Abstract
The intensity and duration of immune responses are controlled by multiple proteins that modulate Toll-like receptor (TLR) signaling. TRAF family member-associated NF-κB activator (TANK) has been implicated in positive regulation of interferon-regulatory factor-3 as well as NF-κB. Here we demonstrate that TANK is not involved in interferon responses, and is a negative regulator of proinflammatory cytokine production induced by TLR signaling. TLR-induced polyubiquitination of TRAF6 was upregulated in Tank−/−macrophages. Notably, Tank−/− mice spontaneously developed fatal glomerulonephritis owing to deposition of immune complexes. Autoantibody production in Tank−/− mice was rescued by antibiotic treatment or the absence of interleukin (IL)-6 or MyD88. These results demonstrate that constitutive TLR signaling by intestinal commensal microflora is suppressed by TANK.
Introduction
Toll-like receptors (TLRs) recognize microbial components and evoke innate as well as adaptive immune responses. Stimulation with TLR ligands induces the production of proinflammatory cytokines and type I interferons (IFNs) in innate immune cells via intracellular signaling cascades1–3. Upon stimulation, TLRs trigger the recruitment of Toll/IL-1R homology (TIR) domain-containing adaptor molecules. One adaptor, MyD88, is essential for the downstream signaling of various TLRs, with the exception of TLR34–6. MyD88 interacts with IL-1R-associated kinase (IRAK)-4, which activates IRAK-1 and IRAK-27. In turn, the IRAKs dissociate from MyD88 and interact with TNFR-associated factor 6 (TRAF6) (http://www.signaling-gateway.org/molecule/query?afcsid=A002312), which acts as a ubiquitin protein ligase. Together with an E2 ubiquitin-conjugating enzyme complex comprised of Ubc13 and Uev1A, TRAF6 catalyzes formation of a lysine 63 (K63)-linked polyubiquitin chain on TRAF6 itself and on NF-κB essential modulator (NEMO). TGF-β-activated kinase 1 (TAK1) is also recruited to TRAF6, and phosphorylates IKK-β and MAP kinase kinase 68. Subsequently, the IκB kinase (IKK) complex, composed of IKK-α, IKK-β and NEMO, is formed. NF-κB binds to IκBα in resting cells and is sequestered in the cytoplasm. Phosphorylation of IκB by the IKK complex leads to its degradation by the ubiquitin-proteasome system, thereby freeing NF-κB to translocate into the nucleus and activate expression of proinflammatory cytokine genes. Activation of the MAP kinase cascade is responsible for AP-1-induced gene expression. In plasmacytoid dendritic cells (pDCs), MyD88-dependent signaling activates the production of type I IFNs via the transcription factor IFN-regulatory factor (IRF)-71, 9.
Multiple proteins control TLR signaling so as to ensure that the strength and duration of TLR signals is appropriate for any given immune response. TLRs have been implicated in the development of autoimmune diseases, and aberrant activation of innate immunity may contribute to rheumatoid arthritis, inflammatory bowel disease (IBD) and systemic lupus erythematosus (SLE)10, 11. Endogenous RNA molecules such as U1snRNP can activate autoreactive B cells and dendritic cells (DCs) via TLR712. Furthermore, duplication of the Tlr7 gene accounts for the autoimmune phenotypes associated with Y chromosome-linked autoimmune accelerator (Yaa) mice13. TLR9 is also involved in the recognition of immune complexes of DNA and anti-double-stranded DNA (dsDNA) antibodies (Abs), together with B cell receptor (BCR)14. Signaling proteins that inhibit TLR signaling include IRAK-M, ST2, single immunoglobulin IL-1 receptor-related (SIGIRR), suppressor of cytokine signaling (SOCS)-1, the tumor suppressor cylindromatosis (CYLD) and A20 15–21. Cells lacking any one of these proteins show elevated production of proinflammatory cytokines in response to TLR stimulation. Furthermore, mice lacking SOCS-1 or A20 display immune disorders that lead to premature death. In addition, the immunosuppressive cytokine IL-10 suppresses colitis development by inhibiting TLR responses22–24. These studies indicate that negative regulation of TLR signaling is important for coordinated innate immune responses.
TRAF family member-associated NF-κB activator (TANK; also known as I-TRAF) was identified as a TRAF-binding protein25, 26. Among the 6 reported TRAF family members, TRAF1, 2, 3, 5 and 6 interact with TANK25–28. TANK has been implicated in positive regulation of NF-κB activation. In addition to TRAF family members, inducible IKK (IKK-i) and TANK-binding kinase 1 (TBK1) are TANK binding partners29, 30. These proteins phosphorylate IRF-3 and IRF-7, which are transcription factors essential for the expression of type I IFN and IFN-inducible genes 31, 32. TBK1 and IKK-i are activated in response to recognition of viruses via TLRs and retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs)33, 34. TRAF3 is required for TBK1 and IKK-i activation downstream of TLRs and RLRs35, 36. It was reported that TANK functions as an adaptor that bridges TRAF3 and TBK1-IKK-i and TANK is required for type I IFN production in response to viral infection or TLR stimulation37. However, the functional roles of TANK in vivo have not yet been clarified.
In the present study, we use Tank-deficient (Tank−/−) mice to demonstrate that TANK is not involved in IFN responses but is a negative regulator of TLR and BCR signaling. Macrophages and B cells from Tank−/− mice show elevated canonical NF-κB activation in response to stimulation of TLRs and BCR. TLR-induced polyubiquitination of TRAF6 was upregulated in Tank−/− macrophages, indicating that TANK suppresses TLR signaling by controlling TRAF ubiquitination. Tank−/− mice spontaneously developed lupus-like autoimmune nephritis. Autoantibody production in Tank−/− mice was abolished in the absence of IL-6 or MyD88, but not TNF. Furthermore, treatment of Tank−/− mice with antibiotics reduced autoantibody production, implying that IL-6 produced by constitutive TLR stimulation resulting from intestinal commensal microflora is important for the development of disease.
Results
Tank−/− mice develop lupus-like nephritis
To investigate the physiological roles of TANK in vivo, we generated Tank−/−mice by homologous recombination in embryonic stem (ES) cells. We targeted exons 3 and 4 of the mouse Tank gene with a neor cassette in ES cells, and established Tank−/−mice (Supplementary Fig. 1a). Homologous recombination of the Tank locus was confirmed by Southern blotting (Supplementary Fig. 1b). Expression of Tank mRNA and TANK protein was abrogated in Tank−/− macrophages (Supplementary Fig. 1c,d). Tank−/−mice were born from interbred Tank+/− mice in Mendelian ratios and grew normally.
