Abstract
BACKGROUND
In several types of cancer, up-regulation of phosphatidylinositol 3-kinase (PI3K)-Akt signaling facilitates tumor cell growth and inhibits apoptosis. Previous reports demonstrate that this pathway promotes growth, survival, and chemotherapy resistance in non-small cell and small cell lung cancer cells. However, the importance of PI3K-Akt signaling has not been explored in pulmonary carcinoids. In this study, our objective was to establish the role of the PI3K-Akt signal transduction pathway in pulmonary carcinoid cells.
STUDY DESIGN
Human pulmonary carcinoid NCI-H727 cells were treated with LY294002 (0-100 μM), a well-known PI3K inhibitor, or transfected with Akt1 siRNA (75 nM). Cellular proliferation was measured by MTT assay for up to 8 days. Western analysis was performed for expression of active, phosphorylated Akt (pAkt), total Akt, Akt1, and the neuroendocrine markers chromogranin A (CgA) and achaete-scute complex-like1 (ASCL1).
RESULTS
Treatment of NCI-H727 cells with LY294002 significantly reduced tumor cell growth (85.3%). Similarly, Akt1 siRNA transfection led to diminished tumor cell proliferation (31.3%). A dose-dependent decrease in CgA and ASCL1 production was observed with both PI3K inhibition and Akt1 RNA interference. Expression of Akt1 was reduced at all time points by transient Akt1 siRNA transfection.
CONCLUSIONS
The PI3K-Akt pathway plays a role in both tumor cell growth and neuroendocrine hormone secretion in human pulmonary carcinoid cells. Inhibition of Akt1, PI3K-Akt signaling, or a downstream mediator of this pathway may provide therapeutic approaches for patients with pulmonary carcinoid tumors.
Keywords: Pulmonary carcinoid, PI3K, Akt, LY294002, NCI-H727
Pulmonary carcinoid tumors are neuroendocrine malignancies that develop in the bronchopulmonary epithelium. These low-grade malignant neoplasms arise from Kulchitsky or enterochromaffin-like cells and have an age-adjusted annual incidence between 0.4 and 0.9 cases per 100,000 people (1). Over the last 30 years, the incidence of pulmonary carcinoids has more than doubled (1). Furthermore, up to 35% of patients present with unlocalized disease (1). The 5-year survival for patients with distant metastases is approximately 26%, compared to 81% for patients with localized tumors (1). Patients with pulmonary carcinoids suffer from symptoms secondary to luminal obstruction and ulceration, such as cough, hemoptysis, pneumonia, chest pain, and dyspnea. Currently, the only potentially curative treatment option for patients with pulmonary carcinoid tumors is surgical resection (2). Effective therapies for patients with unresectable disease are lacking since radiotherapy, systemic chemotherapy, and biotherapy have all shown limited success (3). As a result, innovative therapies are necessary to address patients who present with complex pulmonary carcinoid disease.
One strategy that has been explored in several cancers is manipulation of signaling pathways like the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. Overactivation of Akt signaling has been demonstrated in breast and colon cancer, as well as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) (4-6). PI3K-Akt signaling normally regulates cell survival, proliferation, and motility. Akt, also known as protein kinase B, is the key effector of the pathway and must be phosphorylated at two distinct sites, threonine 308 and serine 473, to be active. PI3K and phosphate-dependent dehydrogenase kinase-1 (PDK1) are responsible for this Akt phosphorylation. Active, phosphorylated Akt (pAkt) then modulates several downstream substrates including, but not limited to various caspases and Forkhead transcription factors. An additional important point about PI3K-Akt signaling is that 3 isoforms of Akt exist: Akt1, 2, and 3 (or protein kinase B-alpha, beta, and gamma, respectively). These isoforms appear to have tissue-specific roles and differential clinical implications (7).
In both NSCLC and SCLC cell lines, PI3K-Akt signaling has been shown to play an important role in cell survival and growth (5, 6). In addition, Akt1 inhibition has been shown to be critical in sensitizing NSCLC and SCLC to chemotherapy and radiation (8, 9). Like pulmonary carcinoids, SCLC is also categorized as a neuroendocrine tumor of the lung. We have previously demonstrated that the PI3K-Akt pathway is significant in another non-pulmonary neuroendocrine tumor, medullary thyroid cancer (10). These observations suggest that this signaling pathway may be important in the tumorigenesis of pulmonary carcinoid cells as well. However, to date, the function of PI3K-Akt signaling in pulmonary carcinoid tumors remains unknown. Therefore, our objective was to establish the role of the PI3K-Akt signal transduction pathway in pulmonary carcinoid cells.
