdR, adrenergic receptor; BR, bradykinin receptor; H+, proton; MR, muscarinic receptor; NE, norepinephrine; NGF, nerve growth factor; NR, neurokinin receptor; NicR, nicotinic receptor; NO, nitric oxide; P2R, purinergic 2 receptor unidentified subtype; P2X and P2Y, purinergic receptors; PG, prostaglandin; SP, substance P; Trk-A, receptor tyrosine kinase A, high affinity receptor for nerve growth factor; TRPs, transient potential channels.
There are at least three lines of evidence that suggest that urothelial cells participate in the detection of both physical and chemical stimuli. First, bladder nerves (afferent and efferent) are localized in close proximity, and some within, the urothelium.16,21-23 In addition, a network of cells with morphologic characteristics similar to those of myofibroblasts or interstitial cells is also detected in the suburothelial space of the bladder in both humans and animals.24-26 These cells, which are extensively linked by gap junctions and have close contacts with nerves, can respond to neurotransmitters, such as ATP released from nerves or urothelial cells, suggesting that they could act as intermediaries in urothelial–nerve interactions.25-27
A second line of evidence suggesting that urothelial cells play a role in sensory function is the expression of numerous receptors/ion channels similar to those found in both nociceptive and mechanoreceptive afferent nerves. Examples of neuronal “sensor molecules” (receptors/ion channels) that have been identified in urothelium include receptors for purines (P2X1–7 and P2Y1,2,4) adenosine (A1, A2a, A2b, and A3), norepinephrine (α and β), acetylcholine (muscarinic and nicotinic), protease-activated receptors (PARs), amiloride and mechanosensitive epithelial sodium channels (ENaC), bradykinin (B1 and B2), neurotrophins (p75, trkA, epidermal growth factor (EGF) family ErbB1-3), corticotrophin releasing factor (CRF), estrogens (Erα and ERβ), endothelins, and various TRP channels (TRPV1, TRPV2, TRPV4, TRPM8, and TRPA1).21,28-36 The expression of various receptors enables the urothelium to respond to a number of “sensory inputs” from a variety of sources. These inputs include increased stretch during bladder filling, soluble factors (many found in the urine) such as EGF or chemical mediators/peptides/transmitters such as substance P (SP), calcitonin gene-related peptide (CGRP), CRF, acetylcholine, adenosine, or norepinephrine released from nerves, inflammatory cells, and even blood vessels.1,15,16,37,38
And finally, these cells secrete a number of transmitters or mediators capable of altering the excitability of sensory neurons including neurotrophins, peptides, ATP, acetylcholine, prostaglandins, prostacyclin, nitric oxide (NO), and cytokines.15,16 For example, urothelial-derived NO can be released in response to mechanical as well as chemical stimulation and may inhibit the activity of bladder afferent nerves.16,39 Release of various factors from the urothelium can also modulate the spontaneous activity of the underlying smooth muscle.27,40
The mechanisms underlying release of chemical mediators from the urothelium, for example, whether all sensory “inputs” stimulate membrane turnover (i.e., vesicular exocytosis) is not well understood. What little is known about the roles and dynamics of membrane-bound cytoplasmic vesicles in urothelial cell physiology is derived from measurements of membrane capacitance and microscopy of fixed tissues and cells. There is evidence that once released, ATP can act as an important autocrine mediator, which can induce membrane turnover as well as enhance both stretch-induced exocytosis and endocytosis.41 Alterations in membrane turnover can not only increase apical surface area but also regulate the number and function of receptors and channels at the cell surface.
Clinical Significance of the Sensory Web
Defects in urothelial sensor molecules and urothelial-cell signaling are likely to contribute to the pathophysiology of bladder diseases. For example, a number of bladder conditions (BPS/IC, spinal cord injury (SCI), chemically induced cystitis) are associated with augmented release of urothelial-derived ATP, which is likely to result in altered sensations or changes in bladder reflexes induced by excitation of purinergic receptors on nearby sensory fibers.5,6,32 ATP can also act in an autocrine manner that would facilitate its own release from urothelial cells.6 Once released, ATP can alter the threshold for activation of ion channels such as TRPV1. This novel mechanism, which likely reflects activation of intracellular protein kinases and phosphorylation of the TRPV1 channel, represents a means by which large amounts of ATP released from damaged or sensitized cells, in response to injury or inflammation, could trigger the sensation of pain. Changes in epithelial signaling/barrier function would not be unique to the urinary bladder because airway epithelia in asthmatic patients as well as keratinocytes in certain types of skin diseases also exhibit a number of similar abnormalities and compromised repair processes.42-44 This is particularly relevant given the high incidence of diseases that include both visceral and somatic dysfunctions, many of which exhibit a decrease in epithelial barrier properties. Taken together, epithelial cells can respond to a number of challenges (including environmental pollutants and mediators released from nerves or nearby inflammatory cells) resulting in altered expression and/or sensitivity of various receptor/channels as well as changes in release of mediators, all of which could impact function.
AFFERENT NEURONS
Properties of Bladder Afferent Neurons
Afferent axons in the pelvic, hypogastric (lumbar splanchnic), and pudendal nerves transmit information from the lower urinary tract to the lumbosacral spinal cord45-47 and studies in several species including cats, rats, and mice have shown some similarities in properties.
The most sensitive afferents are excited by a physiological increase in volume and by detrusor contractions. It is believed that these low threshold afferents have small myelinated axons (A-delta fibers which are larger in diameter and conduct action potentials more rapidly than C-fibers) and that their endings are located in the detrusor smooth muscle. Neurons with this range of conduction velocities are less likely to contain peptides. They have been called “in series tension receptors”48 because they are excited by bladder wall tension caused either by distension or by contraction.
There have been a number of systematic studies recently in mice and guinea pigs that have provided detailed information about the classes of receptors in the pelvic and hypogastric nerves. In the mouse, there are at least four classes of mechanosensitive afferents, which include myelinated and unmyelinated fibers that are distributed in the serosa, muscle, and urothelial layers of the organ. One class has mechanosensitive endings in both the muscle and urothelial layers. The lumbar splanchnic nerves contain principally serosal and muscular afferents whereas all four classes of afferents are present in the pelvic nerve (63% of which are muscular afferents).49 The small myelinated afferents are involved in two processes: (a) sensing bladder volume and (b) reinforcing reflex function by monitoring the contractile state of the detrusor. In particular these afferents, which form the most sensitive distension receptors, are most probably responsible for the sensation of fullness and mediate the normal micturition reflex that involves a spinobulbospinal pathway that passes through the brainstem.
The unmyelinated afferents contain peptides and most appear to terminate within the lamina propria and within the transitional epithelium itself. Many of these afferents discharge within the higher range of physiological bladder volumes and are not usually sensitive to detrusor contraction, possibly because only the former causes stretch of the urinary epithelium. The C-fibers in the urothelium and lamina propria contain peptides such as SP and CGRP, which is a characteristic of one subgroup of afferent C-fibers. These and other C-fiber afferents may mediate the spinal C-fiber micturition reflex seen following cord transection in the cat.50 It is not clear whether they also contribute to normal voiding in this species, but there is increasing evidence that they may be involved in normal bladder control in rats and mice.
Another group of unmyelinated bladder afferent axons does not respond to normal distending volumes but only become active during chemical irritation of the bladder, including high osmolality and high potassium solutions and during inflammation, when they behave like the high volume sensing C-fibers. These have been demonstrated in cats and rats, and are usually called “silent afferents” (meaning that they do not respond to normal distensions, but can become mechanosensitive in inflamed or over-distended tissues). Thus it would be unwise to infer function simply on the basis of conduction velocity. This group of afferents also appears to be sensitive to ATP.
Ultrastructural studies of nerves in the human bladder have found only unmyelinated nerves in the urothelial and immediate suburothelial layer. The small myelinated nerves appear only close to the smooth muscle layers.51 Whether or not the suburothelial nerves become myelinated as they pass toward the serosal surface cannot be ascertained from this study but it would be inadvisable to make deductions about the relative number of C and A-delta fibers in the human based on these observations. Table I shows the properties of afferent fibers classified according to their volume thresholds.
TABLE I.
