Abstract
In addition to the already known differences between adenosine deaminase (ADA) and cytidine deaminase (CDA) in terms of their tertiary structure, the sphere of Zn+2 coordination, and their reverse stereochemical preference, we present evidence that the enzymes also differ significantly in terms of the North/South conformational preferences for their substrates and the extent to which the lack of the O(4’) oxygen affects the kinetics of the enzymatic deamination of carbocyclic substrates. The carbocyclic nucleoside substrates used in this study have either a flexible cyclopentane ring or a rigid bicyclo[3.1.0]hexane scaffold.
Keywords: Adenosine deaminase, cytidine deaminase, carbocyclic nucleosides, bicyclo[3.1.0]hexane nucleosides
INTRODUCTION
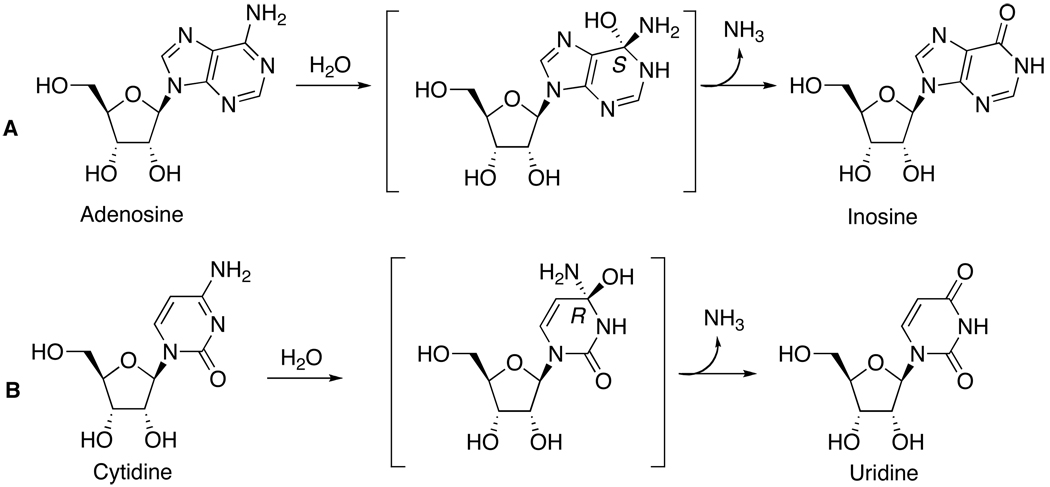
Adenosine deaminase (ADA) and cytidine deaminase (CDA) catalyze the deamination of adenosine and cytidine, respectively, via the formation of unstable hydrated intermediates.[1,2] ADA catalyzes the hydration of the purine ring during the conversion of adenosine to inosine and CDA catalyzes the hydration of the pyrimidine ring during the conversion of cytidine to uridine (Figure 1). Spontaneous deamination of the C4 position of cytidine and of the C6 position of adenosine proceeds with rate constants around 10−10 s−1, whereas the enzymes are able to accelerate the rates of deamination by approximately 12–14 orders of magnitude.[3] These rate enhancements reflect extraordinary transition-state affinities that are among the highest observed for any enzyme. In addition to these important biochemical properties, ADA and CDA are of particular interest as therapeutic targets for the treatment of several diseases, including cancer.[4–8]
Figure 1.
Transition-state, hydrated intermediates formed during the enzymatic hydrolytic deamination of adenosine (A) and cytidine (B).
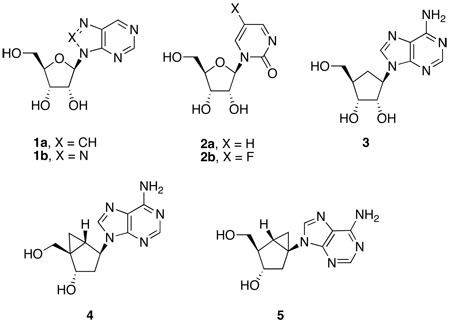
Formation of hydrated intermediates by these enzymes has been confirmed by the crystal structures of inhibitors [nebularine (1a) and zebularine analogues (2a,b)] with ADA and CDA, respectively. These crystal structures revealed the formation of the 1,6-double bond hydrate (6-hydroxyl-1,6-dihydropurine ribonucleoside) from nebularine and the 3,4-double bond hydrate (4-hydroxy-3,4-dihydropyrimidine-2(1H)-one ribonucleoside) from zebularine) at the active sites.[9–13] In both enzymes, the transition-state inhibitors replace the leaving NH3 group with a proton, preventing conversion of the tetrahedral transition-state into product. Remarkably, the presence of these hydrated species at the binding sites of the enzymes suggest that the hydrated species may be associated with extraordinarily high enzyme inhibition affinities by resembling the transition states of the deamination reactions.[14] Indeed, the inhibition constants for the hydrated species of nebularine (1a, Ki = 10−13 M)[1] and zebularine (2a, Ki = 10−12 M)[2] are quite remarkable. The true Ki’s, however, are half of these values since the addition of water to generate the tetrahedral intermediates is stereospecific giving exclusively a single diastereoisomer (Figure 1). In summary, these intermediates appear to capture much of the negative free energy of binding expected of an ideal transition-state analogue for the deamination reactions.
When the X-ray structures of ADA and CDA bound to the hydrates of nebularine (1a) and zebularine (2a) are superimposed, it becomes clear that these enzymes have opposite stereochemical preferences at the C6 (S) carbon for ADA and the C4 (R) carbon for CDA (Figures 1 and 2).[15] This reverse stereochemical preference arises from the fact that the consensus TVHA(G)E sequences of the two proteins curl around opposite sides of the essential zinc. Therefore, because of this near mirror image relationship of the two superimposed binding pockets formed around the zinc and the conserved acid residues, these enzymes receive their substrates from opposite sides.