In this study, we describe the effects of PI3K and Akt1 inhibition on pulmonary carcinoid cells. Suppression of PI3K-Akt signaling with the well-known PI3K inhibitor, LY294002, in vitro resulted in a profound dose-dependent reduction in pulmonary carcinoid cell growth. In addition to inhibiting cell growth, LY294002 also decreased expression of the neuroendocrine tumor markers, chromogranin A (CgA) and achaete-scute complex-like1 (ASCL1). Small-interfering RNA (siRNA) against Akt1 recapitulated the effects of LY294002 on both cell growth and neuroendocrine marker expression, suggesting that PI3K signals through Akt1. These results indicate that PI3K-Akt signaling and Akt1 are involved in cell survival and tumor growth in pulmonary carcinoid cells.
METHODS
Cell Treatment and Growth
NCI-H727 cells, a human pulmonary carcinoid cell line, were obtained from American Type Culture Collection (Manassas, VA) and maintained as previously described (11, 12). For treatment with the PI3K inhibitor, LY294002 (Promega, Madison, WI), NCI-H727 cells were plated onto 100-cm2 dishes for protein isolation or counted using a hemocytometer and plated at a concentration of 30,000 cells/well onto 24-well plates in quadruplicate for cell proliferation measurement. The next day the cells were washed with phosphate buffered saline and treated with LY294002 in phenol-free standard media at concentrations of 0, 25, 50, 75, and100 μM. The concentration of dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO), the solvent for LY294002, was the same in each treatment group, including control (0 μM). For protein analysis, the cells were incubated and isolated after either 48 or 96 hours. To measure cellular proliferation, the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT, Sigma) rapid calorimetric assay was utilized. Every 48 hours, the MTT assay was performed on one 24-well plate by removing the standard medium and replacing it with 250 μL of serum-free media containing a 0.5 mg/mL concentration of MTT. After incubation at 37 °C for 3-4 hours, 750 μL of DMSO was added to each well and mixed gently. Absorbance was then measured at a wavelength of 540 nm using a spectrometer (μQuant; Bio-Tek Instruments, Winooski, VT). On the remaining 24-well plates, the medium was changed and the cells were retreated with the same concentrations of LY294002 every 48 hours until the MTT assay was performed.
siRNA Transfection
NCI-H727 cells also were plated onto 100-cm2 dishes at approximately 30% confluence, and transfection of 75 nM non-specific (NS) or Akt1 siRNAs (Santa Cruz Biotechnology, Santa Cruz, CA) was performed per manufacturers instructions with the reagent Lipofectamine™ 2000 (Invitrogen Corporation, Carlsbad, CA) as described (11). After 24 hours of incubation, the cells were trypsinized and plated in the standard medium onto either 6-well plates for protein isolation or counted with a hemocytometer and plated at a concentration of 30,000 cells/well onto 24-well plates in quadruplicate for the MTT growth assay. Cellular extracts for protein analysis were isolated every 48 hours for up to 6 days. The MTT assay was performed in the same manner as described above.
Western Blotting
After NCI-H727 cells were treated, whole cell lysates were prepared as previously described (11). Total protein concentrations were quantified using a bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, IL). Per manufacturer’s instructions, gel electrophoresis on NuPAGE® Novex® Bis-Tris 10% Mini Gels (Invitrogen) was performed on 30 to 40 μg of denatured cellular extracts. Then, proteins were transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), which were subsequently blocked in milk (5% non-fat dry milk and 0.05% Tween 20 in phosphate buffered saline), and incubated with the appropriate primary antibody overnight at 4°C. The antibody dilutions were: 1:1000 for total Akt, pAkt, Akt1 (Cell Signaling Technology, Beverly, MA), ASCL1 (BD Biosciences, San Diego, CA), CgA (Zymed Laboratories, San Francisco, CA); and 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Trevigen, Gaithersburg, MD). After primary antibody incubation and washing, horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse (Pierce) secondary antibodies were used depending upon the source of the primary antibody. For visualization of the protein signal, Immunstar (Bio-Rad) or SuperSignal West Femto (Pierce) kits were utilized according to the manufacturer’s specifications.