Properties of afferents classed according to volume thresholds
| Sensitivity to distension (cat and rat) |
Natural stimuli | Conduction velocity in cat and rat |
Peptides | Experimental stimuli |
Species | Inflammatory mediators |
P2X3 agonist | Role of TRPV1 | Pathway |
|---|---|---|---|---|---|---|---|---|---|
| Low threshold (LT) | Distension and contraction |
Mainly A-delta (finely myelinated) |
Peptides are relatively sparse |
Distension (D), probing (P), tension (T), stroke (S), chemical (C) |
Cat (D), rat (D,C), mouse (P,T,S,C), guinea pig (P,T,S,C) |
Sensitization (M,R) | Increased firing rate | Normal bladder filling |
Hypogastric pelvic |
| High threshold (HT) |
Distension; some also respond to contraction |
Mainly C (unmyelinated) |
Many peptides | Distension (D), probing (P), tension (T), stroke (S), chemical (C) |
Cat (D), rat (D), mouse (P,T,S,C), guinea pig (P,T,S) |
Sensitization | Increased firing rate, lower threshold |
Nociception | Hypogastric nerve |
| Silent (including nociceptive) (SIL) |
Insensitive to distension unless inflamed |
Mainly C (unmyelinated) |
Many peptides | Distension (D) | Cat (D), rat (D), guinea pig (S,C) |
Appearance of mechanosensitivity (R) |
Appearance of mechanosensitivity |
Nociception | Pelvic |
Urothelial Afferents
The plexus of afferent nerves is thickest in the neck of the bladder and in the initial portion of the urethra, and it becomes progressively less dense in the adjacent regions. It does not extend beyond the equatorial region, and therefore the lamina propria of the cranial region of the bladder has no afferent axons. In contrast, the afferent innervation of the musculature is more diffuse, and appears uniform throughout the bladder. CGRP-immunofluorescence in urothelial afferent axons is enhanced in the surviving axons 5 days after contralateral denervation, a change which may be an early sign of regeneration of these axons.52 In the human bladder, CGRP together with SP and NKA occur only infrequently in nerves in the muscle but are moderately frequent in the suburothelial layer. Also in the human there appears to be another population of CGRP-containing fibers that colocalize with neuropeptide Y (NPY) and galanin (Gal) and some of these synapse on intramural ganglia within the bladder.53-56 There is also recent evidence that nerves cross the basal lamina and enter the basal layers of the human urothelium.52
Sensitivity of Afferent Endings
The term afferent sensitivity refers to the gain of the afferent signal, i.e., the number of impulses that are fired by an afferent ending at any level of distension. Sensitizing mediators are able to increase the size of the sensory signal (the frequency of impulse traffic) at a given level of distension, so the sensations that occur at a particular rate of firing in an afferent occur at lower bladder volumes if the afferent endings have been sensitized.
The sensitivity of afferent endings may be influenced by the release of mediators from different cell types, including possibly the urothelium, myofibroblasts, nerve endings, smooth muscle, mast cells, and other connective tissue cells. It is likely that many or all of these can release ATP, and some may release other mediators including NO, tachykinins (SP, neurokinin A, neurokinin B), growth factors (nerve growth factor [NGF], brain-derived-neurotrophic factor [BDNF], and others) and other endogenous mediators such as nociceptin. The similarity of the properties of the urothelial cells and the C-fiber afferents suggests that the most likely contender for a sensory cell may be a urothelial cell, but it is clear that the afferent endings themselves respond to a variety of stimuli, and that surrounding cells may simply enhance the gain of the transducer.
NGF and TTX-Resistant Na+ Channels
Sensitization of afferents appears to be an important mechanism that leads to reflex hyperexcitability. A number of studies have linked the tetrodotoxin (TTX) resistant sodium channel, sometimes known as Nav1.8 to this process. Sensitizing agents including NGF are known to induce increased expression of this membrane channel; and this appears to be sufficient to change the properties of afferents to lower the threshold for firing and in turn lower the volume threshold for reflex voiding and induce overactive bladder contractions and urgency.57 TTX-resistant Na+ channels (NaV1.8 and NaV1.9) are expressed in SP/CGRP immunoreactive small, C-fiber DRG neurons supplying the bladder.58,59 These neurons also express the trkA receptor, which binds NGF and is necessary for its action. Plasticity of Na+ channels occurs in these neurons after SCI, including an increase in TTX-sensitive Na+ currents, a decrease in TTX-resistant Na+ currents, decreased expression of NaV1.8 channel immunoreactivity, and a small increase in Na 1.9 channel immunoreactivity.60,61
The dependence of bladder afferent neuron sensitization on NGF and on changes in NaV1.8 channel has been shown in experiments using immunoneutralization of NGF or anti-sense oligonucleotide treatment to reduce the expression of these channels in sensory neurons.58,62 More recently in studies of ralfinamide, a drug that interferes with TTX-resistant sodium channels, indicate that this drug reduces inflammatory and neuropathic pain as well as bladder overactivity in rats. The ability of ralfinamide to reduce capsaicin-induced hyperexcitability and tonic activity of rat afferent neurons appears to be due to its action as a sodium channel antagonist.63
In clinical studies the local anesthetic lidocaine and the oral Na+ channel blocker, mexiletine, which operate by reducing excitability in sensitized neurons have been used to treat urge incontinence and hyper-reflexic conditions64-69 with variable degrees of success.
Afferents From the Urethra, Bowel, and Genital Organs
The micturition reflex can be influenced by activation of other sacral afferent pathways,70 including those innervating the urethra, urethral sphincter, colon–rectum, anal canal, and reproductive organs. Facilitatory effects can be elicited in response to electrical stimulation of urethral afferents or by fluid flowing through the urethra; however, contraction of the urethral sphincter reflexly inhibits bladder motility.71 Electrical stimulation of urethral afferent fibers when the bladder is full can evoke strong detrusor contractions sufficient for voiding in spinal intact cats72,73 as well as acute spinalized cats.74 Similarly, using minimally invasive methods to apply electrical stimulation within the proximal urethra via a catheter-mounted electrode, it has been shown that reflex bladder contractions can be generated in humans with complete paraplegia but these do not seem to produce efficient voiding. In chronic SCI cats voiding can be induced by 30–40 Hz electrical stimulation of afferents in the pudendal nerve.75 This type of voiding can be enhanced by blocking the efferent pathways to the external urethral sphincter using very high frequency electrical stimulation of the pudendal nerve. On the other hand low frequency electrical stimulation of the pudendal nerve increases bladder capacity and inhibits reflex micturition. Inhibitory effects are also elicited in response to stimulation of the dorsal nerve of the clitoris.76
Excitability of spinal neurons receiving afferent input from the bladder can also be modulated by input from other pelvic structures such as the colon.77-79 This convergence of sensory information from a number of pelvic organs can occur at the level of the spinal cord. In addition, the expansion of primary axon terminals within the spinal cord can also play a role in altering bladder reflexes.
Bladder afferent neurons contain a number of peptidergic neurotransmitters, and the central distribution of bladder afferent terminals and peptidergic immunoreactive fibers is quite similar. Expression of a number of peptides including CGRP, vasoactive intestinal polypeptide (VIP) as well as pituitary adenylate cyclase activating peptide (PACAP) is altered in primary afferent terminals and may correlate with changes in bladder function following SCI.80-82 There has been considerable interest in the role of tachykinins in the micturition reflex83 and in nociception. Intrathecal treatment of adult rats with intrathecal capsaicin can result in a reversible block of the micturition reflex.84 Further, while normal micturition is not altered following ablation of NK1-R expressing SC neurons using SSP-saporin, the response to a nociceptive stimulus was significantly reduced. These and other studies84,85 suggest that SP and its receptors may play a part in transmission of bladder nociceptive responses at the first synapse in the micturition reflex.
EFFERENT PATHWAYS TO THE BLADDER
Preganglionic Neurons
Parasympathetic preganglionic neurons (PPGN) are located in the lateral part of the sacral intermediolateral gray matter which send dendrites into lateral lamina I of the dorsal horn, the lateral funiculus, and medially into the dorsal gray commissure (DGC). PPGN, which are divided functionally into tonic and phasic types, are cholinergic but also contain opioid peptides and express nitric oxide synthase (NOS).86 PACAP, a peptide present in visceral afferent neurons, enhances the firing of PPGN.87 The DGC contains a group of interneurons, which are likely to be active during micturition88,89 and may influence the function of the PPGN.88-90
At spinal levels L1–L2, both the intermediolateral horn and the DGC contain sympathetic preganglionic neurons whose axons project to the major pelvic ganglion. With ageing, there is selective attrition of these neurons, with reductions in the extent of the dendritic arbors of remaining cells.91,92
In man the preganglionic parasympathetic motor nerves to the bladder and other pelvic organs exit the sacral spinal cord in the anterior roots S2–S4 and course through the pelvic nerves. Stimulating the S3 roots with implanted electrodes designed principally for bladder emptying after SCI93 elicits two principal responses; at low levels of stimulation, the external urethral sphincter, external anal sphincter, and pelvic floor muscles are contracted. At high levels of stimulation, parasympathetic activation contracts the detrusor muscle, leading to efficient emptying of the bladder when the sphincter muscle relaxes.94 Attempts to use extracorporeal magnetic stimulation to achieve the same effect95 have shown insufficient power to activate the small parasympathetic axons at the level of the lumbar-sacral roots.96
Ganglia
The postganglionic autonomic innervation to the lower urinary tract and reproductive organs, along with a substantial part of the extrinsic motor innervation of the lower bowel arises in the pelvic ganglia. There are substantial species differences in organization and neurochemistry of pelvic ganglion cells and their spinal inputs. Within the pelvic plexus there is topographical representation of the pelvic organs. In the female dog, neurons supplying different pelvic organs are located in separate ganglia, which possess a distinctive composition of neuron types and different preganglionic supply.97 Neurons retrogradely labeled from the urinary bladder mainly occur in ganglia located at the vesico-ureteric junction. Autonomic ganglia are also found in the vicinity of the bladder neck, trigone, proximal urethra, and prostate. They receive noradrenergic and cholinergic excitatory innervation and noncholinergic, nonadrenergic inhibitory innervation.98 Knowledge of this distribution allows strategic planning for surgical dissection.