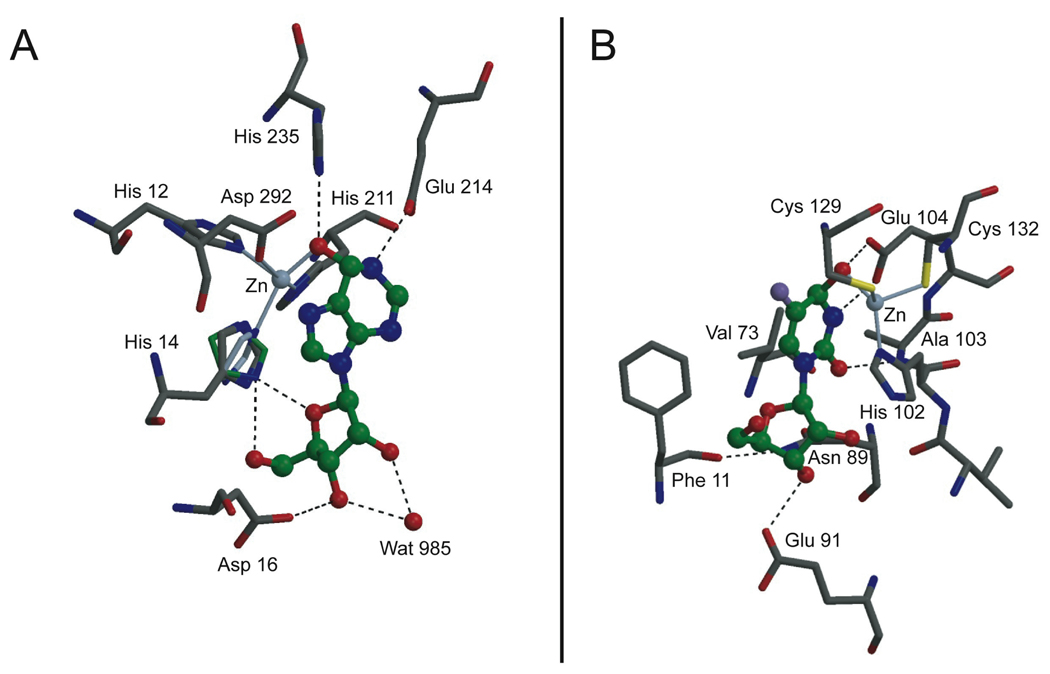
Figure 2.
(A) Bovine ADA in complex with hydrated nebularine.[11] Ligand is shown in ball-and-stick representation, with green carbons. The crystal coordinates of residue His 14 are shown in dark gray. This conformation is suggested to be an average of two rotamers: one (in green) where the Nδ atom makes a bifurcated hydrogen bond to the sugar moiety in the ligand, and one (in light blue-gray) where the zinc atom lies in the plane of the imidazole ring. (B) E. coli CDA in complex with hydrated 5F-zebularine.[13]
Chemical changes in the aglycon of these inhibitors can affect the formation of hydrated intermediates. For example, in the case of ADA, the 100-fold increase in inhibitory potency exhibited by the 8-aza analogue of nebularine (8-azapurine riboside, 1b) over nebularine itself is probably related to the greater tendency of the 8-aza analogue to form a hydrate.[16] Similarly, in the case of CDA, fluorine substitution of zebularine (5-fluorozebularine, 2b), which is expected to promote hydration, helps explain the 10-fold increase in potency of 2b over zebularine.[17] The extent of hydration of these modified bases and those of the natural substrates is known to depend on protonation, which facilitates hydration relative to the neutral species. Counter to the formation of the hydrated species is the loss in resonance energy resulting from hydration, which explains why the existence of hydrated species free in solution are below the limits of detection by direct methods.
In addition to the inverse mirror image relationship between ADA and CDA, there are significant differences in the architecture of the enzyme active sites that have important mechanistic implications: ADA is a monomer and CDA is either a homodimer or a homotetramer. More critically, Zn+2 coordination in ADA is accomplished by five ligands (3 His, 1 Asp, and a hydroxyl group from the transition-state inhibitor or a water molecule when bound to substrates) in a trigonal bipyramidal geometry.[9–11] In the homodimeric E. coli CDA, Zn+2 coordination is achieved with four ligands (2 Cys, 1 His and a hydroxyl group from the transition-state inhibitor or a water molecule when bound to substrates) in a nearly tetrahedral geometry.[13] On the other hand, the three Zn+2 ligands in homotetrameric CDAs from B. subtilis,[18] mouse,[19] and human[20] are all cysteine residues. At the active site environment of these enzymes, which is almost completely sequestered from bulk solvent, the function of Zn+2 is to activate a water molecule to form a hydroxide ion for nucleophilic attack. Hence, the diverse coordination spheres around Zn+2 may affect the water/hydroxide equilibrium differently in each enzyme. In homotetrameric CDAs, the formal negative charge of cysteinate increases the pKa value of the bound water and formation of the Zn-OH− ion becomes less likely than if it were coordinated to a tris-imidazolium zinc as in ADA. However, the excess negative charge in the environment of the Zn+2 in these homotetrameric enzymes is compensated by an Arg residue (Arg56 in B. subtilis and Arg68 in mouse and human CDAs), which lowers the pKa for the zinc-bound water to facilitate nucleophilic attack. In CDA, a key player for this concerted attack is a conserved Glu residue (Glu104 in E. coli, Glu55 in B. subtilis, and Glu67 in mouse and human CDAs) which can act as (1) a general base to abstract a proton from either zinc complex [Zn(Cys)2(His)H2O or Zn(Cys)3H2O], (2) a general acid to protonate N3 of the pyrimidine ring, and (3) a shuttle for proton transfer from the hydroxyl group to the departing ammonia.[18,19,21] In bovine ADA,[11] Glu 214 performs an equivalent role but there are other acidic residues near the active site of ADA (Figure 2A).[9,10,11,22] Asp293, for example (not shown), which is in the vicinity of hydrophobic residues, serves as a hydrogen bond donor to the lone pair of the very basic nitrogen N7. These differences could be important when considering the pKa of adenosine (pKa = 3.5)[23] and cytidine (pKa = 4.2)[23] and their ease of protonation inside the folded structures of ADA and CDA, which can perturb the active site to allow protonation near physiological pH. Intuitively, it should be easier to protonate cytidine than adenosine and that is probably why there are more acidic residues in ADA (Asp16, Glu214, Asp292 and Asp293 in bovine ADA) compared to a solitary Glu in CDA (Glu104 in E. coli, Glu55 in B. subtilis, and Glu67 in mouse and human CDAs). Perhaps too that is the reason why ADA binds to adenosine in a 3’-endo, North conformation (vide infra).[22]
The sugar moiety, has a small effect on the rate of spontaneous deamination but greatly enhances the reactivity of both ADA and CDA by increasing the effective concentration of the altered substrate at the active site.[24,25] This entropic advantage is the direct result of the parts (base and ribose) being properly connected to fit the active site. As far as a direct contribution to catalysis, however, because the sugar ring is distant from the site of the chemical transformation, its role has been considered to be insignificant or at best modest.[26,27] In contrast, studies in our laboratory with carbocyclic nucleosides have revealed that the sugar moiety in ADA seems to play a significant role in catalysis by providing effective electronic communication between the O(4’) and the base through the anomeric effect and also by providing electron-withdrawing activation.[28]
In addition, the crystal structure of the transition-state inhibitor HDPR bound to ADA shows that His 14, one of the Zn+2 ligands, may interact strongly with O(4’) and with the 5’-OH of the sugar (Figure 2A). Therefore, the loss of this interaction resulting from the replacement of the furanose ring of adenosine or 2’-deoxyadenosine with a cyclopentane ring —as in the fermentation product aristeromycin (3)— also helps explains the significant drop in the deamination rate of 3 to 0.58% the rate of adenosine.[28] Obviously the absence of the O(4’) oxygen abolishes the stereoelectronic interplay between the purine ring and the ribofuranosyl moiety, impacting negatively on the propensity of the base to form a covalent hydrate.[29] It has been clearly demonstrated by Chattopadhyaya and coworkers that the electronic properties of the aglycon are effectively transmitted to the pentofuranose moiety through the anomeric effect.[29,30] Because the anomeric effect is more effectively transmitted when the conformation of the sugar is North, protonation of adenosine and 2’-deoxyadenosine is more effective in the North conformation and therefore it is not surprising that in the crystal structures of ADA in complex with 6-hydroxyl-1,6-dihydropurine ribonucleoside, the ribose conformation is in a 3’-endo (North) ring pucker.[22] In the North conformation, the equatorial 3’-hydroxyl group of the ribose is engaged in hydrogen bonding with Asp16, whereas the 2’-hydroxyl does not appear to play an essential role.[9–11,22] This finding agrees with the fact that 2’-deoxyadenosine is as good a substrate for ADA as adenosine. Furthermore, in the North conformation Glu214 is perfectly aligned to donate a proton to N1 of the purine ring, thereby making the C6 carbon more susceptible to hydration (Figure 2A).
