Abstract
Sporadic inclusion body myositis (sIBM) is a common age-related inflammatory myopathy characterized by the presence of intracellular inclusions that contain the amyloid-β (Aβ) peptide, a derivative of the amyloid precursor protein (APP). Aβ is believed to cause Alzheimer's disease (AD), suggesting that a link may exist between the two diseases. If AD and sIBM are linked, then treatments that lower Aβ in brain may prove useful for sIBM. To test this hypothesis, transgenic mice that over express APP in skeletal muscle were treated for 6 months with a variety of nonsteroidal anti-inflammatory drugs (NSAIDs; naproxen, ibuprofen, carprofen or R-flurbiprofen), a subset of which reduce Aβ in brain and cultured cells. Only ibuprofen lowered Aβ in muscle, and this was not accompanied by corresponding improvements in phenotype. These results indicate that the effects of NSAIDs in the brain may be different from other tissues, and that Aβ alone cannot account for skeletal muscle dysfunction in these mice.
Keywords: Amyloid, Sporadic Inclusion Body Myositis, Alzheimer's Disease, Amyloid-β Protein Precursor, Inflammation
Introduction
Sporadic inclusion body myositis (sIBM) is the most common degenerative skeletal muscle disease affecting persons over the age of 50 (Flachenecker, 2006). sIBM pathology includes characteristic rimmed vacuoles, accumulations of filamentous proteins (Askanas and Engel, 2006), inflammation (Dalakas, 2006a), and mitochondrial abnormalities (Oldfors et al., 2006). Although widespread inflammation is an extremely prominent feature of sIBM pathology, various forms of immune suppression have failed to halt the progression of the disease, suggesting that sIBM is driven by a primary degenerative process (Dalakas, 2006b). Currently, there are no effective therapeutics for sIBM.
Alzheimer's disease (AD) is the major neurodegenerative disorder affecting the elderly (Roberson and Mucke, 2006). Although the overall pathology of AD is complex, the disease is believed to be caused by the production and deposition of the 42 amino acid amyloid-β peptide (Aβ42), which is proteolytically derived from the amyloid-β precursor protein (APP) (Hardy, 2006; Wilquet and De Strooper, 2004). It was demonstrated more than a decade ago that both Aβ and an abnormally phosphorylated form of tau are major components of the intracellular inclusions found in the muscle tissue of sIBM patients (Askanas and Engel, 2006). Thus, the proteinaceous deposits in both AD and sIBM share the same characteristic components, raising the intriguing possibility that AD and sIBM may be connected at some level. Unfortunately, there are no clinical studies examining the co-morbidity of the two diseases. Further, the importance of Aβ in AD is backed by strong genetic evidence, whereas the role of Aβ in sIBM primarily rests on descriptive studies of human pathology.
Although a comprehensive clinical comparison of the two diseases is not available, good evidence for a role of APP and/or Aβ in the pathogenesis of sIBM comes from several other sources. Although over expression is not likely required for disease, APP is often over expressed in the disease state (Li et al., 2006; Sarkozi et al., 1993). The in vitro over expression of APP in muscle cells leads to a range of degenerative changes resembling various facets of sIBM pathology (Askanas et al., 1996), including the induced over expression of other proteins found within the inclusion bodies (Wojcik et al., 2006). The over expression of a C-terminal fragment (CTFβ) of APP (Fukuchi et al., 1998; Jin et al., 1998) or the full length APP molecule itself (Moussa et al., 2006; Sugarman et al., 2002) also leads to the manifestation of vacuolar changes, centric nuclei, lymphocytic infiltration, and amyloid deposition in skeletal muscle. This is accelerated by the introduction of a presenilin 1 (PS1) mutation that increases the relative amount of Aβ42, and the muscle weakening phenotype is highly correlated with increasing amounts of this peptide (Kitazawa et al., 2006). The fact that the over expression of APP recapitulates many facets of the disease phenotype in mice, and that this phenotype is worsened by a selective augmentation of the most pathogenic Aβ fragment, is perhaps the strongest evidence for a causal role of APP and Aβ in sIBM.
Given that there is some evidence that AD and sIBM are connected, we considered the possibility that potential AD therapeutics may be effective against sIBM. Chronic use of non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) is widely acknowledged to reduce the risk of AD (McGeer et al., 1996). Our group and others have published reports demonstrating that a subset of NSAIDs act to selectively modulate Aβ42 production, both in cell culture and in APP transgenic mice (Eriksen et al., 2003; Lim et al., 2000; Weggen et al., 2001). For example, ibuprofen is effective at lowering Aβ42 production and reversing AD-like pathology in the brains of Tg2576 transgenic mice, whereas naproxen is ineffective. These compounds likely target the γ-secretase enzyme, possibly through allosteric modulation, by shifting cleavage sites to generate slightly shorter secreted peptides (Beher et al., 2004; Eriksen et al., 2003; Lleo et al., 2004; Weggen et al., 2001). Similarly, other compounds can act in the opposite manner, increasing the proportion of longer fragments at the expense of the shorter ones (Kukar et al., 2005). In this study, we assessed the effectiveness of NSAIDs in an sIBM model, the T7A6 mouse (Sugarman et al., 2002).
Materials and Methods
Transgenic Mice and NSAID Treatment
We selected candidate NSAIDs based on our past experience with the effects of these compounds both in vitro and in vivo (Kukar et al., 2005; Kukar et al., 2007; Weggen et al., 2001). Ibuprofen was included as a positive control for an Aβ42 lowering agent and as the most commonly used (and best tolerated) NSAID that inhibits both COX1 and COX2 in humans; carprofen is the roughly equivalent veterinary NSAID used in several species, including rodents. Naproxen was included as an NSAID that inhibits COX1/2 but has no effect on Aβ42 production (Weggen et al., 2001). R-flurbiprofen inhibits Aβ42 production and only weakly inhibits the COX enzymes (Eriksen et al., 2003). Prior to beginning the mouse study, we evaluated the effects of each NSAID in cultured human neuroglioma cells, following a protocol similar to that which we have used successfully in the past (Kukar et al., 2005). Briefly, H4 neuroglioma cells over expressing Swedish mutant APP (APPΔNL695) were grown in OptiMEM (Invtrogen; Carlsbad, CA) supplemented with 1% fetal bovine serum. Confluent 6-well cultures were incubated overnight with carprofen (Cayman Chemical; Ann Arbor, MI), naproxen (from either Cayman Chemical or Sigma-Aldrich; St. Louis, MO), ibuprofen (either the sodium salt, >98% GC pure, or (S)-(+) form; Cayman Chemical or Sigma-Aldrich), or R-flurbiprofen (Cayman Chemical), each at a final concentration of 100 μM. Compounds were added to cell culture from 50 mM stock dissolved in dimethyl sulfoxide (DMSO), and NSAID treated wells (n = 8 – 14 / compound) were compared to those treated with a matched concentration of DMSO vehicle (n = 22). We chose to compare different preparations and sources of ibuprofen and naproxen, as these would be our positive and negative controls for the mouse study. After treatment, conditioned media were collected, EDTA added to a final concentration of 5mM (to inhibit Aβ degradation), and secreted Aβ measured by ELISA (Kukar et al., 2005; McGowan et al., 2005). We observed no significant difference between suppliers or preparations of naproxen or ibuprofen.
