Abstract
Aim
The urothelium, or epithelial lining of the lower urinary tract (LUT), is likely to play an important role in bladder function by actively communicating with bladder nerves, smooth muscle, and cells of the immune and inflammatory systems. Recent evidence supports the importance of non-neuronal cells that may extend to both the peripheral and central processes of the neurons that transmit normal and nociceptive signals from the urinary bladder. Using cats diagnosed with a naturally occurring syndrome termed feline interstitial cystitis (FIC), we investigated whether changes in physiologic parameters occur within 3 cell types associated with sensory transduction in the urinary bladder: 1) the urothelium, 2) identified bladder dorsal root ganglion (DRG) neurons and 3) grey matter astrocytes in the lumbosacral (S1) spinal cord. As estrogen fluctuations may modulate the severity of many chronic pelvic pain syndromes, we also examined whether 17β-estradiol (E2) alters cell signaling in rat urothelial cells.
Results
We have identified an increase in nerve growth factor (NGF) and substance P (SP) in urothelium from FIC cats over that seen in urothelium from unaffected (control) bladders. The elevated NGF expression by FIC urothelium is a possible cause for the increased cell body size of DRG neurons from cats with FIC, reported in this study. At the level of the spinal cord, astrocytic GFAP immuno-intensity was significantly elevated and there was evidence for co-expression of the primitive intermediate filament, nestin (both indicative of a reactive state) in regions of the FIC S1 cord (superficial and deep dorsal horn, central canal and laminae V-VIl) that receive input from pelvic afferents. Finally, we find that E2 triggers an estrus-modifiable activation of p38 MAPK in rat urothelial cells. There were cyclic variations with E2-mediated elevation of p38 MAPK at both diestrus and estrus, and inhibition of p38 MAPK in proestrous urothelial cells.
Conclusion
Though urothelial cells are often viewed as bystanders in the processing of visceral sensation, these and other findings support the view that these cells function as primary transducers of some physical and chemical stimuli. In addition, the pronounced activation of spinal cord astrocytes in an animal model for bladder pain syndrome (BPS) may play an important role in the pain syndrome and open up new potential approaches for drug intervention.
Keywords: bladder epithelium, nerve growth hormone, sensor function, spinal cord astrocytes
INTRODUCTION
Though the urinary bladder urothelium has classically been thought of as a passive barrier to ions/solutes, recent studies have revealed that these cells exhibit specialized sensory and signaling properties.1 Evidence suggests that the urothelium exhibits both “sensor” (expressing receptors/ion channels capable of responding to thermal, mechanical, and chemical stimuli) as well as “transducer” (ability to release chemicals) properties. Modification of the urothelium in a number of pathologic conditions can result in altered release of urothelial-derived mediators, which can activate local sensory nerves. Additionally, our finding that 17-β-estradiol triggers an estrus-modifiable activation of p38 mitogen-activated protein kinase (MAPK) in bladder urothelial cells suggests that signaling by these cells can be altered by hormonal stimuli.
These functions also may be altered by diseases affecting the urinary bladder. For example, elevated levels of neurotrophin (NT) nerve growth factor (NGF) have been reported in a number of bladder pathologies, including bladder pain syndrome/interstitial cystitis (BPS/IC).2–4 In cats diagnosed with a comparable disease (feline interstitial cystitis, FIC), we report increased urothelial expression of NGF and a trend toward neuronal dorsal root ganglion (DRG) hypertrophy. The importance of non-neuronal cells may extend to both the peripheral and central processes of the neurons that transmit normal and nociceptive signals from the urinary bladder. In this article, we report significant changes in the immunointensity and morphology of FIC spinal cord glial cells (astrocytes) in regions to which pelvic afferents project. These and other findings suggest that alterations in “non-neuronal cell” signaling may contribute to the sensory abnormalities in a number of pelvic disorders.
ANATOMY AND BARRIER FUNCTION OF THE UROTHELIUM
The urothelium is the epithelial lining of the lower urinary tract (LUT) between the renal pelvis and the urinary bladder (Fig. 1). It is composed of at least three layers: a basal cell layer attached to a basement membrane; an intermediate layer; and a superficial or apical layer composed of large hexagonal cells (25–250 μm in diameter) known as “umbrella cells.”5,6 The umbrella cells are covered on nearly 70–80% of their apical surface by crystalline proteins called uroplakins, which assemble into hexagonal plaques.7,8 Uroplakins and other urothelial cellular differentiation markers, such as cytokeratin-20, are not expressed in the stratified epithelium of the urethra.9 In some species, the umbrella cells, and perhaps also the intermediate cells, extend projections to the basement membrane.7,10
Fig. 1.
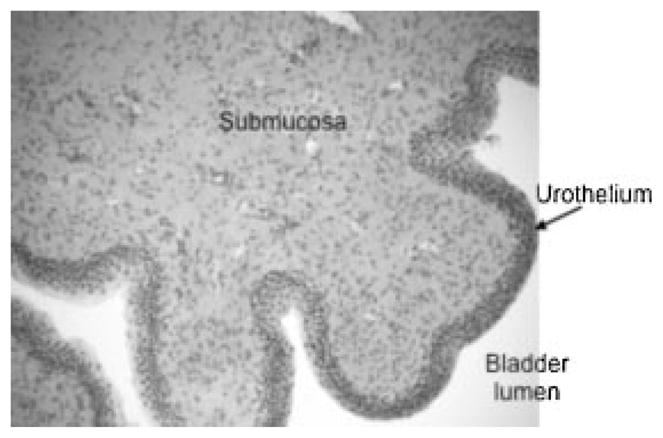
Cross-section of urinary bladder wall. H&E stained cross-section of the cat urinary bladder wall.
The ability of the bladder to maintain its barrier function despite large alterations in urine volume and increases in pressure during bladder filling and emptying is conferred by several unique features of the umbrella cells. These features include tight-junction complexes composed of multiple proteins, such as the claudins, which reduce the movement of ions and solutes between cells and specialized lipid molecules, and uroplakin proteins in the apical membrane, which reduce the permeability of the cells to small molecules (water, urea, protons).7,8,11 A sulfated polysaccharide glycosaminoglycan (GAG) or mucin layer covers the apical surface. This layer is thought to act as a non-specific anti-adherence factor and as a defense mechanism against infection.9,12
During bladder filling, the umbrella cells become flat and squamous. This shape change is accompanied by vesicular traffic (i.e., exocytosis/endocytosis) that adds additional membrane to the apical surface. The resultant overall increase in surface area allows the bladder to accommodate increasing volumes of urine during filling without compromising its barrier function.13–16 There is evidence that stretch-induced exocytosis depends on activation of the epidermal growth factor receptor (EGFR).17 There is also interest in the role of exocytosis/endocytosis (vesicular recycling) in modulating urothelial release of neurotransmitters/mediators, as well as a multitude of receptors, channels, and other proteins expressed by urothelial cells.