The bladder wall itself contains intramural ganglia, and small clusters of autonomic ganglion cells are present in the adventitial connective tissue and among the detrusor muscle bundles. There is species variation in the extent of intramural innervation of the bladder; ganglia are present in many species such as the guinea pig,99 while the rat bladder contains the postsynaptic innervation alone.100 The ganglia are found throughout the bladder wall and vary considerably in size.54,101 They show immunoreactivity to VIP, NOS, NPY, and Gal in varying amounts. However, they do not contain enkephalin (ENK), SP, CGRP, or somatostatin (Som),53 suggesting that cell bodies of sensory neurons are not located in the intramural ganglia. Postganglionic sympathetic nerves, identified with antibodies to TH and NPY, also synapse on these neurons. Nicotinic receptors have been identified on intramural nerve cell bodies within the bladder.102 α1-Adrenergic facilitatory receptors are present in bladder parasympathetic ganglia.103
Terminal Nerve Fibers
The majority of nerves running in the detrusor stain positively for acetylcholinesterase and for vesicular acetylcholine transferase (VAChT)101,104 and are thought to be parasympathetic. Putative postganglionic sympathetic fibers immunoreactive for TH or NPY are rare in the detrusor, although they are moderately frequent in the suburothelium.55 Nonetheless, presynaptic α1-adrenergic facilitatory receptors are present on efferent parasympathetic nerve terminals in the bladder wall.105,106 The parasympathetic efferents release acetylcholine to stimulate muscarinic receptors. However, the presence of additional substances allows immunohistochemical subclassification of nerve fibers, and raises the question as to whether additional transmitters other than ACh have a role in normal micturition function or disease pathophysiology.
Pelvic Organ Interactions at the Efferent Neural Level
Bladder and Outlet
Neural coordination of physiological and behavioral functions depends on convergence within the nervous system of information from relevant areas, and convergence could result in collateral effects of pathology in one organ affecting function elsewhere. There is extensive convergence of pelvic organ input107,108 at the levels of the spinal cord, dorsal column nuclei, solitary nucleus, medullary reticular formation, and thalamus.109 Convergent processing underpins the coherent functioning of systems controlled by efferent outflows diverging from a common starting point, as exemplified by the synergic coordination of bladder and urethra required for normal voiding. The fundamental role of supraspinal mechanisms in lower urinary tract synergy is well recognized. However, synergic lower urinary tract function may also be a feature of the peripheral innervation, independent of central nervous system (CNS) coordination. In the female minipig, preganglionic pelvic nerve stimulation evokes a pressure increase in the bladder and a pressure decrease in the urethra.110 It remains to be determined whether this observation reflects coherent activation of separate motoneurons (excitatory to the bladder, inhibitory to the outlet), or whether postganglionic motoneurons send branches which supply both bladder and urethra.
Bladder and Bowel/Uterus
Clinicians are familiar with the detrimental effect of bowel disorders on lower urinary tract activity. Physiologically, the efferent limb of the micturition reflex is inhibited by afferent input from the rectum;111 thus, rectal distension inhibits bladder activity via glycinergic and GABAergic mechanisms in rats.112 Dual labeling studies show that many neurons in Barrington’s nucleus supply both colon and bladder, with smaller populations supplying the two organs separately. At the level of the major pelvic ganglion, double-labeled cells are relatively infrequent, but processes of colonic-retrograde-labeled cells often surround cell bodies of equivalent cells for the bladder. Dual-labeled cells in the spinal cord are rare.113
In addition to efferent input, local reflexes may contribute to the inhibition of detrusor activity, probably driven by interstitial cells,114 so that peripheral autonomous activity increases as a result of bladder distension.115,116 This has been proposed to signify the presence of a regional regulatory influence117 and a peripheral “pacemaker”118 and various mechanisms for the propagation of activity within the bladder wall.119 In the clinical context, processes affecting peripheral innervation would then predispose to emergence of inappropriate detrusor activity during urine storage (through loss of inhibitory fibers), associated with inefficient bladder emptying (through loss of excitatory fibers).
NEURAL CONTROL OF FEMALE PELVIC FLOOR MUSCLES AND RHABDOSPHINCTERS
Structural Elements of the Pelvic Floor
The pelvic floor120 in women is a bowl-shaped structure comprised of bone, muscle, and connective tissue. The rim of the bowl is formed by the bones of the pelvic girdle (sacrum, ileum, ischium, and pubis). The “inside and bottom” of the bowl is lined with striated muscle: the iliococcygeus and pubococcygeus (which together comprise the levator ani) as well as the coccygeus, and puborectalis muscles. The muscles are attached to the bone and to each other with various connective tissue supports. These three components, bone, muscle, and connective tissue provide support of the pelvic viscera (i.e., rectum, vagina, and bladder) but also allow for excretory and sexual functions.
Innervation of the Female Levator Ani Muscles
The levator ani muscle of the pelvic floor is innervated by the levator ani nerve in human,121 squirrel monkey,122-124 dog,125 cat (Karicheti and Thor, unpublished observations), and rat.126 The levator ani nerve primarily arises from sacral spinal roots (e.g., S3–S5 in humans) and travels along the intrapelvic face of the levator ani muscle with a high degree of variability in branching patterns.121 In humans, there is some controversy whether or not the pudendal nerve also innervates the levator ani muscle.127,128 This is not the case in other species (rat, cat, dog, squirrel monkey) where hodological studies show: (1) a marked loss of levator ani muscle mass and a decrease in levator ani myocyte diameter following transection of the levator ani nerve124,126 but no change in levator ani muscle mass or myocyte diameter following pudendal neurectomy,124,126 (2) the existence of only a single motor endplate zone at the point of levator ani nerve insertion into the levator ani muscles,124,126 (3) absence of contractions of levator ani muscles upon electrical stimulation of pudendal nerve efferent fibers (Thor and Karicheti, unpublished observations), and (4) phenotypically distinctive motor neuron labeling following application of nerve tracers to the pudendal and levator ani nerves.125,129-134
Role of the Levator Ani Innervation in Pelvic Organ Prolapse in Monkeys
Because the pelvic floor is responsible for providing support of the viscera, and because one might expect contraction of pelvic floor muscles to be necessary for adequate support, damage to the levator ani innervation and subsequent muscle flaccidity might be expected to promote pelvic organ prolapse (POP). To test this expectation, the levator ani muscles were bilaterally denervated in squirrel monkeys,122 which is a species that shows age and parity correlated POP similar to humans.135 Surprisingly, these monkeys showed no POP following this procedure for 2–3 years after surgery, despite showing statistically significant decreases in levator ani muscle mass and myocyte diameter. However, a slight increase in bladder and cervical descent with abdominal pressure was seen on MRI evaluation compared to nulliparous controls. Of possible significance was the finding that, after a single birth, 2 of 4 bilateral levator ani neurectomy animals showed POP, which is unusual. Thus, these experiments indicate that, in the absence of childbirth, the pelvic floor muscle plays a minor role in providing visceral support and suggests that the connective tissue plays the major role.
Innervation of Urethral and Anal Rhabdosphincters
At the level of the pelvic floor, the urethra and rectum are surrounded by intimately associated bands of striated muscle fibers; the urethral and anal rhabdosphincters, respectively. The muscles do not have “dedicated” attachments to skeletal structures and thus act as a true sphincters (i.e., contraction produces virtually no movement except constriction of the lumen). Extensive studies of the urethral rhabdosphincter, anal rhabdosphincter, bulbocavernosus, and ischiocavernosus muscles have shown that these muscles are innervated by the pudendal nerve,121,129,131-134,136,137 which originates from the sacral roots and passes along the lateral surface of the internal obturator and coccygeus muscles, through Alcock’s canal, to eventually approach these muscles laterally from the extrapelvic surface of the pelvic floor.