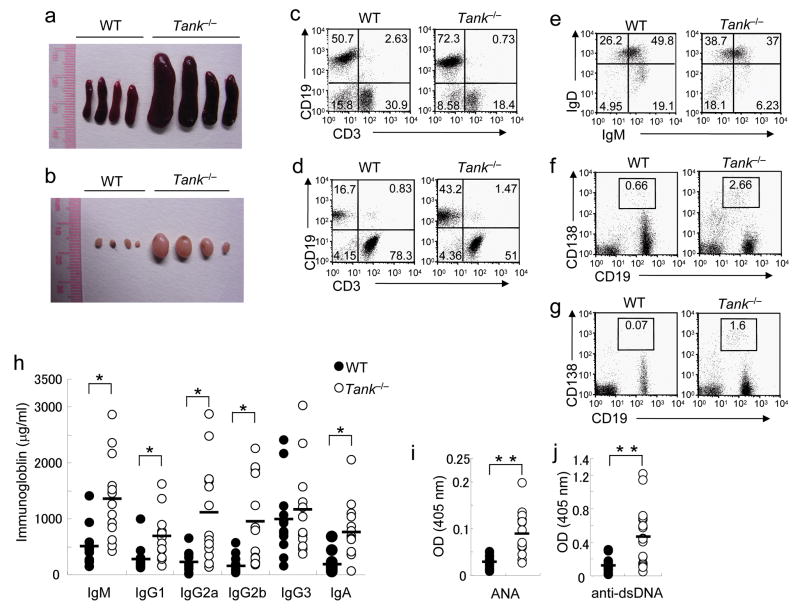
Tank−/− mice displayed splenomegaly and lymphadenopathy (Fig. 1a,b). Flow cytometric analysis revealed a higher percentage of CD19+ B cells in the spleen and lymph nodes (LNs) of Tank−/− mice (Fig. 1c,d). IgMlowIgDhigh mature B cells accumulated in the spleen of Tank−/− mice (Fig. 1e). The percentage of CD19+CD138+ plasma cells was also highly increased in the spleen and LNs of Tank−/− mice (Fig. 1f,g). In contrast, the percentage of FoxP3+CD4+ regulatory T cells did not differ between wild-type and Tank−/− mice (Supplementary Fig. 2a). Consistent with the increased B cell populations, basal serum concentrations of IgM, IgG1, IgG2a, IgG2b and IgA were significantly elevated by 1.2–6.2-fold in Tank−/− mice compared with wild-type mice (Fig. 1h). Importantly, anti-nuclear Ab (ANA) and anti-dsDNA Abs were detected in the sera of Tank−/− mice (Fig. 1i,j)
Figure 1. Increased Ig and autoantibody production due to B cell abnormalities in Tank−/− mice.
(a,b) Images of representative spleens (a) and inguinal LNs (b) from 6-month-old wild-type (WT) and Tank−/− mice. (c–g) Expanded plasma cell populations in spleens and LNs of Tank−/− mice. The percentages of B cells and T cells in spleens (c) and LNs (d), expression of IgM and IgD on splenic B cells (e) and expression of CD138 and CD19 on cells in spleens (f) and LNs (g), from WT and Tank−/− mice were analyzed by FACS. (h) Basal titers of Ig isotypes in sera from nonimmunized 3-month-old WT (n = 13) and Tank−/− (n = 13) mice were measured by ELISA. (i, j) ANA (i) and anti-dsDNA Abs (j) in sera from 12-month-old WT (n = 12) and Tank−/− (n = 12) mice were measured by ELISA. *, P<0.005, **, P<0.001, versus Tank−/− cells.
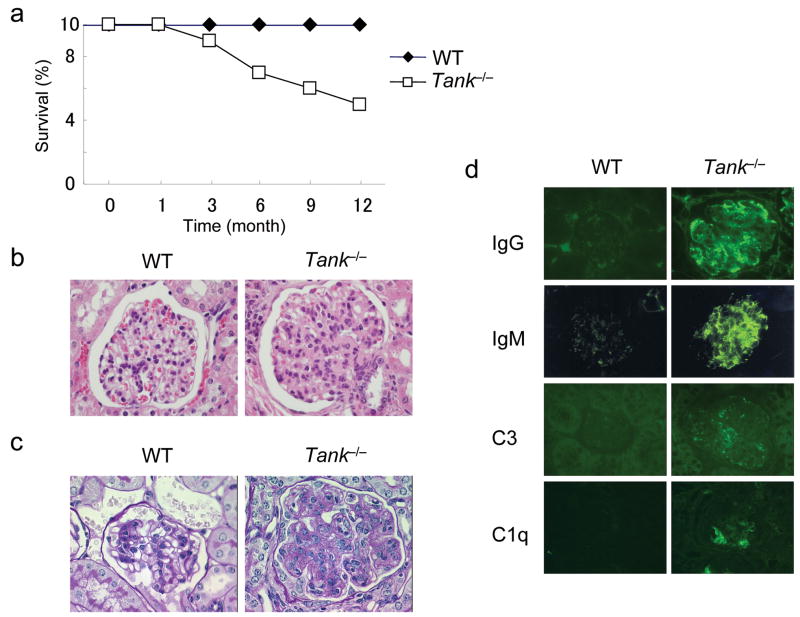
Tank−/− mice spontaneously started to die at 3 months after birth and about 50% of Tank−/− mice had died by 12 months after birth (Fig. 2a). Histological studies revealed that 24 week old Tank−/− mice exhibited glomerulonephritis with mesangial cell proliferation and expansion of the mesangial matrix (Fig. 2b,c). The glomerular structure was devastated in terminally ill Tank−/− mice, suggesting that renal failure was the cause of death (data not shown). Although infiltration of lymphocytes was observed in the liver and lungs of Tank−/− mice, no histological changes were detected in their intestine, heart or joints (data not shown). In addition, deposition of IgG, IgM and complement components C3 and C1q was observed in the glomeruli of Tank−/− mice (Fig. 2d). Such depositions are characteristic of lupus-like nephritis, and suggest that deposition of immune complexes of autoantibodies is the cause of the glomerulonephritis in Tank−/− mice.