Data analysis
Using SPSS statistical software (version 10.0, SPSS Inc, Chicago, IL), t-tests were used for statistical comparisons between treatment groups. A p value of less than 0.05 was considered statistically significant.
RESULTS
PI3K Inhibition Suppressed NCI-H727 Cell Growth
PI3K-Akt pathway inhibition has been shown to suppress growth in several cancer lines, including both non-pulmonary and pulmonary tumors such as NSCLC and SCLC (5-7). In addition, PI3K inhibitors have shown in vitro effectiveness in certain neuroendocrine tumors (10). However, the role of PI3K-Akt signaling in the growth of pulmonary carcinoid tumors has not yet been elucidated. To measure cell viability, we utilized the MTT assay over 8 days on NCI-H727 cells treated with LY294002 (0-100 μM). We observed a profound dose-dependent decrease in NCI-H727 human pulmonary carcinoid cancer cell growth (Fig. 1A). At 6 and 8 days, cell proliferation was significantly inhibited compared to control was seen even at 10 μM LY294002, the lowest treatment concentration (p<0.005). After 8 days of treatment with 100 μM LY294002, pulmonary carcinoid tumor cell growth was reduced by 85.3% relative to untreated cells (p<0.0001). Therefore, PI3K-Akt signaling appeared to play a significant role in pulmonary carcinoid cell growth.
Figure 1.
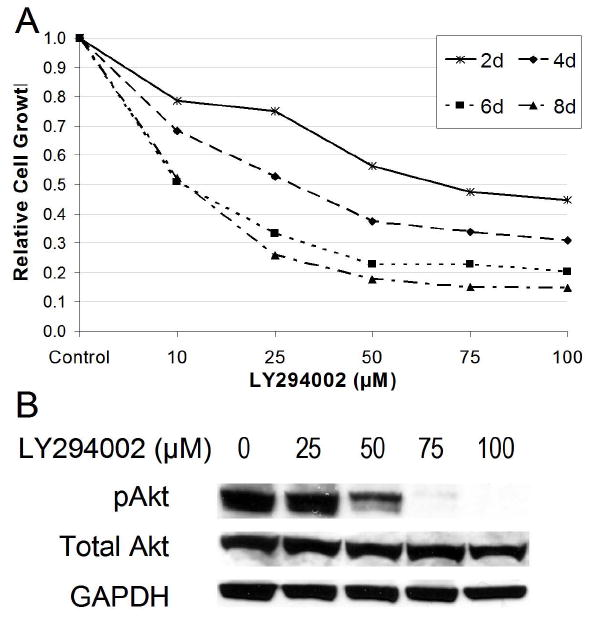
PI3K inhibition caused dose-dependent reduction of pulmonary carcinoid cell growth and Akt phosphorylation (pAkt) in vitro. A, NCI-H727 cells were treated with the indicated concentrations of LY294002, a PI3K inhibitor, over an 8 day time period. A MTT growth assay was done to measure cell viability (bars represent SE from 4 separate experiments). B, Western blot analysis of NCI-H727 cells shows that inhibition of PI3K-Akt signaling by LY294002 at 96 hours suppressed the levels of active, pAkt (Ser473) with no effect on Total Akt. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
In order to determine the effectiveness of our PI3K inhibition with LY294002 in pulmonary carcinoid NCI-H727 cells, western blotting was performed for activation of Akt. Figure 1B illustrates the effects of LY294002 treatment on Akt phosphorylation (pAkt) at serine 473. Treatment of NCI-H727 cells with LY294002 caused a dose-dependent decrease in the levels of pAkt. We observed no effects on the levels of Total Akt (Fig. 1B). These results suggested that LY294002 successfully inhibited the PI3K. Furthermore, the growth suppression observed in our MTT experiment was likely due to inhibition of PI3K by LY294002.