Segmental Activation of Urethral and Anal Rhabdosphincters
Rhabdosphincter motor neurons can be activated via segmental138-142 and descending pathways.139,143,144 The segmental inputs can be activated by stretch receptors and nociceptors in the bladder or urethra or genitalia.145-148 Electrophysiological studies138,140-142,149,150 show that stimulation of either pelvic nerve or pudendal nerve afferent fibers can activate polysynaptic spinal segmental reflexes that can be recorded at a latency of about 10 msec from electrodes placed on pudendal nerve efferent fibers or inserted directly into the urethral or anal rhabdosphincter muscles.
Previously, the afferent inputs from the urinary bladder were emphasized as being of primary importance for activation of the segmental reflex by pelvic nerve stimulation and is often referred to as the “guarding reflex” or “continence reflex.” However, recent studies are placing greater emphasis on urethral afferent fibers146,148,151 mediating spinal reflex activation of the urethral rhabdosphincter.
Supraspinal Activation of Rhabdosphincters and Pelvic Floor Muscles
Supraspinal activation of urethral and anal rhabdosphincter motor neurons includes voluntary inputs (i.e., corticospinal),152 as well as involuntary reflexic inputs (e.g., during coughing, sneezing, vomiting) presumably from nucleus retroambiguus in the caudal medulla.144,153-157
Rhabdosphincter motor neurons are unique among somatic motor neurons in receiving input from the paraventricular hypothalamus,143 although the function of this input has not been determined. In addition, their input from brainstem serotonergic and noradrenergic neurons is among the densest in the spinal cord.158-161 Finally, rhabdosphincter motor neurons also receive input from the “L region” of the pons that might be important for maintaining continence, since a lesion in this area produced continuous incontinence in a cat.162
Pharmacology of Urethral and Anal Rhabdosphincters
The preferential association of norepinephrine and serotonin terminals158-161 led to extensive studies of noradrenergic and serotonergic control of rhabdosphincter function and eventual clinical studies of duloxetine, a norepinephrine and serotonin reuptake inhibitor, as a treatment for stress urinary incontinence (SUI).147,163-167 Elegant studies in humans using magnetic stimulation of brain and sacral nerve roots168 have indicated that duloxetine increases the excitability of rhabdosphincter motor neurons to both supraspinal and segmental inputs and to double urethral pressure responses to sacral nerve root magnetic stimulation. Importantly, duloxetine’s ability to increase urethral rhabdosphincter activity did not interfere with the inhibition of sphincter activity during voiding (i.e., bladder–sphincter synergy was well-maintained). Similar clinical results have been seen with S,S-reboxetine, a selective norepinephrine reuptake inhibitor.169,170 More recent data on the effect of the selective noradrenergic reuptake inhibitor, [S,S]-reboxetine in the treatment of SUI, indicate that the serotonergic activity of duloxetine may be redundant and that effects are dependent solely on noradrenergic reuptake inhibition.169,170 This approach of increasing synaptic levels of serotonin and/or norepinephrine is logical since, it has been shown that noradrenergic and serotonergic terminals associated with rhabdosphincter motor neurons show an age-dependent decrease in density in rats.171
Multiple adrenergic receptor subtypes play a role in control of the rhabdosphincter, and the results with norepinephrine reuptake inhibitors indicate that these receptors can be activated by endogenous norepinephrine in anesthetized cats.149 Strong evidence exists that α1 adrenoceptors excite rhabdosphincter motor neurons.149,172-174 On the other hand, strong evidence exists that α2 adrenoceptor stimulation has the opposite effect, i.e., inhibition of rhabdosphincter activity.149,175 Importantly, the innervation of the urethral and anal smooth muscle (i.e., the hypogastric nerve) shows similar adrenergic pharmacology—an enhancement of activity by α1 adrenoceptors149,172,176 and inhibition of activity by α2 adrenoceptors.149,172,177
Multiple subtypes of serotonin (5-hydroxytryptamine, 5-HT) receptors are also involved in modulating rhabdosphincter motor neuron excitability. Strong evidence exists that 5-HT2 receptors can excite sphincter motor neurons.178 Indeed duloxetine’s facilitatory effects on rhabdosphincter activity in anesthetized cats are mediated in part through activation of 5-HT2 receptors.147 Both 5-HT2A and 5-HT2C receptor agonists increase rhabdosphincter EMG activity in dogs, guinea pigs, and rats.179,180 Recent in vitro rat spinal cord slice patch clamp studies show that part of this effect may be directly on rhabdosphincter motor neurons, as opposed to interneurons,174 since 5-HT induces a direct depolarization of rhabdosphincter motor neurons. Interestingly, SP, a peptide transmitter that is colocalized with 5-HT in raphe spinal nerve terminals, also produces direct depolarization of rhabdosphincter motor neurons in rat spinal cord slices,181 and thyrotropin releasing hormone (TRH), another peptide transmitter colocalized with 5-HT in nerve terminals, induces excitation of rat sphincter activity182,183 in vivo.
Abnormal Lower Urinary Tract Function
Dysfunction of neural control may underpin a wide range of clinical urinary tract problems. On the afferent side, neural dysfunction will alter reflex activity and influence sensation, which can be enhanced, reduced, or altered (e.g., with the emergence of pain instead of usual bladder filling sensations). Although no consensus has been reached on the fundamental causes of BPS/IC, existing data suggest pathophysiological mechanisms including epithelial dysfunction, mast cell activation, and/or neurogenic inflammation.184
On the efferent side, motor activity within the components of the lower urinary tract (bladder and outlet) can be increased, reduced, or uncoordinated. For example, detrusor overactivity can arise in neuropathic conditions, secondary to bladder outlet obstruction. Several observations on structural and functional properties of the bladder have been made in individuals with detrusor overactivity. These can include patchy denervation within the bladder wall, exaggerated spontaneous myogenic activity, or changes in smooth muscle ultrastructure. A common ultrastructural feature of the overactive detrusor is the emergence of protrusion junctions and ultraclose abutments between the smooth muscle cells.185 Preclinical studies in animal models of OAB/detrusor overactivity contribute further to our understanding of the importance of neural control in this condition. The main models relevant to OAB include (i) instillation of irritative agents into the bladder during cystometry, (ii) partial bladder outflow obstruction, (iii) the spontaneously hypertensive rat, (iv) SCI, and (v) other CNS lesions similar to those responsible for bladder dysfunction in humans.186
SUI is characterized by a reduction in outflow resistance during urinary storage due to weakness in the urethral sphincter mechanism. It is often associated with weakness of the pelvic floor and urethral musculature, but peripheral nerve dysfunction is also implicated, in particular pudendal nerve damage following childbirth in women.187 Animal models have contributed to understanding of the importance of the peripheral innervation of the urethra in SUI, with development of disease models associated with pudendal nerve crush and vaginal distension initiating neuropathic nerve injury in rats188 and mice.189 The role of pharmacological neuromodulation in the treatment of SUI has shown that urethral function and incontinence can be improved by augmenting somatic neuronal discharge using the serotonergic (5HT) and the noradrenergic (NE) reuptake inhibitor duloxetine or SS-reboxetine (discussed above).163,190
The significance of neural control mechanism in lower urinary tract dysfunction is further implied by a number of comorbid conditions that have been associated with BPS/IC and incontinence. Patients with BPS/IC and SUI are more likely to report depressive symptoms. BPS/IC has also been associated with other chronic pain conditions, especially fibromyalgia.191-194 Based on recent improvements in understanding of pain processing pathways in the CNS, and in particular the role of limbic structures, especially the anterior cingulate cortex, hippocampus and amygdala, in chronic and affective pain perception, a condition termed limbic associated pelvic pain has been proposed to explain the concurrence of these various chronic pain conditions. This limbic dysfunction is manifest both as an increased sensitivity to nociceptive afferents from pelvic organs, and as an abnormal efferent innervation of pelvic musculature, which undergoes tonic contraction as a result of limbic efferent stimulation, generating a further sensation of pain. The nociceptive afferents from these pelvic organs then follow the medial pain pathway back to the sensitized, hypervigilant limbic system. Chronic stimulation of the limbic system by pelvic pain afferents again produces an efferent contraction of the pelvic muscles, thus perpetuating the cycle.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Apodaca G. The uroepithelium: Not just a passive barrier. Traffic. 2004;5:117–28. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol. 2000;278:F867–74. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 3.Hicks M. The mammalian urinary bladder: An accomodating organ. Biol Rev. 1975;50:215–46. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 4.Liang FX, Riedel I, Deng FM, et al. Organization of uroplakin subunits: Transmembrane topology, pair formation and plaque composition. J Biochem. 2001;355:13–8. doi: 10.1042/0264-6021:3550013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: Implications for wound healing. Am J Physiol. 2006;291:F9–21. doi: 10.1152/ajprenal.00035.2006. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Keay S, DeDeyne P, et al. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951–6. [PubMed] [Google Scholar]