Transgenic mice over expressing APPΔNL695 in skeletal muscle (Sugarman et al., 2002), maintained at the University of Kentucky, were treated with NSAIDs in their food for 6 months, starting at ~12 months of age. This is the age when this mouse strain begins to show symptoms that might be considered roughly analogous to the earliest stage of sIBM in humans, when an individual might normally see a clinician for diagnosis of a problem. We selected the T7A6 rather than the APP × PS1 cross (Kitazawa et al., 2006) since the presence of a PS1 mutation could interfere with NSAID effectiveness (Weggen et al., 2003). Treatment groups were: ibuprofen (sodium salt, Sigma-Aldrich; 25 mg/kg/day; n = 18; 9M, 9F), naproxen (Sigma-Aldrich; 25 mg/kg/day; n = 15; 7M, 8F), R-flurbiprofen (Cayman Chemical; 10 mg/kg/day; n = 18; 7M, 11F), carprofen (Cayman Chemical; 10 mg/kg/day; n = 16; 7M, 9F) or control diet (n = 15; 7M, 8F). A group of wild type littermates (n = 17; 8M, 9F), also consuming control diet, were included as an additional comparison group, to assess both normal motor performance and the appearance of normal skeletal muscle at comparable ages. All diets were formulated by Bio-Serv (Frenchtown, NJ). NSAID doses were selected based on data indicating the maximum dose that could be administered without significant group attrition, and that also had demonstrated efficacy in mouse models of brain Aβ pathology (Kukar et al., 2007; Weggen et al., 2001). Under this rationale, lower doses are unlikely to be informative, since they lack evidence of efficacy. Dosage was calculated based on average food consumption determined over 5 days at the start of the study (Male, n = 9: 3.67 +/- 0.23 g/day ; Female, n = 7: 3.66 +/- 0.31 g/day). Long term delivery in food is a more reliable route of administration, since these compounds have poor aqueous solubility and effective treatment by gavage is logistically unworkable for a study of this size (Eriksen et al., 2003). We and others have successfully used this method of delivery for chronic NSAID treatment in mice (Kukar et al., 2007; Lim et al., 2000).
At the completion of the study, we performed a survival analysis across the different treatment groups to determine if there was any evidence of toxicity. A total of 13 animals died of unknown causes during the course of the study. There was no indication of mortality associated with any specific treatment group (χ2(4) = 8.29, p<0.1). Animals that died before the completion of the study were not included in the analysis. We monitored body weights and observed the mice several times per week for the duration of the study (a necessity due to the regular motor testing and delivery of compound in food), and saw no evidence of systematic health problems (including abnormal variations in body weight) in any treatment group.
Tissue Preparation and Histology
At the end of the study, mice were euthanized by CO2 asphyxiation followed by decapitation, approved methods for euthanasia as recommended by the veterinarians comprising the 1986 Panel on Euthanasia for the U.S. Public Health Service. The brain was cut in half along the longitudinal fissure, and one hemibrain drop fixed in 10% buffered formalin and the other frozen at -80°C for biochemical analysis. Skeletal muscles from one hind limb were formalin fixed, and the other frozen, as above; analyses were performed on the quadriceps and gastrocnemius. Fixed skeletal muscle was embedded in paraffin according to standard protocol, and sectioned at 10 μm using a standard rotary microtome. Sections were stained with standard Hemotoxylin and Eosin, and Engel-Gomori trichrome, as described (Sugarman et al., 2002). Antibodies (4G8; Covance; Denver, PA) for immunohistochemistry were used at 10 μg/ml. Visualization of immunoreactivity was performed using the avidin-biotin peroxidase method with a mouse on mouse kit to eliminate background mouse IgG activity (Vector Labs; Burlingame, CA), using 3,3’-diaminobenzidine as substrate. Some sections were counterstained with thioflavin S, to visualize fibrillar Aβ deposits.
Immunohistochemical amyloid load was determined by imaging quadriceps sections immunostained with 4G8 at 40x final total magnification (the entire section is visible at this magnification, guarding against selection bias) in a subset of animals (n = 4 / group, 2M / 2F). Each section was positioned alongside an adjacent section on the same slide; one section had the primary antibody omitted to control for background immunoreactivity. All slides were processed simultaneously, and all experimental groups were equally represented in the processing run, to minimize staining variability across slides. Digital images were collected in a single run, without adjusting lighting or time of exposure from the initial settings. A uniform adjustment to brightness and contrast was applied to all images, and data were then collected using Scion Image. The total pixel area of each section was determined, and 4G8 positive pixels counted following conversion to a binary image. Final data were expressed as a percentage of the total area corrected to background signal in the adjacent, antibody omitted section.
Assays and Western Blotting
Soluble pools of Aβ in tissue samples were measured using a standard, well characterized extraction procedure, followed by measurement of the amount of Aβ by ELISA. Details of this procedure, and the antibodies used, have been published (Das et al., 2003; Kukar et al., 2005; McGowan et al., 2005; Murphy et al., 2007; Murphy et al., 2003; Murphy et al., 1999; Murphy et al., 2000). Briefly, tissue was homogenized using an AHS200 PowerMax homogenizer, with complete protease inhibitor cocktail (Amresco; Solon, OH). Muscle was homogenized in 2% (w/v) SDS in double distilled H20. Endogenous rodent Aβ in brain was measured following extraction in 0.2% diethylamine / 50 mM NaCl, as described (Eckman et al., 2003; Ramsden et al., 2003). Supernatants were collected following centrifugation at 20,000 × g for 30 minutes at 4°C, to pellet insoluble material. We did not do further extractions in formic acid, because we determined in a previous study that the residual amount of Aβ is negligible in these mice (Sugarman et al., 2002). We could not detect Aβ from skeletal muscle homogenized in aqueous buffer (either standard PBS or TBS). SDS fractions were diluted 1:20 in AC buffer [0.02 M sodium phosphate buffer (pH = 7), 0.4 M NaCl, 2 mM EDTA, 0.4% Block Ace (Serotec; Raleigh, NC), 0.2% BSA, 0.05% CHAPS, and 0.05% NaN3]; DEA supernatants were first neutralized by the addition of a 1/10 volume of 0.5 M Tris-HCl (pH = 6.2). Standard curves were prepared from recombinant Aβ42, and oligomeric Aβ standards were prepared by standard methods (LeVine, 2004). Standards and samples were run at least in duplicate. The sandwich ELISA was conducted using antibodies Ab9 (human sequence Aβ1-16), 4G8 (against Aβ17-24), and 2.1.3 (end specific for Aβ42). A single site sandwich ELISA (4G8/4G8) was used to detect the presence of oligomeric Aβ (LeVine, 2004). 384-well plates (Immulon 4HBX) were coated with 0.5 μg / well of antibody, and blocked with SynBlock (Serotec, as per the manufacturer's instructions). Detection was performed using biotinylated-4G8, followed by an incubation with 0.1 μg / ml of neutravidin-HRP (Pierce Biotechnologies; Rockford, IL) for 1-2 hours. Following development with 3,3',5,5'-Tetramethylbenzidine reagent (TMB; Kirkegaard & Perry Laboratories; Gaithersburg, MD), plates were stopped with 6% o-phosphoric acid and read at 450 nm using a BioTek multiwell plate reader.
