Abstract
Previously we showed that anti-Aβ peptide immunotherapy significantly attenuated Alzheimer’s-like amyloid deposition in the central nervous system of aged canines. In this report we have characterized the changes that occurred in the humoral immune response over 2.4 years in canines immunized repeatedly with aggregated Aβ1–42 (AN1792) formulated in alum adjuvant. We observed a rapid and robust induction of anti-Aβ antibody titers, which were associated with an anti-inflammatory T helper type 2 (Th2) response. The initial antibody response was against dominant linear epitope at the N-terminus region of the Aβ1–42 peptide, which is identical to the one in humans and vervet monkeys. After multiple immunizations the antibody response drifted toward the elevation of antibodies that recognized conformational epitopes of assembled forms of Aβ and other types of amyloid. Our findings indicate that prolonged immunization results in distinctive temporal changes in antibody profiles, which may be important for other experimental and clinical settings.
Keywords: Alzheimer’s disease, immunotherapy, amyloid beta, large animal model, aged beagles, conformational antibodies
Introduction
Alzheimer’s Disease (AD) is associated with progressive cognitive decline, neuronal loss and the accumulation of senile plaques and neurofibrillary tangles in affected regions of the brain (Braak and Braak, 1991; Mirra et al., 1991). The amyloid beta peptide (Aβ) is a major component of senile plaques, and the original amyloid cascade hypothesis (Hardy and Higgins, 1992; Selkoe, 1996) proposed that the accumulation of amyloid plaques was the principle factor in AD pathogenesis, however the cascade hypothesis has evolved to include neurotoxic small soluble Aβ aggregates or oligomers (Golde et al., 2006; Lesne et al., 2006; Lue et al., 1999; Mucke et al., 2000; Walsh et al., 2002a; Walsh et al., 2002b). Thus, therapeutic interventions currently being tested are targeted towards slowing production, accumulation or increasing clearance of pathological Aβ species (Selkoe, 2007; Selkoe and Schenk, 2003).
Based on the success of immunotherapy in transgenic mice (Janus et al., 2000; Morgan et al., 2000; Petrushina et al., 2007; Schenk et al., 1999; Seabrook et al., 2007), a clinical trial was initiated in AD patients who were immunized with aggregated Aβ42 (AN1792) formulated in QS-21 adjuvant. However, a subset of patients (6%) developed aseptic meningoencephalitis (Orgogozo et al., 2003; Schenk, 2002), and the clinical trial was halted with patients receiving only 1–3 injections of AN1792 instead of 6 proposed in the Phase IIa protocol. Evaluation of the complete study cohort revealed limited cognitive benefits and “less worsening” of clinical outcome measures (Gilman et al., 2005). In spite of the failure of AN1792 there is still considerable interest in active and passive immunotherapeutic approaches for AD. (http://www.clinicaltrials.gov/).
Aged dogs show a decline in learning and memory, which correlates with a progressive increase in Aβ pathology in the brain (Cummings et al., 1996; Head et al., 1998; Head et al., 2000; Milgram et al., 2002; Milgram et al., 1994; Selkoe, 1996). We recently reported that immunizing aged beagles with aggregated Aβ1–42 was associated with a significant reduction in brain Aβ (Head et al., 2008), however, there were only minor improvements in learning and memory observed. Similar outcomes have now been confirmed in a recent study of 8 AD patients in the AN1792 trial who have come to autopsy. These patients showed significantly less Aβ plaque deposition, however, none of the immunized patients showed any slowing of dementia and all eventually progressed to end stage disease (Holmes et al., 2008).
In this report we describe changes in the humoral response in individual dogs after immunization with Aβ1–42 over 2.4 years. We observed that the initial antibody response was primarily against linear epitopes, however after multiple immunizations the antibody response drifted toward antibodies that recognized conformational epitopes of Aβ, as well as other types of amyloid. This is the first report on the effects of long-term active immunization with Aβ1–42 peptide on the humoral immune response in a large animal model of Aβ-pathogenesis, and the results provide new insights into changes that occur in response to repeated immunization with the full-length peptide.
Materials and Methods
Animals
The longitudinal study included 20 beagles (Head et al., 2008). Briefly, the ages of the animals ranged from 8.4–12.4 years at the beginning of the study (18 females, 2 males). All animals were thoroughly examined prior to inclusion in the study and were determined to be in good health. On the basis of baseline cognitive test results, 9 animals were assigned to be immunized with Aβ, 6 received the adjuvant alum only and 5 received saline only injections. As we reported earlier (Head et al., 2008), in the first year of study one saline injected dog was removed from the study because of an oronasal fistula. A second animal developed blindness (aggregated Aβ-immunized group) and was maintained on the study but could not complete the cognitive testing protocol. A third animal in alum control group was removed in the second year of the treatment because of mammary carcinoma that spread into the lymphatic system. In last year of the study, a fourth animal from Aβ-immunized group was removed after poor recovery from a CSF tap. However, the treatment overall was well tolerated by the animals and no symptoms of adverse events were noted. All procedures were conducted in accordance with IACUC approved animal protocols and the NIH Policy on Humane Care and Use of Laboratory Animals.