ROLES FOR UROTHELIAL CELLS IN VISCERAL SENSATION
Although urothelial cells are often viewed as bystanders in the process of visceral sensation, recent evidence has supported the view that these cells function as primary transducers of some physical and chemical stimuli and are able to communicate with underlying cells, including bladder nerves, smooth muscle, and inflammatory cells.
There are at least three lines of evidence suggesting that urothelial cells participate in the detection of both physical and chemical stimuli. First, recent studies have shown that bladder nerves (sensory afferent and autonomic efferent) are localized in close proximity to, and some within, the urothelium. Peptidergic, P2X-, and TRPV1-immunoreactive nerve fibers presumed to arise from afferent neurons in the lumbosacral DRG are distributed throughout the urinary bladder musculature as well as in a plexus beneath, and extending into, the urothelium.1,18 In humans with neurogenic detrusor overactivity (NDO), intravesical administration of resiniferatoxin, a C-fiber afferent neurotoxin, reduces the density of TRPV1 and P2X3 immunoreactive suburothelial nerves, indicating that these are sensory nerves.19,20 Studies have also revealed evidence that both adrenergic (tyrosine hydroxylase) and cholinergic (vesicular choline transporter, VAChT) nerves are in close proximity to the urothelium.21,22
A network of cells with morphologic characteristics similar to those of myofibroblasts or interstitial cells are also detected in the suburothelial space of the bladder in both humans and animals.23,24 These cells, which are extensively linked by gap junctions and have close contacts with nerves, can respond to neurotransmitters such as adenosine triphosphate (ATP) released from nerves or urothelial cells, suggesting that they could act as intermediaries in urothelial–nerve interactions.23–26 Thus, the anatomic substrates for bidirectional urothelial–neural communication exist within the urinary bladder.
A second line of evidence suggesting that urothelial cells play a role in sensory function is the expression of numerous receptors/ion channels that are linked to mechano- or nociceptive sensations. Examples of neuronal “sensor molecules” (receptors/ion channels) that have been identified in the urothelium include receptors for purines (P2X1–7 and P2Y1,2,4), adenosine (A1, A2a, A2b, and A3), norepinephrine (α and β), acetylcholine (muscarinic and nicotinic), protease-activated receptors (PARs), amiloride mechanosensitive channels (ENaC), bradykinin (B1 and B2), NTs (p75, trkA, EGF family ErbB1-3), corticotrophin-releasing factor (CRF2), estrogens (ERα and ERβ), endothelins, and various TRP channels (TRPV1, TRPV2, TRPV4, TRPM8, and TRPA1).17,18,27–38 The expression of these various receptors enables the urothelium to respond to a number of “sensor inputs” from a variety of sources. These inputs include increased stretch during bladder filling; soluble factors (many found in the urine), such as EGF, or chemical mediators/peptides/transmitters, such as substance P (SP), calcitonin gene-related peptide (CGRP), CRF, acetylcholine, adenosine, or norepinephrine released from nerves; inflammatory cells; and even blood vessels.1,7,14
The third line of evidence suggesting a sensory role for urothelial cells is the secretion of a number of transmitters or mediators capable of modulating, activating, or inhibiting sensory neurons. These include NTs, peptides, ATP, acetylcholine, prostaglandins, prostacyclin, nitric oxide (NO), and cytokines.1,14,37,39 For example, studies have shown that urothelial-derived NO can be released in response to mechanical and chemical stimulation and may either facilitate or inhibit the activity of bladder afferent nerves.1,40 There are also studies that demonstrate that the urothelium, via release of various soluble factors, can modulate the spontaneous activity of the underlying smooth muscle.25,41 However, the mechanism underlying this release, including whether all sensory “inputs” stimulate membrane turnover (i.e., vesicular exocytosis) is not well understood. What little is known about the roles and dynamics of membrane-bound cytoplasmic vesicles in urothelial cell physiology is taken from measurements of membrane capacitance and microscopy of fixed tissues and cells. In this regard, there is evidence that once released, ATP can act as an important autocrine mediator that can induce membrane turnover and enhance both stretch-induced exocytosis and endocytosis.42 Alterations in membrane turnover can not only increase apical surface area (as described above) but also influence the number and function of receptors and channels at the cell surface.
UROTHELIAL SENSOR TARGETS AND MEDIATORS: ROLE IN PATHOLOGY
ATP and the Urothelium
Since the first report of distension-evoked ATP release from the urothelium,33 there is now abundant evidence supporting a role for urothelial-derived release of ATP in autocrine and paracrine signaling within the LUT. ATP is released from both the apical and basolateral urothelial surfaces in response to bladder stretch and can act via stimulation of P2X2 and P2X3 urothelial receptors to stimulate exocytosis.42 The expression of both P2X and P2Y receptors in nerve fibers and myofibroblasts in close proximity to the bladder lumen, and the sensitivity of these cells to ATP, suggest that basolateral ATP release from the urothelium may also influence function of myofibroblasts and bladder nerves.43,44 There is also evidence that the amiloride-sensitive apical sodium channel, ENaC, may be involved in mechanotransduction by controlling basolateral release of ATP.45 In addition, intercellular communication mediated by gap junctions in myofibroblasts could provide a mechanism for long-distance spread of signals from the urothelium to the detrusor muscle.25 Adenosine is also produced and released by the urothelium and may play an important role in modulating sensory afferent function and smooth muscle contraction.37
Pathology results in augmented release of ATP from the bladder urothelium, which can cause painful sensations via excitation of purinergic receptors on nearby sensory fibers.31,46 This type of non-cholinergic mechanism is likely to play an important role in a number of bladder pathologies such as BPS/IC, which is chronic and characterized by urgency, frequency, and bladder pain upon filling.47–50 Consistent with findings in patients with BPS/IC,46 studies of FIC in cats also revealed an augmented stretch-evoked release of urothelium-derived ATP and changes in purinergic receptor profiles in urothelial cells,31,51 suggesting that urothelial sensor molecules are altered by disease.