Total cyclooxygenase (COX) activity was assayed from skeletal muscle extracted in 200 mg / mL (w/v) of TBST (100 mM Tris-HCl, 50 mM NaCl, 0.1% Tween-20, complete protease inhibitor cocktail; pH = 8.0) in a subset (n = 6-7 / group; 3 M + 3-4 F) of mice. Supernatants (10 μL / sample) were run in comparison to a linear range of resorufin standards, and corrected to background readings (identical except with arachidonic acid omitted), per the manufacturer's instructions (Cayman Chemical). Assays were allowed to develop for one minute at room temperature (~25°C) after addition of arachidonic acid, and read at excitation / emission wavelengths of 530 / 590 nm. Total COX activity was expressed as nmol / min / mL.
For immunoblotting, samples were first separated by SDS-PAGE (10-20% Tris-HCl; BioRad Criterion) under reducing conditions and transferred to PVDF membranes. Equivalent amounts of protein (100 μg) were loaded per lane, as determined by standard BCA assay (Pierce). Membranes were blocked over night with 1% BSA and 2% BlockAce in PBS, and probed for APP and APP fragments using monoclonal antibody 22C11 (Millipore; Billerica, MA) at 0.5 μg/mL. For oligomeric Aβ, following SDS-PAGE (4-12% Bis-Tris; BioRad Criterion), transfer was to nitrocellulose membranes, which were then boiled in PBS for 5 minutes, blocked as above, and then simultaneously probed with antibodies 4G8 (2.5 μg / mL) and 6E10 (against Aβ1-16, 2.5 μg/ml; Covance). Primary antibody incubation (1 hour at 25°C) was followed by detection with HRP-conjugated rabbit anti-mouse IgG (0.1 μg/mL, 30 minutes at 25°C; Rockland Immunochemicals, Gilbertsville, PA). Blots were probed with rabbit anti-GAPDH (Abcam), to control for loading variation. Immunoblots were visualized following application of SuperSignal® West Dura enhanced chemiluminescence reagent (Pierce). Densitometry data were collected using Scion Image.
Motor Performance
Motor coordination and balance were evaluated using a standard rota-rod apparatus (Columbus Instruments; Columbus, OH) (Sugarman et al., 2002). Mice were placed on a rotating spindle, which accelerated over 30 seconds to a top speed of 30 rpm (out to a maximum retention time of 120 seconds). The latency to fall was recorded automatically by an infrared sensor. Mice were also tested on a wire suspension task which we have used successfully in the past (Lewis et al., 2000; Murphy et al., 1995). We validated this as a useful test on untreated T7A6 mice prior to the start of this study. The mouse was allowed to grasp (with its forepaws) a plastic coated wire suspended ~45 cm above a cushioned counter surface, and the latency to fall recorded. For each task, data were collected over 5 trials, and the median score used for subsequent analyses.
Data Analysis
Data were analyzed using SPSS® for Windows, SigmaPlot®, and Microsoft Office Excel®. Group comparisons were performed by multiple linear regression analyses or general linear model ANOVA, followed by post-hoc analyses using Dunnett's test for multiple comparisons to a control group, with an overall type I error rate of 5% (α = 0.05). Single group contrasts were performed using Student's t-test. Nonparametric comparisons were made using Kruskal-Wallis one-way ANOVA or the Mann-Whitney test.
Results
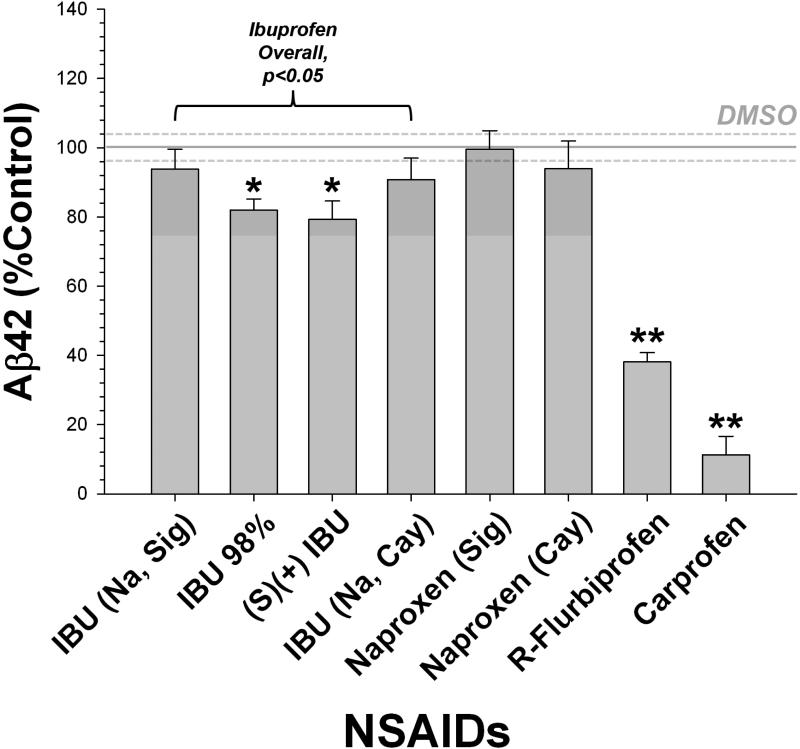
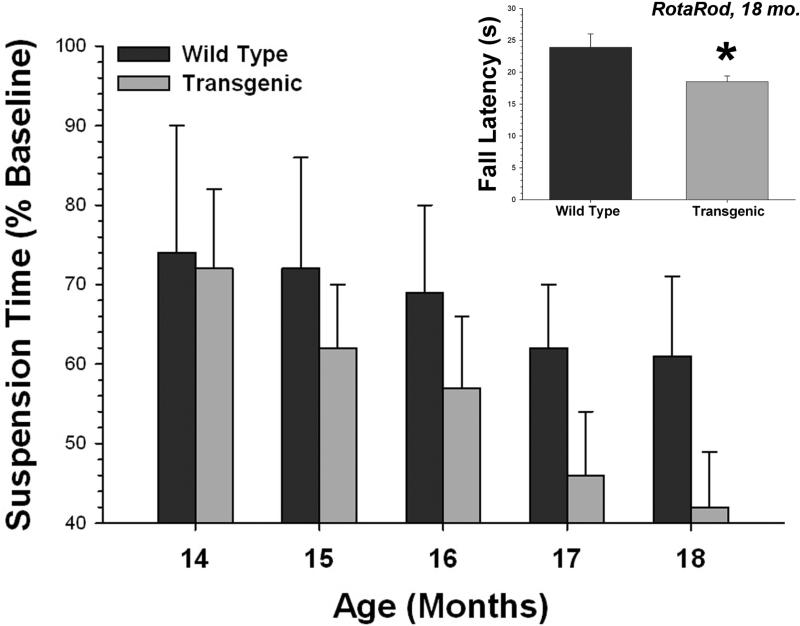