Immunization
Aggregated Aβ1–42 immunogen was prepared as previously described (Head et al., 2008). Briefly, Aβ1–42 was prepared by adding 1ml of phosphate buffered saline (PBS pH 7.5) to 1 mg of peptide, and incubated overnight at 37°C in a water bath with moderate shaking prior to conjugation with the adjuvant. To prepare Aβ for immunization, 0.5 mg of aggregated Aβ suspension was mixed with 50 μl of 2% aluminum hydroxide (Alum, Accurate Chemical, Westbury, NY) and 450 μl of PBS. Control animals either received alum only in saline (n=6) or saline only (n=5). Animals were immunized subcutaneously in the back of the neck and monitored for adverse reactions. After 2 weeks, animals were boosted with an additional injection. Following the first 2 injections, animals received a single injection each month for a period of 2.4 years. Blood from experimental animals was collected prior to the start of the study (pre-bleed) and immediately prior to each vaccination monthly for the first 6 months and every 6 months after that (Fig. 1A). Blood was collected in 10 cc red top tubes, centrifuged and the supernatant (serum) was used for the anti-Aβ antibody analyses.
Fig. 1.
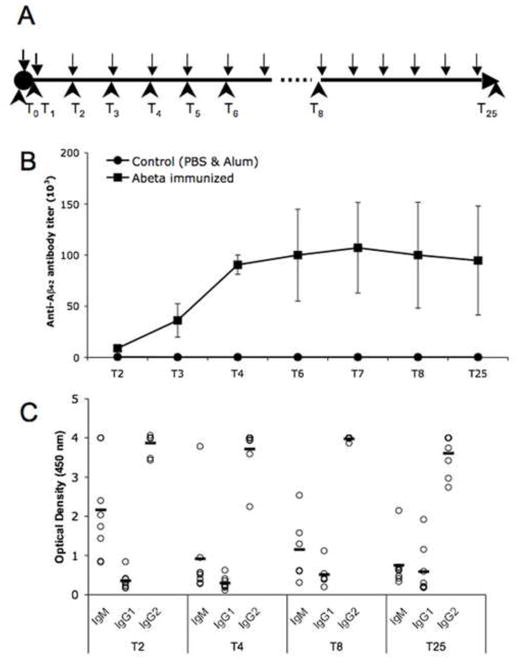
Immunization of aged canines with aggregated Aβ1–42 induces a robust and relatively uniform antibody response. (A) Time line of injections (black arrows) and blood collection (black arrowheads) in older canines. (B) Anti-Aβ antibody levels detected with ELISA reached a plateau after 4 injections and were maintained until the end of the study. (C) Anti-Aβ antibodies induced by vaccination were primarily of the IgG2 (anti-inflammatory) isotype. Open circles represent the data for individual animals, lanes are the value of a mean. (T= blood collection time points). Bars represent means, and error bars represent SEM.
Anti-Aβ antibody titers and linear epitope mapping
The titers of anti-Aβ antibodies were measured by ELISA as previously described (Cribbs et al., 2003; Head et al., 2008). Briefly, 96-well plates (Immulon 2HB, Thermo Fisher Scientific, Waltham, MA) were coated with 2.5 μM of Aβ1–42 protein in carbonate coating buffer (CCB) pH 9.6 (Sigma-Aldrich) and incubated over night at 4°C. The wells were washed and blocked with 3% non-fat dry milk for 1 hour at 37°C with shaking. After washing, serial dilutions of all serum samples were added to the wells and plates were incubated for 1 hour at 37°C with shaking. After washing, HRP-conjugated rabbit anti-dog IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added at 1:2000 dilution for 1 hour at 37°C with shaking, wells were washed, and Ultra-TMB ELISA substrate (Pierce, Rockford, IL) was added to develop the reaction for 15 min. The reaction was stopped by adding 2 N H2SO4 and plates were analyzed on a Synergy HT Spectrophotometer (Bio-Tek Instruments, Winooski, VT) at 450 nm. The sera end-point titer was defined as the maximal sera dilution in which the OD for the antibodies was 3 times higher than the OD values of the blank wells. To calculate anti-Aβ antibody concentration, we used the mouse monoclonal 6E10 antibody (Covance, Princeton, New Jersey) as a standard and calculated dog antibody concentrations using automatic KD4 software (Bio-Tek Instruments). B-cell linear epitope mapping was performed as described previously (Cribbs et al., 2003). Briefly, small overlapping 15- and 17-mer peptides (Invitrogen) spanning the Aβ1–42 peptide sequence as shown in Fig. 2 were coated on ELISA plates as above and individual dog sera from different time points were serially diluted and added to the wells. Serum binding was detected with HRP-conjugated rabbit anti-dog antibodies, followed by Ultra-TMB ELISA substrate as above.