As expected, the absence of the O(4’) oxygen also caused a large decline in the deamination rates of two conformationally locked North-dAdo (4) and South-dAdo (5) carbocyclic nucleosides to 1% and 0.01%, respectively, of the values observed for adenosine.[28] Significantly, despite this considerable drop in activity, the enzyme still showed a 100-fold preference for North-dAdo (4) over South-dAdo (5), and the former compound was in turn preferred by nearly 2-fold over the flexible aristeromycin (3). Based on these findings we postulated that the preference of ADA for North-dAdo resulted principally from the correct equatorial disposition of the 3’-OH engaged in effective hydrogen bonding with Asp16, as observed in the crystal structure.[11] In summary, the combined factors responsible for the low deamination rate of carbocyclic adenosine nucleosides are the reduced level of hydration of the base and the missing interaction of the O(4’) oxygen with His 14. The removal of the O(4’) oxygen appears to be so critical that even a North-locked version of the 8-azaadenosine failed to show a comparable enhancement in the rate of deamination as it was observed for the corresponding nucleoside.[31]
Intriguingly, CDA has a totally different preference for the sugar pucker of substrates and inhibitors, and, as opposed to ADA, the ribose and 2’-deoxyribose glycons adopt the antipodal 2’-endo South conformation.[13,18–20] In the crystal structure of hydrated 5-fluorozebularine (2b) at the active site of E. coli CDA, it is the axial 3’-OH that engages in hydrogen bonding with Glu91, while the 2’-OH does not interact with the protein (Figure 2B).[13] The lack of involvement of the 2’-OH is in agreement with the known experimental fact that cytidine and 2’-deoxycytidine are equally good substrates for CDA. Also, in the South conformation, Glu104 (E.coli CDA) is in perfect alignment to provide the critical protonation at N3 of the pyrimidine ring.[13] More importantly, because of the mirror image relationship of the binding pockets of ADA and CDA, the ribose (or 2’-deoxyribose) ring of the substrates are in opposite orientations relative to the zinc’s coordination sphere; in the case of ADA, His 14 can interact with O(4’) (Figure 2A), whereas in the case of CDA from E. Coli a similar interaction with His 102 is not possible (Figure 2B). Furthermore, other CDAs have all cysteine residues around the zinc.
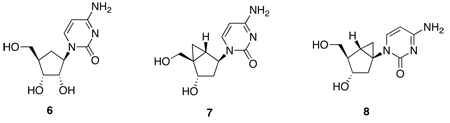
In the present investigation we confirm ADA’s preference for North conformer substrates by utilizing the conformationally locked carbocyclic nucleosides North-dAdo (4) and South-dAdo (5). We demonstrate the antipodal preference of CDA for South conformer substrates employing a similar approach with an equivalent set of carbocyclic nucleosides having a flexible cyclopentane ring (carbodine, 6), or conformationally locked North and South versions of cytidine [North-dCyd (7) and South-dCyd (8)] built on a bicyclo[3.1.0]hexane platform.[32,33] We also highlight how the important architectural differences between ADA and CDA can help explain the changes in catalysis observed in going from ribose (or 2’-deoxyribose) nucleosides to their carbocyclic isosteres in these two mechanistically similar enzymes.
EXPERIMENTAL RESULS
Kinetic analysis of locked carbocyclic adenosine analogues, North-dAdo (4) and SouthdAdo (5), as substrates of ADA
ADA activity was measured spectrophotometrically as described for the natural substrate adenosine following the decrease in absorbance at 264 nm.[34] An initial comparison between North-dAdo and South-dAdo analogues (Figure 3) was performed using ADA from bovine spleen (bs). The experiments were run in 0.1 M phosphate buffer (pH 7.4) at 25 °C using 60 µM substrate (essentially identical to conditions for CDA studies, vide infra).
Figure 3.
Bovine spleen ADA (~0.5 units) deamination of South-dAdo (▲) and NorthdAdo (○) at 60 µM determined by measuring the decrease in absorbance at 264 nm (25 °C).
Relative rates (Table 1) were calculated using initial rates following the first ~10% of reaction (Figures 1S and 2S, Supporting Information). Despite the difference in temperature (37 °C vs. 25 °C) and substrate concentration (50 µM vs. 60 µM) used in these experiments, compared to our previous published results,[28] the conclusion is the same: the North analogue is clearly a better substrate than the South analogue by more than two orders of magnitude (Figure 2, Table 1). Table 1 also shows that the two ADA enzymes from calf-intestine (ci) and bovine serum (bs) behaved very similarly toward these substrates.
TABLE 1.
Relative rates for the North-dAdo and South-dAdo analogues compared to the normal ADA substrates with ADA from calf-intestine (ci) and bovine serum (bs)
| ADA (ci)[28] (50 µM, 37 °C) |
ADA (ci) (60 µM, 25 °C) |
ADA (bs) (60 µM, 25 °C) |
|
|---|---|---|---|
| Rel. Rates | Rel. Rates | Rel. Rates | |
| Ado | 100 | 100 | 100 |
| dAdo | 121 | 124 | 119 |
| North-dAdo | 0.990 | 1.210 | 1.380 |
| South-dAdo | 0.010 | 0.007 | 0.008 |
Due to the relatively long incubation time of South-dAdo with ADA (~22 hours), an enzyme control (same conditions) was monitored for activity loss during the course of the reaction. This sample showed no significant change in activity when compared with a second enzyme control kept at 4 °C.