We performed two validation tests at the start of this study. We evaluated the NSAIDs to be used as treatments in the mouse diet in a well characterized cell culture model of Aβ secretion (H4 neuroglioma cells, clone 15x) that we have used previously to identify modulators of γ-secretase (Kukar et al., 2005). This experiment had two primary goals. First, we wanted to determine that our positive and negative controls (ibuprofen and naproxen, respectively) were adequate. Second, we were interested in the effects of carprofen, a veterinary NSAID used to treat mild pain and inflammation in rodents (and other species), which has not been previously reported as a modulator of Aβ production. Overall, we detected a strong Aβ42 lowering effect of NSAIDs (Fig. 1), with ibuprofen and R-flurbiprofen acting as lowering agents and naproxen being ineffective. These results are identical to previous published studies. There were no differences between forms or sources of either ibuprofen or naproxen. Surprisingly, carprofen was the most potent of the compounds tested in this system, reducing Aβ42 by more than 90%. Our second validation test was performed to determine if the wire suspension task was able to detect a decline in motor function in the T7A6 mice. We compared the rate of decline in wire suspension ability in a group of mice followed longitudinally across the age range to be used in this study; the T7A6 mice show a significant decline in performance, whereas their wild type littermates did not (Fig. 2).
Fig. 1.
Evaluation of effects of NSAIDs on cultured H4 neuroglioma (15x) cells. Control levels of Aβ42 are indicated by the solid line, and the error range (s.e.m.) by dotted lines on either side. Aβ42 was selectively reduced by ibuprofen, R-flurbiprofen, and carprofen treatment; naproxen was ineffective (Dunnett's test; * = p<0.05, ** = p<0.01). There were no differences detected between sources or formulations of ibuprofen or naproxen.
Fig. 2.
Wire suspension ability in T7A6 mice. Normal T7A6 mice show a gradual decline in motor function, starting at ~15 months of age, whereas the WT mice do not [T7A6, r = 0.28, p<0.005, n = 23 (11F, 12M); WT, r = 0.09, p<0.35, n = 20 (9F, 11M)]. This is qualitatively similar to the rota-rod deficit seen at the same end-point, ~18 months of age (inset, * = p<0.02).
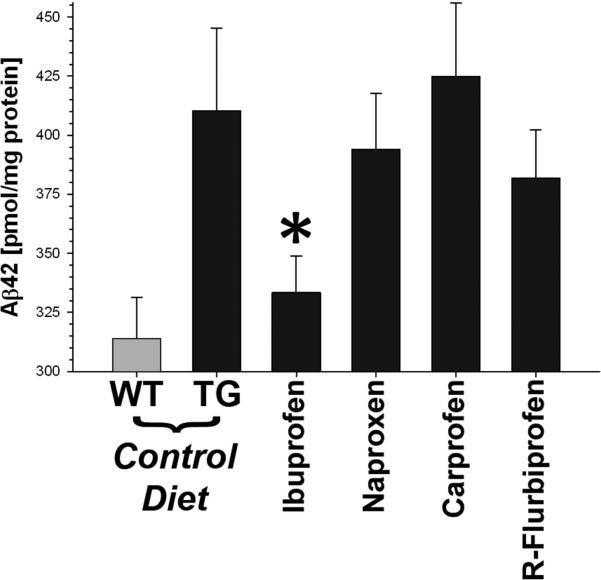
At the end of the study, we compared the amount of Aβ in skeletal muscle (Fig. 3). The mouse quadriceps were extracted in 2% SDS, and the amount of Aβ42 determined by sandwich ELISA. As expected, the T7A6 mice had significantly more Aβ42 in their muscle as compared to wild type littermates (p<0.006). Ibuprofen treated mice showed a significant decrease in the amount of Aβ42 as compared to transgenic controls (p<0.05). The amount of Aβ42 in the ibuprofen treated mice was reduced to that of wild type littermate control mice. No effect was observed for the other three NSAIDs. The total amount of Aβ was also higher in T7A6 mice relative to WT littermates (p<0.02), but was unaffected by any NSAID (p<0.2), indicating that the effect of ibuprofen may be selective for Aβ42, as previously reported (Weggen et al., 2001). Based on the obtained Aβ values, we calculated the effective power of the experimental design at a 92% chance to detect a 10% difference between groups.
Fig. 3.
Skeletal Muscle Aβ42 in NSAID treated mice. T7A6 transgenic mice had more Aβ42 in their quadriceps as compared to wild type littermate controls. Ibuprofen was the only NSAID that was effective at lowering Aβ42 (* = p<0.05, Dunnett's test). Number of subjects / group: Control (WT), n = 8M / 9F; Control (TG), n = 7M / 8F; Ibuprofen, n = 9M / 9F; Naproxen, n = 7M / 8F; Carprofen, n = 7M / 9F; R-Flurbiprofen, n = 7M / 11F.