Fig. 2.
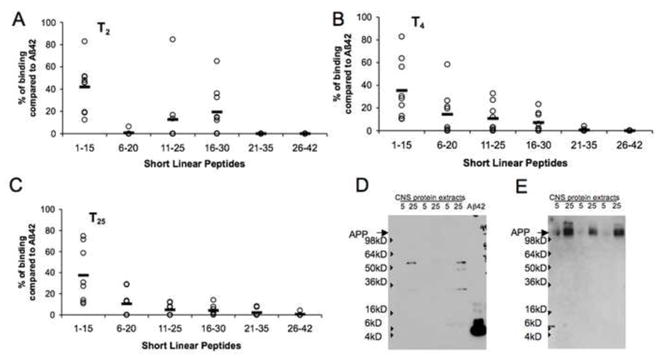
Fine epitope mapping of anti-Aβ antibodies with short linear peptides revealed a major linear epitope induced against the N-terminus (peptide 1–15) of the Aβ1–42 peptide. Linear epitopes were detected by ELISA after 2 (A), 4 (B) and 25 (C) injections. (D) Immune dog serum does not detect canine APP in a Western blot assay, while it recognizes Aβ1–42 peptide. Brain extracts from three different dogs were loaded on a gel at 5 or 25μg per well. (E) membrane from (D) was striped and re-probed with monoclonal 6E10 antibody to demonstrate the presence of APP protein. Open circles represent the individual animals data, lines are the value of the mean.
Anti-Aβ Antibody Isotype Determination
To determine the specific isotypes of induced anti-Aβ antibodies generated by immunized dogs we followed the protocol established by Head et al. (Head et al., 2006) with some modifications. Briefly, sera from individual dogs collected at different time points was serially diluted, and applied on ELISA plates coated with Aβ1–42 peptide as above. To detect Aβ-specific canine IgM, IgG1, IgG2, IgA and IgE antibody subclasses we used anti-dog Ig subclass-specific HRP-conjugated secondary antibodies (Bethyl Laboratories, Montgomery, TX).
Detection of conformational selective antibodies
To detect conformationally-dependent antibodies in dog serum we established several new protocols.
Competition ELISA
To determine the presence of conformationally-sensitive anti-Aβ1–42 antibodies we used a competition ELISA. Serum collected at time points 2, 4 or 25 was diluted to a saturation point (saturation point was defined as maximal dilution of the serum at which it still falls within the plateau OD value of 4.0 on the Aβ ELISA) and mixed with sets of 15- and 17-mer short linear peptides spanning the Aβ1–42 sequence (12.5 μM final concentration for each peptide), with 12.5 μM Aβ1–42 or without Aβ (control). Samples were rotated overnight at 4°C in 10mM Tris Buffered Saline +0.1% Tween-20 (TTBS) with 0.3% of non-fat dry milk. After the incubation, samples were pipetted onto the ELISA plates pre-coated with Aβ1–42, and all of the bound antibodies were detected with HRP-conjugated rabbit anti-dog antibodies as above. Data are presented as the percent of inhibition of binding to Aβ1–42, which was calculated as follows:
where X – is the OD at 450 nm of binding of control (no competing peptides) serum to Aβ1–42
Y – is the OD at 450 nm of binding of serum to Aβ1–42 on ELISA plate after incubation with the set of the short peptides or full-length Aβ1–42.