From the data in Table 1 it is also clear that the effect of the 4’(O) is critical for the ADA reaction. However, despite the >100-fold difference between the natural substrates and the two locked analogues, ADA shows a definitive preference for the North conformation, in agreement with the conformation of the sugar ring observed in the crystal structures of inhibitors bound to ADA.[9–11,22]
Kinetic analysis of locked carbocyclic cytidine analogues, North-dCyd (7) and South-dCyd (8), as substrates of CDA
Cytidine deaminase activity was measured spectrophotometrically as described for the natural substrate cytidine (Δε282 = −3600).[35] The deamination of 2’-deoxycytidine (dCyd) was monitored at 280 nm (Δε280 = −5000; difference in extinction coefficients between dCyd and 2’-deoxyuridine at 280 nm[36]). The extinction coefficients for the locked North- and South-dCyd derivatives were based on that of 2’-deoxycytidine (λmax = 272, ε272 = 9000[36]). However, a comparison of the UV spectrum of dCyd with that of South-dCyd (8) revealed a complete 4 nm shift to longer wavelengths in the region of 270 to 290 nm (Figure 3S, Supporting Information). Therefore, the enzymatic deamination of both locked derivatives was monitored by the decrease in absorbance at 284 nm rather than 280 nm (Δε284 = −5000). The λmax was also correspondingly shifted by 4 nm (λmax = 276, ε276 = 9000). Because the locked compounds were such poor substrates, it was necessary to use a higher concentration of enzyme and follow the reaction for longer periods of time to determine their deamination rates relative to cytidine.
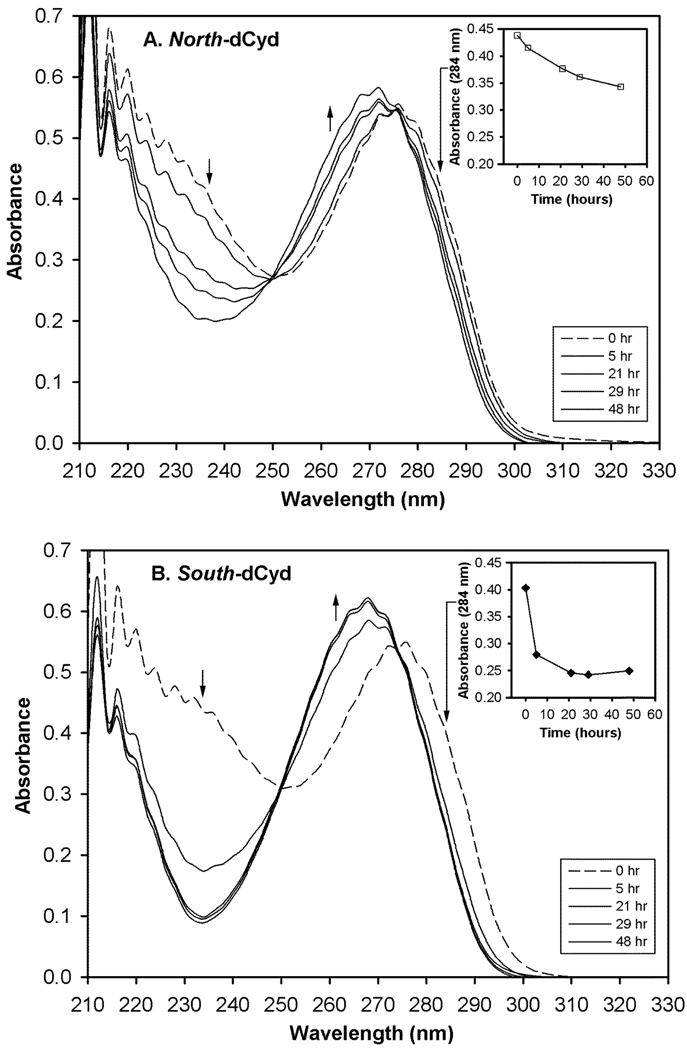
The deamination of both North-dCyd (62 µM) and South-dCyd (61 µM) was followed over the course of two days at a CDA concentration of ~900 nM in phosphate buffer (100 mM) at pH 6.8. Controls containing the analogues in the absence of enzyme showed no change over the same time period. UV spectra of enzyme/buffer control samples were subtracted from all raw spectra of the actual CDA/analogue samples. All spectra represent an average of 5 sequential runs (Figure 4). The deamination of both compounds proceeded with a single isobestic point at 250 nm, indicative of direct enzymatic conversion of substrate to product. It is also clear that while the deamination of South-dCyd was complete well within the experimental time frame (48 hours), the deamination of North-dCyd had not yet reached completion (see insets).
Figure 4.
Full UV spectrum for the enzymatic deamination of North- and South-dCyd over time. A clean isobestic point at 250 nm is maintained throughout the course of the reaction. The inset corresponds to the drop in absorbance at 284 nm (long arrow) as a function of time. The short arrows indicate either the increase (↑) or decrease (↓) in absorbance at that point over time. A) North-dCyd (□). B) South-dCyd (♦).
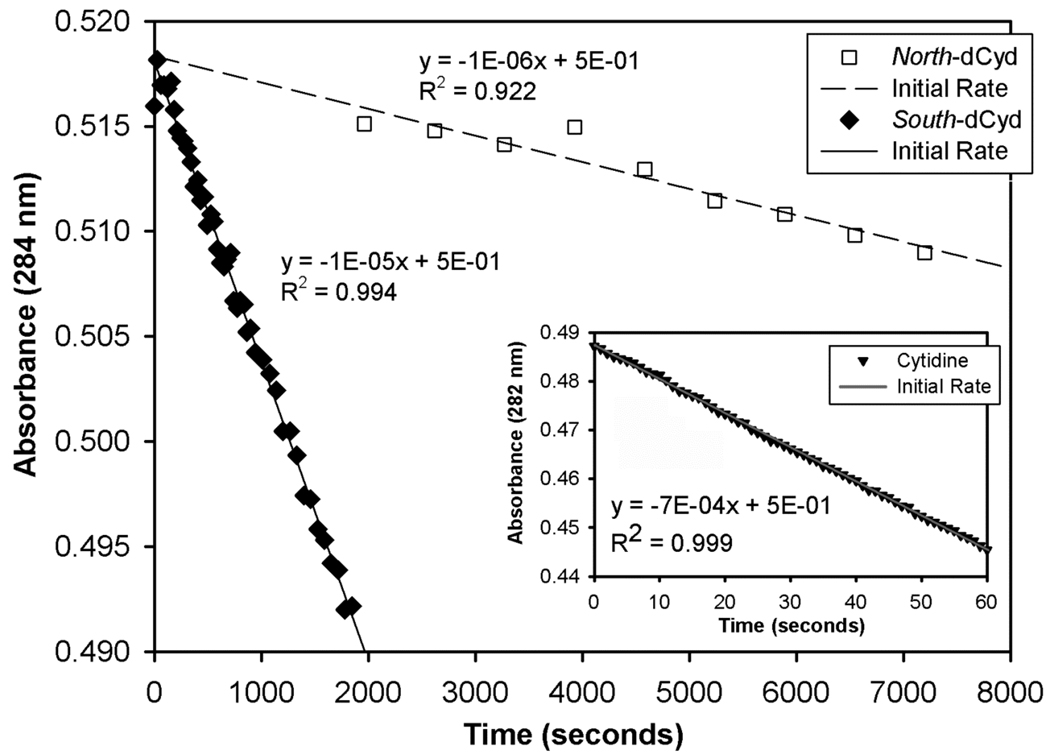
Initial rates for CDA (900 nM) catalyzed deamination of South-dCyd (○) and North-Cyd (□), each at 70 µM, were also determined by a linear fit to initial rate data (Figure 5, Table 2). The inset shows the initial rate data for the CDA (0.9 nM) catalyzed deamination of the natural substrate cytidine (Δ), also at 70 µM. This data shows that the deamination of South-dCyd is roughly an order of magnitude faster than that of North-dCyd.