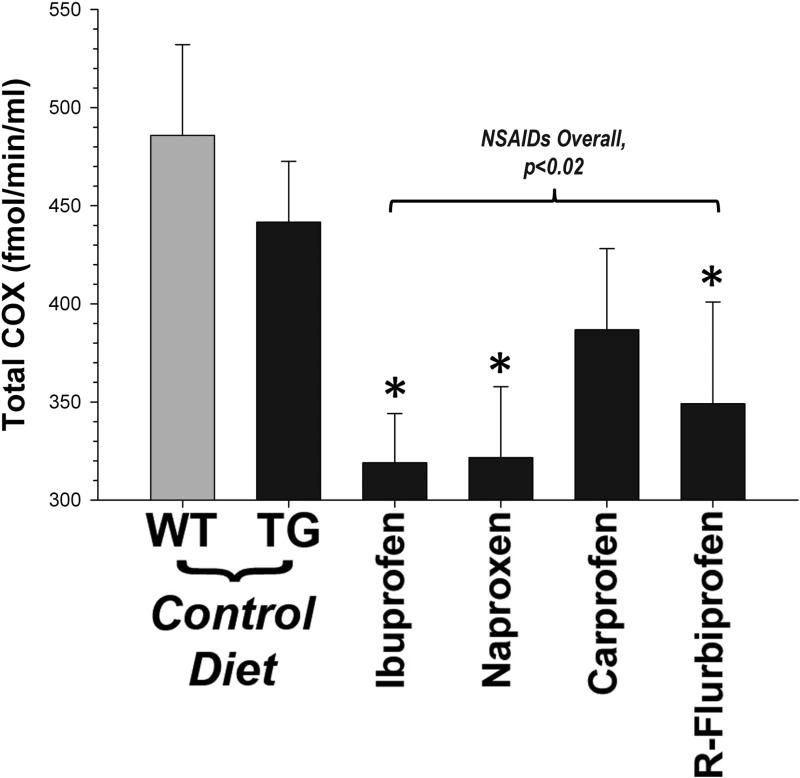
We performed two control experiments to verify NSAID effectiveness. First, we measured endogenous rodent Aβ in DEA extracts from the brains of treated mice. The R-flurbiprofen group had the lowest levels of brain endogenous rodent Aβ42 compared to the other groups (26.3 ± 1.8 vs. 31.1 ± 1.0 pmol/mg total protein; p<0.01), as expected based on previous studies (Eriksen et al., 2003). Second, we measured cyclooxygenase activity. COX activity in muscle was not elevated in transgenic mice relative to controls, but overall was significantly reduced by NSAID treatment (p<0.02; Fig. 4). All four NSAIDs showed approximately equal efficacy: ibuprofen (~31%), naproxen (~31%), and R-flurbiprofen (~25%) all significantly reduced COX activity in muscle. Although carprofen treatment also reduced COX activity (~17%), this did not reach statistical significance. We were unable to distinguish between COX1 and COX2 inhibition in this assay. There was no apparent relationship between COX inhibition and Aβ levels. For instance, the amount of COX activity was nearly identical in naproxen versus ibuprofen treated mice, but only ibuprofen was effective at reducing Aβ42.
Fig. 4.
COX activity in treated mice. NSAID treatment was effective at reducing total COX activity in skeletal muscle (p<0.02). Ibuprofen, naproxen and R-flurbiprofen were equally effective at these dosages (* = p<0.05, Dunnett's test).
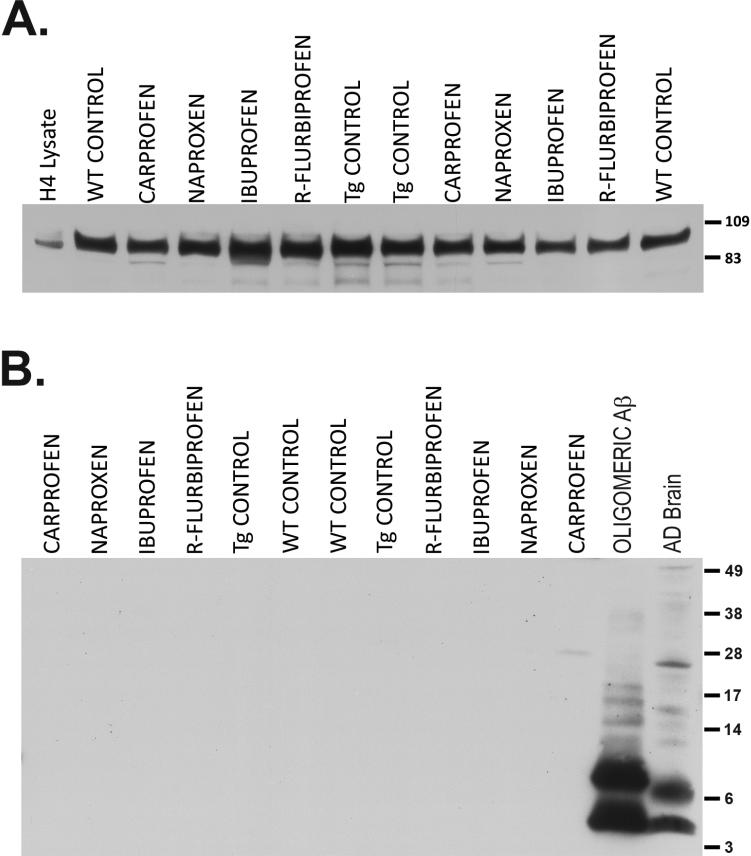
In order to guard against the possibility that NSAID treatment might affect transgene expression, we evaluated the levels of full length APP in the same extracts (Fig. 5A). As expected, APP over expression was relatively modest (1.31 ± 0.03 fold increase over WT; p<0.005) in the T7A6 mice (Sugarman et al., 2002). Although effects at the mRNA level cannot be ruled out, NSAID treatment had no effect on the amount of full length APP protein, an effect we have noted previously (Kukar et al., 2005). We were unable to consistently detect oligomeric Aβ in skeletal muscle from T7A6 mice using a single site ELISA (LeVine, 2004), even in animals as old as 24 months (data not shown). Spiking the samples with synthetic Aβ oligomers (~400 pM) confirmed that the assay was capable of detecting oligomeric Aβ and that such oligomers were not completely dissociated during homogenization of the tissue. This most likely indicates that the concentration of oligomeric Aβ in T7A6 muscle falls below the sensitivity threshold of the assay. We were similarly unable to see either oligomeric or monomeric Aβ by immunoblot (Fig. 5B), suggesting that the various species of Aβ can only reliably be detected by immunoassay or other, more sensitive techniques in this mouse line. These data also indicate that higher order forms of Aβ are not a feature of the pathology in the T7A6 mouse.
Fig. 5.
APP and Aβ in skeletal muscle. Equal quantities of protein (100 μg) from SDS extracted quadriceps were separated by SDS-PAGE. (A) Expression of full length APP (antibody 22C11 against the N-terminus of APP). Lysate (10 μg) from H4 neuroglioma cells over expressing human APP was run as a marker control. T7A6 mice over express human APP (~1.3x) as compared to wild type littermates. NSAID treatment did not alter the expression of APP protein. (B) Oligomeric Aβ (antibodies 6E10 + 4G8). Synthetically prepared oligomeric Aβ (3 pmol) and SDS extract from an AD case (~100 μg protein) were run as positive controls (note the appearance of monomeric Aβ slightly above the 3 kDa marker). Neither monomeric nor oligomeric Aβ was detectable in skeletal muscle from T7A6 mice.