Competition Dot Blots
To determine if dog antibodies recognized different conformations of Aβ1–42 in addition to linear epitopes, competition dot blots were used. Control and immunized dog serum samples from time points T2, T4 and T25 were diluted to the saturation concentrations in TTBS with 5% milk. Half of the prepared serum was pre-incubated with the same set of competing short linear Aβ peptides described previously (12.5 μM final concentration for each peptide) on a rotator in 4°C overnight, while the other half was used as a control (without competition). Aβ conformational species for the dot blot assay were prepared as follows, Aβ monomers were freshly prepared by dissolving Aβ1–42 in ice cold CCB, pH 9.6 followed by 30 sec sonication in a water bath sonicator FS20 (Fisher Scientific) and used immediately, while Aβ aggregates were allowed to assemble for 24 hours in PBS, pH 7.5 overnight at 37°C with shaking. One μl of each Aβ species was blotted in triplicate onto 0.2μm pore nitrocellulose Protran membranes (Whatman Schleicher & Schuell, Florham, NJ). Another series of dot blots were also tested, which contained pre-defined Aβ monomers, Aβ pre-fibrillar aggregates, Aβ fibrils, α-synuclein pre-fibrillar aggregates, and islet amyloid polypeptide (iAPP) pre-fibrillar aggregates (Anguiano et al., 2002; Lin et al., 2007; Necula et al., 2007). All membranes were allowed to air dry for at least 30 minutes and submerged in TTBS for all washes and 5% milk/TTBS for 1 hour blocking steps and antibody incubations. Dot blot membranes were incubated overnight with either dog serum alone, serum that was pre-incubated with competing linear peptides, or series of control antibodies. Control blots were probed with the following: mouse monoclonal 20.1 Aβ1–8 specific (kind gift from Dr. W. Van Nostrand, Stony Brook, NY, 1:5000) and A11 (oligomeric proteins specific - rabbit 1.8 mg/ml stock used at 1:5,000). Our secondary antibodies were HRP-conjugates of IgG goat anti-rabbit or goat anti-mouse (1:5000, BioRad Laboratories, Hercules, CA), or rabbit anti-dog (1:5000, Sigma-Aldrich, St. Louis, MO) used as needed. HRP reactivity was visualized with Supersignal Chemiluminscent Substrate (Pierce Biotechnology Inc., Rockford, IL) on Hyperfilm ECL (Amersham Bioscience, Piscataway, NJ). Immunoblots were quantified using Image J software to obtain optical density (OD) measures. Blots from the same serum time points were all assayed together and exposed on the same sheet of film, thus OD measures are comparable between samples across different time points. Control blots probed only with the secondary antibodies did not produce a detectable signal.
Western blot analysis
Immune dog serum was tested for the recognition of canine APP by Western blot assay. Briefly, 5 or 25 μg of total protein from canine brain RIPA fraction (Head et al., 2008) or 100 ng of Aβ1–42 peptide were electrophoresed on a bis-tris 4–12% polyacrylamide gel (Bio-Rad) and proteins were transferred onto nitrocellulose membranes. Membranes were blocked for 1 hour at room temperature with 5% non-fat dry milk in PBS pH 7.4 with 0.1% Tween 20. Membranes were probed over night at 4°C with immune canine sera followed by HRP conjugated rabbit anti-dog IgGs (Bethyl Lab) at 1:10,000 dilution. Bands were visualized using SuperSignal West Pico kit (Pierce). Membranes were then striped in stripping buffer (Pierce) and re-probed with monoclonal 6E10 antibody at 1:2,000 dilution followed by goat anti-mouse IgGs at 1:20,000. To detect different Aβ1–42 species on a Western blot, 200–400 ng of monomeric, aggregated or Aβ1–42 peptide stored in CCB, pH 9.6, were used with the same conditions described above. Aβ1–42 forms were detected with monoclonal 6E10 or 4G8 antibodies (Covance).
Immunohistochemical analysis
Immune dog sera were also used to determine if dog antibodies recognized dense core plaques on brain sections from APP Tg2576 mice (Hsiao et al., 1996). Briefly, 18 month old mice APP/Tg and wild type mice were overdosed with 100 mg/kg Nembutal, intracardially perfused with ice-cold PBS, pH 7.4, brains were collected and fixed over night in 4% paraformaldehyde. Coronal sections 40 μm thick were mounted on glass slides and air-dried. After autofluorescence quenching, immune dog sera from time point 25 was diluted to the saturation point and added to the slides alone or together with the set of short linear peptides described previously (12.5 μM each) or Aβ1–42 peptide (12.5 μM). After over night incubation at 4°C, slides were washed and biotinylated rabbit anti-dog antibodies (Bethyl Lab) were added at 1:250 dilution, followed by streptavidin-Alexa fluor 555 (Thermo Fisher Scientific) at 1:250. Slides were washed and subsequently stained with 0.5% Thioflavin S (Sigma) as described (Petrushina et al., 2007). Sections were analyzed on an Olympus IX70 (Japan) microscope using LaserSharp 2000 software and individual images were captured for each wavelength at 40× magnification. Control slices from wild type mice were used to establish the background fluorescence. Sera from control dogs or secondary antibodies only did not produce any detectable fluorescence.
Data Analysis
Our preliminary analysis of cognitive test scores (baseline and during treatment) and Aβ neuropathology established that the two control groups (alum only and saline injected) could be combined into one control group for comparison with the aggregated Aβ immunized animals. Subsequently, either t-tests or repeated measures ANOVA were used to compare groups. Post hoc comparisons used the Bonferroni correction. All analyses used SPSS for Windows.