Figure 2. Development of lethal glomerulonephritis in Tank−/− mice.
(a) The survival of WT (n = 10) and Tank−/− (n = 10) mice was monitored for 1 year. (b,c) Kidney sections from 6-month-old WT and Tank−/− mice were stained with hematoxylin and eosin (H&E) (b) or Periodic acid-Schiff (PAS) (c). (d) Kidney sections from 6-month-old WT and Tank−/− mice were stained with FITC-labeled anti-mouse IgG, IgM, C3 and C1q.
TANK is a negative regulator of TLR responses
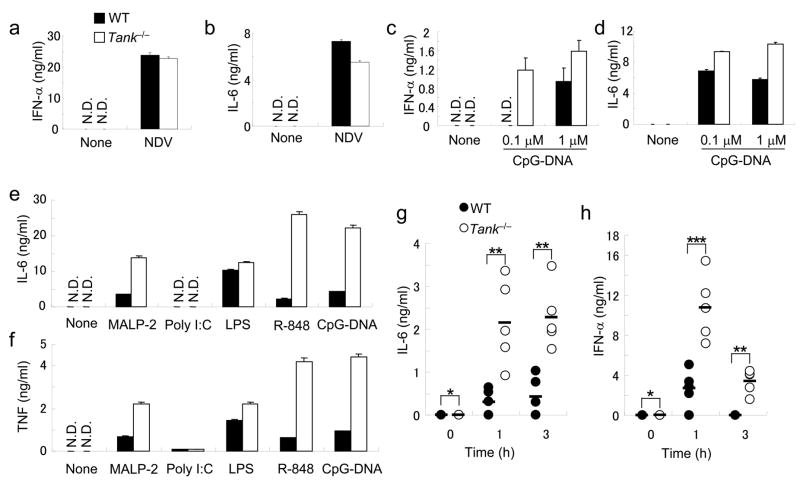
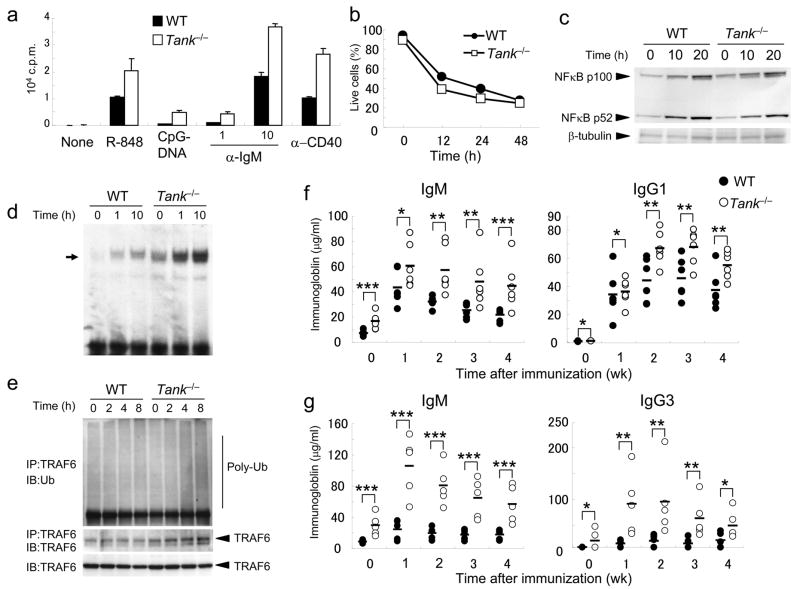
Then, we examined the type I IFN responses of Tank−/− cells to virus infection. In contrast to the results obtained by in vitro studies, IFN-β production in response to Newcastle disease virus (NDV) infection did not differ between wild-type and Tank−/− conventional DCs (cDCs) derived from bone marrow cells (Fig. 3a). Wild-type and Tank−/− cDCs also produced similar amounts of IL-6 (Fig. 3b). NDV is recognized by RIG-I in cDCs, indicating that TANK is not essential for the activation of signaling pathways by RLRs. TRAF3 has been shown to be activated downstream of TLR7 and TLR9 in pDCs. However, bone marrow pDCs induced by Flt3 ligand from Tank−/− mice produced increased, rather than decreased, amounts of IFN-α and IL-6 in response to A/D-type CpG-DNA (Fig. 3c,d). Collectively, these results indicate that TANK is not essential for type I IFN responses.
Figure 3. Enhanced proinflammatory cytokine production in response to TLR stimulation in Tank−/− mice.
(a,b) BM-DCs from wild-type and Tank−/− mice were infected with NDV for 24 h. The concentrations of IFN-α (a) and IL-6 (b) in the culture supernatants were measured by ELISA. (c,d) Flt3L-DCs from wild-type and Tank−/− mice were stimulated with 0.1 or 1μM CpG-DNA for 24 h. The concentrations of IFN-α (c) and IL-6 (d) in the culture supernatants were measured by ELISA. (e,f) Peritoneal macrophages from wild-type and Tank−/− mice were stimulated with MALP-2 (10 ng/ml), poly I:C (100 μg/ml), LPS (100 ng/ml), R-848 (10 nM) or CpG-DNA (1 μM) for 24 h. The concentrations of IL-6 (e) and TNF (f) in the culture supernatants were measured by ELISA. (g, h) Wild-type (n = 5) and Tank−/− (n = 5) mice were intraperitoneally injected with 30 nmol of R-848. Sera were collected and the concentrations of IL-6 (g) and IFN-α (h) were determined by ELISA. Data represent the means ± s.d. of triplicate assays. Similar results were obtained in three independent experiments. *, P < 0.05, **, P < 0.01 and ***, P < 0.005, versus Tank−/− mice.
Next, we examined the production of proinflammatory cytokines in macrophages in response to a set of TLR ligands, including MALP-2 (TLR6-TLR2), poly I:C (TLR3), LPS (TLR4), R-848 (TLR7) and CpG-DNA (TLR9). The production of IL-6 and TNF in response to these TLR ligands, except poly I:C, were highly increased in Tank−/− peritoneal macrophages compared with wild-type cells (Fig. 3e,f). Of note, the enhanced cytokine production in response to LPS stimulation in Tank−/− macrophages was less severe compared to that induced by other TLR ligands. cDCs from Tank−/− mice also showed excessive cytokine production in response to these TLR ligands (data not shown).
