Introduction
Arsenic trioxide (ATO) is an effective therapeutic agent for acute promyelocytic leukemia (APL) (Evens et al, 2004). In APL, ATO induces differentiation at low concentrations, while inducing apoptosis at higher concentrations (Miller, et al 2002). In addition, ATO-induced apoptosis in APL is mediated through the mitochondrial apoptotic pathway, resulting in part from the production of reactive oxygen species (ROS) such as hydrogen peroxide (Dai, et al 1999, Yi, et al 2002).
High intracellular levels of glutathione (GSH) confer resistance to ATO in part through the detoxification of ROS. Compounds that promote ROS and/or deplete protective metabolites such as GSH are able to sensitize tumor cells to oxidative cytolysis. Buthionine sulfoximine (BSO), a selective inhibitor of gamma glutamylcysteine synthetase, is known to effectively deplete cellular GSH (Davison, et al 2003, Gartenhaus, et al 2002). We evaluated herein the cytotoxic activity and cell death pathways induced by ATO alone and combined with BSO in non-Hodgkin’s lymphoma (NHL) cell lines and primary lymphoproliferative cells.
Results
ATO-induced apoptosis in lymphoma cell lines and primary cells
With ATO 10µM, approximately 15–30% apoptosis was seen in Ramos, HF1, and SUDHL4 cell lines (Figure 1A). NHL cell lines were subsequently treated with BSO (100µM) or ATO (2µM) alone or in combination. Minimal apoptosis was seen with BSO or ATO alone, while BSO combined with ATO was highly synergistic inducing over 75% apoptosis in all cell lines (Figure 1B).
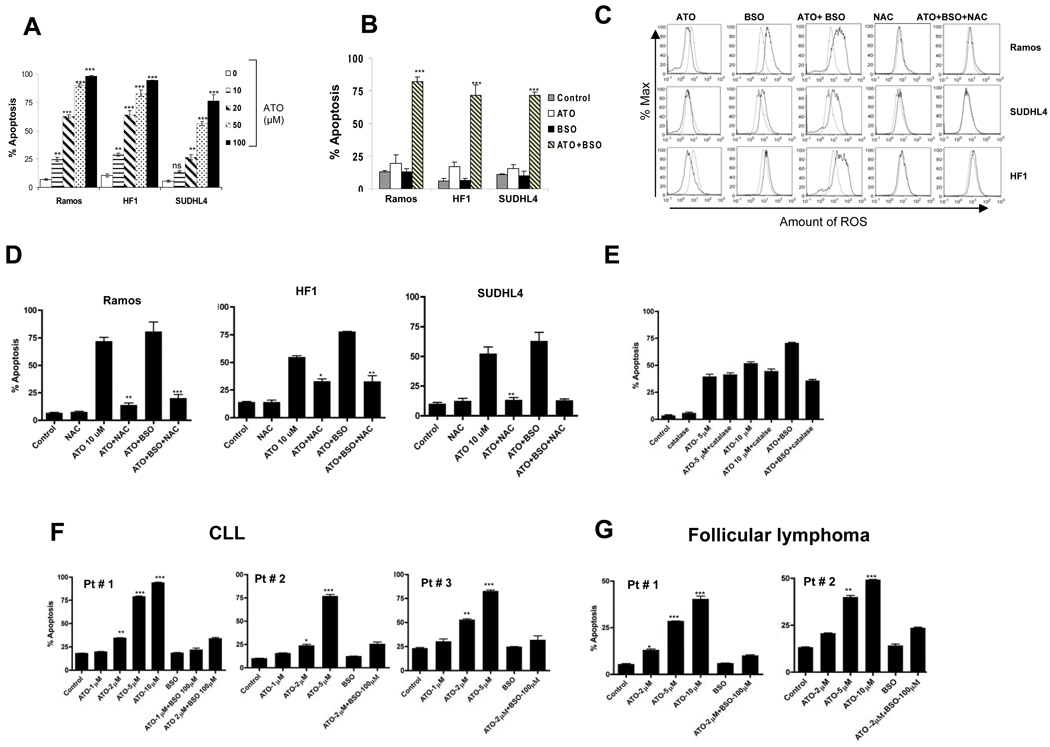
Figure 1. Arsenic trioxide (ATO)-induced apoptosis in lymphoma cells with or without buthionine sulfoximine (BSO).