Acetylcholine and the Urothelium
There is evidence that the urothelium expresses the full complement of muscarinic receptors, as well as the enzymes necessary for the synthesis and release of acetylcholine.39,52 Further, the urothelium is able to release acetylcholine following both chemical and mechanical stimulation.39 Once released, urothelial-derived acetylcholine is likely to exert effects via a number of sites including smooth muscle and nerves, as well as urothelial-associated muscarinic (or nicotinic) receptors; the latter could contribute to feedback mechanisms modifying urothelial function.39,53 In addition, stimulation of urothelial cholinergic receptors elicits release of mediators such as NO and ATP, which could alter bladder sensation by stimulating nearby sensory afferent nerves.54 Release of urothelial-derived mediators can also induce spread of Ca2+-transients that begin near the urothelial–suburothelial interface and spread to the detrusor smooth muscle, raising the possibility that the urothelium may initiate the generation of spontaneous, non-voiding contractions in the urinary bladder.25,26 Thus, targeting muscarinic receptors and/or urothelial release mechanisms may play an important role in the treatment of a number of bladder disorders. Accordingly, recent evidence suggests that botulinum toxins prevent the release of transmitters from the urothelium, which may suggest that urothelial-released mediators contribute to sensory urgency.55
Estrogen and the Urothelium
The steroid hormone 17β-estradiol (E2) is a key regulator of growth, differentiation, and function in a wide array of target tissues. Its predominant biologic effects are mediated through two distinct estrogen receptors (ER); ERα and ERβ.56 In addition to the well-established classical (“genomic”) pathway, which involves interaction with an estrogen response element on the promoter region of the target gene, E2 can exert rapid “non-genomic” effects,57 which involve putative estrogen-binding proteins in the cell membrane and cytoplasm.58 Rapid effects of estrogen involve activation of distinct signal transduction cascades, such as the MAPK pathways. The p38 MAPK pathway can be activated in response to chemical and physical stress and has therefore been termed a “stress-activated kinase.”59
To investigate the possibility that E2 may trigger an estrus-modifiable activation of p38 MAPK in the urothelium, which expresses both ERα and ERβ,60 urothelial cells isolated from rats in diestrus, proestrus, and estrus phases61 were treated with vehicle (control) or E2 (17-β estradiol; 10 nM; 60 min). As shown in Figure 2, we noted that treatment with E2 resulted in an increase in p38 MAPK phosphorylation that was significant in urothelial cells isolated from estrus rats (P < 0.05 compared to unstimulated cells). We also saw some increase in p38 MAPK phosphorylation in diestrus rats, but a slight decrease in proestus rats. During proestrus, there is a surge in estrogen followed by progesterone61 that could desensitize the response to E2. We hypothesized that the inhibitory effect we saw in proestrus rats may be related to a recent finding of an inhibitory role for the G protein-coupled receptor 30 (GPR30) in mediating E2-induced effects in human urothelial cells.62,63 Our finding of a phase-related p38 MAPK activation may suggest autocrine activation, possibly mediated by NGF, with a cyclical variation peaking in the proestrus phase. Further studies are needed to elucidate the full range of the influences of alterations in ovarian hormones on LUT structure and function, which potentially may be important in a number of bladder dysfunctions, such as urethra and pelvic floor weakness, detrusor instability, bladder pain syndrome, and even underactive detrusor.
Fig. 2.
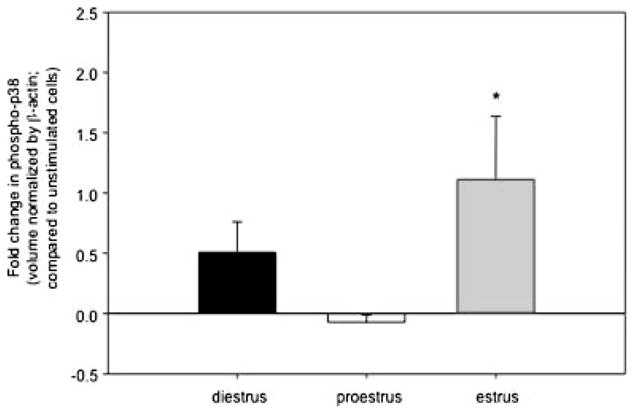
E2 triggers an estrus-modifiable activation of p38 MAP kinase in rat urothelial cells. Urothelial cells isolated from rats in diestrus, proestrus, and estrus phases were treated with 10 nM of β-estradiol (or vehicle, saline) for 60 min. Levels of phospho-p38 in cellular whole cell lysates were determined after Western blotting. *P < 0.05; Mann–Whitney rank sum test; n = 2–4.
UROTHELIAL RESPONSE TO INJURY
A variety of local and distant events can result in damage to the urothelium. For example, local factors, such as tissue pH, mechanical or chemical trauma, or bacterial infection can degrade the barrier function.64,65 Basal cells, which are thought to be precursors for other cell types, normally exhibit a slow (3–6 month) turnover rate—in fact, the slowest turnover of any mammalian epithelial cells.10,64 While neither urine-derived factors nor cyclic mechanical changes contribute to urothelial differentiation, injury readily accelerates proliferation. For example, protamine sulfate, which selectively damages the umbrella cell layer, rapidly induces both proliferation and differentiation to restore the barrier.66a The initiation of urothelial proliferation is also thought to involve up-regulation of growth factors. Besides NGF,66b fibroblast, epidermal, and transforming growth factors have been shown to initiate urothelial proliferation.67,68
BPS/IC, spinal cord injury, and even external environmental events can also change the urothelial barrier.65,69–72 When the barrier is compromised, water, urea, and toxic substances can pass into the underlying tissue (neural/muscle layers) resulting in urgency, frequency, and pain during bladder filling and voiding. In some pathologic conditions, the disruption of the urothelial barrier is associated with ultrastructural changes and alterations in the levels of chemical mediators, such as NO and ATP. Disruption of urothelial-barrier integrity has also been linked to the expression of substances such as anti-proliferative factor (APF), a frizzled-8 protein detected in the urine of patients with BPS/IC. Bladder epithelial cells obtained from these symptomatic patients also secrete APF.73,74 Further, purified APF treatment of urothelial cells from normal patients decreases the expression of adhesion and tight junction proteins and slows urothelial growth.
Disruption of urothelial barrier function by more remote pathologic conditions may be mediated by neural and/or hormonal mechanisms. For example, spinal cord transection in rats leads to a rapid alteration in the urothelial barrier, including ultrastructural changes and increased permeability.69 The changes were blocked by pretreatment with a ganglionic blocking agent, suggesting an involvement of efferent autonomic pathways on bladder urothelium in the acute effects of spinal cord injury. Based on recent reports that various stimuli induce urothelial cells to release chemical mediators that can in turn modulate the activity of afferent nerves, other types of urothelial–neural interactions are also likely.1,7 This has raised the possibility that the urothelium may have a role in sensory mechanisms in the urinary tract.
NGF also influences bladder responses to injury. NGF belongs to the family of NTs, which in mammals include the NT-3, NT-4/5 and brain-derived neurotrophic factor (BDNF).75 The biologic effects of NTs are mediated by a common pan-NT low-affinity receptor (p75NTR) and three high-affinity tyrosine kinase-transducing receptors (trkA, trkB, trkC). NGF-induced signals are mediated by p75 and trkA receptors. Activation of p75NTR alone is reported to promote apoptosis, whereas NGF promotes cell survival by acting on trkA receptors.76 NGF was the first NT to be characterized, based on its ability to stimulate growth, differentiation, survival, and maintenance of peripheral sensory and sympathetic neurons during development and after injury.77 In adults, NGF modulates the sensitivity and plasticity of the nociceptive system.78–80 It also plays a role in central sensitization in nociception, which is likely to involve the synthesis and release of nociceptive peptides including SP and CGRP.81,82
In the periphery, NGF is produced and utilized by several non-neuronal cell types including immune inflammatory cells, epithelial cells, and smooth muscle cells; because of these functions, NGF is better described as a pleiotropic factor.83 A specific “epitheliotrophic”84 role for NGF is seen in the epidermis, where it is endogenously synthesized and plays an important role in wound healing.85,86 Numerous inflammatory cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-α and IL-6, can induce NGF production in non-neuronal cell types, such as fibroblasts, endothelial cells, and glial cells.