- 7.Acharya P, Beckel J, Ruiz WG, et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol. 2004;287:F305–18. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 8.Parsons CL, Greenspan C, Moore SW, et al. Role of surface mucin in primary antibacterial defense of bladder. Urology. 1977;9:48–52. doi: 10.1016/0090-4295(77)90284-9. [DOI] [PubMed] [Google Scholar]
- 9.Parson CL, Boychuk D, Jones S, et al. Bladder surface glycosaminoglycans: An epithelial permeability barrier. J Urol. 1990;143:139–42. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 10.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis) J Urol. 1991;145:732–5. doi: 10.1016/s0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang E, Truschel ST, Apodaca G. Analysis of hydrostatic pressure-induced changes in umbrella cell surface area. Methods. 2003;30:207–17. doi: 10.1016/s1046-2023(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 12.Truschel ST, Wang E, Ruiz WG, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–43. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balestreire EM, Apodaca G. Apical EGF receptor signaling: Regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell. 2007;18:1312–23. doi: 10.1091/mbc.E06-09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J, Huang H, Zhang ZT. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer Res. 2002;62:4157–63. [PubMed] [Google Scholar]
- 15.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int. 2007;72:1057–64. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 16.Birder LA, deGroat WC. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat Clin Prac Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson G, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 18.Conrads TP, Tocci GM, Hood BL, et al. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem. 2006;281:37836–43. doi: 10.1074/jbc.M604581200. [DOI] [PubMed] [Google Scholar]
- 19.Keay SK, Zhang CO, Shoenfelt J, et al. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9–14. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- 20.Keay SK, Szekely Z, Conrads TP, et al. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004;101:11803–8. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birder LA, Nealen ML, Kiss S, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–70. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jen PY, Dixon JS, Gosling JA. Immunohistochemical localization of neuromarkers and neuropeptides in human fetal and neonatal urinary bladder. Br J Urol. 1995;75:230–5. doi: 10.1111/j.1464-410x.1995.tb07317.x. [DOI] [PubMed] [Google Scholar]
- 23.Kunze A, Neuhaus J, Stolzenburg JU. Quantitative immunohistochemical study of the innervation of the guinea-pig lower urinary tract. BJU Int. 2006;98:424–9. doi: 10.1111/j.1464-410X.2006.06235.x. [DOI] [PubMed] [Google Scholar]
- 24.Ost D, Roskams T, Van der Aa F, et al. Topography of the vanilloid receptor in the human bladder: More than just the nerve fibers. J Urol. 2002;168:293–7. [PubMed] [Google Scholar]
- 25.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–43. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- 26.Brading AF, McCloskey KD. Mechanisms of disease: Specialized interstitial cells of the urothelium: An assessment of current knowledge. Nat Clin Prac Urol. 2005;2:546–54. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda Y, Fry C, Hayashi F, et al. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol. 2006;293:F1018–25. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra B, Gever J, Barrick SR, et al. Expression and function of rat urothelial P2Y receptors. Am J Physiol. 2008;294:F821–9. doi: 10.1152/ajprenal.00321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chopra B, Barrick SR, Meyers S, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–71. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmcol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 31.Chess-Williams R. Muscarinic receptors of the urinary bladder: Detrusor, urothelial and prejunctional. Auton Autocoid Pharmacol. 2002;22:133–45. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 32.Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol. 2003;285:F423–9. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rat urinary bladder epithelial cells by hydrostatic pressure changes—A possible sensory mechanism? J Physiol. 1997;505:503–11. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckel JM, Kanai AJ, Lee SJ, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol. 2006;290:F103–10. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 36.Carattino MD, Sheng S, Kleyman TR. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem. 2005;280:4393–401. doi: 10.1074/jbc.M413123200. [DOI] [PubMed] [Google Scholar]
- 37.LeBerge J, Malley SE, Zvarova K, et al. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- 38.Hanna-Mitchell AT, Beckel J, Barbadora S, et al. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: Possible implications for physiology and pathophysiology. Scand J Urol Nephrol. 1995;175:43–53. [PubMed] [Google Scholar]
- 40.Templeman L, Chapple CR, Chess-Williams R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol. 2002;167:742–5. doi: 10.1016/S0022-5347(01)69137-7. [DOI] [PubMed] [Google Scholar]
- 41.Wang EC, Lee JM, Ruiz WG, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–22. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosse Y, Pare PD, Seow CY. Airway wall remodeling in asthma: From the epithelial layer to the adventitia. Curr Allergy Asthma Rep. 2008;8:357–66. doi: 10.1007/s11882-008-0056-0. [DOI] [PubMed] [Google Scholar]
- 43.Hendrix S. Neuroimmune communication in skin: Far from peripheral. J Invest Dermatol. 2008;128:260–1. doi: 10.1038/sj.jid.5701171. [DOI] [PubMed] [Google Scholar]
- 44.Lumplin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nat Rev. 2007;445:858–65. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 45.Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 46.de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165–87. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- 47.Morrison JFB. Neural connections between the lower urinary tract and the spinal cord. In: Torrens M, Morrison JFB, editors. The physiology of the lower urinary tract. Springer-Verlag; Berlin: 1987. pp. 53–85. [Google Scholar]
- 48.Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2007;99:244–53. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Groat WC, Booth AM, Yoshimura N, et al. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system, Vol. 3, Chapter 8, Nervous control of the urogenital system. Harwood Academic Publishers; London, UK: 1993. pp. 227–89. [Google Scholar]
- 51.Wiseman OJ, Brady CM, Hussain IF, et al. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol. 2002;168:2040–5. doi: 10.1016/S0022-5347(05)64291-7. [DOI] [PubMed] [Google Scholar]
- 52.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–55. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 53.Smet PJ, Edyvane KA, Jonavicius J, et al. Neuropeptides and neurotransmitter-synthesizing enzymes in intrinsic neurons of the human urinary bladder. J Neurocytol. 1996;25:112–24. doi: 10.1007/BF02284790. [DOI] [PubMed] [Google Scholar]
- 54.Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- 55.Drake MJ, Hedlund P, Mills IW, et al. Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest. 2000;80:1491–9. doi: 10.1038/labinvest.3780158. [DOI] [PubMed] [Google Scholar]
- 56.Gu J, Blank MA, Huang WM, et al. Peptide-containing nerves in human urinary bladder. Urology. 1984;24:353–7. doi: 10.1016/0090-4295(84)90209-7. [DOI] [PubMed] [Google Scholar]
- 57.Dmitrieva N, McMahon SB. Sensitization of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura N, Seki S, Novakovic SD, et al. The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci. 2001;21:8690–6. doi: 10.1523/JNEUROSCI.21-21-08690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett DL, Dmietrieva N, Priestley JV, et al. TrkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurons in the rat. Neurosci Lett. 1996;206:33–6. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurons innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–76. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Black JA, Cummins TR, Yoshimura N, et al. Tetrodotoxin-resistant sodium channels Na(v)1.8/SNS and Na(v)1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res. 2003;963:132–8. doi: 10.1016/s0006-8993(02)03957-4. [DOI] [PubMed] [Google Scholar]