(A) Ramos, HF1, and SUDHL4 cells were incubated with increasing concentrations of ATO (10µM–100µM) for 18 hours. Percentage apoptosis was measured by annexinV/propidium iodide (PI) staining and analyzed by flow cytometry as described in Materials and Methods. Dose dependent apoptosis was seen in all cell lines, with effective dose for 50% apoptosis (ED50) between 10–20µM for Ramos and HF-1 and 20– 50µM for SUDHL4. P values represent respective concentrations compared with control. (B) Ramos, HF1, and SUDHL4 cells were treated with 2µM ATO, 100µM BSO alone, or ATO/BSO combined for 48 hours. The percentage of apoptotic cells was determined by AnnexinV/PI staining and analyzed by flow cytometry as described. P values compare ATO + BSO vs ATO and BSO alone. In all three cell lines, there was >75% apoptosis when BSO was added to ATO. P values represent respective concentrations compared with control and single agent alone.(C) ROS was measured in Ramos, HF1, and SUDHL4 cells. Cells were incubated with ATO alone (10µM) or ATO 2µM combined with BSO 100µM for 16 hours. Increase in ROS was measured by staining with H2DCF-DA and analyzed by flow cytometry as described in Materials and Methods. Peak shift to the right denotes an increase in ROS production. P value for level of ROS production was <0.05 for control vs ATO/BSO and for ATO/BSO vs ATO+BSO+NAC for all three cell lines. (D) N-acetylcysteine (NAC) attenuated ATO- and ATO/BSO-induced apoptosis. Ramos, HF1, and SUDHL4 cells were pretreated with NAC 10mM for 4 hours followed by incubation with ATO 10µM or ATO 2µM and BSO 100µM, and in combination with NAC for 48 hours. Percentage apoptosis was determined as previously described. P values compared ATO+NAC vs ATO and ATO+BSO+NAC vs ATO+BSO. *P<0.05, **P<0.01, and ***P<0.001. (E) Ramos cells were pre-incubated with 1000 units of catalase following treatment with the indicated concentrations of ATO or ATO/BSO for 48 hours. Apoptosis was measured by annexinV/PI staining. (F) Peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of three CLL patients and from (G) two patients with follicular lymphoma as described in the Materials and Methods. Cells were incubated with indicated concentrations of ATO or ATO/BSO for 24 hours (all CLL cases), 48 hours (follicular lymphoma patient #1), and 72 hours (follicular lymphoma patient #2). Percentage apoptosis was determined as described before. P values compare ATO vs control. *P<0.05, **P<0.01, and ***P<0.001.
We next measured ROS prior to and after ATO and/or BSO. ATO alone induced minimal ROS, while ATO/BSO combined resulted in pronounced ROS production. To determine ROS-dependence, we co-incubated cells with the antioxidant, N-acetylcysteine (NAC). Pretreatment with NAC significantly reduced ROS levels in cells treated with ATO/BSO (Figure 1C). Furthermore, NAC blocked ATO/BSO-induced apoptosis as well as ATO alone (Figures 1D). Catalase did not inhibit ATO-induced apoptosis, while ATO/BSO-induced apoptosis was significantly reduced (Figure 1E). These data suggest that ATO-induced apoptosis is attributed primarily through the depletion of GSH, while ATO/BSO induced apoptosis is more prominently ROS-mediated.