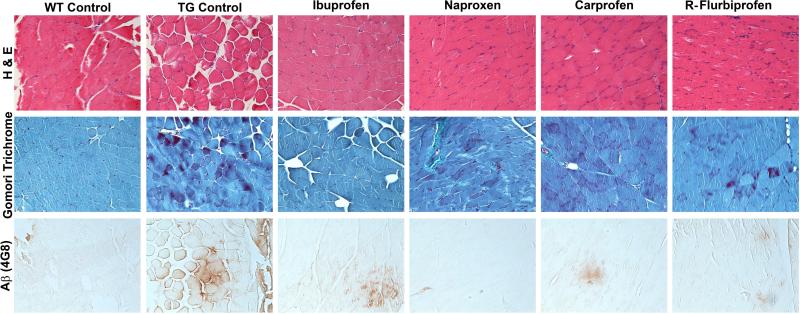
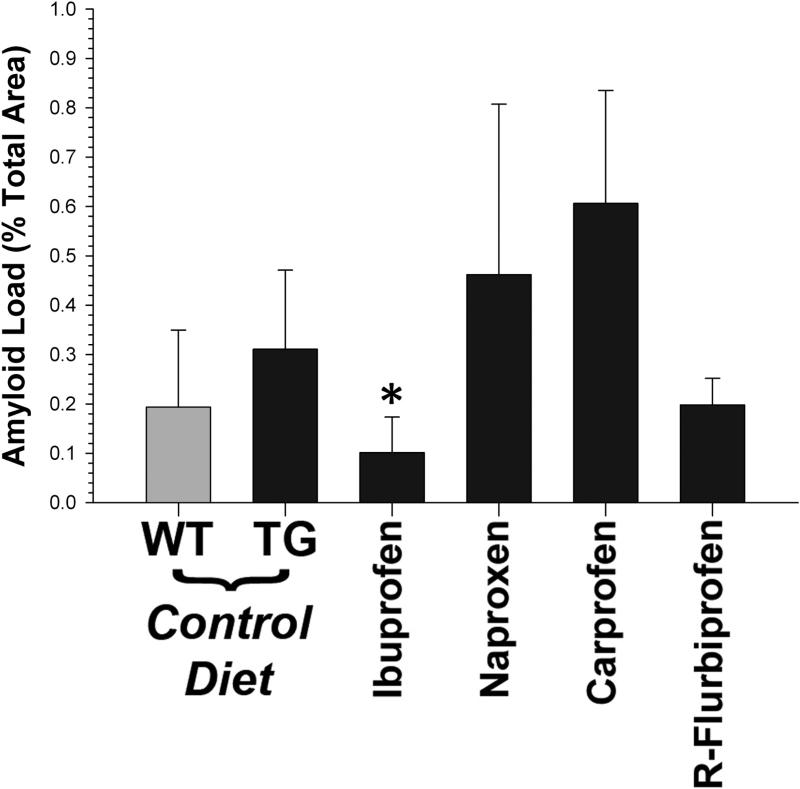
We examined the histopathology in both treated and untreated mice (Fig. 6). At the end point of this study (~18 months of age), the pathology in the T7A6 was relatively mild. There were occasional centric nuclei, and some irregular fibers. Signs of inflammation were not prominent, and we did not see strong evidence of immune cell infiltration. Although Aβ immunoreactivity was present in the T7A6 mice, it was represented for the most part by diffusely immunoreactive fibers. Aβ immunoreactivity was not positive for thioflavin S, indicating that extensive β-sheet structure was absent. We did not see myofibrillar inclusions in these animals. We did not observe any striking differences between groups. We therefore measured the amount of 4G8 immunoreactivity in a subset of animals to determine if we could detect the effect of ibuprofen. Immunohistochemical amyloid load was low overall in the T7A6 mice (0.39 ± 0.10%), but was approximately 2x fold higher than WT mice (~0.2%; Fig. 7). A comparison across treatment groups indicated that ibuprofen significantly (p<0.04) lowered 4G8 signal.
Fig. 6.
Histopathology in T7A6 mice (magnification, 200x). Sections were treated with Hematoxylin and Eosin (H&E; top panel), Engel-Gomori trichrome stain (middle panel) and antibody 4G8 against Aβ (bottom panel). Histopathology in the T7A6 mice was relatively mild, with H&E and Engel-Gomori stains showing infrequent centric nuclei, and occasional irregular fibers. 4G8 immunoreactivity was sparse, and consisted of small areas of diffusely immunoreactive fibers, and rare punctate inclusions in isolated fibers.
Fig. 7.
Estimated amyloid load, as determined from 4G8 immunoreactivity. Positive pixels are expressed as a percentage of the total number of pixels in the section. Consistent with the immunoassay data, ibuprofen was the only NSAID effective at lowering Aβ immunoreactivity in T7A6 muscle (* = p<0.04).
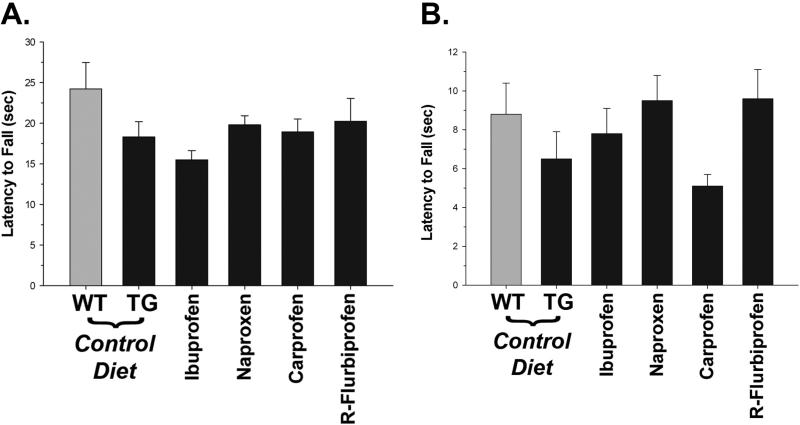
Finally, we evaluated the mice on tests of motor performance. Although T7A6 mice showed some impairments relative to wild type littermate controls (c.f. Fig. 2), treatment with NSAIDs did not improve their performance on either the rota-rod or wire suspension (Figs. 8A and 8B, respectively). Although overall rota-rod scores remained significantly lower (p<0.05) in the transgenic mice, wire suspension scores were surprisingly higher than expected. This resulted in the transgenic mice overall scoring nearly as well as wild type littermate controls. To determine why these results differed from our earlier observations, we standardized the data to baseline performance (not shown). This also did not reveal any differences between NSAIDs, although there was some evidence of a slight improvement (~2 seconds) in the wire suspension across all treated groups (p<0.008, by Mann-Whitney test). A similar, albeit smaller, increase was seen on the rota-rod test, but this did not reach statistical significance. This small improvement in the treated mice may be due to general anti-inflammatory activity or mild analgesic effects of the NSAIDs, since there was no evidence of significant correlation between Aβ42 and rota-rod (r = -0.03) or wire suspension (r = - 0.09) scores.
Fig. 8.