- 62.Seki S. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–74. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 63.Yamane H, de Groat WC, Sculptoreanu A. Effects of ralfinamide, a Na(+) channel blocker, on firing properties of nociceptive dorsal root ganglion neurons of adult rats. Exp Neurol. 2007;208:63–72. doi: 10.1016/j.expneurol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castleden CM, Duffin HM, Clarkson EJ. In vivo and in vitro studies on the effect of sodium antagonists on the bladder in man and rat. Age Ageing. 1983;12:249–55. doi: 10.1093/ageing/12.3.249. [DOI] [PubMed] [Google Scholar]
- 65.Lapointe SP, Wang B, Kennedy WA, et al. The effects of intravesical lidocaine on bladder dynamics of children with myelomeningocele. J Urol. 2001;165:2380–2. doi: 10.1016/S0022-5347(05)66209-X. [DOI] [PubMed] [Google Scholar]
- 66.Yokoyama O, Komatsu K, Kodama K, et al. Diagnostic value of intravesical lidocaine for overactive bladder. J Urol. 2000;164:340–3. [PubMed] [Google Scholar]
- 67.Reuther K, Aagaard J, Jensen KS. Lignocaine test and detrusor instability. Br J Urol. 1983;55:493–4. doi: 10.1111/j.1464-410x.1983.tb03355.x. [DOI] [PubMed] [Google Scholar]
- 68.Sethia KK, Smith JC. The effect of pH and lignocaine on detrusor instability. Br J Urol. 1987;60:516–8. doi: 10.1111/j.1464-410x.1987.tb05032.x. [DOI] [PubMed] [Google Scholar]
- 69.Chalfin SA, Bradley WE. The etiology of detrusor hyperreflexia in patients with intravesical obstruction. J Urol. 1982;127:938–42. doi: 10.1016/s0022-5347(17)54139-7. [DOI] [PubMed] [Google Scholar]
- 70.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol. 2008;294:R1880–90. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.deGroat WC, Fraser MO, Yoshiyama M. Neural control of the urethra. Scand J Urol Nephrol. 2001;207:35–43. doi: 10.1080/003655901750174872. [DOI] [PubMed] [Google Scholar]
- 72.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999;517:599. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 75.Tai C, Wang J, Wang X, et al. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–7. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 76.Floyd K, McMahon SB, Morrison JF. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol. 1982;322:45. doi: 10.1113/jphysiol.1982.sp014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660–72. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 78.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–64. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Case-control study of medical comorbidities in women with interstitial cystitis. J Urol. 2008;179:2222–5. doi: 10.1016/j.juro.2008.01.172. [DOI] [PubMed] [Google Scholar]
- 80.Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204:777–90. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Shehab SA, Spike RC, Todd AJ. Do central terminals of intact myelinated primary afferents sprout into the superficial dorsal horn of rat spinal cord after injury to a neighboring peripheral nerve? J Comp Neurol. 2004;474:427–37. doi: 10.1002/cne.20147. [DOI] [PubMed] [Google Scholar]
- 82.Zvara P, Braas KM, May V, et al. A role for pituitary adenylate cyclase activating poly peptide (PACAP) in detrusor hyperreflexia after spinal cord injury. Ann NY Acad Sci. 2006;1070:622–8. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- 83.Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Peptides. 2001;15:1–18. doi: 10.1016/s0167-0115(01)00285-3. [DOI] [PubMed] [Google Scholar]
- 84.Seki S, Erickson KA, Seki M, et al. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol. 2005;288:F466–73. doi: 10.1152/ajprenal.00274.2004. [DOI] [PubMed] [Google Scholar]
- 85.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 86.Vizzard MA, Erdman SL, Forstermann U, et al. Differential distribution of nitric oxide synthase in neural pathways to the urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res. 1994;646:279–91. doi: 10.1016/0006-8993(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 87.Ishizuka O, Alm P, Larsson B, et al. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–14. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 88.Vera PL, Nadelhaft I. Anatomical evidence for two spinal ‘afferent-interneuron-efferent’ reflex pathways involved in micturition in the rat: A ‘pelvic nerve’ reflex pathway and a ‘sacrolumbar intersegmental’ reflex pathway. Brain Res. 2000;883:107–18. doi: 10.1016/s0006-8993(00)02732-3. [DOI] [PubMed] [Google Scholar]
- 89.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–33. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 90.Sie JA, Blok BF, de Weerd H, et al. Ultrastructural evidence for direct projections from the pontine micturition center to glycine-immunoreactive neurons in the sacral dorsal gray commissure in the cat. J Comp Neurol. 2001;429:631–7. doi: 10.1002/1096-9861(20010122)429:4<631::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 91.Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the rat hypogastric ganglion. J Neurocytol. 1996;25:555–63. doi: 10.1007/BF02284823. [DOI] [PubMed] [Google Scholar]
- 92.Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the paracervical ganglion of nulliparous and multiparous rats. Brain Res. 1998;780:245–52. doi: 10.1016/s0006-8993(97)01199-2. [DOI] [PubMed] [Google Scholar]
- 93.Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977;40:358–69. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brindley GS. The first 500 patients with sacral anterior root stimulator implants: General description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 95.Lin VW, Wolfe V, Frost FS, et al. Micturition by functional magnetic stimulation. J Spinal Cord Med. 1997;20:218–26. doi: 10.1080/10790268.1997.11719472. [DOI] [PubMed] [Google Scholar]
- 96.Bycroft JA, Craggs MD, Sheriff M, et al. Does magnetic stimulation of sacral nerve roots cause contraction or suppression of the bladder? Neurourol Urodyn. 2004;23:241–5. doi: 10.1002/nau.20009. [DOI] [PubMed] [Google Scholar]
- 97.Li MZ, Masuko S. Target specific organization and neuron types of the dog pelvic ganglia: A retrograde-tracing and immunohistochemical study. Arch Histol Cytol. 2001;64:267–80. doi: 10.1679/aohc.64.267. [DOI] [PubMed] [Google Scholar]
- 98.Brading AF, Greenland JE, Mills IW, et al. Blood supply to the bladder during filling. Scand J Urol Nephrol Suppl. 1999;201:25–31. [PubMed] [Google Scholar]
- 99.Gabella G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990;261:231–7. doi: 10.1007/BF00318664. [DOI] [PubMed] [Google Scholar]
- 100.Gabella G, Berggren T, Uvelius B. Hypertrophy and reversal of hypertrophy in rat pelvic ganglion neurons. J Neurocytol. 1992;21:649–62. doi: 10.1007/BF01191726. [DOI] [PubMed] [Google Scholar]
- 101.Dixon JS, Gilpin SA, Gilpin CJ, et al. Intramural ganglia of the human urinary bladder. Br J Urol. 1983;55:195–8. doi: 10.1111/j.1464-410x.1983.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 102.De Biasi M, Nigro F, Xu W. Nicotinic acetylcholine receptors in the autonomic control of bladder function. Eur J Pharmacol. 2000;393:137–40. doi: 10.1016/s0014-2999(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 103.Keast JR, Kawatani M, de Groat WC. Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by alpha 1- and alpha 2-adrenoceptors. Am J Physiol. 1990;258:R44–50. doi: 10.1152/ajpregu.1990.258.1.R44. [DOI] [PubMed] [Google Scholar]
- 104.Ek A, Alm P, Andersson KE, et al. Adrenergic and cholinergic nerves of the human urethra and urinary bladder. A histochemical study. Acta Physiol Scand. 1977;99:345–52. doi: 10.1111/j.1748-1716.1977.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 105.Somogyi GT, Tanowitz M, de Groat WC. Prejunctional facilitatory alpha 1-adrenoceptors in the rat urinary bladder. Br J Pharmacol. 1995;114:1710–6. doi: 10.1111/j.1476-5381.1995.tb14961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szell EA, Yamamoto T, de Groat WC, et al. Smooth muscle and parasympathetic nerve terminals in the rat urinary bladder have different subtypes of alpha(1) adrenoceptors. Br J Pharmacol. 2000;130:1685–91. doi: 10.1038/sj.bjp.0703475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foreman RD. Integration of viscerosomatic sensory input at the spinal level. Prog Brain Res. 2000;122:209–21. doi: 10.1016/s0079-6123(08)62140-8. [DOI] [PubMed] [Google Scholar]
- 108.Kaddumi EG, Hubscher CH. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J Physiol. 2006;572:393–405. doi: 10.1113/jphysiol.2005.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci. 1994;14:6796–814. doi: 10.1523/JNEUROSCI.14-11-06796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalmose AL. Bladder and urethral responses to pelvic nerve stimulation in the pig. Scand J Urol Nephrol Suppl. 2002;25:34–45. doi: 10.1080/003655902320765944. [DOI] [PubMed] [Google Scholar]