Primary chronic lymphocytic leukemia (CLL) and follicular lymphoma cells were treated with increasing concentrations of ATO +/− BSO (Figure 1F and 1G). Apoptosis in primary CLL cells was approximately 75% with 5µM of ATO alone. Interestingly, significantly less cell death was seen compared with the same concentrations (5–10µM ATO) in NHL cell lines (Figure 1A). Further, the addition of BSO to ATO in primary CLL or follicular lymphoma cells did not enhance apoptosis compared with ATO alone. We hypothesized that low intracellular GSH content might explain the higher sensitivity of primary cells to ATO alone and the lower sensitivity to ATO/BSO. We found that GSH levels in CLL cells were 4–5 fold lower compared with levels seen in Ramos or HF1 cells (data not shown).
ATO alone, but not ATO/BSO, requires Bax, Bak, and Δψm to induce cell death
We investigated whether ATO-induced apoptosis is dependent on Bax translocation. As shown in Figure 2A, ATO alone redistributed Bax from the cytosol to the mitochondria (evident at 6 hours) with release of cytochrome C in Ramos cells. Of note, treatment of cells with combined ATO/BSO did not affect Bax translocation or change in mitochondrial membrane potential (Δψm), suggesting an alternative cell death pathway.
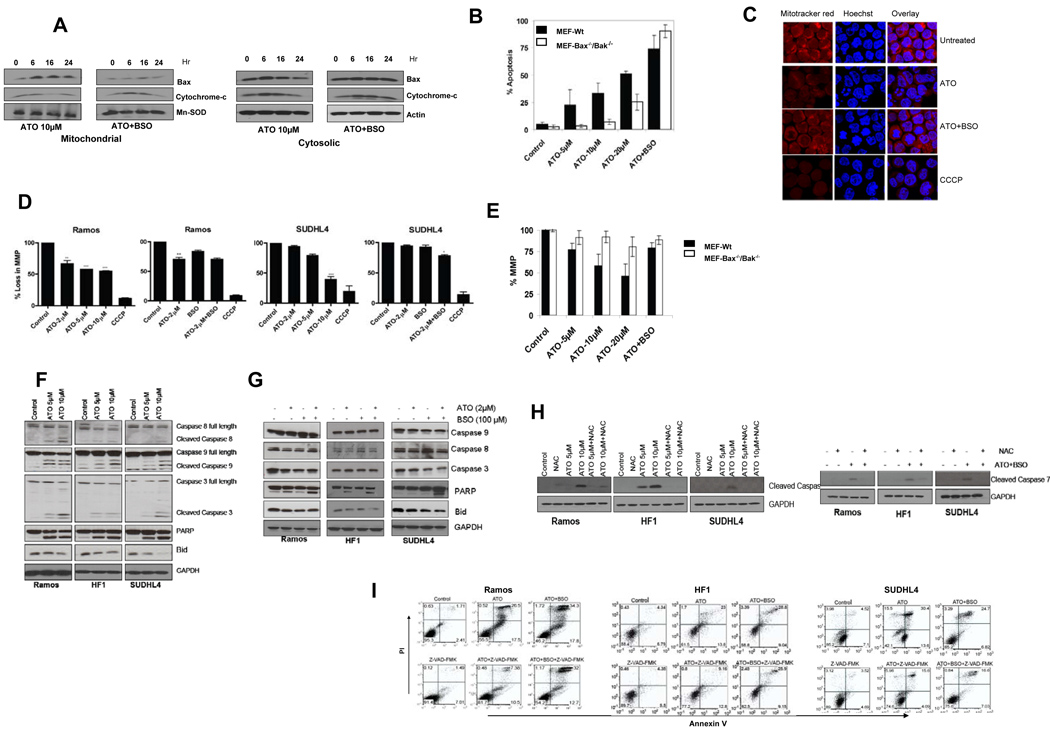
Figure 2. ATO, but not ATO/BSO-induced apoptosis, was mediated by Bax translocation, mitochondrial depolarization, and caspase activation.