Akt1 Knockdown Suppressed the Proliferation of NCI-H727 Cells
Previous studies implicate Akt1 in tumor cell survival in NSCLC and SCLC (8, 9). Therefore, we sought to determine if the effects of LY294002 treatment were mediated by Akt1, a specific isoform of Akt. We performed the MTT cellular proliferation assay over 6 days on NCI-H727 cells transiently transfected with 75 nM Akt1 siRNA. Inhibition of Akt1 protein production by siRNA treatment resulted in significant growth suppression of pulmonary carcinoid cells (Fig. 2A). We observed 27% and 31% reductions in the growth of Akt1 siRNA trnsfected cells at 4 and 6 days, respectively, compared to the lipofectamine (Lipo) control (p ≤ 0.001 for both). These MTT data suggested that PI3K-Akt signaling and Akt1 play a definite role in the proliferation of pulmonary carcinoid cells in vitro.
Figure 2.
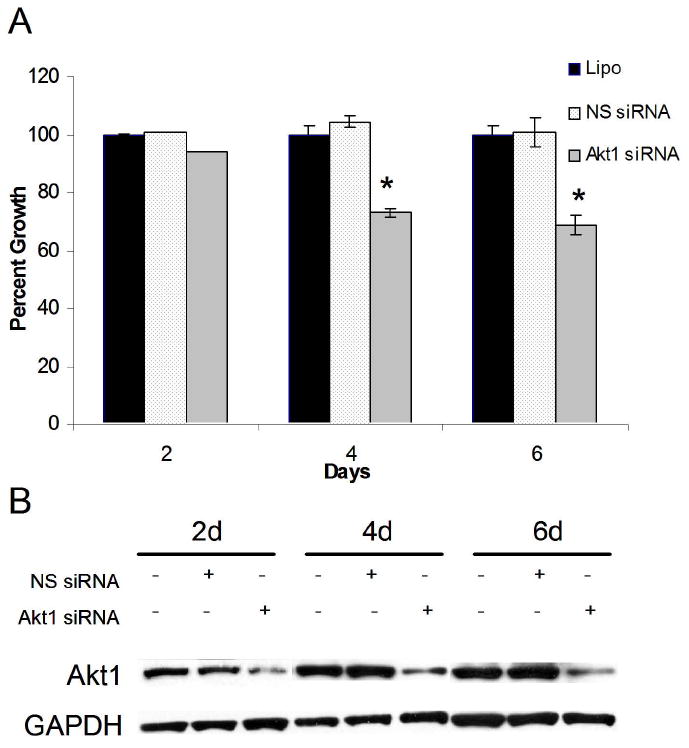
Pulmonary carcinoid cell growth was inhibited by Akt1 knockdown in vitro. A, Akt1 and non-specific (NS) siRNA were transfected into NCI-H727 cells using Lipofectamine 2000® (Lipo). Cellular proliferation was measured by MTT growth assay every 2 days for up to 6 days. Significant growth reduction was observed in cell transfected with Akt1 siRNA (* p ≤ 0.001) B, Transfection of pulmonary carcinoid cells with Akt1 siRNA also led to decreased Akt1 protein production at all time points. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Next, we performed western blot analysis for Akt1 protein levels in order to confirm that our RNA interference blocked translation of Akt1 mRNA. Cellular lysates were prepared at 2, 4, and 6 days after transient transfection of NCI-H727 cells with Akt1 siRNA. Expression of Akt1 was reduced relative to the two control groups, lipofectamine alone and NS siRNA (Fig. 2B). These reductions in Akt1 protein levels corresponded directly with the degree of growth reduction observed by MTT assay (Fig. 2A). Thus, Akt1 seemed to be mediating some of the growth effects seen with LY294002 treatment.
PI3K and Akt1 Inhibition Decreased CgA and ASCL1 Levels
As a neuroendocrine tumor, pulmonary carcinoid tumors frequently excrete excess bioactive amines and peptides, such as chromogranin A (CgA), that can be used diagnostically and as markers of tumor progression. Our previous studies on carcinoids also have examined a basic helix-loop-helix transcription factor that regulates the neuroendocrine phenotype, ASCL1 (11, 12). Therefore, in NCI-H7272 cells, we wanted to determine whether PI3K-Akt signaling regulates CgA and ASCL1 expression. Western blot analysis of LY294002-treated pulmonary carcinoid cells at 2 (Fig. 3A) and 4 days (Fig. 3B) revealed dose-dependent reductions in CgA and ASCL1 protein levels. At a concentration of 100 μM, CgA was significantly suppressed at 2 days and undetectable at 4 days. Similarly, ASCL1 levels were nearly or totally absent at both 2 and 4 days after treatment with the same concentration of LY294002. These data indicated that PI3K-Akt signaling is involved in the expression of neuroendocrine markers in pulmonary carcinoid cells.