Results
Immunization with aggregated Aβ42 formulated in alum adjuvant induced rapid and uniform antibody response of anti-inflammatory Th2 type
Dogs in the control group (alum or saline only) had very low endogenous serum titers (~1:100–1:400) at all time points tested. Actively immunized dogs rapidly and uniformly responded to the vaccination (Fig. 1B). The average titers of anti-Aβ antibodies after the first two injections were 8,850±3,593 and reached a plateau after 4 injections (titers 90,625±9,375). The same levels of antibody titers were maintained until the end of the experiment (94,685±26,566) (Fig. 1B) in response to continued monthly vaccinations.
We next characterized the isotypes of Aβ specific immunoglobulins induced after immunization as an indirect measure of anti-inflammatory Th2-type versus pro-inflammatory Th1-type immune responses (Finkelman et al., 1990). We used anti-canine isotype-specific antibodies from Bethyl Laboratories, which are well validated and highly specific in serological testing (German et al., 1998; Peters et al., 2004). After two injections with aggregated Aβ formulated in alum adjuvant, anti-Aβ antibodies were primarily of the IgG2 isotype (Fig. 1C), and this isotype remained dominant throughout the study. As we expected, initial injections also induced high levels of T-cell independent antibodies of the IgM isotype, which slowly diminished after subsequent immunizations (Fig. 1C). Anti-Aβ antibodies of IgG1 isotype were also detected in the immunized dogs throughout the experiment, but they represented a minor fraction relative to other isotypes (Fig. 1C). Anti-Aβ antibodies of IgA and IgE isotypes were not detected in the immune serum (data not shown). In mouse vaccination studies, the production of IgG1 antibodies is primarily induced by anti-inflammatory Th2-type cytokines, whereas the production of IgG2a antibodies reflects the involvement of pro-inflammatory Th1-type cytokines (Finkelman et al., 1990). In humans, the antibody subclasses are not as restricted, but the Th2 type of the immune response is typically characterized by the presence of IgG4, IgE and IgG2 isotypes, while Th1 type is characterized by IgG1, IgG2 and IgG3 isotypes (Purkerson and Isakson, 1992; Rodriguez et al., 1996). A comparative alignment of the complete genome sequence has suggested closer homology between canine and human sequences than between that of human and mouse (Kirkness et al., 2003). To date, canine genes encoding heavy chains for IgA, IgE, IgD and four heavy chains for IgGs have been identified (Patel et al., 1995; Rogers et al., 2006; Tang et al., 2001).
Regarding the adjuvant used in the canine study, alum is a well characterized Th2 anti-inflammatory adjuvant both in mice and in humans (Asuni et al., 2006; Cribbs et al., 2003; Durham et al., 1999; Gordon, 1993; Kool et al., 2008). As mentioned above, in mice, the IgG1 isotype represents the contribution of a Th2-type response, while the IgG2a isotype a product of the proinflammatory Th1-type response. In canines, we observed the opposite situation. After immunization with alum as the adjuvant, which induces an anti-inflammatory Th2 type response, canines responded with predominantly IgG2 antibodies (see also (Head et al., 2006)), which suggest that in canines the IgG2 subclass of antibodies is a measure of the anti-inflammatory response, while the IgG1 subclass is of pro-inflammatory phenotype, which more closely resembles the isotype preferences in humans.
Dominant linear anti-Aβ-specific B-cell epitopes is identical to the ones reported for humans and monkeys
We next characterized linear anti-Aβ-specific B-cell epitopes and compared the response of dogs with immunized mice, monkeys and humans (Cribbs et al., 2003; Lee et al., 2005; Lemere et al., 2004). Thus, to identify the linear B-cell antigenic determinant(s) within the Aβ1–42 peptide we utilized series of six overlapping short peptides, spanning the entire Aβ sequence, for epitope mapping with direct ELISA, which include five 15-mer peptides and one 17-mer peptide. We analyzed the relative binding of antisera after 2 (Fig. 2A), 4 (Fig. 2B) or the 25th (final) injections (Fig. 2C) at saturation dilutions from the immune dogs. Initial injections induced antibodies that recognized the N-terminus of the Aβ1–42 peptide (peptide 1–15; 41.83% of binding compared with full-length Aβ42), as well as the middle sequence of Aβ (peptides 11–25 and 16–30; 12.78% and 19.5%, respectively, compared to binding to the full-length Aβ42) (Fig. 2A). Similar results were obtained in our preliminary experiments with young and old beagles (Head et al., 2006). Subsequent vaccinations led to a maturation and shift of B-cell epitopes away from the middle of Aβ1–42 sequence toward the N-terminus of Aβ (Fig. 2B and 2C). Thus, after 4 injections, the 6–20 peptide became the second leading epitope after the N-terminal region (14.38% of binding compared to Aβ42). Antibodies binding regions at 11–25 and 16–30 dropped to 10.66% and 7.31% after 4 injections and to 4.93% and 4.28% after 25 injections. It is important to note, however, that at each time point tested the major B-cell epitope was located in the N-terminus of the Aβ1–42 peptide (peptide 1–15) consistently in all animals. As reported by Lemere and colleagues in immunized vervets (Lemere et al., 2004) and by Lee and colleagues in humans (Lee et al., 2005), antibodies from immunized dogs also do not detect full-length canine APP by Western blot (Fig. 2D), despite canine APP being detectable with the monoclonal antibody 6E10 (Fig. 2E).