NGF levels are increased in the bladder smooth muscle and urothelium in animal models for bladder outlet obstruction, spinal cord injury, and cyclophosphamide-induced inflammation.87,88 Increased NGF also has been identified in the urine of patients with overactivity and idiopathic sensory urgency, and in those diagnosed with BPS/IC.89 In fact, NGF has been proposed as a potential biomarker for certain bladder disorders due to the possible link between elevated NGF levels in tissue and urine and overactivity and painful inflammatory conditions.90 An antibody to NGF is currently in phase II clinical trials (ClinicalTrials.gov identifier NCT00601484) for BPS/IC-associated pain.91
As mentioned, cats diagnosed with FIC exhibit a number of similarities to BPS/IC in humans. We therefore compared NGF levels and associated receptors in urothelium from healthy and affected cats. Our investigation revealed a significant (P < 0.05) between-group difference, with higher NGF levels in FIC urothelium than in control (normal) urothelium (Table I). In addition, we also found increased expression of the trkA receptor and, to a lesser extent, in the low affinity p75 receptor (Table I). The trend for a greater increase in trkA expression over p75 suggests that NGF autocrine activity may be involved in urothelial cells survival and possibly in repair of the urothelium. In animals, increased target NGF expression has been linked with increased urinary frequency and unstable bladder contractions, and intravesical NGF produces a hyperreflexia suggestive of inflammatory pain, likely by sensitizing bladder afferents.92,93 Though the mechanism has not been established, our previous reports of increased spontaneous smooth muscle contractility and increased mechanical hypersensitivity in FIC support a possible role for augmented NGF.
TABLE I.
Levels of NGF (and Corresponding Receptors), Substance P, and Phospho p38 in Normal and FIC Cat Urothelium
| Normal | FIC | |
|---|---|---|
| NGF (pg/μg protein) | 5.814 ± 0.989 | 13.814 ± 1.510* |
| trkA (volume normalized by β-actin) | 941.870 ± 358.172 | 1,461.788 ± 470.851 |
| p75 (volume normalized by β-actin) | 49.737 ± 13.774 | 63.562 ± 14.156 |
| SP (pg/mg wet tissue weight) | 1.495 ± 0.726 | 2.827 ± 0.512* |
| Phospho-p38 (volume normalized by β-actin) | 32.438 ± 18.009 | 69.217 ± 18.194 |
Protein (mucosal) lysates from normal and FIC cat urinary bladders (n = 3–7 for each) were analyzed for NGF and SP by ELISA; trkA, p75, and phospho-p38 by Western immunoblotting.
Values are presented as units ± SEM. Statistical significances (*P < 0.05) between the groups were explored using the Student’s t-test.
Influence of Target-Organ Expression of NGF
NGF is internalized by the nerve endings of NGF-dependent neurons and is retrogradely transported to the neuronal soma.94 The presence of NGF and its associated receptors in the neuronal somata of subpopulations of adult sensory neurons has been reported.95 NGF has been associated with a number of features of neuroplasticity, including augmented cell size (following uptake from the target organ). Previous studies in cats with FIC have revealed an increased sensitivity to the TRPV1 agonist, capsaicin, and an increase in membrane capacitance (suggesting an increase in cell size).96 To determine if augmented mucosal NGF is associated with hypertrophy of identified DRG neurons, we examined the cell body diameter profile in bladder DRG neurons from FIC and normal cats. As shown in Figure 3, there was a rightward-shift in the cell soma diameter profile in the pathologic state. NGF is reported to cause an enlargement in the perikarya of adult DRG cells both in vitro94 and in vivo.97 The physiologic significance of the observed increase in DRG cell-body size in our findings is unknown but might occur to accommodate a larger cytosolic environment necessary for increased metabolic processes driven by NGF and other trophic factors.
Fig. 3.
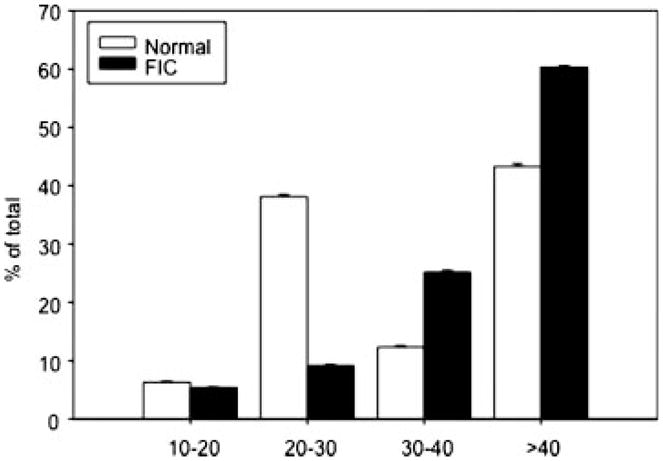
Diameter distribution of identified DRG neurons in FIC and normal cats. Mean number (±SEM) of identified (fast-blue dye labeled) DRG neurons in a minimum of 10 sections of the S1 DRG from FIC (n = 4) and normal (n = 4) animals.
One effect of increased NGF expression in the FIC bladder could be to increase sensitivity of the urothelial cells and sensory afferents. For example, NGF is reported to increase the intracellular content of SP in sensory neurons,98,99 and also can increase SP expression/release in non-neuronal cells. The expression of the neuropeptide SP was significantly higher in FIC urothelium (Table I), which may be as a consequence of the higher expression levels of NGF. We previously have reported increased excitability of low-threshold bladder afferents to distension and changes in electrical properties of neurons from FIC cats.100 Taken together, these findings may explain, in part, changes in bladder afferent activity in chronic bladder disorders such as BPS/IC.
MODULATION OF NEURONAL SIGNALING BY SPINAL CORD GLIA
Recent evidence has shown that spinal cord activation of glial cells (astrocytes and microglia) may be involved in both the development and maintenance of central sensitization in various chronic pain conditions.101 At the level of the spinal cord, the first relay site in the transmission of nociceptive information from the periphery to the brain,102,103 dorsal horn glial cells may be activated by neuropeptides/neurotransmitters released from primary afferent nerve terminals, such as SP, CGRP, NO, purines, glutamate, opioid peptides, and the chemokines fractalkine or neuractin. Activation may result in altered cell morphology, changes in receptor expression, or release of factors by glial cells, which in turn can lead to changes in neuronal function and ultimately enhance nociceptive transmission.101 There is evidence that microglia may mediate the activation of astrocytes seen in both somatic and visceral pain pathologies—the “neuropathic pain triad.”104 Generally, microglial activation is transient, while astrocytic activation is much longer lasting. However, activation of either of the two cell types promotes pain.101,105 Although most reports of the contribution of glia to nociception come from studies of somatic rather than visceral forms of chronic pain, there is now increasing evidence pointing to a role for glial cells as key modulators during visceral inflammatory pain.