Figure 3.
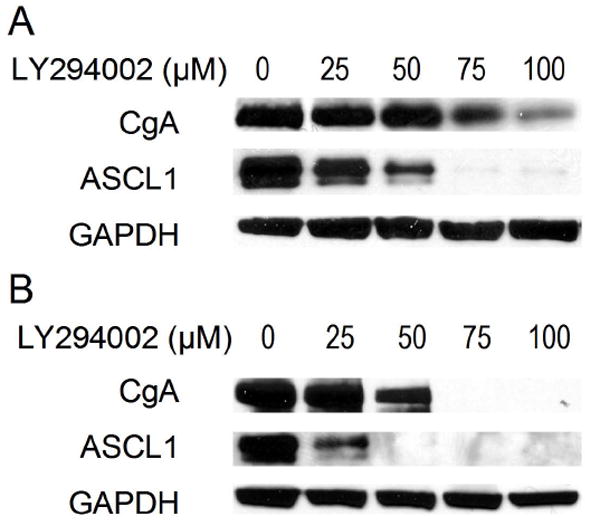
LY294002 treatment decreased levels of the neuroendocrine tumor markers, achaete-scute complex-like1 (ASCL1) and chromogranin A (CgA) in a dose-dependent manner. Western blot data at 2 (A) and 4 (B) days revealed these changes in ASCL1 and CgA expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
After establishing the effects of PI3K inhibition on CgA and ASCL1 expression, we wanted to verify that Akt1 inhibition also suppressed neuroendocrine marker expression in pulmonary carcinoid cells. At 2, 4, and 6 days after transient transfection of Akt1 siRNA into NCI-H727 cells, CgA levels were decreased (Fig. 4). Similar to the reduction in CgA protein levels observed with PI3K inhibition, our siRNA experiment showed that CgA levels were lowest at 4 days after Akt1 inhibition. Expression of ASCL1 also was suppressed by transfection of NCI-H727 cells with Akt1 siRNA at all three time points examined (Fig. 4). When combined with the results of the LY294002 experiments, our findings suggested that the PI3K-Akt pathway mediates the neuroendocrine phenotype of pulmonary carcinoid cells at least in part through Akt1.
Figure 4.
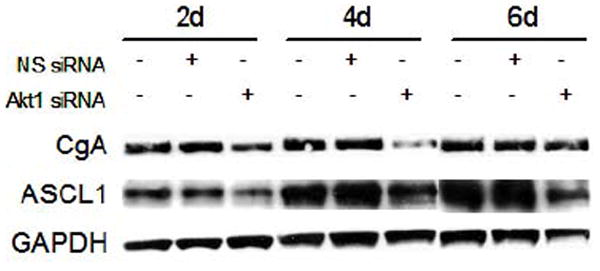
Lysates from NCI-H727 pulmonary carcinoid cells transfected with Akt1 siRNA were analyzed by western blotting. Non-specific (NS) siRNA was used as a control. Cells were transfected at day 0, and whole cell lysates were isolated every 2 days for 6 days. Achaete-scute complex-like1 (ASCL1) and chromogranin (CgA) expression was reduced at all time points. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
DISCUSSION
The principal findings of this study were that inhibition of PI3K and Akt1 in pulmonary carcinoid cells (NCI-H727) significantly reduced cellular proliferation and neuroendocrine marker expression in vitro. LY294002, a well-described PI3K inhibitor, was used to treat NCI-H727 cells. In response to this treatment, cell growth and active pAkt expression was reduced in a dose-dependent manner (Fig. 1A and 1B). In addition, LY294002 treatment led to profoundly decreased levels of the neuroendocrine tumor markers ASCL1 and CgA (Fig. 3A and 3B). RNA interference (RNAi) against Akt1 mRNA also decreased pulmonary carcinoid cell viability as well as ASCL1 and CgA protein levels (Figs. 2A and 4). Our results demonstrate that the PI3K-Akt signaling and Akt1, specifically, mediate pulmonary carcinoid cell survival and neuroendocrine phenotype.