- 111.Kruse MN, Mallory BS, Noto H, et al. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol. 1992;262:R478–84. doi: 10.1152/ajpregu.1992.262.3.R478. [DOI] [PubMed] [Google Scholar]
- 112.Miyazato M, Sugaya K, Nishijima S, et al. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J Urol. 2004;171:1353–6. doi: 10.1097/01.ju.0000099840.09816.22. [DOI] [PubMed] [Google Scholar]
- 113.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: Substrates for pelvic visceral coordination. Eur J Neurosci. 2003;18:3311–24. doi: 10.1111/j.1460-9568.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 114.Lagou M, Gillespie JI, Andersson KE, et al. Bladder volume alters cholinergic responses of the isolated whole mouse bladder. J Urol. 2006;175:771–6. doi: 10.1016/S0022-5347(05)00140-0. [DOI] [PubMed] [Google Scholar]
- 115.Lagou M, Gillespie J, Kirkwood T, et al. Muscarinic stimulation of the mouse isolated whole bladder: Physiological responses in young and ageing mice. Auton Autacoid Pharmacol. 2006;26:253–60. doi: 10.1111/j.1474-8673.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 116.Drake M, Gillespie J, Hedlund P, et al. Muscarinic stimulation of the rat isolated whole bladder: Pathophysiological models of detrusor overactivity. Auton Autacoid Pharmacol. 2006;26:261–6. doi: 10.1111/j.1474-8673.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 117.Grol S, van Koeveringe GA, de Vente J, et al. Regional differences in sensory innervation and suburothelial interstitial cells in the bladder neck and urethra. BJU Int. 2008;102:870–7. doi: 10.1111/j.1464-410X.2008.07752.x. [DOI] [PubMed] [Google Scholar]
- 118.de Jongh R, van Koeveringe GA, van Kerrebroeck PE, et al. Damage to the bladder neck alters autonomous activity and its sensitivity to cholinergic agonists. BJU Int. 2007;100:919–29. doi: 10.1111/j.1464-410X.2007.07129.x. [DOI] [PubMed] [Google Scholar]
- 119.Finney SM, Stewart LH, Gillespie JI. Cholinergic activation of phasic activity in the isolated bladder: Possible evidence for M3- and M2-dependent components of a motor/sensory system. BJU Int. 2007;100:668–78. doi: 10.1111/j.1464-410X.2007.07021.x. [DOI] [PubMed] [Google Scholar]
- 120.Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann NY Acad Sci. 2007;1101:266–96. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 121.Barber MD, Bremer RE, Thor KB, et al. Innervation of the female levator ani muscles. Am J Obstet Gynecol. 2002;187:64–71. doi: 10.1067/mob.2002.124844. [DOI] [PubMed] [Google Scholar]
- 122.Pierce LM, Coates KW, Kramer LA, et al. Effects of bilateral levator ani nerve injury on pelvic support in the female squirrel monkey. Am J Obstet Gynecol. 2008;198:e581–8. doi: 10.1016/j.ajog.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 123.Pierce LM, Rankin MR, Foster RT, et al. Distribution and immunohistochemical characterization of primary afferent neurons innervating the levator ani muscle of the female squirrel monkey. Am J Obstet Gynecol. 2006;195:987–96. doi: 10.1016/j.ajog.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 124.Pierce LM, Reyes M, Thor KB, et al. Innervation of the levator ani muscles in the female squirrel monkey. Am J Obstet Gynecol. 2003;188:1141–7. doi: 10.1067/mob.2003.329. [DOI] [PubMed] [Google Scholar]
- 125.Thuroff JW, Bazeed MA, Schmidt RA, et al. Regional topography of spinal cord neurons innervating pelvic floor muscles and bladder neck in the dog: A study by combined horseradish peroxidase histochemistry and autoradiography. Urol Int. 1982;37:110–20. doi: 10.1159/000280804. [DOI] [PubMed] [Google Scholar]
- 126.Bremer RE, Barber MD, Coates KW, et al. Innervation of the levator ani and coccygeus muscles of the female rat. Anat Rec. 2003;275:1031–41. doi: 10.1002/ar.a.10116. [DOI] [PubMed] [Google Scholar]
- 127.Grigorescu BA, Lazarou G, Olson TR, et al. Innervation of the levator ani muscles: Description of the nerve branches to the pubococcygeus, iliococcygeus, and puborectalis muscles. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:107–16. doi: 10.1007/s00192-007-0395-8. [DOI] [PubMed] [Google Scholar]
- 128.Wallner C, van Wissen J, Maas CP, et al. The contribution of the levator ani nerve and the pudendal nerve to the innervation of the levator ani muscles; a study in human fetuses. Eur Urol. 2008;54:1136–42. doi: 10.1016/j.eururo.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 129.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–49. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 130.Pierce LM, Reyes M, Thor KB, et al. Immunohistochemical evidence for the interaction between levator ani and pudendal motor neurons in the coordination of pelvic floor and visceral activity in the squirrel monkey. Am J Obstet Gynecol. 2005;192:1506–15. doi: 10.1016/j.ajog.2004.10.607. [DOI] [PubMed] [Google Scholar]
- 131.Roppolo JR, Nadelhaft I, de Groat WC. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol. 1985;234:475–88. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- 132.Thor KB, Morgan C, Nadelhaft I, et al. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–79. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 133.Ueyama T, Mizuno N, Nomura S, et al. Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol. 1984;222:38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]
- 134.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol. 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 135.Coates KW, Galan HL, Shull BL, et al. The squirrel monkey: An animal model of pelvic relaxation. Am J Obstet Gynecol. 1995;172:588–93. doi: 10.1016/0002-9378(95)90577-4. [DOI] [PubMed] [Google Scholar]
- 136.Pacheco P, Camacho MA, Garcia LI, et al. Electrophysiological evidence for the nomenclature of the pudendal nerve and sacral plexus in the male rat. Brain Res. 1997;763:202–8. doi: 10.1016/s0006-8993(97)00408-3. [DOI] [PubMed] [Google Scholar]
- 137.Pacheco P, Martinez-Gomez M, Whipple B, et al. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989;490:85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- 138.Bradley WE, Teague CT. Electrophysiology of pelvic and pudendal nerves in the cat. Exp Neurol. 1972;35:378–93. doi: 10.1016/0014-4886(72)90162-8. [DOI] [PubMed] [Google Scholar]
- 139.Mackel R. Segmental and descending control of the external urethral and anal sphincters in the cat. J Physiol. 1979;294:105–22. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McMahon SB, Morrison JF, Spillane K. An electrophysiological study of somatic and visceral convergence in the reflex control of the external sphincters. J Physiol. 1982;328:379–87. doi: 10.1113/jphysiol.1982.sp014271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rampal G, Mignard P. Behaviour of the urethral striated sphincter and of the bladder in the chronic spinal cat. Pflugers Arch. 1975;353:33–42. doi: 10.1007/BF00584509. [DOI] [PubMed] [Google Scholar]
- 142.Rampal G, Mignard P. Organization of the nervous control of urethral sphincter. Pflugers Arch. 1975;353:21–31. doi: 10.1007/BF00584508. [DOI] [PubMed] [Google Scholar]
- 143.Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincters in the cat. Brain. 1987;110:1323–44. doi: 10.1093/brain/110.5.1323. [DOI] [PubMed] [Google Scholar]
- 144.Miller AD, Nonaka S, Siniaia MS, et al. Multifunctional ventral respiratory group: Bulbospinal expiratory neurons play a role in pudendal discharge during vomiting. J Auton Nerv Syst. 1995;54:253–60. doi: 10.1016/0165-1838(95)00018-s. [DOI] [PubMed] [Google Scholar]
- 145.Bors E. Bulbocavernosus reflex. J Urol. 1959;82:128–30. doi: 10.1016/S0022-5347(17)65843-9. [DOI] [PubMed] [Google Scholar]
- 146.Chen Z, Anderson DL, Faison WL, et al. Biphasic urethral sphincter responses to acetic acid infusion into the lower urinary tract in anesthetized cats. J Urol. 2001;166:1539–48. [PubMed] [Google Scholar]
- 147.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–24. [PubMed] [Google Scholar]
- 148.Thor KB, Muhlhauser MA. Vesicoanal, urethroanal, and urethrovesical reflexes initiated by lower urinary tract irritation in the rat. Am J Physiol. 1999;277:R1002–12. doi: 10.1152/ajpregu.1999.277.4.R1002. [DOI] [PubMed] [Google Scholar]
- 149.Danuser H, Bemis K, Thor KB. Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther. 1995;274:820–5. [PubMed] [Google Scholar]
- 150.Thor KB, Hisamitsu T, Roppolo JR, et al. Selective inhibitory effects of ethylketocyclazocine on reflex pathways to the external urethral sphincter of the cat. J Pharmacol Exp Ther. 1989;248:1018–25. [PubMed] [Google Scholar]
- 151.Hurtado EA, Smith PP, Smith CP, et al. Urethral afferent signaling leads to activation of the external urethral sphincter and abdominal wall muscles. Neurourol Urodyn. 2008;27:105. [Google Scholar]
- 152.Nakagawa S. Onuf’s nucleus of the sacral cord in a South American monkey (Saimiri): Its location and bilateral cortical input from area 4. Brain Res. 1980;191:337–44. doi: 10.1016/0006-8993(80)91285-8. [DOI] [PubMed] [Google Scholar]