We subsequently assessed the role of TANK in cytokine responses to stimulation with TLR ligands in vivo. We chose R-848, because the enhancement in cytokine production in Tank−/− macrophages was most pronounced after stimulation with R-848. We injected R-848 into the peritoneum of 8-week-old mice, and measured serum concentrations of IL-6 and IFN-α one and three hours later. Tank−/− mice contained significantly higher amounts of these serum cytokines at both time points compared with wild-type mice (Fig. 3g,h). Taken together, these results indicate that TANK is a negative regulator of TLR-mediated responses, but not an essential positive regulator of type I IFN responses in vivo.
TANK controls TRAF6 ubiquitination
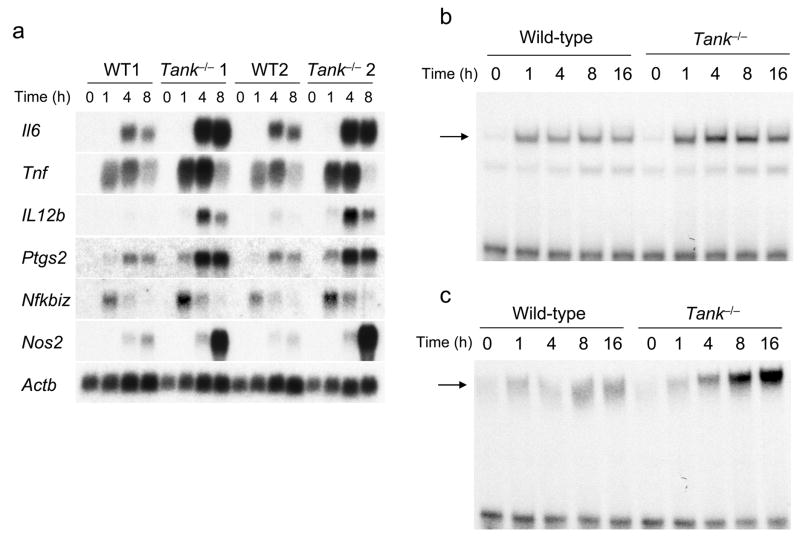
We examined whether the increased cytokine production in Tank−/− macrophages was evident at the level of transcription. In response to R-848 stimulation, wild-type macrophages showed induction of Il6, Tnf, Il12b, Ptgs2, Nfkbiz and Nos2 gene expression. The expression of these genes was enhanced in Tank−/− macrophages in response to R-848 stimulation (Fig. 4a), indicating that initial TLR-induced gene expression is enhanced in Tank−/− macrophages. Next, we analyzed the activation of the transcription factors NF-κB and AP-1 by electrophoretic mobility shift assays (EMSA). In response to R-848 stimulation, activation of NF-κB and AP-1 was enhanced in Tank−/− macrophages compared to wild-type macrophages (Fig. 4b,c).
Figure 4. TANK negatively regulates the activation of NF-κB and AP-1 as well as gene expression in response to TLR7 stimulation in macrophages.
(a) Peritoneal macrophages from wild-type (WT) and Tank−/− mice were stimulated with 10 nM R-848 for the indicated periods. Total RNA was extracted and subjected to Northern blot analyses for the expression of Il6, Tnf, Il12b, Ptgs2, Nfkbiz and Nos2. The same membranes were rehybridized with an Actb probe. Data of two independent experiments (lanes marked 1 and 2 represent distinct experiments) are shown. (b,c) Wild-type and Tank−/− macrophages were stimulated with R-848 (10 μM) for the indicated periods. Nuclear extracts were prepared, and the NF-κB (b) and AP-1 (c) DNA-binding activities were determined by EMSA using NF-κB- and AP-1-specific probes. The arrows indicate the induced NF-κB and AP-1 complexes. The results are representative of three independent experiments.
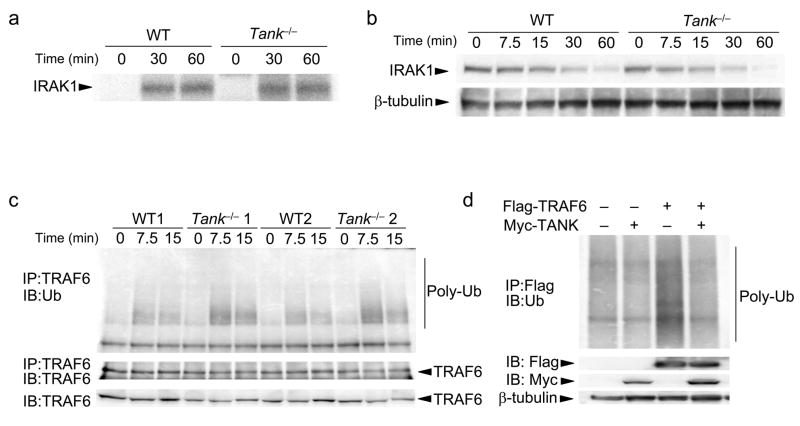
The above-described results indicate that TANK negatively regulates TLR-induced activation of NF-κB and AP-1. Activation of IRAK-1 in response to R-848 was not enhanced in Tank−/− macrophages (Fig. 5a). Furthermore, IRAK-1 was degraded after R-848 stimulation with similar kinetics in wild-type and Tank−/− macrophages (Fig. 5b), indicating that TANK regulates signaling downstream of IRAKs. TANK has been reported to interact with the TRAF family members TRAF1, 2, 3, 5 and 6. Among these, TRAF6 is needed for TLR signaling. Since TRAF6 is ubiquitinated in response to TLR stimulation, we examined whether TANK modifies the ubiquitination of TRAF6. As shown in Fig. 5c, induction of TRAF6 ubiquitination in response to R-848 stimulation was enhanced in Tank−/− macrophages compared with wild-type cells. Reciprocally, overexpression of TANK in HEK293 cells inhibited the ubiquitination of TRAF6 (Fig. 5d). Taken together, these results indicate that TANK inhibits TLR-induced NF-κB and AP-1 activation by suppressing TRAF6 ubiquitination.
Figure 5. TANK controls TRAF6 ubiquitination in response to TLR7 stimulation in macrophages.