Cats with FIC offer an opportunity to examine potential roles for glial cells in chronic bladder pathology. We compared the immune-intensity of glial fibrillary acidic protein (GFAP; an intermediate filament protein found in the astrocytes) of healthy versus FIC cats in lower lumbrosacral (S1) spinal cord regions, which receive sensory afferent input from pelvic viscera. As shown in Figures 4 and 5, there were significant (P < 0.05) increases in GFAP immuno-intensity (Fig. 4) and changes in morphology (Fig. 5a,b, representative images) in all regions of interest—the superficial and deep dorsal horn, near the central canal (lamina X), and laminae V–VII. This is an indicative of a reactive state of the astrocytes that is further supported by co-expression of GFAP and nestin, a more primitive intermediate filament106 in FIC spinal cord astrocytes (Fig. 5c, representative image). Astrocytes contribute to the regulation of local ion (notably K+) and pH homeostasis. In addition, these cells are involved in the clearance of synaptically released neurotransmitters, such as glutamate and GABA.107 These multifunctional cells are key players in what is termed the tripartite synapse, which comprises pre- and post-synaptic neurons and extra-synaptic astrocytic contacts. These cells have the potential to modify synaptic transmission and plasticity.108 Alterations in the physiology of astrocytes in response to inflammatory conditions, such as BPS/IC, may enhance synaptic transmission, laying the “physiologic” groundwork for the state of chronic pain. Pharmacologic interventions aimed at targeting neuronal–glial and glial–glial interactions may provide new targets for pharmacologic management of a number of bladder disorders.
Fig. 4.
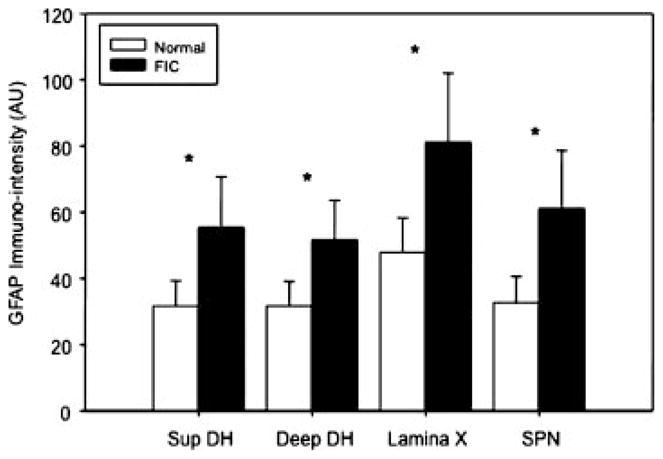
Increased GFAP immuno-intensity from FIC as compared to unaffected cat spinal cord. GFAP immuno-intensity levels in the S1 spinal cord superficial and deep dorsal horn; lamina × (surrounding the central canal) and the sacral parasympathetic nucleus (SPN region) from cats with FIC are higher compared with similar regions from unaffected cats (unpaired t-test; n = 4 each, FIC and unaffected).
Fig. 5.
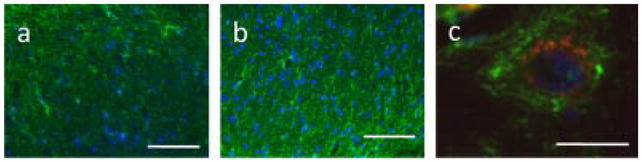
GFAP immuno-intensity in FIC and normal cat spinal cord. The immunointensity of glial fibrillary acidic protein (GFAP), an intermediate filament protein found in astrocytes, is significantly elevated in lower lumbosacral (S1) FIC spinal cord. a: Representative image of astrocytes (green—GFAP astrocytic marker; blue-4′,6-diamidino-2-phenylindole (DAPI)—used to stain cell nuclei) in S1 spinal cord dorsal horn (DH) region from a healthy cat as compared with similar S1 DH spinal cord region from an FIC cat (in b). c: FIC astrocytes (GFAP, green) co-express the intermediate filament, nestin (red), indicating a reactive state (blue—DAPI, nuclear marker). Scale bars: (a,b) 100 μm; (c), 10 μm.
SUMMARY
Recent studies suggest that there may be a number of common or shared mechanisms in bladder pain syndrome associated with other functional pain syndromes.47 For example, patients with bladder pain syndrome also suffer a variety of co-morbid symptoms and disorders, which can include irritable bowel syndrome (IBS), endometriosis, vulvodynia, fibromyalgia, rheumatoid arthritis, and even asthma. Though the etiology of these syndromes is incompletely understood, several factors may play an important role, such as changes in urothelial or epithelial sensor/barrier function, neurogenic inflammation, and even autoimmune involvement.109,110 There is evidence that defects in urothelial sensor molecules and urothelial-cell signaling are likely to contribute to the pathogenesis of bladder diseases.
Changes in epithelial signaling/barrier function are not unique to the urinary bladder. For example, airway epithelia in asthmatic patients, as well as keratinocytes in certain types of skin diseases, also exhibit a number of similar abnormalities and compromise repair processes.111 This is particularly relevant given the high incidence of associated diseases that can include both visceral and somatic conditions, many of which exhibit a shared loss of epithelial barrier function. In addition, glial cells (astrocytes and microglia) placed at the interface of communication between the periphery and the CNS may be major players in resetting and modulating these lines of communication with deleterious effects. Taken together, these non-neuronal cells can respond to a number of challenges, including environmental pollutants and mediators released from nerves or nearby inflammatory cells, resulting in altered expression and/or sensitivity of various receptors/channels, as well as changes in release of mediators, all of which could impact function.
METHODOLOGY
Animals
All procedures were conducted in accordance with Institutional Animal Care and Use Committee policies. Healthy and FIC adult cats were used for this study. All cats with FIC were obtained as donations from clients due to a history of chronic recurrent stranguria, hematuria, pollakiuria, and/or urination in inappropriate locations and were evaluated at The Ohio State University (OSU) Veterinary Teaching Hospital. Evaluation consisted of a complete physical examination (including body weight), complete blood count, serum biochemical analysis, urinalysis, urine bacteriological culture, and cystoscopy. Cystoscopy was performed using a 9-F rigid pediatric cystoscope (Karl Storz, Endoscopy America, Culver City, CA) in female cats and a 3-F flexible fiber optic cystoscope (Five Star Medical, San Jose, CA) in male cats. The diagnosis of FIC was based on compatible history and consideration of standard National Institutes of Health inclusion and exclusion criteria after the results of the above laboratory tests were obtained, including the presence of submucosal petechial hemorrhages (glomerulations) at cystoscopy. Healthy, age-matched cats obtained from commercial vendors and determined to be free of disease and signs referable to the LUT according to the same diagnostic criteria as cats with FIC were used as controls. All cats were housed in stainless steel cages in the OSU animal facilities and allowed to acclimate to their environment for at least 3 months before the study. For identification of bladder DRG somata, usual surgical aseptic precautions were employed to expose the bladder dome, which was injected with a retrogradely transported fluorescent dye (4% fast blue), in deeply anesthetized cats. Two to four weeks after injection, the animals were anesthetized and sacrificed via intracardiac perfusion first with Krebs buffer followed by 8% paraformaldehyde fixation. Serial frozen (10 μm) sections were mounted on slides (superfrost). The diameter of fast-blue labeled neurons in a ganglion was estimated from counts at moderate-to-high magnification of positively stained profiles in a minimum of 10 sections separated by 100 μm. All cell counts are presented as the mean number of identified neurons per 10 sections ± SEM from each ganglion. For the size distributions shown in Figure 3, the values reported are the percentage of identified neurons counted.