Figure 5.
Initial time course data for the CDA (900 nM) catalyzed deamination of North-dCyd (□) and South-dCyd (♦) followed at 284 nm (Δε284 = −5000). The inset shows initial time course data for the catalyzed deamination of the natural substrate cytidine (▼) by CDA (0.9 nM) monitored at 282 nm (Δε282 = −3600).
TABLE 2.
Relative deamination rates for carbocyclic cytidine derivatives.
| Substrate | Relative Rates |
|---|---|
| dCyd | 270 |
| Cytidine | 100 |
| Carbodine (6) | 25 |
| South-dCyd (8) | 0.001 |
| North-dCyd (7) | 0.0001 |
Kinetic analysis of plain carbocyclic cytidine (carbodine, 8) as a substrate of CDA
Concentrations of the plain carbocyclic cytidine analogue (carbodine) were measured at λmax, 275 nm (ε275 = 9300[37]), and enzymatic deamination was monitored by the increase in absorbance at 267 nm for the uridine analogue (Δε267 = 2800). The value for Δε267 was calculated using the experimentally determined extinction coefficient for carbodine at 267 nm (ε = 7900) and the reported extinction coefficient for carbocylic uridine at 267 nm (ε = 10,700[38]).
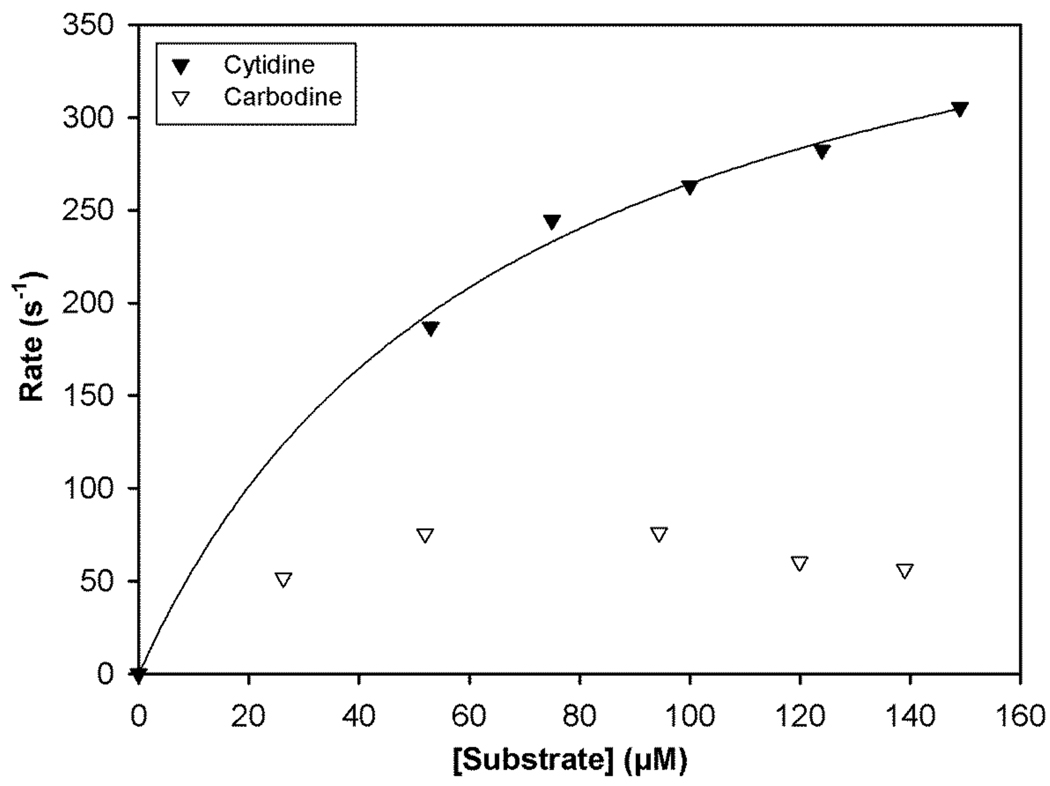
Figure 6 shows a Michaelis-Menten plot for both cytidine and carbodine. Interestingly, initial rates for carbodine deamination were only ~4-fold lower than those of cytidine over the observed substrate concentration range (Table 2). At higher concentrations of carbodine, there appeared to be a slight decrease in enzymatic activity, but the source of this deviation was not investigated further.
Figure 6.
Michaelis-Menten plot for enzyme catalyzed deamination of cytidine (▼) and carbodine (∇). The solid line represents a non-linear regression fit of the cytidine data to the Michaelis-Menten equation.
When the relative rates for all five compounds determined at 70 µM were adjusted for differences in enzyme concentration (Table 2) it can be appreciated that the flexibility of the cyclopentane in carbodine allows it to be a better substrate for CDA. In fact, the best substrate of the locked analogues (South-dCyd) is still dismally poor relative to carbodine despite having the required South conformation. These preferences are definitely different from those of ADA where the flexible, carbocyclic analogue (aristeromycin, 3) was intermediate between the better North-dAdo and poorer South-dAdo substrates. It may also be worth noting that dCyd is a slightly better substrate than cytidine (kcat ~3-fold faster[39]), consistent with our results (Table 2).