(a) Peritoneal macrophages from wild-type (WT) and Tank−/− mice were stimulated with 10μM R-848 for the indicated periods. Cell lysates were prepared and immunoprecipitated with anti-IRAK1. The kinase activities in the immunoprecipitates were measured using an in vitro kinase assay. (b) Macrophages from wild-type and Tank−/− mice were stimulated with 10 μM R-848 for the indicated periods. Whole cell lysates were subjected to immunoblot analysis with anti-IRAK1. Immunoblots ofβ-tubulin are shown as a loading control. (c) Cell lysates of macrophages treated with R-848 for the indicated periods were immunoprecipitated with anti-TRAF6, followed by immunoblot analysis with anti-Ub. Immunoblots of TRAF6 are shown as a loading control. Data of two independent experiments are shown. (d) HEK293 cells were cotransfected with Flag-TRAF6 and Myc-TANK. Cell lysates were immunoprecipitated with anti-Flag, followed by immunoblot analysis with anti-Ub. Immunoblots of β-tubulin are shown as a loading control. The data shown are representative of three independent experiments.
TANK is involved in BCR and CD40 signaling
Next, we investigated the responses of Tank−/− B cells to mitogens such as TLR ligands and crosslinking of BCR and CD40. After stimulation with R-848, CpG-DNA, anti-IgM or anti-CD40, Tank−/− B cells proliferated much more than wild-type B cells (Fig. 6a). On the other hand, the amounts of splenic B cell death following culture without a mitogen were comparable between wild-type and Tank−/− mice (Fig. 6b), indicating that TANK is not involved in the control of B cell apoptosis. In response to anti-CD40, B cells activate both canonical and non-canonical NF-κB. The non-canonical pathway is characterized by processing of the NF-κB2 precursor protein p100 to generate p52. As shown in Fig. 6c, activation of non-canonical NF-κB in response to CD40 stimulation was similar in wild-type and Tank−/− B cells. In contrast, NF-κB DNA-binding activity was enhanced in Tank−/− B cells compared with wild-type B cells (Fig. 6d), and the band was supershifted by anti-p65 and anti-p50 (data not shown). Ubiquitination of TRAF6 after anti-CD40 stimulation was also enhanced in Tank−/− B cells (Fig. 6e). Furthermore, BCR stimulation also induced enhanced activation of NF-κB and ubiquitination of TRAF6 in Tank−/− B cells (Supplementary Fig. 3a,b). Further, the expression of cyclin D2, an NF-κB-inducible protein, was higher in Tank−/− B cells than in wild-type B cells after stimulation with anti-CD40 or anti-IgM (Supplementary Fig. 4). These data suggest that TANK is involved in canonical, but not non-canonical, NF-κB activation pathways in B cells.
Figure 6. Enhanced activation of B cells in Tank−/− mice.
(a) Purified splenic B cells were cultured with R-848 (10 nM), CpG-DNA (10 nM), anti-IgM (1, 10μg/ml) or anti-CD40 (1 μg/ml) for 48 h. The samples were pulsed with [3H]-thymidine (1 μCi) for the last 16 h. [3H]-thymidine incorporation was measured using a β-scintillation counter. (b) Splenic B cells were cultured in the absence of cytokines for the indicated periods. The viability of the cells was determined by annexin V staining followed by flow cytometric analysis. (c) B cells from wild-type and Tank−/− mice were stimulated with 5μg/ml anti-CD40 for the indicated periods, and the processing of p100 to p52 in whole cell lysates was detected by immunoblot analysis. Immunoblots of β-tubulin are shown as a loading control. (d) B cells from wild-type and Tank−/− mice were stimulated with 5μg/ml anti-CD40 for the indicated periods. Nuclear extracts were prepared and the NFκB DNA-binding activity was determined by EMSA. The arrow indicates the induced NF-κB complex. (e) Cell lysates of splenic B cells treated with 5μg/ml anti-CD40 for the indicated periods were immunoprecipitated with anti-TRAF6, followed by immunoblot analysis with anti-Ub. Immunoblots of TRAF6 are shown as a loading control. The data shown are representative of three independent experiments. (f) Mice were immunized with nitrophenol-chicken γ-globulin, and nitrophenol (NP)-specific IgM and IgG1 production was measured by ELISA at 1, 2, 3 and 4 weeks after immunization. The data for 5 representative mice per genotype are shown. (g) Mice were immunized with trinitrophenol-Ficoll, and trinitrophenol (TNP)-specific IgM and IgG3 production was measured at 1, 2, 3 and 4 weeks after immunization. The data for 5 representative mice per genotype are shown. *, P > 0.05, **, P < 0.05 and ***, P < 0.01, versus Tank−/− mice.
In contrast to B cells, wild-type and Tank−/− T cells proliferated to a similar degree after stimulation with anti-CD3 or anti-CD3 together with anti-CD28 (Supplementary Fig. 2b). When stimulated with phorbol myristate acetate (PMA) and ionomycin in vitro, similar proportions of wild-type and Tank−/− CD4+ T cells produced IFN-γ or IL-17 (Supplementary Fig. 2c), suggesting that TANK does not play a role in the development of T helper type 1 (TH1) or TH-17cells.
To explore the influence of TANK deficiency on Ab responses in vivo, wild-type and Tank−/− mice were immunized with a T cell-dependent antigen, NP-CGG, or a T cell-independent antigen, TNP-Ficoll. The NP-specific IgG1 and IgM titers were elevated in Tank−/− mice compared with wild-type mice (Fig. 6f). The TNP-specific IgG3 and IgM titers were also augmented in Tank−/− mice compared with wild-type mice (Fig. 6g). The difference between wild-type and Tank−/− mice was more severe in response to TNP-Ficoll immunization, suggesting that TANK may be more critical for T cell-independent than for T cell-dependent immune responses in vivo.