Tissue Preparation
Whole rat bladder and the bladder mucosa were homogenized separately in HBSS (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5.6 mM glucose, and 10 mM Hepes, pH 7.4) containing complete protease inhibitor cocktail (1 tablet/10 ml, Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Sigma, 1:100). The homogenate was centrifuged (13,000g; 15 min). The membrane protein fraction was prepared by suspending the membrane pellets in lysis buffer containing 0.3 M NaCl, 50 mM Tris–HCl (pH 7.6), and 0.5% Triton X-100 and the same concentration of protease inhibitors as above. The suspensions were incubated on ice and centrifuged (13,000 rpm; 15 min at 4°C). The protein concentrations of the combined supernatants were determined using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL).
NGF ELISA
Cytosol lysates were diluted with 4 volumes of D-PBS (0.02% KCl, 0.8% NaCl, 0.02% KH2PO4, 0.115% Na2HPO4, 0.0133% CaCl2 2H2O, and 0.01% MgCL2 6H2O, pH 7.35, Sigma). Following acidification (1 N HCl to pH 2.0–3.0) and neutralization (1 N NaOH to pH 7.5–8.0), the samples were assayed in duplicate by ELISA (Promega, Madison, WI) according to manufacturer instructions. Plates were read at 450 nm using a SpectraFluor Plus (Tecan, Maennedorf, Switzerland). The tissue NGF values were normalized against the protein concentrations of each sample and are expressed as pg/mg protein.
TrkA and p75 Immunoblotting
After denaturation (100°C for 5 min), lysate from each sample was separated on an SDS–PAGE gel using a standard Western protocol. Proteins were transferred to polyvinylidene fluoride membranes, blocked with 5% Milk TBS-T (1 hr), rinsed in TBS-T, and incubated (overnight at 4°C) with primary antibody [rabbit anti-TrkA or p75, Santa Cruz (Santa Cruz, CA) and Upstate (Waltham, MA), respectively] in 5% Milk TBS-T. The membranes were then incubated with secondary antibody (anti-rabbit IgG HRP, Santa Cruz) for 1 hr in 5% Milk TBS-T, developed with ECL Plus (Amersham, Piscataway, NJ), and exposed to film. The volume of each band was determined using a Personal Densitometer SI (Molecular Probes, Carlsbad, CA). The membranes were stripped (membrane recycling kit from Alpha Diagnostic International, San Antonio, TX) and reprobed overnight with rabbit anti-β-actin (Abcam, Cambridge, MA) as a loading control. Two immunoreactive bands (140 and 70 kDa) were observed by Western immunoblotting for TrkA. Both bands were blocked by the peptide; therefore, the volumes of both bands were totaled in our analysis. A single immunoreactive band was observed for p75 (75 kDa) and for β-actin (43 kDa).
p38 Immunoblotting
After denaturation at 100°C for 5 min, lysate from each sample was separated on an SDS–PAGE gel using a standard Western protocol. Western blots were probed with rabbit anti-phospho-p38 (Cell Signaling, Beverly, MA) diluted in 5% BSA TBS-T, followed by the secondary antibody, goat anti-rabbit IgG HRP (Santa Cruz) diluted in 5% Milk TBS-T. The membranes were stripped using a membrane recycling kit from Alpha Diagnostic International and reprobed using rabbit anti-p38 (in 5% BSA, Cell Signaling). Finally, the Western blots were stripped a second time and reprobed using rabbit anti-β-actin (Abcam Limited, Cambridgeshire, UK) and goat anti-rabbit IgG HRP (Santa Cruz). The volume of each band was determined using a Personal Densitometer SI (Molecular Probes, Eugene, OR) and a single immunoreactive band was observed for phospho-p38 at 38 kDa.
Substance P
The mucosa was stripped from underlying smooth muscle in both FIC and normal urinary bladder. Tissues were placed in 0.5 M boiling acetic acid for 10 min and homogenized with a Tissue Tearor (BioSpec Products, Brattlesville, OK). After centrifugation (14,000g, 15 min) the SP was extracted from the supernatants using C-18 SPE cartridges following the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI). SP was measured in a SP EIA kit from Cayman Chemical and is expressed as pg/mg wet tissue weight.
Immunohistochemistry
The lower lumbosacral spinal cord (S1) was dissected from deeply anesthetized (induction with 2% halothane; maintained with α-chloralose 60–70 mg/kg) cats diagnosed with FIC (n = 4) and healthy cats (n = 4). After removal of tissue, animals were humanely sacrificed and the spinal cord was post-fixed, placed in 30% sucrose solution, and subsequently frozen. Transverse cryo-sections (6 μm) were prepared and mounted on glass slides (Fisher Scientific, Pittsburgh, PA) in preparation for immunohistochemistry. GFAP (1:200, Sigma) antibody was used to identify astrocytes. Reactive astrocytes were identified by positive staining for Nestin (1:100, Abcam). FITC- and CY3-conjugated secondary antibodies were used to visualize binding. DAPI fluorescent stain (1:5,000, Molecular Probes) was used to detect cell nuclei. Non-specific staining was assessed in the absence of primary antibodies. Tissue was viewed on a Zeiss Axioplan microscope with an attached Leica DC 200 digital camera. Images were visualized, saved, and subsequently analyzed using C. Imaging software (Hamamatsu, PA).
Acknowledgments
This work was supported by NIH grants R37 DK54824 and R01 DK57284 (to L. Birder) and P50 DK64539 (L. Birder and C. Buffington), and K01 DK080184 (A.T. Hanna-Mitchell).
Grant sponsor: NIH; Grant numbers: R37 DK54824, R01 DK57284, P50 DK64539, K01 DK080184.
Footnotes
Conflicts of interest: none.