DISCUSSION
In addition to the structural differences between ADA and CDA that were discussed earlier, and their opposite stereochemical preferences in forming the hydrated intermediates, our studies confirm that the conformational preferences of ADA and CDA for substrates built as locked carbocyclic nucleosides are also opposite between North and South. The preferences that we have determined agree well with the conformations of conventional nucleosides, substrate or inhibitors, observed in the crystal structures of the complexes. However, in terms of reactivity, the differences that separate these two seemingly similar enzymes are staggering. For ADA, the North-South-dAdo analogues mimic the natural substrates better than the North-South-dCyd analogues are able to mimic the natural substrates for CDA. In other words, the North-South-dAdo analogues are better substrates for ADA than the North-South-dCyd analogues are for CDA. We know this by comparing how well these analogues work relative to the normal substrates: North-dAdo is only ~100 times worse than adenosine, but South-dCyd is nearly ~100,000 times worse than cytidine. In each case, we are making a comparison to the normal substrate, so we are addressing structural differences between the normal substrate and the carbocyclic substrates for their respective enzymes. For both ADA and CDA it is clear that the removal of the O(4’) oxygen reduces the capacity of the substrate to undergo efficient deamination. However, why are the two enzymes so different in terms of the impact of the missing O(4’)? In the case of ADA, despite the ~100-fold drop in the rate of deamination caused by the missing oxygen, the enzyme clearly prefers North-dAdo (4) over South-dAdo (5). In view of the absence of stereoelectronic effects and the key interaction between O(4’) and His 14, the selection of North-dAdo as the better substrate by ADA appears to be driven exclusively by the North conformation of the locked sugar and its ability to reproduce the important network of hydrogen bonds seen with the natural substrates bound in the 3’-endo, North conformation. All things being equal in terms of the missing O(4’)-effect, the rate of deamination of the conformationally flexible aristeromycin (3), lies between the rates of deamination of the rigid North-dAdo and rigid South-dAdo and supports ADA’s preference for the North conformer.
The situation with CDA is completely different. Not only CDA has the opposite preference for the South conformer (which it deaminates ~100,000-fold less efficiently than cytidine), as inferred from the 10-fold faster rate of deamination of South-dCyd (8) over North-dCyd (7) (Table 2), but also the flexible carbocyclic carbodine (6) is a very good substrate, just 4-fold less efficient than cytidine!
Because formation of the hydrated intermediates is key for the deamination reactions to go forward efficiently, we need to address the impact that the sugar puckering and the O(4’) have on the protonation of the substrates which is an important step prior to the nucleophilic attack by Zn-OH− at the active site in both enzymes. In order to explain these differences, we propose the following arguments:
The pKa of adenosine (pKa = 3.5) is 0.7 log units lower than that of cytidine (pKa = 4.2) and hence it is much harder to protonate. This is probably why there are more acidic residues in ADA (Glu214, Asp292 and Asp293) in direct contact with the base.
The O(4’) anomeric effect is more effectively transmitted when the conformation of the sugar is North; therefore, protonation of the base in adenosine and 2’-deoxyadenosine is facilitated when both nucleosides adopt the North conformation at the active site of ADA.
The influence of the O(4’) anomeric effect in driving the conformation of 2’-deoxyadenosine to the North at the active site of ADA has to be critical because this nucleoside is more stable in the South conformation due to the gauche effect between the 3’-OH and O(4’).[29]
In the absence of O(4’), the combined loss of anomeric effect and the lack of interaction with His 14 significantly reduces the deamination rate of carbocyclic adenosine analogues by ADA, and the preference for the North substrate is driven solely by the shape of the binding pocked of ADA which accommodates the North conformer more effectively.
Aristeromycin (3) has a ring pucker that favors a conformation away from the North[40] orientation and hence there is an additional penalty to shift the flexible cyclopentane ring to the North conformation required by ADA.
The pKa of cytidine is higher, and a single solitary Glu residue in CDA is able to perform the protonation of the base.
Because of the higher pKa of cytidine, the O(4’) anomeric effect may not be critical in facilitating the protonation of cytosine at the active site of CDA. The 3’-OH/O(4’) gauche effect, particularly in 2’-deoxycytidine, drives the conformation to the South[28] to which CDA can bind effectively.
The diminished role of the O(4’) anomeric effect facilitating protonation of cytidine substrates by CDA agrees with the fact the flexible carbocyclic nucleoside carbodine (6) is a good substrate. Protonation of carbodine is even easier due to its higher pKa, which is ~0.45 log units higher than native cytidine nucleosides.[41] Therefore, the only penalty paid during the binding of carbodine at the active site of CDA is that of changing its flexible conformation[42] to the South.
According to the crystal structure of CDA bound to either substrates or inhibitors, there is no interaction between the O(4’) oxygen of the sugar with the ligands around the zinc’s coordinating sphere. Therefore, changing from ribose (or 2’-deoxyribose) to cyclopentane is not as important and the small decrease in the deamination rate of carbodine reflects only the absence of anomeric effect and its role in facilitating hydration.
South-dCyd (8) is a poorer substrate relative to carbodine (6) because of the well known tendency in this class of South bicyclo[3.1.0]hexane nucleosides to force the base into the more stable, unnatural syn conformation.[43] This conformation is further stabilized by an intramolecular hydrogen bond between the 5’-OH and the pyrimidine carbonyl in a binding pocket sequestered from bulk water. The fusion of the cyclopropane ring adjacent to the C—N bond significantly increases the barrier of rotation around the glycosyl bond and the ensuing penalty to rotate the glycosyl bond into the anti range to fit the enzyme is severe.[43]
Despite the severity of the penalty that the South-dCyd substrate must pay to rotate the cytosine base into the required anti range, the enzyme still recognizes the South substrate more effectively than the North counterpart even when the latter favors the anti conformation.[43] The North conformer simply has the wrong sugar pucker and does not fit effectively at the active site of CDA.
The behavior of carbodine as a good substrate for CDA agrees with previous studies form our laboratory showing that the carbocyclic analogue of zebularine experienced only 16-fold drop in potency as a CDA inhibitor.[45]
In summary, we believe that the staggering differences in the rates of deamination of carbocyclic analogues of adenosine and cytidine by ADA and CDA are the result of complex, antipodal stereoelectronic interactions that further confirm the lack of evolutionary homology between them.
EXPERIMENTAL SECTION
Enzyme Assays
Cytidine, 2’-deoxycytidine, potassium phosphate (monobasic and dibasic) and adenosine deaminase (bovine spleen and calf intestinal) were obtained from Sigma. Cytidine deaminase was purified as previously described.[34] All assays were performed in 1 cm quartz cuvettes at 25 °C in 0.1 M phosphate buffer (pH 6.8) on an HP 8452a diode array spectrophotometer. Relative kinetic rates were determined using initial rates (following the first ~10% of reaction), corrected for any differences in enzyme concentration. Substrate concentrations were either 60 µM (adenosine analogues) or 70 µM (cytidine analogues). Deamination of substrate analogues (60 µM) were also followed to completion, where possible. Control experiments were also run to test the stability of the analogues and the enzyme solutions individually, under the same conditions. All of the other experimental information is included in the main text under results, which was necessary to help interpret the figures.