Signal transduction pathways, such as PI3K-Akt, are activated by growth factor receptors and are known to regulate cell growth, survival, motility, and differentiation (4, 7). As a consequence, these pathways are often dysregulated during tumorigenesis. Thus, a significant amount of research has focused on identifying suitable targets in these pathways for anticancer drug development. The PI3K-Akt pathway is one of many pathways that is being explored and has been shown to have a critical function in the neoplastic process of NSCLC, SCLC, and neuroendocrine cancers (5, 6, 10, 14). Moreover, Akt1 has been recognized as an attractive target for directed therapies in these and other tumors (8, 9, 15). A group from Korea has shown that Akt1 siRNA can be delivered in an aerosolized form that effectively suppresses lung tumor progression in murine models of NSCLC (15). Therefore, investigation into the role of PI3K-Akt signaling in pulmonary carcinoid cells is necessary to establish if targeted approaches would be promising in patients with advanced pulmonary carcinoid tumors.
We utilized two complimentary approaches to assess the importance of PI3K-Akt signaling in pulmonary carcinoid NCI-H727 cells. First, we treated pulmonary carcinoid cells with LY294002, a PI3K inhibitor. The degree of growth inhibition observed with this treatment corresponded directly with the level of pAkt suppression by LY294002 (Fig. 1A and 1B). At concentrations of LY294002 greater than 10 μM, where the drug’s actions are most selective, considerable cytotoxicity was seen. In other neuroendocrine tumors, such as SCLC and medullary thyroid cancer, LY294002 has similarly resulted in growth suppression (6, 10). Furthermore, treatment of pulmonary carcinoid cells with this PI3K inhibitor decreased levels of ASCL1 and CgA (Fig. 3A and 3B). These data confirm our previous in vitro work with PI3K-Akt signaling in medullary thyroid cancer cells where inhibition of this pathway also reduced expression of neuroendocrine tumor markers (10). However, because LY294002 has been shown to inhibit related enzymes, these studies do not definitively establish that the PI3K-Akt pathway is responsible for cell death and neuroendocrine marker suppression in pulmonary carcinoid cells (17).
In order to address this issue, we attempted to inhibit translation of a specific Akt isoform using RNAi technology. This particular Akt isoform has previously been identified as an oncogenic molecule in other pulmonary tumor cell lines (8, 9). However, the significance of Akt1 in pulmonary carcinoid cells has not been explored. In this study, targeting Akt1 by siRNA considerably reduced the levels of Akt1 protein detectable by Western blot analysis as expected (Fig. 2B). Interestingly, pulmonary carcinoid cell growth and expression of neuroendocrine tumor markers also were inhibited by the Akt1 siRNA treatment (Figs. 2A and 4). To our knowledge, this finding is the first to suggest that Akt1 may be involved in neuroendocrine tumor marker expression. In prostate cancer cells, the PI3K-Akt signaling pathway appears to be critical for neuroendocrine differentiation (14). However, Akt1 has not been specifically identified as playing a part in this process. Others have shown that Akt1 controls migration and invasion by modulating downstream molecules in breast cancer cells (15). These same investigators also have linked expression of Akt1 to progression of metastatic disease in patients with breast cancer (15). Further investigation is needed to clarify the role of Akt1 in the migration and invasion of pulmonary carcinoid cells. Nonetheless, our data support the current evidence that Akt1 is involved in tumor cell survival (8, 9, 15, 18).