Besides linear Aβ-specific epitopes repetitive Aβ immunization also induced conformational Aβ-selective antibodies
To detect the possible presence of conformational Aβ-selective antibodies in the immune dog serum, we used a competitive ELISA modified from previously published protocol (Cribbs et al., 2003). Immune dog serum collected at time points 2, 4 and 25 were diluted to the saturation point and incubated overnight with either the complete mixture of 6 short linear peptides described above, with Aβ42, or left without competitive inhibition. At time point 2, 4 and 25 the binding of canine immune serum to Aβ1–42 was inhibited by 93.7±0.6%;, 95.57±0.78% and 92.27±1.48%, respectively, by pre-incubation with Aβ1–42 (Fig. 3A). At time point 2, the mixture of short linear peptides inhibited the binding of immune serum to Aβ1–42 by 73.45±5.25%, while at time point 4 there was less inhibition (36.58±7.55%). Similar inhibition of dog antibody binding was detected at the end of the experiment (34.92±6.89%) suggesting the stabilization of the immune responses in dogs after 4 injections. Importantly, individual dogs showed different levels of inhibition of binding to Aβ by short peptides (from 2.0% to 76.3% at time point 4), which suggests large individual variability in the presence of additional non-linear (conformational) specific antibodies. We thus hypothesized that the Aβ1–42 peptide preparations that were used to coat the ELISA plates contained monomers as well as aggregates. To address this issue, we analyzed our Aβ1–42 preparations used for ELISA experiments in parallel with defined monomeric and aggregated Aβ1–42 species by Western blot (Fig. 3B). As predicted, Aβ1–42 aggregates of molecular weights between 90–120 kD were also readily detected in Aβ1–42 preparations in CCB with two different monoclonal antibodies 6E10 and 4G8.
Fig. 3.
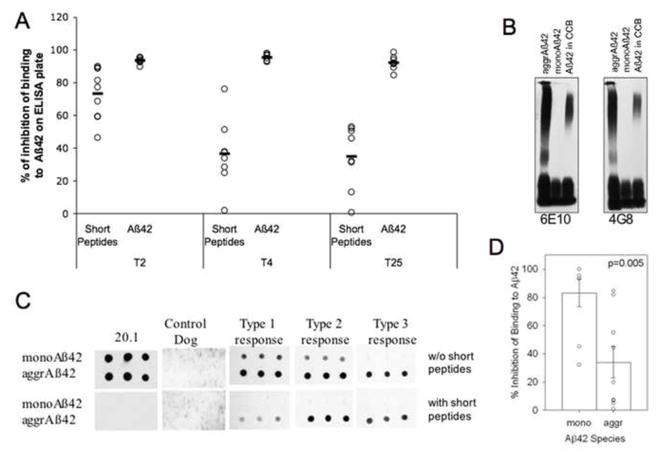
Conformational sensitive antibodies were induced in dogs after 4 injections with aggregated Aβ1–42 peptide and persisted until the end of the experiment. (A) Canine immune serum after 2, 4 or 25 injections was pre-incubated with short linear peptides (see experimental design section) or Aβ1–42 peptide and the % of inhibition of binding to Aβ1–42 on an ELISA plate was measured. (B) Aβ1–42 peptide in CCB forms high molecular weight aggregates, which were detected with monoclonal antibodies 6E10 and 4G8 in a Western blot assay. (C) Immune canine serum at T4 was incubated with short linear peptides or left without inhibition, and probed against monomeric and aggregated Aβ1–42 peptide spotted on a membrane. As controls, N-terminal Aβ specific mouse monoclonal 20.1 antibody or serum collected from a PBS injected dog reveal positive and negative staining, respectively. (D) Resulting dot blots from (C) were analyzed using Image J software and presented as a percent of inhibition of binding to monomeric or aggregated Aβ with short linear peptides. Pictures of representative dot blots are shown. (CCB= carbonate coating buffer; T=blood collection time points; mono= monomeric Aβ1–42 peptide; aggr= aggregated Aβ1–42 peptide). Open circles represent the individual animals data, lines and bars are the value of the mean, error bars are the SEM.