(A) Ramos cells were treated with ATO (2µM or 10µM) alone or combined with 100µM BSO for the indicated period of time. Mitochondrial and cytosolic fractions were separated as described in Materials and Methods and analyzed by Western blotting for pro-apoptotic Bax and cytochrome c proteins. Mn-SOD and actin were used as internal controls for mitochondrial and cytosolic extracts, respectively. There is release of cytochrome c from the mitochondria to the cytosol over time with ATO alone with translocation of Bax to the mitochondria (left panel), while in the presence of BSO, there was no change in mitochondrial Bax or cytosolic cytochrome c, suggesting an alternate cell death pathway. (B) Apoptosis was measured in wild type and Bax−/−/Bak−/− MEFs. Cells were plated the night before the treatment. Cells were treated with the indicated concentrations of ATO and ATO/BSO for 24 hours followed by annexinV/PI staining and flow cytometry. (C) SUDHL4 cells were treated with ATO or ATO/BSO for 16 hours followed by incubation with 200nM mitotracker red and 1ug/ml Hoechst stain for 30 minutes at 37°C followed by washing with HBSS and fixed in 3.7 % formaldehyde. Fixed cells were used for confocal microscopy. (D) Mitochondrial membrane potential was measured in Ramos and SUDHL4 cells. Cells were treated with indicated concentrations of ATO vs ATO/BSO for 16 hours, followed by staining with Tetramethylrhodamine (TMRE) as described in materials and methods. Cells were analyzed by flow cytometry. 3-chlorophenylhydrazone (CCCP, Invitrogen) was used as a positive control. (E) Mitochondrial membrane potential was measured in wild type and Bax−/−/Bak−/− MEFs. Cells were plated the night before the treatment and treated with the indicated concentrations of ATO and ATO/BSO for 6 hr and stained with TMRE followed by flow cytometry.Unless indicated, p values represent comparison with control. Abbreviations: Hr, hours; Mito-Bax, mitochondrial-related Bax. *P<0.05, **P<0.01, and ***P<0.001, unless otherwise indicated. (F) Ramos, HF1, and SUDHL4 cells were treated for 16 hours with increasing concentrations of ATO or (G) using BSO (100µM) in combination with lower dose ATO (2µM) for 16 hours. Western blots of whole cell extracts were performed to detect caspase, PARP, and BID proteins. GAPDH was used as internal control. (H) Western blot of cleaved caspase7 in cells treated with ATO or ATO?BSO with and without NAC. (I) Ramos, SUDHL4, and HF1 cells were pre-treated with the pan-caspase inhibitor, Z-VAD-FMK (50µM) for 4 hours, followed by treatment with ATO 10µM or ATO 2µM combined with BSO 100µM for 48 hours. Percentage apoptosis was determined by flow cytometry after staining the cells with annexin V/PI.
To further examine the role of Bax translocation and Δψm, we used immortalized wild type and Bax−/−Bak−/− mouse embryonic fibroblasts (MEFs). Treatment of wild type MEFs with ATO alone resulted in enhanced apoptosis compared with Bax−/−Bak−/− MEFs, while ATO/BSO induced similar apoptosis in wild type and Bax−/−Bak−/− MEFs (Figure 2B). Further, we stained cells with mitotracker red and Hoechst followed by confocal microscopy. Treatment of cells with ATO alone resulted in Δψm loss as indicated by the loss of red stain in Hoechst-stained blue cells (Figure 2C). We also determined the loss of Δψm by tetramethylrhodamine (TMRE) staining (Figure 2D). Treatment of cells with ATO/BSO did not show significant Δψm change indicating mitochondrial-independent cell death in these cells. Moreover, compared with changes in Δψm in wild-type MEFs and the Bax−/−Bak−/− double knockout MEFs, there was a substantial difference in Δψm in wild type and Bax−/−Bak−/− double knockout MEFs following ATO treatment, while ATO/BSO did not show any difference (Figure 2E). Altogether, these studies implicate the dependence of Bax and loss of Δψm in NHL cells treated with ATO alone, but not with ATO/BSO.