Intestinal microflora in autoimmunity of Tank−/− mice
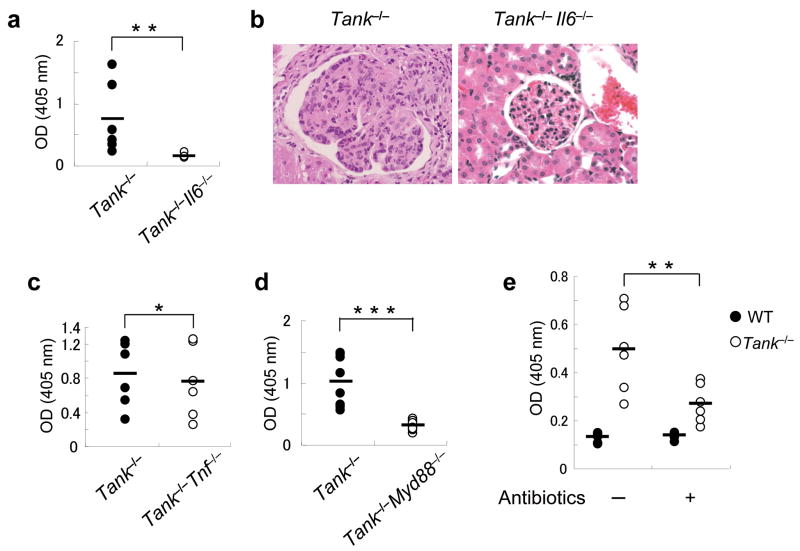
Proinflammatory cytokines play critical roles in the development of autoimmune diseases. Overproduction of IL-6 and TNF in mice results in the development of mesangioproliferative glomerulonephritis and chronic polyarthritis, respectively. To investigate whether IL-6 or TNF is involved in disease pathogenesis in Tank−/− mice, we generated mice lacking IL-6 or TNF on the Tank−/− genetic background. The titers of anti-dsDNA Abs were significantly lower in Tank−/−IL-6−/− than in Tank−/− 5 month old mice (Fig. 7a). Moreover, IL-6 deficiency rescued the glomerulonephritis that developed in Tank−/− mice (Fig. 7b). On the other hand, TNF deficiency did not significantly alter the amount of anti-dsDNA Ab production in Tank−/− mice (Fig. 7c). To examine whether MyD88 deficiency protects against the disease progress, we crossed Tank−/− mice with MyD88−/− mice. The anti-dsDNA Ab titers in 5-month-old Tank−/−MyD88−/− mice were significantly lower than in Tank−/− mice (Fig. 7d), indicating that TLR and/or IL-1R family members are critical for the autoimmunity caused by TANK deficiency. The next question we addressed was how TLR and/or IL-1R signaling was activated to cause IL-6 production. Intestinal microflora has been shown to be involved in the pathogenesis of autoimmune diseases, such as colitis in IL-10-deficient mice. Therefore, we orally treated Tank−/− mice with a combination of antibiotics to clear the intestinal microflora. As shown in Fig. 7e, the antibiotic treatment significantly ameliorated the production of anti-dsDNA Abs, suggesting that continuous stimulation of TLRs by intestinal microflora contributes to the generation of autoantibodies in the absence of TANK.
Figure 7. Antibiotic treatment as well as deficiency of MyD88 or IL-6 ameliorates autoantibody production in Tank−/− mice.
(a) Anti-dsDNA Abs in sera from 5-month-old Tank−/− (n = 6) and Tank−/−IL-6−/− (n = 6) mice. (b) H&E staining of kidney sections from Tank−/− and Tank−/−IL-6−/− mice. (c,d) Anti-dsDNA Abs in Tank−/− (n = 6) and Tank−/−Tnf−/− (n = 6) mice (c), Tank−/− (n = 6) and Tank−/−MyD88−/− (n = 6) mice (d) were measured by ELISA. (e) Oral treatment with antibiotics reduces the serum anti-dsDNA Ab concentrations in Tank−/− mice. WT (n = 6) and Tank−/− (n = 6) mice were given drinking water containing ampicillin (1 g/L), neomycin (1 g/L), vancomycin (0.5 g/L) and metronidazole (1 g/L) after birth. Control wild-type (n = 6) and Tank−/− (n = 6) mice received untreated drinking water. The serum anti-dsDNA Ab concentrations were measured by ELISA at 16 weeks of age.
Discussion
In the present study, we generated Tank−/− mice and showed that TANK is essential for negative regulation of canonical NF-κB signaling. Tank−/− mice displayed enhanced activation of macrophages and B cells in response to TLR ligands and antigens, culminating in the development of fatal immune complex-mediated renal failure. Although TANK has been shown to positively regulate TBK1 and IKK-i-mediated type I IFN production by in vitro studies, analyses of Tank−/− mice revealed that TANK was not needed for activation of the type I IFN pathway downstream of RLRs or TRIF. TANK forms a family with NAK-associated protein 1 (NAP1) and similar to NAP1 TBK1 adaptor (SINTBAD)38, 39, which are comprised of an N-terminal coiled-coil domain and a TBK1-binding domain. NAP1 and SINTBAD have also been implicated in the activation of TBK1 and IKK-i downstream of virus sensors. Knockdown of NAP1, SINTBAD or TANK by siRNA has been linked to impaired IFN responses. Hence, it is possible that these three proteins function redundantly in the activation of TBK1 and IKK-i.
Although previous studies showed that TANK is a positive regulator of NF-κB, our results clearly demonstrate that TANK is critical for the negative regulation of canonical NF-κB via suppression of TRAF6 ubiquitination. K63-type ubiquitination is important for the activation of TAK1 via TAB2 and TAB3 in TLR signaling, and TANK may inhibit TRAF6 ubiquitination by directly binding to TRAF6 in response to TLR stimulation. Although A20 and CYLD have been identified as deubiquitinases40–42, TANK does not harbor a deubiquitination enzyme domain. Immunoprecipitation experiments revealed that overexpressed A20 or CYLD failed to co-immunoprecipitate with overexpressed TANK, suggesting that TANK may suppress ubiquitination of TRAF6 independently of A20 or CYLD (data not shown). Further studies are required to assess the precise mechanism through which TANK modifies TRAF6. In addition, canonical NF-κB activation in response to BCR and CD40 stimulation was augmented in Tank−/− B cells. Consistently, proliferation of B cells in response to TLR and BCR stimulation was highly elevated in Tank−/− mice. In TCR signaling, TRAF2 and TRAF6 were reported to participate in NF-κB activation downstream of Bcl10 and MALT143. Given that TANK suppresses the polyubiquitination of TRAF6 in response to TLR stimulation in macrophages, it is possible that TANK suppresses BCR and CD40 signaling by regulating the activation of TRAF proteins in B cells. On the other hand, activation of non-canonical NF-κB activation was not enhanced in Tank−/− B cells, and it was reported that TRAF3 mainly controls non-canonical NF-κB activation in B cells44. Hence, these observations suggest that TANK is not involved in signaling downstream of TRAF3. Further, TRAF2 can control non-canonical NF-κB as well as marginal zone B cell development. The relationship between TANK and TRAF2 needs to be further explored in future.