To further confirm the presence of conformational selective antibodies in immunized dogs, we also used a competitive dot blot assay. Monomeric or aggregated Aβ1–42 species were spotted on a nitrocellulose membrane, and after blocking, immune dog sera from time point 4, pre-incubated overnight with set of short linear peptides as described above or left uninhibited, were added to the membranes. The type of binding of individual animal serum samples to the Aβ species could be divided into three types: (1) dog antibodies recognizing monomeric and aggregated Aβ1–42 forms, and binding to both Aβ species was evenly inhibited with the short linear peptides; (2) dog antibodies that bind to monomeric and aggregated Aβ, but short linear peptides inhibited binding was limited to only the monomeric Aβ1–42 species, and (3) dog antibodies recognizing only aggregated Aβ1–42, and short linear peptides were modestly effective in inhibiting the binding of serum to aggregated Aβ (Fig. 3C; representative blots are shown). Overall, competing short peptides inhibited the binding of immune serum to monomeric Aβ forms by 83%, while the same peptides were effective in inhibiting the binding to aggregated Aβ forms by 37% (Fig. 3D), which suggests the presence of a large pool of conformation sensitive antibodies in immune dog serum collected after 4 injections.
Dogs have high individual variability in conformational selective anti-Aβ antibodies
To further characterize the specificity of conformational antibodies generated in immunized dogs we used dot blot assays and serum samples collected at the end of the study after 25 vaccinations. Monomeric, pre-fibrillar and fibrillar forms of Aβ, as well as defined pre-fibrillar forms of proteins of non-Aβ origin, alpha-synuclein and islet APP (Kayed et al., 2007), were used. Dogs immunized with aggregated Aβ1–42 showed widespread binding to different conformational species of Aβ, alpha-synuclein and islet APP (Fig. 4; representative blots are shown). One animal showed the largest response to monomeric Aβ1–42 with a lower response to other Aβ1–42 conformations as well as non-Aβ proteins of pre-fibrillar conformation (Fig. 4A). Two dogs generated antibodies that recognized all Aβ1–42 species independently of conformation, but did not detect non-Aβ proteins of pre-fibrillar conformation (Fig. 4B). Three animals developed antibodies that recognize monomeric and fibrillar Aβ1–42, while the response to pre-fibrillar Aβ1–42 or other pre-fibrillar proteins was significantly lower (Fig. 4C). Finally, two animals developed antibodies that primarily recognized aggregated forms of proteins of both Aβ and non-Aβ origin, and were less responsive to monomeric Aβ1–42 (Fig. 4D). Thus, animals could be loosely categorized into groups including monomeric Aβ-sensitive, Aβ-specific, monomeric and fibrillar Aβ-sensitive and aggregated proteins sensitive. As expected, control A11 antibody was specific for pre-fibrillar assemblies of Aβ, as well as oligomeric forms of peptides of non-Aβ origin, while the mouse monoclonal 20.1 antibody was Aβ specific independent of the conformation (Fig. 4E).
Fig. 4.
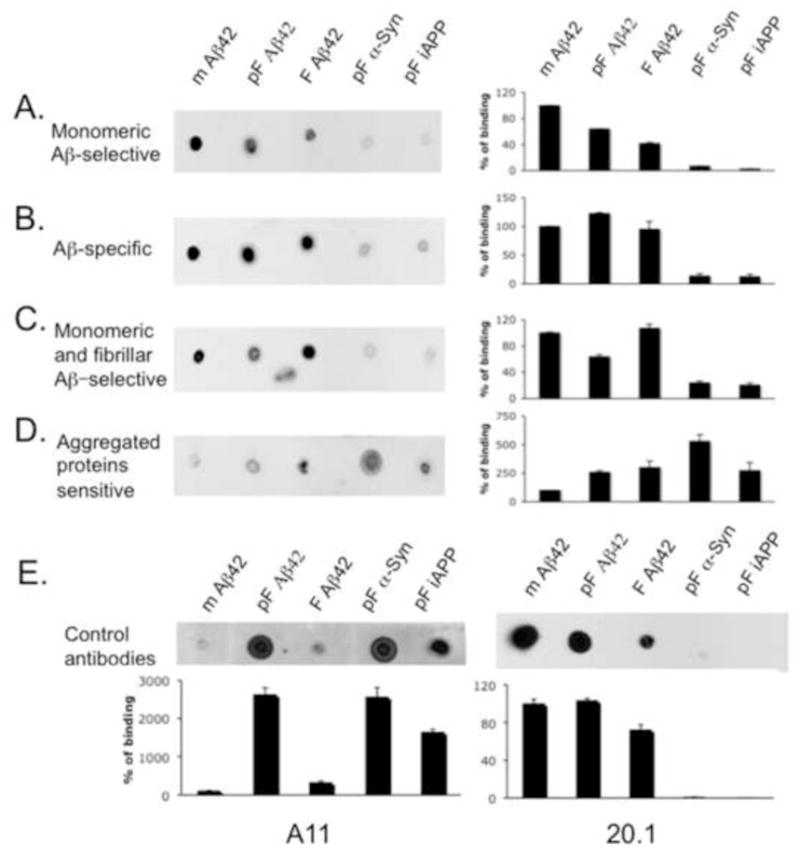