- 153.Boers J, Ford TW, Holstege G, et al. Functional heterogeneity among neurons in the nucleus retroambiguus with lumbosacral projections in female cats. J Neurophysiol. 2005;94:2617–29. doi: 10.1152/jn.00370.2005. [DOI] [PubMed] [Google Scholar]
- 154.Vanderhorst VG, Holstege G. Caudal medullary pathways to lumbosacral motoneuronal cell groups in the cat: Evidence for direct projections possibly representing the final common pathway for lordosis. J Comp Neurol. 1995;359:457–75. doi: 10.1002/cne.903590308. [DOI] [PubMed] [Google Scholar]
- 155.Vanderhorst VG, Holstege G. Nucleus retroambiguus projections to lumbosacral motoneuronal cell groups in the male cat. J Comp Neurol. 1997;382:77–88. doi: 10.1002/(sici)1096-9861(19970526)382:1<77::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 156.Kenton K, Brubaker L. Relationship between levator ani contraction and motor unit activation in the urethral sphincter. Am J Obstet Gynecol. 2002;187:403–6. doi: 10.1067/mob.2002.123939. [DOI] [PubMed] [Google Scholar]
- 157.Enck P, Vodusek DB. Electromyography of pelvic floor muscles. J Electromyogr Kinesiol. 2006;16:568–77. doi: 10.1016/j.jelekin.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 158.Kojima M, Matsuura T, Kimura H, et al. Fluorescence histochemical study on the noradrenergic control to the anterior column of the spinal lumbosacral segments of the rat and dog, with special reference to motoneurons innervating the perineal striated muscles (Onuf’s nucleus) Histochemistry. 1984;81:237–41. doi: 10.1007/BF00495633. [DOI] [PubMed] [Google Scholar]
- 159.Kojima M, Takeuchi Y, Goto M, et al. Immunohistochemical study on the distribution of serotonin fibers in the spinal cord of the dog. Cell Tissue Res. 1982;226:477–91. doi: 10.1007/BF00214778. [DOI] [PubMed] [Google Scholar]
- 160.Kojima M, Takeuchi Y, Goto M, et al. Immunohistochemical study on the localization of serotonin fibers and terminals in the spinal cord of the monkey (Macaca fuscata) Cell Tissue Res. 1983;229:23–36. doi: 10.1007/BF00217878. [DOI] [PubMed] [Google Scholar]
- 161.Rajaofetra N, Passagia JG, Marlier L, et al. Serotoninergic, noradrenergic, and peptidergic innervation of Onuf’s nucleus of normal and transected spinal cords of baboons (Papio papio) J Comp Neurol. 1992;318:1–17. doi: 10.1002/cne.903180102. [DOI] [PubMed] [Google Scholar]
- 162.Holstege G, Griffiths D, de Wall H, et al. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–61. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 163.Dmochowski RR, Miklos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259–63. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]
- 164.Millard RJ, Moore K, Rencken R, et al. Duloxetine vs placebo in the treatment of stress urinary incontinence: A four-continent randomized clinical trial. BJU Int. 2004;93:311–8. doi: 10.1111/j.1464-410x.2004.04607.x. [DOI] [PubMed] [Google Scholar]
- 165.Miyazato M, Kaiho Y, Kamo I, et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol. 2008;295:F264–71. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Norton PA, Zinner NR, Yalcin I, et al. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187:40–8. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 167.van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111:249–57. doi: 10.1111/j.1471-0528.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 168.Boy S, Reitz A, Wirth B, et al. Facilitatory neuromodulative effect of duloxetine on pudendal motor neurons controlling the urethral pressure: A functional urodynamic study in healthy women. Eur Urol. 2006;50:119–25. doi: 10.1016/j.eururo.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 169.Klarskov N, Scholfield D, Soma K, et al. Measurement of urethral closure function in women with stress urinary incontinence. J Urol. 2009;181:2629–33. doi: 10.1016/j.juro.2009.01.114. [DOI] [PubMed] [Google Scholar]
- 170.Zinner N, Scholfield D, Soma K, et al. A phase 2, 8-week, multicenter, randomized, double-blind, placebo controlled, parallel group study evaluating the efficacy, tolerability and safety of [s,s]-reboxetine (PNU-165442G) for stress urinary incontinence in women. J Urol. 2008;279:569–70. [Google Scholar]
- 171.Ranson RN, Dodds AL, Smith MJ, et al. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003;972:149–58. doi: 10.1016/s0006-8993(03)02521-6. [DOI] [PubMed] [Google Scholar]
- 172.Danuser H, Thor KB. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the alpha 1 adrenergic receptor antagonist prazosin. J Urol. 1995;153:1308–12. [PubMed] [Google Scholar]
- 173.Gajewski J, Downie JW, Awad SA. Experimental evidence for a central nervous system site of action in the effect of alpha-adrenergic blockers on the external urinary sphincter. J Urol. 1984;132:403–9. doi: 10.1016/s0022-5347(17)49637-6. [DOI] [PubMed] [Google Scholar]
- 174.Burgard EC, Portbury S, Thor KB. Electrophysiology of motor neurons innervating the urethral sphincter of female rats. Soc Neurosci. 2008 Abstract 582.8. [Google Scholar]
- 175.Downie JW, Espey MJ, Gajewski JB. Alpha 2-adrenoceptors not imidazole receptors mediate depression of a sacral spinal reflex in the cat. Eur J Pharmacol. 1991;195:301–4. doi: 10.1016/0014-2999(91)90551-z. [DOI] [PubMed] [Google Scholar]
- 176.Ramage AG, Wyllie MG. A comparison of the effects of doxazosin and terazosin on the spontaneous sympathetic drive to the bladder and related organs in anaesthetized cats. Eur J Pharmacol. 1995;294:645–50. doi: 10.1016/0014-2999(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 177.Krier J, de Groat WC. Effects of clonidine on the lumbar sympathetic pathways to the large intestine and urinary bladder of the cat. Eur J Pharmacol. 1979;59:47–53. doi: 10.1016/0014-2999(79)90023-2. [DOI] [PubMed] [Google Scholar]
- 178.Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol. 1996;118:150–4. doi: 10.1111/j.1476-5381.1996.tb15378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Conlon K. Identification of 5-HT2C-mediated mechanisms involved in urethral sphincter reflexes. Abstr Soc Neurosci. 2005;48:14. doi: 10.1111/j.1464-410X.2011.10756.x. [DOI] [PubMed] [Google Scholar]
- 180.Mbaki Y, Ramage AG. Investigation of the role of 5-HT(2) receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol. 2008;155:343–56. doi: 10.1038/bjp.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ogier R, Tribollet E, Suarez P, et al. Identified motoneurons involved in sexual and eliminative functions in the rat are powerfully excited by vasopressin and tachykinins. J Neurosci. 2006;26:10717–26. doi: 10.1523/JNEUROSCI.3364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holmes GM, Rogers RC, Bresnahan JC, et al. Differential effects of intrathecal thyrotropin-releasing hormone (TRH) on perineal reflexes in male rats. Physiol Behav. 1997;61:57–63. doi: 10.1016/s0031-9384(96)00317-4. [DOI] [PubMed] [Google Scholar]
- 183.Kimura Y, Hamada K, Taniguchi N, et al. CNS-mediated influence of TRH and its analog, NS-3, on the function of the rabbit lower urinary tract. J Auton Nerv Syst. 1996;60:1–11. doi: 10.1016/0165-1838(96)00021-5. [DOI] [PubMed] [Google Scholar]
- 184.Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69:24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- 185.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J Urol. 1993;150:1668–80. doi: 10.1016/s0022-5347(17)35868-8. [DOI] [PubMed] [Google Scholar]
- 186.McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147:S62–79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Torrisi G, Sampugnaro EG, Parralardo EM, et al. Postpartum urinary stress incontinence: Analysis of the associated risk factors and neurophysiological tests. Minerva Ginecol. 2007;59:491–8. [PubMed] [Google Scholar]
- 188.Damaser MS, Broxton-King C, Ferguson C, et al. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 189.Lin YH, Liu G, Daneshgari F. A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn. 2008;27:353–8. doi: 10.1002/nau.20509. [DOI] [PubMed] [Google Scholar]
- 190.Katofiasc MA, Nissen J, Audia JE, et al. Comparison of the effects of serotonin selective, norepinephrine selective, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats. Life Sci. 2002;71:1227–36. doi: 10.1016/s0024-3205(02)01848-9. [DOI] [PubMed] [Google Scholar]
- 191.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Case-control study of medical comorbidities in women with interstitial cystitis. J Urol. 2008;179:2222–5. doi: 10.1016/j.juro.2008.01.172. [DOI] [PubMed] [Google Scholar]
- 192.Clauw DJ, Schmidt M, Radulovic D, et al. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–31. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 193.Alagiri M, Chottiner S, Ratner V, et al. Interstitial cystitis: Unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–7. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 194.Brand K, Littlejohn G, Kristjanson L, et al. The fibromyalgia bladder index. Clin Rheumatol. 2007;26:2097–103. doi: 10.1007/s10067-007-0626-x. [DOI] [PubMed] [Google Scholar]
- 195.Fenton BW. Limbic associated pelvic pain: A hypothesis to explain the diagnostic relationships and features of patients with chronic pelvic pain. Med Hypotheses. 2007;69:282–6. doi: 10.1016/j.mehy.2006.12.025. [DOI] [PubMed] [Google Scholar]