Supplementary Material
ACKNOWLEGMENT
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by grant GM-18325 (DW and GKS). The authors thank Dr. Megan Peach of the LMC for helping with the analysis and display of the crystal structures.
Footnotes
In honor of and in celebration of Morris J. Robins' 70th birthday
REFERENCES
- 1.Jones W, Kurz LC, Wolfenden R. Transition-state stabilization by adenosine deaminase: 1,6-addition of water to the purine ribonucleoside, the enzyme’s affinity for 6-hydroxy-1,6-dihydropurine ribonucleoside, and the effective concentration of substrate water at the active site. Biochemistry. 1989;28:1242–1247. doi: 10.1021/bi00429a043. [DOI] [PubMed] [Google Scholar]
- 2.Frick L, Yang C, Marquez VE, Wolfenden R. Binding of pyrimidin-2-one ribonucleoside by cytidine deaminase as the transition-state analogue of 3,4-dihydrouridine and the contribution of the 4-hydroxyl group to its binding affinity. Biochemistry. 1989;28:9423–9430. doi: 10.1021/bi00450a027. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder GK, Wolfenden R. Rates of spontaneous disintegration of DNA and the rate enhancements produced by DNA glycosylases and deaminases. Biochemistry. 2007;46:13638–13647. doi: 10.1021/bi701480f. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal RP, Spector T, Parks RE. Tight-binding inhibitors—IV. Inhibition of adenosine deaminases by various inhibitors. Biochem. Pharmacol. 1979;26:259–267. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- 5.Glazer RI. Adenosine deaminase inhibitors: Their role in chemotherapy and immunosuppression. Cancer Chemother. Pharmacol. 1980;4:227–235. doi: 10.1007/BF00255266. [DOI] [PubMed] [Google Scholar]
- 6.Smyth JF, Prentice HG, Proctor S, Hoffbrand AV. Deoxycoformycin in the Treatment of Leukemias and Lymphomasa. Ann. N.Y. Acad. Sci. 1985;451:123–128. doi: 10.1111/j.1749-6632.1985.tb27102.x. [DOI] [PubMed] [Google Scholar]
- 7.Laliberte J, Marquez VE, Momparler RL. Potent inhibitors for the deamination of cytosine arabinoside and 5-aza-2'-deoxycytidine by human cytidine deaminase. Cancer Chemother. Pharmacol. 1992;30:7–11. doi: 10.1007/BF00686478. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire M, Momparler LF, Bernstein M, Marquez VE, Momparler RL. Enhancement of antineoplastica acion of 5-aza-2’-deoxycytidine by zebularine on L1210 leukemia. Anti-Cancer Drugs. 2005;16:301–308. doi: 10.1097/00001813-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DK, Rudolph FB, Quiocho FA. Atomic structure of adenosine deaminase complexed with transition-state analog: understanding catalysis and immunodeficiency mutations. Science. 1991;252:1278–1284. doi: 10.1126/science.1925539. [DOI] [PubMed] [Google Scholar]
- 10.Sharff AJ, Wilson DK, Chang Z, Quiocho FA. Refined 2.5 Å structure of murine adenosine deaminase at pH 6.0. J. Mol. Biol. 1992;226:917–921. doi: 10.1016/0022-2836(92)91040-v. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita T, Nishio N, Nakanishi I, Sato A, Fujii T. Structure of bovine adenosine deaminase complexed with 6-hydroxy-1,6-dihydropurine. Acta Cryst. 2003;D59:299–303. doi: 10.1107/s090744490202190x. [DOI] [PubMed] [Google Scholar]
- 12.Xiang S, Short SA, Wolfenden R, Carter CW., Jr Transition-state selectivity for a single hydroxyl group during catalysis by cytidine deaminase. Biochemistry. 1995;34:4516–4523. doi: 10.1021/bi00014a003. [DOI] [PubMed] [Google Scholar]
- 13.Betts L, Xiang S, Short SA, Wolfenden R, Carter CW., Jr Cytidine deaminase. The 2.3 Å crystal structure of an enzyme:transition-state complex. J. Mol. Biol. 1994;235:635–656. doi: 10.1006/jmbi.1994.1018. [DOI] [PubMed] [Google Scholar]
- 14.According to high level calculations it appears that the zebularine 3,4-hydrate may be unstable and that proton transfer from the Zn-OH group to Glu-104 in E. coli leads to an alkoxide-like inhibitor that functions a the real tansition state. Guo H, Rao N, Xu Q, Guo H. Origin of tight binding of a near-perfect transition-state analogue by cytidine deaminase: Implications for enzyme catalysis. J. Am. Chem. Soc. 2005;127:3191–3197. doi: 10.1021/ja0439625.