References
- 1.Birder LA, DeGroat WC. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat Clin Pract. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steers WD, Tuttle JB. Mechanisms of disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3:101–10. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- 3.Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–41. [PubMed] [Google Scholar]
- 4.Lowe EM, Anand P, Terenghi G, et al. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–7. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- 5.Staehelin LA, Chlapowski FJ, Bonneville MA. Lumenal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images. J Cell Biol. 1972;53:73–91. doi: 10.1083/jcb.53.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob J, Ludgate CM, Forde J, et al. Recent observations on the ultrastructure of human urothelium. 1. Normal bladder of elderly subjects. Cell Tissue Res. 1978;193:543–60. doi: 10.1007/BF00225350. [DOI] [PubMed] [Google Scholar]
- 7.Apodaca G. The uroepithelium: Not just a passive barrier. Traffic. 2004;5:117–28. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Acharya P, Beckel JM, Ruiz WG, et al. Distribution of the tight junction proteins ZPO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:F305–18. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 9.Romih R, Korosec P, de Mello WJ, Jr, et al. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–68. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 10.Martin BF. Cell replacement and differentiation in transitional epithelium: A histological and autoradiographic study of the guinea-pig bladder and ureter. J Anat. 1972;112:433–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–74. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 12.Parsons CL, Mulholland SG, Anwar H. Antibacterial activity of bladder surface mucin duplicated by exogenous glycosaminoglycan (heparin) Infect Immun. 1979;24:552–7. doi: 10.1128/iai.24.2.552-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282:F179–90. doi: 10.1152/ajprenal.2002.282.2.F179. [DOI] [PubMed] [Google Scholar]
- 14.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int. 2007;72:1057–64. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 15.Lewis S, de Moura J. Apical membrane area of rabbit urinary bladder increases by fusion of intracellular vesicle: An electrophysiological study. J Membr Biol. 1984;82:123–36. doi: 10.1007/BF01868937. [DOI] [PubMed] [Google Scholar]
- 16.Truschel ST, Wang E, Ruiz WG, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–46. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balestreire EM, Apodaca G. Apical epidermal growth factor receptor signaling: Regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell. 2007;18:1312–23. doi: 10.1091/mbc.E06-09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–60. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 19.Apostolidis A, Brady CM, Yiangou Y, et al. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–5. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Brady CM, Apostolidis A, Yiangou Y, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–53. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Lips KS, Wunsch J, Zarghooni S, et al. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007;51:1042–53. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Jen PY, Dixon JS, Gosling JA. Immunohistochemical localization of neuromarkers and neuropeptides in human fetal and neonatal urinary bladder. Br J Urol. 1995;75:230–5. doi: 10.1111/j.1464-410x.1995.tb07317.x. [DOI] [PubMed] [Google Scholar]
- 23.Brading AF, McCloskey KD. Mechanisms of disease: Specialized interstitial cells of the urinary tract—An assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546–54. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 24.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–43. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda Y, Fry C, Hayashi F, et al. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–25. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Kanai AJ. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: Role of mucosal muscarinic receptors. Am J Physiol. 2008;295:F454–61. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopra B, Barrick SR, Meyers S, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–71. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra B, Gever J, Barrick SR, et al. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol. 2008;294:F821–9. doi: 10.1152/ajprenal.00321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 30.Chess-Williams R. Muscarinic receptors of the urinary bladder: Detrusor, urothelial and prejunctional. Auton Autocoid Pharmacol. 2002;22:133–45. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 31.Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–9. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 32.Beckel JM, Kanai A, Lee S-J, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103–10. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rat urinary bladder epithelial cells by hydrostatic pressure changes—A possible sensory mechanism? J Physiol. 1997;505:503–11. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattino MD, Passero CJ, Steren CA, et al. Defining an inhibitory domain in the α-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2007;294:F47–52. doi: 10.1152/ajprenal.00399.2007. [DOI] [PubMed] [Google Scholar]
- 35.Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 36.LeBerge J, Malley SE, Zvarova K, et al. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- 37.Yu W, Zacharia LC, Jackson EK, et al. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291:C254–65. doi: 10.1152/ajpcell.00025.2006. [DOI] [PubMed] [Google Scholar]
- 38.Murray E, Malley SE, Qiao LY, et al. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172:2434–9. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- 39.Hanna-Mitchell AT, Beckel JM, Barbadora S, et al. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: Possible implications for physiology and pathophysiology. Scand J Urol Nephrol. 1995;175:43–53. [PubMed] [Google Scholar]
- 41.Templeman L, Chapple CR, Chess-Williams R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol. 2002;167:742–5. doi: 10.1016/S0022-5347(01)69137-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang EC, Lee JM, Ruiz WG, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–22. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sui G-P, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int. 2006;97:1327–31. doi: 10.1111/j.1464-410X.2006.06200.x. [DOI] [PubMed] [Google Scholar]
- 44.Fry C, Sui GP, Kanai AJ, et al. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914–9. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- 45.Du S, Araki I, Mikami Y, et al. Amiloride-sensitive ion channels in urinary bladder epithelium involved in mechanosensory transduction by modulating stretch-evoked adenosine triphosphate release. Urology. 2007;69:590–5. doi: 10.1016/j.urology.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Keay S, DeDeyne P, et al. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951–56. [PubMed] [Google Scholar]
- 47.Nickel JC. Interstitial cystitis—An elusive clinical target? J Urol. 2003;170:816–7. doi: 10.1097/01.ju.0000081996.84687.ac. [DOI] [PubMed] [Google Scholar]
- 48.Parsons CL, Greenberger M, Gabal L, et al. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–7. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 49.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 50.Gillenwater JY, Wein AJ. Summary of the national institute of arthritis, diabetes, and kidney diseases workshop on interstitial cystitis. J Urol. 1998;140:205. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 51.Birder LA, Ruan HZ, Chopra B, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–91. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 52.Zarghooni S, Wunsch J, Bodenbenner M, et al. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci. 2007;80:2308–13. doi: 10.1016/j.lfs.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 53.Hanna-Mitchell A, de Groat W, Kanai A, et al. The correlation of vesicular traffic and transmitter release in bladder urothelial cells: Involvement of urothelial muscarinic receptors and overactive bladder. International Continence Society Annual Meeting; Rotterdam, Netherlands. August 20–24, 2007; Poster 69. [Google Scholar]
- 54.Kullman FA, Artim DE, Birder LA, et al. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–87. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chancellor MB, Fowler CJ, Apostolidis A, et al. Drug insight: Biological effects of botulinum toxin A in the lower urinary tract. Nat Clin Pract Urol. 2008;5:319–28. doi: 10.1038/ncpuro1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–72. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 57.Seval Y, Cakmak H, Kayisli UA, et al. Estrogen-mediated regulation of p38 mitogen-activated protein kinase in human endometrium. J Clin Endocrinol Metab. 2006;91:2349–57. doi: 10.1210/jc.2005-2132. [DOI] [PubMed] [Google Scholar]