The disease caused by the absence of TANK was characterized by glomerulonephritis due to deposition of immune complexes in the glomeruli. In addition, anti-dsDNA Abs and ANA were present in high concentrations in Tank−/− mice. These observations indicate that Tank−/− mice may represent a mouse model of lupus-like immune diseases. The phenotypes of Tank−/− mice are reminiscent of mice overexpressing IL-6 in B cells45, which are characterized by lymphadenopathy and plasmacytosis culminating in the development of severe glomerular nephritis. IL-6 is a pleiotropic cytokine responsible for fever, acute-phase protein expression, osteoclast activation and the development of TH-17 and plasma cells. Indeed, Tank−/− macrophages showed enhanced production of proinflammatory cytokines including IL-6 and TNF in response to TLR stimulation. Furthermore, Tank−/− mice failed to produce autoantibodies and did not develop glomerulonephritis in the absence of IL-6. These results indicate that IL-6 is essential for the development of the Tank−/− B cells that are responsible for the production of autoantibodies. In contrast, Tank−/− T cells responded normally to TCR stimulation. Given that TANK is critical for inhibiting BCR-induced B cell activation, it is possible that the lack of TANK in B cells is important for the generation of autoimmune nephritis via aberrant activation of B cells in response to antigen stimulation.
The generation of anti-dsDNA Abs in Tank−/− mice was significantly decreased in response to oral treatment with antibiotics or in the absence of MyD88, suggesting that TLR signaling is critical for the development of autoimmune diseases in Tank−/− mice. Although various proteins have been identified as negative regulators of TLR signaling, few mice lacking any single one of these proteins spontaneously develop autoimmune diseases spontaneously, with the exception of mice lacking A20. A20−/− mice spontaneously develop multiorgan inflammation and premature lethality, which can be rescued by MyD88 deficiency46, 47. Unlike Tank−/− mice, A20−/− mice do not develop immune complex-mediated glomerulonephritis. A20 controls TNFR in addition to TLR signaling, and the responses to TNF were not altered in Tank−/− cells. TNF is involved in the pathogenesis of organ-specific autoimmune diseases, such as rheumatoid arthritis and Crohn’s disease48. Hence, the differences in the signaling pathways regulated by A20 and TANK may explain the differences in the types of autoimmune disease caused by A20 or TANK deficiency.
Since oral treatment with antibiotics ameliorated autoantibody production in Tank−/− mice, constitutive stimulation of TLRs by intestinal microflora seems to be responsible for the generation of autoimmunity in the absence of TANK. Bone marrow transfer experiments revealed that hematopoietic cells were responsible for the lethality in Tank−/− mice (data not shown). Intestinal microflora contribute to the pathogenesis of IBD48, 49, and the colitis observed in IL-10-deficient mice was rescued by the absence of MyD8824, suggesting that TLR signaling is involved in the pathogenesis of IBD. Since TLRs are expressed on intestinal DCs and are responsible for sensing microbes in the intestine, it is possible that TANK controls the production of certain cytokines in intestinal tissues. Further studies are required to understand why TANK deficiency causes autoimmune nephritis but not colitis.
In addition, the antigen-specific humoral immune responses to haptens were enhanced in Tank−/− mice. This may be due to the enhanced DC and B cell activation in response to antigens and the adjuvant in Tank−/− mice. It will be interesting to explore whether inhibition of TANK expression in certain cell types is beneficial for inducing antigen-specific immune responses in vivo. Modification of TANK may be useful in vaccinations when administered together with an adjuvant.
In summary, the results of the present study clearly demonstrate that TANK is a negative regulator of TLR and BCR responses. Future studies involving cell-type specific deletion of TANK will clarify the complex interplay between immune cells needed to prevent the development of autoimmune diseases.
Methods
Generation of Tank−/− mice
The Tank gene was isolated from genomic DNA extracted from ES cells (GSI-I) by PCR. The targeting vector was constructed by replacing a 2.0-kb fragment encoding the Tank ORF with a neomycin-resistance gene cassette (neor), and a herpes simplex virus thymidine kinase (HSV-TK) driven by the PGK promoter was inserted into the genomic fragment to facilitate negative selection. After transfection of the targeting vector into ES cells, G418 and gancyclovir doubly-resistant colonies were selected, screened by PCR and further confirmed by Southern blotting. Homologous recombinants were microinjected into blastocysts from C57BL/6 female mice, and heterozygous F1 progenies were intercrossed to obtain Tank−/− mice. Tank−/− mice under the 129Sv × C57BL/6 background and their littermate controls were used for the experiments.
Mice and cells
MyD88−/−, and Tnf−/− mice were described previously4, 23. Il6−/− mice were provided by T. Yasui (Osaka University, Osaka, Japan). All animal experiments were carried out with the approval of the Animal Research Committee of the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan). At 3 days after injection of 2 ml of 4.0% thioglycolate medium (Sigma), peritoneal exudate cells were isolated from the peritoneal cavities of mice by washing with ice-cold Hank’s buffered salt solution (Invitrogen). Resting B cells were isolated from splenocyte single-cell suspensions by positive selection with anti-B220 magnetic beads (Miltenyi Biotec). T cells were isolated from splenocyte single-cell suspensions by positive selection with anti-Thy1.2 magnetic beads (Miltenyi Biotec). The cell purities were confirmed to be >90% by flow cytometric analysis.
Reagents
MALP-2 was provided as described previously7. LPS from Salmonella minnesota Re-595 was purchased from Sigma-Aldrich. Poly I:C was purchased from Amersham Biosciences. R-848 was provided by the Pharmaceuticals and Biotechnology Laboratory of the Japan Energy Corporation. The CpG oligonucleotide was synthesized as described previously7. Polyclonal anti-IRAK1 was described previously7.
Measurement of cytokines and autoantibodies
The concentrations of cytokines in culture supernatants and sera were measured by ELISA. The ELISA kits for mouse TNF and IL-6 were purchased from R&D Systems. The ELISA kit for mouse IFN-α was purchased from PBL Biomedical Laboratories. The ELISA kits for mouse anti-dsDNA Abs and ANA were purchased from Alpha Diagnostic International. Serum Ig concentrations were determined as described previously50.
Histological analysis
Formalin-fixed tissues were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS). For detection of renal IgG deposits, kidneys were rapidly frozen in liquid nitrogen and 2-μm cryostat sections were fixed in 100% acetone for 15 min. The sections were incubated with FITC-conjugated goat anti-mouse IgG (ICN Biomedicals), FITC-conjugated donkey anti-mouse IgM (Jackson ImmunoResearch), FITC-conjugated sheep anti-human C3c complement (Thermo Electron Corporation) or FITC-conjugated anti-mouse C1q (RmC7H8) (Cedarlane Laboratories) at 10 mg/ml overnight at 4°C.