- 15.Carter CW., Jr The nucleoside deaminases for cytidine and adenosine: structure, transition state stabilization, mechanism, and evolution. Biochimie. 1995;77:92–98. doi: 10.1016/0300-9084(96)88110-7. [DOI] [PubMed] [Google Scholar]
- 16.Seewach DS, Krawczyk SH, Acevedo OL, Townsend LB. Rapid communication: Inhibition of adenosine deaminase by azapurine ribonucleosides. Biochem. Pharmacol. 1992;44:1697–1700. doi: 10.1016/0006-2952(92)90061-m. [DOI] [PubMed] [Google Scholar]
- 17.McCormack JJ, Marquez VE, Liu PS, Vistica DT, Driscoll JS. Inhibition of cytidine deaminase by 2-oxopyrimidine riboside and related compounds. Biochem. Pharmacol. 1980;29:830–832. doi: 10.1016/0006-2952(80)90566-3. [DOI] [PubMed] [Google Scholar]
- 18.Johansson E, Mejlhede N, Neuhard J, Larsen S. Crystal structure of the tetrameric cytidine deaminase from Bacillus subtilis at 2.0 Å resolution. Biochemistry. 2002;41:2563–2570. doi: 10.1021/bi011849a. [DOI] [PubMed] [Google Scholar]
- 19.Teh A-H, Kimura M, Yamamoto M, Tanaka N, Yamaguchi I, Kumasaka T. The 1.48 Å resolution crystal structure of the homotetrameric cytidine deaminase from mouse. Biochemistry. 2006;45:7825–7833. doi: 10.1021/bi060345f. [DOI] [PubMed] [Google Scholar]
- 20.Chung S, Fromme JC, Verdine GL. Structure of human cytidine deaminasebound to a potent inhibitor. J. Med. Chem. 2005;48:658–660. doi: 10.1021/jm0496279. [DOI] [PubMed] [Google Scholar]
- 21.Carlow DC, Carter CW, Jr, Mejlhede N, Neuhard J, Wolfenden R. Cytidine deaminase from B. subtilis and E. coli: Compensating effects of changing zinc coordination and quaternary structure. Biochemistry. 1999;38:12258–12265. doi: 10.1021/bi990819t. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Quiocho FA. Complexes of adenosine deaminase with two potent inhibitors: X-ray structures in four independent molecules at pH of maximum activity. Biochemistry. 1998;37:8314–8324. doi: 10.1021/bi980324o. [DOI] [PubMed] [Google Scholar]
- 23.See http://www.steve.gb.com/science/nucleic_acids.html for listings of nucleoside pKa’s
- 24.Kati WM, Acheson SA, Wolfenden R. A transition state in pieces: Major contributions of entropic effects to ligand binding by adenosine deaminase. Biochemistry. 1992;31:7356–7366. doi: 10.1021/bi00147a021. [DOI] [PubMed] [Google Scholar]
- 25.Carlow D, Wolfenden R. Substrate connectivity in the transition state for cytidine deaminase. Biochemistry. 1998;37:11873–11878. doi: 10.1021/bi980959n. [DOI] [PubMed] [Google Scholar]
- 26.Erion MD, Reddy MR. Calculation of relative hydration free energy differences for heteroaromatic compounds: use in the design of adenosine deaminase and cytidine deaminase inhibitors. J. Am. Chem. Soc. 1998;120:3295–3304. [Google Scholar]
- 27.Carlow DC, Short SA, Wolfenden R. Complementary truncations of a hydrogen bond to ribose involved in transition-state stabilization by cytidine deaminase. Biochemistry. 1998;37:1199–1203. doi: 10.1021/bi971731n. [DOI] [PubMed] [Google Scholar]
- 28.Marquez VE, Russ P, Alonso R, Siddiqui MA, Hernandez S, George C, Nicklaus MC, Dai F, Ford H., Jr Synthesis of conformationally restricted carbocyclic nucleosides. The role of the O4'-oxygen in the key hydration step of adenosine deaminase. Helv. Chim. Acta. 1999;82:2119–2129. [Google Scholar]
- 29.Plavec J, Tong W, Chattopadhyaya J. How do gauche and anomeric effects drive the pseudorotational equilibrium of the pentofuranose moiety of nucleosides? J. Am. Chem. Soc. 1993;115:9734–9746. [Google Scholar]
- 30.Thibaudeau C, Plavec J, Chattopadhyaya J. Quantitation of the pD dependent thermodynamics of the N⇋S pseudorotational equilibrium of the pentofuranose moiety in nucleosides gives a direct measurement of the strength of the tunable anomeric effect and the pKa of the nucleobase. J. Org. Chem. 1996;61:266–286. [Google Scholar]
- 31.Hernandez S, Ford H, Jr, Marquez VE. Is the anomeric effect an important factor in the rate of adenosine deaminase catalyzed hydrolysis of purine nucleosides? A direct comparison of nucleoside analogues constructed on ribose and carbocyclic templates with equivalent heterocyclic bases selected to promote protonation. Bioorg. Med. Chem. 2002;10:2723–2730. doi: 10.1016/s0968-0896(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 32.Marquez VE, Siddiqui MA, Ezzitouni A, Russ PL, Wang J, Wagner RW, Matteucci MD. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem. 1996;39:3739–3747. doi: 10.1021/jm960306+. [DOI] [PubMed] [Google Scholar]
- 33.Ezzitouni A, Barchi JJ, Jr, Marquez VE. Conformationally locked carbocyclic nucleosides built on a bicyclo[3.1.0]hexane template with a fixed southern conformation. Synthesis and antiviral activity. J. Chem. Soc. Perkin Trans. 1997;1:1073–1078. [Google Scholar]
- 34.Cercignani G, Allegrini S. On the validity of continuous spectrophotometric assays for adenosine deaminase activity: A critical reappraisal. Anal. Biochem. 1991;192:312–315. doi: 10.1016/0003-2697(91)90541-z. [DOI] [PubMed] [Google Scholar]
- 35.Cohen RM, Wolfenden R. Cytidine deaminase from Escherichia coli: Purification, properties and inhibition by the potential transition state analog 3,4,5,6-tetrahydrouridine. J. Biol. Chem. 1971;246:7561–7565. [PubMed] [Google Scholar]
- 36.Sober HA. In: Handbook of Biochemistry: Selected Data for Molecular Biology. Sober HA, editor. Cleveland: Chemical Rubber Company; 1968. pp. G46–G50. [Google Scholar]
- 37.Shealy YF, O’Dell CA. Carbocyclic analogs of cytosine nucleosides. J. Heterocycl. Chem. 1980;17:353–358. [Google Scholar]
- 38.Shealy YF, O’Dell CA. Synthesis of carbocyclic analogs of uracil nucleosides. J. Heterocycl. Chem. 1976;13:1015–1020. [Google Scholar]
- 39.Ashley GW, Bartlett PA. Purification and properties of cytidine deaminase from Escherichia coli. J. Biol. Chem. 1984;259:13615–13620. [PubMed] [Google Scholar]
- 40.Thibaudeau C, Kumar A, Bekiroglu S, Matsuda A, Marquez VE, Chattopadhyaya J. NMR conformation of (−)-beta-D-aristeromycin and its 2’-deoxy and 3’-deoxy counterparts in aqueous solution. J. Org. Chem. 1998;63:5447–5462. [Google Scholar]
- 41.Froehler BC, Ricca DJ. Triple helix formation by oligodeoxynucleotides containing the carbocyclic analogs of thymidine and 5-methyl-2’-deoxycytidine. J. Am. Chem. Soc. 1992;114:8320–8322. [Google Scholar]
- 42.Although there is no crystal structure of (−)-carbodine, we could assume that its conformation mirrors that of aristeromycin which in the solid state is almost a perfect East conformation (P = 89°) and in solution it is in a dynamic equilibrium between 35° < P < 65° and 128° < P < 131° (ref. 40).
- 43.Marquez VE, Ben-Kasus T, Barchi JJ, Jr, Green KM, Nicklaus MC, Agbaria R. Experimental and structural evidence that herpes 1 kinase and cellular DNA polymerase(s) discriminate on the basis of sugar pucker. J. Am. Chem. Soc. 2004;126:543–549. doi: 10.1021/ja037929e. [DOI] [PubMed] [Google Scholar]
- 44.Jeong LS, Buenger G, McCormack JJ, Cooney DA, Hao Z, Marquez VE. Carbocyclic analogues of the potent cytidine deaminase inhibitor 1-(β-D-ribofuranosyl)-1,2-dihydropyrimidine-2-one (Zebularine) J. Med. Chem. 1998;41:2572–2578. doi: 10.1021/jm980111x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.