- 58.Song RX, Santen RJ. Membrane initiated estrogen signaling in breast cancer. Biol Reprod. 2006;75:9–16. doi: 10.1095/biolreprod.105.050070. [DOI] [PubMed] [Google Scholar]
- 59.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 60.Teng J, Wang ZY, Bjorling DE. Estrogen-induced proliferation of urothelial cells is modulated by nerve growth factor. Am J Physiol. 2002;282:F1075–83. doi: 10.1152/ajprenal.00215.2001. [DOI] [PubMed] [Google Scholar]
- 61.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 62.Filardo EQJ, Pang Y, Graeber C, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 63.Teng J, Wang ZY, Prossnitz ER, et al. The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology. 2008;149:4024–34. doi: 10.1210/en.2007-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hicks M. The mammalian urinary bladder: An accommodating organ. Biol Rev Camb Philos Soc. 1975;50:215–46. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 65.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 66.66a Lavelle J, Meyers S, Ramage R, et al. Bladder permeability barrier: Recovery from selective injury of surface epithelial cells. Am J Physiol. 2002;283:F242–53. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]; 66b Teng J, Wang Z-Y, Bjorling DE. Estrogen-induced proliferation of urothelial cells is modulated by nerve growth factor. Am J Physiol. 2002;282:F1075–83. doi: 10.1152/ajprenal.00215.2001. [DOI] [PubMed] [Google Scholar]
- 67.de Boer WI, Vermeij M, Diez de Medina SG, et al. Functions of fibroblast and transforming growth factors in primary organoid-like cultures of normal human urothelium. Lab Invest. 1996;75:147–56. [PubMed] [Google Scholar]
- 68.Bassuk JA, Cochrane K, Mitchell ME. Induction of urothelial cell proliferation by fibroblast growth factor-7 in RAG1-deficient mice. Adv Exp Med Biol. 2003;539:623–33. doi: 10.1007/978-1-4419-8889-8_40. [DOI] [PubMed] [Google Scholar]
- 69.Apodaca G, Kiss S, Ruiz WG, et al. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol. 2003;284:F966–76. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 70.Buffington CA, Westropp JL, Chew DJ, et al. Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg. 2006;8:261–8. doi: 10.1016/j.jfms.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chai TC, Keay S. New theories in interstitial cystitis. Nat Clin Pract Urol. 2004;1:85–9. doi: 10.1038/ncpuro0057. [DOI] [PubMed] [Google Scholar]
- 72.Lavelle JP, Meyers SA, Ruiz WG, et al. Urothelial pathophysiological changes in feline interstitial cystitis: A human model. Am J Physiol Renal Physiol. 2000;278:F540–53. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- 73.Conrads TP, Tocci GM, Hood BL, et al. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem. 2006;281:37836–43. doi: 10.1074/jbc.M604581200. [DOI] [PubMed] [Google Scholar]
- 74.Keay SK, Szekely Z, Conrads TP, et al. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004;101:11803–8. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sendtner M. Molecular biology of neurotrophic factors. Baillieres Clin Neurol. 1995;4:575–91. [PubMed] [Google Scholar]
- 76.Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–94. [PubMed] [Google Scholar]
- 77.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 78.Mendell LM, Albers KM, Davis BM, et al. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–61. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 79.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 80.Petruska JC, Mendell LM. The many functions of nerve growth factor: Multiple actions on nociceptors. Neurosci Lett. 2004;361:168–71. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430–6. doi: 10.1016/j.expneurol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz G, Banos JE. The effect of endoneurial nerve growth factor on calcitonin gene-related peptide expression in primary sensory neurons. Brain Res. 2005;1042:44–52. doi: 10.1016/j.brainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Micera A, Lambiase A, Stampachiacchiere B, et al. Nerve growth factor and tissue repair remodeling: trkA (NGFR) and p75 (NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18:245–56. doi: 10.1016/j.cytogfr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Botchkarev VA, Metz M, Botchkareva NV, et al. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 act as “epitheliotrophins” in murine skin. Lab Invest. 1999;79:557–72. [PubMed] [Google Scholar]
- 85.Li AK, Koroly MJ, Schattenkerk ME, et al. Nerve growth factor: Acceleration of the rate of wound healing in mice. Proc Natl Acad Sci USA. 1980;77:4379–81. doi: 10.1073/pnas.77.7.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsuda H, Koyama H, Sato H, et al. Role of nerve growth factor in cutaneous wound healing: Accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim JC, Kim DB, Seo S, et al. Nerve growth factor and vanilloid receptor expression, and detrusor instability, after relieving bladder outlet obstruction in rats. BJU Int. 2004;94:915–8. doi: 10.1111/j.1464-4096.2003.05059.x. [DOI] [PubMed] [Google Scholar]
- 88.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–84. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 89.Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydro-distension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007;70:463–8. doi: 10.1016/j.urology.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 90.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int. 2008;102:1440–4. doi: 10.1111/j.1464-410X.2008.07757.x. [DOI] [PubMed] [Google Scholar]
- 91.Vastag B. Monoclonals expand into neural disorders. Nat Biotechnol. 2006;24:595–6. doi: 10.1038/nbt0606-595. [DOI] [PubMed] [Google Scholar]
- 92.Seki S, Sasaki K, Fraser MO, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–74. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 93.Chuang YC, Fraser MO, Yu Y, et al. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–9. [PubMed] [Google Scholar]
- 94.Yasuda T, Sobue G, Ito T, et al. Nerve growth factor enhances neurite arborization of adult sensory neurons: A study in single-cell culture. Brain Res. 1990;524:54–63. doi: 10.1016/0006-8993(90)90491-s. [DOI] [PubMed] [Google Scholar]
- 95.Sobue G, Yasuda T, Mitsuma T, et al. Nerve growth factor receptor immunoreactivity in the neuronal perikarya of human sensory and sympathetic nerve ganglia. Neurology. 1989;39:937–41. doi: 10.1212/wnl.39.7.937. [DOI] [PubMed] [Google Scholar]
- 96.Sculptoreanu A, de Groat WC, Buffington CA, et al. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol. 2005;193:437–43. doi: 10.1016/j.expneurol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Yip HK, Rich KM, Lampe PA, et al. The effects of nerve growth factor and its antiserum on the postnatal development and survival after injury of sensory neurons in rat dorsal root ganglia. J Neurosci. 1984;4:2986–92. doi: 10.1523/JNEUROSCI.04-12-02986.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–4. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 99.Adler JE, Kessler JA, Black IB. Development and regulation of substance P in sensory neurons in vitro. Dev Biol. 1984;102:417–25. doi: 10.1016/0012-1606(84)90206-9. [DOI] [PubMed] [Google Scholar]
- 100.Roppolo JR, Tai C, Booth AM, et al. Bladder Adelta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol. 2005;173:1011–5. doi: 10.1097/01.ju.0000145591.35569.9e. [DOI] [PubMed] [Google Scholar]
- 101.Ren K, Dubner R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–9. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 103.Peters A, Palay SL, Webster H. The fine structure of the nervous system: Neurons and their supporting cells. 3. New York: Oxford University Press; 1991. [Google Scholar]
- 104.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 105.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Tamagno I, Schiffer D. Nestin expression in reactive astrocytes of human pathology. J Neurooncol. 2006;80:227–33. doi: 10.1007/s11060-006-9181-6. [DOI] [PubMed] [Google Scholar]
- 107.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 108.Araque A, Parpura V, Sanzgiri RP, et al. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 109.Hendrix S. Neuroimmune communication in skin: Far from peripheral. J Invest Dermatol. 2008;128:260–1. doi: 10.1038/sj.jid.5701171. [DOI] [PubMed] [Google Scholar]
- 110.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. 2007;4:484–91. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 111.Bosse Y, Paré PD, Seow CY. Airway wall remodeling in asthma: From the epithelial layer to the adventitia. Curr Allergy Asthma Rep. 2008;8:357–66. doi: 10.1007/s11882-008-0056-0. [DOI] [PubMed] [Google Scholar]