Motor performance in T7A6 mice. NSAIDs did not affect either (A) rota-rod or (B) wire suspension performance at the end of the study. There were no significant differences between individual NSAIDs.
Discussion
In this study, we treated T7A6 mice with NSAIDs over a range of ages starting with the approximate onset of motor impairment and muscle pathology (at 12 months) and ending with a clear pathologic phenotype (at 18 months). We selected NSAIDs based on their published effectiveness (or lack thereof) at lowering Aβ in various mouse models of brain amyloid deposition (Eriksen et al., 2003; Kukar et al., 2007). A similar strategy has recently been pursued with some success using anti-Aβ immunotherapy in a similar mouse model of sIBM amyloid pathology (Kitazawa et al., 2009). We reasoned that if Aβ42 was primarily responsible for driving the pathology in mouse skeletal muscle (Kitazawa et al., 2006), then a targeted reduction of Aβ42 by treatment with γ-secretase modulators would reverse the pathologic phenotype over time. Of the four NSAIDs tested, only ibuprofen significantly lowered Aβ42 in mouse skeletal muscle. Further, although this reduction brought the amount of Aβ42 into the same range as wild type controls, ibuprofen treated mice did not show any improvement in motor function. This outcome suggests that Aβ42 alone cannot account for aspects of skeletal muscle dysfunction, at least in the T7A6 transgenic mouse line.
That ibuprofen was the only effective NSAID was a somewhat surprising result, since of the three NSAIDs capable of reducing Aβ42 production in cultured cells, it was the weakest (~20% reduction). Both R-flurbiprofen (~60%) and carprofen (~90%) were much more effective in the cell based assay, and yet had no effect on the levels of Aβ42 in skeletal muscle. Although overall NSAIDs were effective at lowering the activity of their major target, COX, carprofen was the least effective in this model. It is possible that carprofen was delivered at too low a dose. However, R-flurbiprofen is also very effective at lowering Aβ42 in cell based assays (Eriksen et al., 2003), is effective at lowering Aβ42 in brain (Kukar et al., 2007), and is a relatively weak COX inhibitor (Geisslinger et al., 1994). Further, even though R-flurbiprofen is a poor COX inhibitor, it faired at least as well as both ibuprofen and naproxen in mouse muscle, suggesting that all four compounds were delivered within a somewhat comparable effective range. We have previously shown that inhibitory potency of an NSAID against the COX enzymes is not related to its ability to lower Aβ42, either in vitro or in vivo (Weggen et al., 2001). These data also show that the effect of NSAIDs in brain or in cultured cells does not necessarily predict universal effectiveness as an Aβ lowering agent, and also that ibuprofen may have some unique properties among this class of compounds.
Reduction of Aβ42 in skeletal muscle did not have any bearing on other aspects of the T7A6 phenotype, including motor function. The most straight forward explanation for this finding is that Aβ42, at least in its soluble form, does not account for the phenotype in these animals. This differs from other studies where Aβ42 was found to be negatively correlated with muscle function (Kitazawa et al., 2006), and where the removal of Aβ lead to an improvement (Kitazawa et al., 2009). However, the sIBM-like phenotype in the T7A6 line is more modest than in other APP over expressing lines (Kitazawa et al., 2006; Moussa et al., 2006), with both frank inclusion bodies and inflammation being far less pronounced. One possibility is that higher molecular weight forms of Aβ, such as oligomers and fibrils, are the key drivers of pathology. Since these forms of Aβ are more toxic, their clearance should result in an improvement in the pathologic phenotype. An evaluation of the relationship between oligomeric Aβ and skeletal muscle function, both in vitro and in vivo, would be useful for determining if toxic properties of Aβ are a general phenomenon or are unique to the central nervous system.
A second possibility is that the concurrent presence of significant inflammatory changes, such as immune cell infiltration, or large numbers of inclusion bodies themselves, are strong contributors to the pathologic phenotype and a restoration to normal function is seen when these features are cleared (Kitazawa et al., 2009). This would suggest that the relationship between Aβ42 and tissue pathology, both in brain and skeletal muscle, is not a simple one, and that the development of other aspects of pathology contribute significantly to the final phenotype. Transgenic mice that over express APP recapitulate many facets of the sIBM clinical phenotype, and are the best available evidence for a causal role for Aβ in sIBM. However, because protein over expression in general can lead to cellular inclusions, the issue is difficult to resolve. It is worth noting that the over expression of other proteins in mice can also mimic aspects of sIBM (Page et al., 2009; Weihl et al., 2007), although these (gelsolin and valosin-containing polypeptide, respectively) may have some disease relevance in their own right. Nevertheless, it is possible that some component of the T7A6 phenotype can be attributed solely to APP over expression, as it has multiple, poorly understood functions (Hardy, 2009).
The strongest evidence for the role of Aβ42 in AD is genetic. However, there is an absence of such evidence for sIBM. There has not been a systematic investigation into whether or not mutations or genetic conditions known to cause AD and AD-like pathology also cause sIBM (Murphy and Golde, 2006; Needham et al., 2007). Remarkably, there are no clinical studies that examine the co-morbidity of AD and sIBM. If there is an underlying connection between AD and sIBM – one that reflects a major role for the Aβ peptide – it might be expected that individuals with AD might also show a significant increase in the incidence of sIBM. No one knows the answer to this question. With large differences in both incidence and prevalence, it is unlikely that an association between AD and sIBM would have been noticed in the early stages of AD without formal study, even in cases of familial, inherited AD. Also, if sIBM developed after the onset of AD in the same individual, it would likely be overlooked. Many elderly demented patients are bed ridden and suffer significant, complex health issues, and a systematic connection between the two diseases would almost certainly be missed. These studies are clearly needed, and would go a long way to resolve the issue of whether or not the two disease processes are truly shared.
Acknowledgements
Funding provided by NIH (NS058382, AG005119, RR020171, HL086341) and the Myositis Association. All work was approved by the University of Kentucky IACUC. Thanks to Ela Patel (University of Kentucky) for assistance with the histology, and Dr. Elizabeth Head (University of Kentucky) for helpful discussion. Additional thanks to Todd Golde (Mayo Clinic Jacksonville) for providing antibodies, and Dr. Frank M. LaFerla (University of California at Irvine) for providing the T7A6 mouse line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement / Conflicts of Interest
The authors have no real or perceived conflicts of interest pertaining to this work.