RNA hybridization
Peritoneal macrophages were treated with 10 nM R-848 for 0, 1, 4 and 8 h, and total RNA was extracted using the TRIzol reagent (Invitrogen). The extracted RNA was electrophoresed, transferred to nylon membranes and hybridized with various cDNA probes. To detect the expression of Tank mRNA, a 319-bp fragment (350–669) of Tank cDNA was used as a probe. The same membranes were rehybridized with an Actb probe.
In Vitro Kinase Assay
Peritoneal macrophages stimulated with 10 nM R-848 were lysed and immunoprecipitated with anti-IRAK-1 antibody. Then, IRAK-1 activity was measured by an in vitro kinase assay, as described previously7.
Immunoblot analysis
Peritoneal macrophages were treated with 10 nM R-848 for various times. The cells were then lysed in a lysis buffer comprising 1.0% Nonidet-P40, 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 1 mM EDTA and a protease inhibitor cocktail (Roche). The cell lysates were separated by SDS-PAGE and analyzed by immunoblots. Polyclonal anti-TANK (2141) was purchased from Cell Signaling. Polyclonal anti-TRAF6 (sc-7221), monoclonal anti-Ub (F-7), monoclonal anti-β-tubulin (D-10) and anti-cyclin D2 (34B1-3) were obtained from Santa Cruz Biotechnology.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from peritoneal macrophages (4 × 106) stimulated with 10 nM R-848 as described previously7. The nuclear extracts were then incubated with or without Abs against NF-κB p65 or p50 (Santa Cruz), and further incubated with a specific probe for NF-κB DNA-binding sites, before being electrophoresed and visualized by autoradiography.
Immunoblot, immunoprecipitation and in vivo ubiquitination assays
Peritoneal macrophages (4 × 106) were stimulated with 10 nM R-848 for various times. Immunoblotting and immunoprecipitation were carried out as described previously7. For detection of in vivo ubiquitination of TRAF6, cell lysates were boiled in 1% SDS at 90°C for 10 min to remove noncovalently attached proteins, followed by immunoprecipitation with anti-TRAF6 in 0.1% SDS lysis buffer in the presence of protease inhibitors. Ubiquitin was detected by immunoblot analysis.
B and T cell proliferation assays
Purified splenic B cells (5 × 104) were cultured in 96-well plates for 48 h with various concentrations of R-848, CpG-DNA, anti-IgM (Jackson ImmunoResearch) or anti-CD40 (HM40-3, PharMingen). Purified splenic T cells were stimulated with plate-bound anti-CD3 (1 or 5 μg/ml; 2C11, Pharmingen) alone or anti-CD3 (1 μg/ml) plus anti-CD28 (1 μg/ml; 37.51, Pharmingen) for 48 h. The samples were pulsed with 1 μCi of [3H]thymidine for the last 16 h and the 3H uptake was measured using a β-scintillation counter (Packard).
In vivo immunization and ELISA
Mice were immunized intraperitoneally with 50 μg of nitrophenol-chicken γ-globulin (Biosearch Technologies) precipitated with Imject alum (Pierce) or with 25 μg of trinitrophenol-Ficoll (Biosearch Technologies). Antigen- and isotype-specific antibodies in sera collected from peripheral blood at various time points were measured by ELISA on plates coated with nitrophenol-BSA or trinitrophenol-BSA. Antibodies against mouse IgM, IgG1, IgG2a, IgG2b, IgG3 and IgA were purchased from Southern Biotechnology.
Cell viability
Purified splenic B cells (1 × 106) were cultured in RPMI medium containing 10% FCS for various periods. Cell viability was assessed using annexin V-indocarbocyanine (BioVision) and a FACSCalibur (Becton Dickinson).
Construction of TANK expression plasmids
A murine full-length TANK cDNA was obtained by PCR from a murine cDNA library, and cloned into the Myc-pcDNA3 vector.
Statistical analysis
Statistical significance was calculated with the two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We are grateful to T. Yasui (Research Institute for Microbial Diseases, Osaka University) for providing Il6−/− mice and plasmids. We thank all the colleagues in our laboratory, E. Kamada for secretarial assistance, and Y. Fujiwara, M. Kumagai and R. Abe for technical assistance. We thank S. Sato for helpful discussions. This work was supported by the Special Coordination Funds of the Japanese Ministry of Education, Culture, Sports, Science and Technology, and grants from the Ministry of Health, Labour and Welfare in Japan, the Global Center of Excellence Program of Japan, and the NIH (P01 AI070167).
Footnotes
Author contributions
T. K., O.T. and S.A. designed the research and analyzed data. T.K. generated the Tank−/− mice and performed most of the experiments. Y.T., Y.I. and T.T. carried out histological examination of kidneys. H.K. provided advice. T.K., O.T. and S.A. prepared the manuscript.
Disclosure
The authors declare that they have no competing financial interests.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 7.Kawagoe T, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19:11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 14.Viglianti GA, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 15.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 17.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 18.Wald D, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa R, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 20.Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 21.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 26.Rothe M, et al. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci U S A. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin AI, et al. TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol Cell Biol. 1999;19:6665–6672. doi: 10.1128/mcb.19.10.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, et al. Downstream regulator TANK binds to the CD40 recognition site on TRAF3. Structure. 2002;10:403–411. doi: 10.1016/s0969-2126(02)00733-5. [DOI] [PubMed] [Google Scholar]
- 29.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. Embo J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura F, Kawai T, Nakanishi K, Akira S. NF-kappaB activation through IKK-i-dependent I-TRAF/TANK phosphorylation. Genes Cells. 2000;5:191–202. doi: 10.1046/j.1365-2443.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 33.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 36.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 37.Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 38.Sasai M, et al. Cutting Edge: NF-kappaB-activating kinase-associated protein 1 participates in TLR3/Toll-IL-1 homology domain-containing adapter molecule-1-mediated IFN regulatory factor 3 activation. J Immunol. 2005;174:27–30. doi: 10.4049/jimmunol.174.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. Embo J. 2007;26:3180–3190. doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 41.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 42.Trompouki E, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 44.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suematsu S, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 47.Turer EE, et al. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 49.Elson CO, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 50.Sato S, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.