The therapeutic implications of the results of this study are multiple. In solid and hematologic malignancies, several researchers have postulated that manipulation of the PI3K-Akt signaling pathway could inhibit tumor growth in humans (6, 7, 10, 14, 15, 19-21). Currently, clinical trials are being conducted to examining the efficacy of various PI3K-Akt pathway inhibitors. However, these compounds are not specific to any particular Akt isoform. Our data suggest that Akt1 is an important therapeutic target. Though we have not investigated the role of Akt1 in relation to the radiosensitivity of pulmonary carcinoids here, other researchers have demonstrated that Akt1 inhibition sensitizes SCLC cells, another radiation-resistant pulmonary neuroendocrine tumor, to radiotherapy (8). Pulmonary carcinoid tumors also typically have low response rates to chemotherapy. In NSCLC, RNAi against Akt1 enhances the chemosensitivity of these tumors (9). Furthermore, aerosol delivery of Akt1 siRNA may be a feasible noninvasive method of delivery in patients with pulmonary tumors (16). Taken together, the data collected here and by others provide support for therapeutically targeting Akt1 in patients with pulmonary carcinoid tumors.
This study showed that PI3K-Akt signaling and Akt1 are important in pulmonary carcinoid tumor cell growth and neuroendocrine tumor marker expression. Besides surgical resection, few alternative therapies for patients with these tumors exist, since chemotherapy and external beam radiation are generally ineffective. Thus, developing effective and selective pharmacologic inhibitors of PI3K and Akt1 may present new treatment options for patients with unresectable pulmonary carcinoid disease.
Acknowledgments
Funded in part by the American College of Surgeons Resident Research Scholarship and George H.A. Clowes Jr. Memorial Research Career Development Award; NIH grants T32 CA009614 Physician Scientist Training in Cancer Medicine, R21 CA117117, and R01 CA109053; American Cancer Society Research Scholars Grant 05-08301TBE; Carcinoid Cancer Foundation Research Grant; and the Society of Surgical Oncology Clinical Investigator Award.
Abbreviations
- PI3K
phosphatidylinositol 3-kinase
- pAkt
phosphorylated Akt
- siRNA
small interfering RNA
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- ASCL1
achaete-scute complex-like 1
- CgA
chromogranin A
- RNAi
RNA interference
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Author Contributions Study conception and design: Pitt, Chen, Kunnimalaiyaan
Acquisition of data: Pitt
Analysis and interpretation of Data: Pitt, Chen, Kunnimalaiyaan
Drafting of manuscript: Pitt
Critical revision: Pitt, Chen, Kunnimalaiyaan
Presented at the 94th Annual American College of Surgeons Clinical Congress, San Francisco, CA, October 14th, 2008
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008 doi: 10.1634/theoncologist.2008-0207. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;133(1):5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 5.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 6.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–22. [PubMed] [Google Scholar]
- 7.Nicholson KM, Anderson NG. The protein kinase B/Akt signaling pathway in human malignancy. Cellular Signaling. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 8.Toulany M, Kehlbach R, Florczak U, et al. Targeting Akt1 enhances radiation toxicity of human tumor cells by inhbitin DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther. 2008;7(7):1172–81. doi: 10.1158/1535-7163.MCT-07-2200. [DOI] [PubMed] [Google Scholar]
- 9.Lee MW, Kim DS, Min NY, Kim HT. Akt1 inhibition by RNA interference sensitizes human non-small cell lung cancer cells to cisplatin. Int J Cancer. 2008;122(10):2380–84. doi: 10.1002/ijc.23371. [DOI] [PubMed] [Google Scholar]
- 10.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–15. doi: 10.1016/j.surg.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates Notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–51. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 12.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Langerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 13.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4:910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Huang J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapapmycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282(6):3571–83. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. PNAS. 2006;103(11):4134–9. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Jere D, Jin H, et al. Poly(ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesis. Am J Resp Crit Care Med. 2008;178:60–73. doi: 10.1164/rccm.200707-1022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenzweig KE, Youmell MB, Palayoor ST, Price BD. Radiosensitization of human tumor cells by the phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin Cancer Res. 1997;3:1149–56. [PubMed] [Google Scholar]
- 18.Bleeker FE, Felicionii L, Buttitta F, et al. AKT1E17K in human solid tumors. Oncogene. 2008;27:5648–50. doi: 10.1038/onc.2008.170. [DOI] [PubMed] [Google Scholar]
- 19.Mandal M, Younes M, Swan EA, et al. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–9. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang HJ, Jin X, Wang S, et al. A small molecule compound inhibits AKT pathway in ovarian cancer cell lines. Gynecol Oncol. 2006;100:308–17. doi: 10.1016/j.ygyno.2005.08.044. [DOI] [PubMed] [Google Scholar]