References
- Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Aβ, protein misfolding, and proteasome inhibition. Neurology. 2006;66:S39–48. doi: 10.1212/01.wnl.0000192128.13875.1e. [DOI] [PubMed] [Google Scholar]
- Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci U S A. 1996;93:1314–9. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected Non-steroidal Anti-inflammatory Drugs and Their Derivatives Target γ-Secretase at a Novel Site: EVIDENCE FOR AN ALLOSTERIC MECHANISM. J Biol Chem. 2004;279:43419–43426. doi: 10.1074/jbc.M404937200. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Inflammatory, immune, and viral aspects of inclusion-body myositis. Neurology. 2006a;66:S33–8. doi: 10.1212/01.wnl.0000192129.65677.87. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Sporadic inclusion body myositis--diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006b;2:437–47. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ-/- knock-out mice. J Neurosci. 2003;23:8532–8. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer's disease β-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–4. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J Clin Invest. 2003;112:440–9. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachenecker P. Epidemiology of neuroimmunological diseases. J Neurol. 2006;253:v2–v8. doi: 10.1007/s00415-006-5001-3. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Pham D, Hart M, Li L, Lindsey JR. Amyloid-β deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol. 1998;153:1687–93. doi: 10.1016/s0002-9440(10)65682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisslinger G, Lotsch J, Menzel S, Kobal G, Brune K. Stereoselective disposition of flurbiprofen in healthy subjects following administration of the single enantiomers. Br J Clin Pharmacol. 1994;37:392–4. doi: 10.1111/j.1365-2125.1994.tb04295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–34. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Jin LW, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM. Transgenic mice over-expressing the C99 fragment of βAPP with an α-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol. 1998;153:1679–86. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Green KN, Caccamo A, LaFerla FM. Genetically augmenting Aβ42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am J Pathol. 2006;168:1986–97. doi: 10.2353/ajpath.2006.051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Vasilevko V, Cribbs DH, LaFerla FM. Immunization with amyloid-β attenuates inclusion body myositis-like myopathology and motor impairment in a transgenic mouse model. J Neurosci. 2009;29:6132–41. doi: 10.1523/JNEUROSCI.1150-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ42 production. Nat Med. 2005;11:545–50. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- Kukar T, Prescott S, Eriksen JL, Holloway V, Murphy MP, Koo EH, Golde TE, Nicolle MM. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., 3rd Alzheimer's β-peptide oligomer formation at physiologic concentrations. Anal Biochem. 2004;335:81–90. doi: 10.1016/j.ab.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–5. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Li J, Yin C, Okamoto H, Jaffe H, Oldfield EH, Zhuang Z, Vortmeyer AO, Rushing EJ. Proteomic analysis of inclusion body myositis. J Neuropathol Exp Neurol. 2006;65:826–33. doi: 10.1097/01.jnen.0000228204.19915.69. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Hsaio Ashe K, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT. Nonsteroidal anti-inflammatory drugs lower Aβ42 and change presenilin 1 conformation. Nat Med. 2004;10:1065–6. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–9. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa CE, Fu Q, Kumar P, Shtifman A, Lopez JR, Allen PD, Laferla F, Weinberg D, Magrane J, Aprahamian T, Walsh K, Rosen KM, Querfurth HW. Transgenic expression of βAPP in fast-twitch skeletal muscle leads to calcium dyshomeostasis and IBM-like pathology. Faseb J. 2006 doi: 10.1096/fj.06-5763fje. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Aβ solubility and deposition during AD progression and in APPxPS1 knock-in mice. Neurobiol Dis. 2007;27:301–11. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Das P, Nyborg AC, Rochette MJ, Dodson MW, Loosbrock NM, Souder TM, McLendon C, Merit SL, Piper SC, Jansen KR, Golde TE. Overexpression of nicastrin increases Aβ production. Faseb J. 2003;17:1138–40. doi: 10.1096/fj.02-1050fje. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Golde TE. Inclusion-body myositis and Alzheimer disease: two sides of the same coin, or different currencies altogether? Neurology. 2006;66:S65–8. doi: 10.1212/01.wnl.0000192108.02654.ac. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Hickman LJ, Eckman CB, Uljon SN, Wang R, Golde TE. γ-Secretase, evidence for multiple proteolytic activities and influence of membrane positioning of substrate on generation of amyloid beta peptides of varying length. J Biol Chem. 1999;274:11914–23. doi: 10.1074/jbc.274.17.11914. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Rick JT, Milgram NW, Ivy GO. A simple and rapid test of sensorimotor function in the aged rat. Neurobiol Learning Memory. 1995;64:181–186. doi: 10.1006/nlme.1995.1057. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Uljon SN, Fraser PE, Fauq A, Lookingbill HA, Findlay KA, Smith TE, Lewis PA, McLendon DC, Wang R, Golde TE. Presenilin 1 regulates pharmacologically distinct γ-secretase activities. Implications for the role of presenilin in γ-secretase cleavage. J Biol Chem. 2000;275:26277–84. doi: 10.1074/jbc.M002812200. [DOI] [PubMed] [Google Scholar]
- Needham M, Mastaglia FL, Garlepp MJ. Genetics of inclusion-body myositis. Muscle Nerve. 2007;35:549–61. doi: 10.1002/mus.20766. [DOI] [PubMed] [Google Scholar]
- Oldfors A, Moslemi AR, Jonasson L, Ohlsson M, Kollberg G, Lindberg C. Mitochondrial abnormalities in inclusion-body myositis. Neurology. 2006;66:S49–55. doi: 10.1212/01.wnl.0000192127.63013.8d. [DOI] [PubMed] [Google Scholar]
- Page LJ, Suk JY, Bazhenova L, Fleming SM, Wood M, Jiang Y, Guo LT, Mizisin AP, Kisilevsky R, Shelton GD, Balch WE, Kelly JW. Secretion of amyloidogenic gelsolin progressively compromises protein homeostasis leading to the intracellular aggregation of proteins. Proc Natl Acad Sci U S A. 2009;106:11125–30. doi: 10.1073/pnas.0811753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate β-amyloid levels in male rat brain. J Neurochem. 2003;87:1052–5. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer's disease. Science. 2006;314:781–4. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozi E, Askanas V, Johnson SA, Engel WK, Alvarez RB. β-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993;4:815–8. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Sugarman MC, Yamasaki TR, Oddo S, Echegoyen JC, Murphy MP, Golde TE, Jannatipour M, Leissring MA, LaFerla FM. Inclusion body myositis-like phenotype induced by transgenic overexpression of βAPP in skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:6334–9. doi: 10.1073/pnas.082545599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid-β 42 production by direct modulation of γ-secretase activity. J Biol Chem. 2003;278:31831–7. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Weihl CC, Miller SE, Hanson PI, Pestronk A. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet. 2007;16:919–28. doi: 10.1093/hmg/ddm037. [DOI] [PubMed] [Google Scholar]
- Wilquet V, De Strooper B. Amyloid-β precursor protein processing in neurodegeneration. Curr Opin Neurobiol. 2004;14:582–8. doi: 10.1016/j.conb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, McFerrin J, Paciello O, Askanas V. AbetaPP-overexpression and proteasome inhibition increase αB-crystallin in cultured human muscle: relevance to inclusion-body myositis. Neuromuscul Disord. 2006;16:839–44. doi: 10.1016/j.nmd.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]