ATO-induced apoptosis is caspase-dependent
With ATO alone, there was increased caspase 9 and caspase 3 cleavage in all lymphoma cell lines at 5–10µM (Figure 2F). ATO also induced PARP and BID activation. These findings suggest involvement of both cell death pathways using ATO alone, although with more prominent activation of the intrinsic cascade. In contrast, minimal activation of the intrinsic cascade was observed with ATO/BSO (Figure 2G). However, increase in cleaved caspase 7 (Figure 5C) and PARP cleavage was observed following treatment of each of the three cell lines with both ATO and ATO/BSO (2H). To further test the dependence of the caspases in ATO–related apoptosis (+/−BSO), cell lines were pre-treated with the pan-caspase inhibitor, Z-VAD-FMK. Z-VAD–FMK effectively blocked apoptosis with ATO alone in all cell lines, while much less inhibition was seen in ATO/BSO-treated cells (Figure 2I).
Discussion
In the present study, we found that ATO alone induced apoptosis in NHL cell lines through ROS, Bax, and caspase-dependent pathways, although relatively high in vitro concentrations of ATO were required for effect. Addition of BSO to ATO resulted in highly synergistic cell death with apoptosis that occurred through a Bax- and caspase-independent pathway. Furthermore, we discovered critical differences between primary lymphoma/leukemia cells and in vitro cell lines. BSO did not enhance ATO-related cell death in primary cells, which was likely due to higher basal levels of intracellular GSH in cell lines compared with primary cells. Continued investigation of arsenic-based therapy, including new arsenical compounds that are able to overcome known mechanisms of ATO resistance (e.g., GSH-redox mechanisms) (Diaz, et al 2008, Tsimberidou, et al 2009) and through novel methods of arsenic delivery (Chen, et al 2009), for the treatment of lymphoma is warranted.
Acknowledgements
We would like to thank members of Flow Cytometry and cell imaging facility of Northwestern University.
Supported in part from grants from the National Cancer Institute (AME, K23 CA109613-A1 and LCP, R01CA121192 and a Merit review grant from the Department of Veterans Affairs.
References
- Chandra J, Tracy J, Loegering D, Flatten K, Verstovsek S, Beran M, Gorre M, Estrov Z, Donato N, Talpaz M, Sawyers C, Bhalla K, Karp J, Sausville E, Kaufmann SH. Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and -independent imatinib resistance. Blood. 2006;107:2501–2506. doi: 10.1182/blood-2005-07-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ahn R, Van den Bossche J, Thompson DH, O'Halloran TV. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther. 2009;8:1955–1963. doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- Diaz Z, Mann KK, Marcoux S, Kourelis M, Colombo M, Komarnitsky PB, Miller WH., Jr A novel arsenical has antitumor activity toward As2O3-resistant and MRP1/ABCC1-overexpressing cell lines. Leukemia. 2008;22:1853–1863. doi: 10.1038/leu.2008.194. [DOI] [PubMed] [Google Scholar]
- Evens AM, Lecane P, Magda D, Prachand S, Singhal S, Nelson J, Miller RA, Gartenhaus RB, Gordon LI. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–1273. doi: 10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- Evens AM, Tallman MS, Gartenhaus RB. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk Res. 2004;28:891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Gartenhaus RB, Prachand SN, Paniaqua M, Li Y, Gordon LI. Arsenic trioxide cytotoxicity in steroid and chemotherapy-resistant myeloma cell lines: enhancement of apoptosis by manipulation of cellular redox state. Clin Cancer Res. 2002;8:566–572. [PubMed] [Google Scholar]
- Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- Tsimberidou AM, Camacho LH, Verstovsek S, Ng C, Hong DS, Uehara CK, Gutierrez C, Daring S, Stevens J, Komarnitsky PB, Schwartz B, Kurzrock R. A phase I clinical trial of darinaparsin in patients with refractory solid tumors. Clin Cancer Res. 2009;15:4769–4776. doi: 10.1158/1078-0432.CCR-08-2984. [DOI] [PubMed] [Google Scholar]
- Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X. The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis. 2002;7:209–215. doi: 10.1023/a:1015331229263. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]