After 25 injections, dogs were categorized into 4 separate groups according to the conformational specificity of the antibodies produced: (A) monomeric Aβ-selective antibodies (n=1); (B) Aβ-specific antibodies independently of conformation (n=2); (C) monomeric and fibrillar Aβ forms selective antibodies (n=3); and (D) aggregated proteins sensitive antibodies (n=2). (E) Control oligomer-specific A11 antibody or N-terminal Aβ specific mouse monoclonal antibody 20.1 show predicted binding to pre-fibrillar proteins (A11) or all Aβ forms (20.1) respectively. Canine antibodies (A–D) as well as control antibodies (E) were analyzed using dot blots spotted with monomeric Aβ1–42, pre-fibrillar Aβ1–42, fibrillar Aβ1–42 forms as well as pre-fibrillar forms of a-synuclein and islet APP. The percent of binding of immune serum to monomeric Aβ1–42 was considered to be at 100%. The percent of binding to other Aβ-related and non-related aggregates was calculated after Image J analysis of the dot blots. Pictures of representative dot blots are shown. (mAβ1–42= monomeric Aβ1–42 peptide; pF Aβ1–42= pre-fibrillar Aβ1–42 peptide; F Aβ1–42= fibrillar Aβ1–42 peptide; pF α-Syn= pre-fibrillar alpha-synuclein protein; pF iAPP= pre-fibrillar islet amyloid polypeptide). Bars represent means, the error bars represent SEM.
Conformational-sensitive antibodies undergo maturation process after repeated Aβ injections
The serum from time points 2 and 4 were retrospectively compared to detect a “maturation” process or shift of the antibody preferences for conformational epitopes. After two injections (T2), most of the dogs produced predominately monomeric Aβ sensitive antibodies (Fig. 5). Also, some minor recognition of aggregated Aβ, as well as non-Aβ aggregated proteins was observed (Fig. 5), probably as a result of initial production of lower specificity IgM isotype antibodies, or due to the presence of low titers of autoantibodies reported recently by Dr. Wyss-Coray’s group (Britschgi et al., 2009). In dogs that produced primarily monomeric Aβ sensitive antibodies at T25, time point 4 showed preferential binding to monomeric Aβ form as well, with some minor binding to aggregated Aβ forms (Fig. 5A). Animals that produced Aβ-specific antibodies at T25, showed higher preferences towards pre-fibrillar and fibrillar Aβ at time point 4, which became even more pronounced at the end of the study (Fig. 5B). Dogs that produced monomeric and fibrillar Aβ sensitive antibodies at T25, initially favored the fibrillar form of Aβ, and over time a strengthening of the response to monomeric Aβ conformations was detected (Fig. 5C). Lastly, dogs that produced antibodies against aggregated proteins, regardless of protein sequence at T25, showed a gradual shift from monomeric and pre-fibrillar Aβ antibodies towards increasing levels of antibodies against fibrillar Aβ and aggregates of alpha-synuclein and islet APP (Fig. 5D).
Fig. 5.
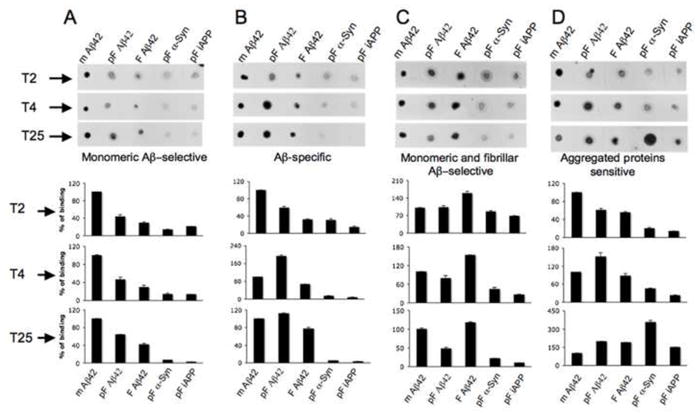
Progression of conformational sensitive antibodies in defined groups during the course of immunization. (A) Monomeric Aβ-selective antibodies. (B) Aβ-specific antibodies independent of conformation. (C) Monomeric and fibrillar Aβ-selective antibodies. (D) Aggregated proteins sensitive antibodies (independent of sequence). Canine immune serum after 2, 4 or 25 injections was analyzed by dot blots. The percent of binding of immune serum to monomeric Aβ1–42 was considered as 100%. The percent of dog antibody binding to other Aβ-related and non-related aggregates was calculated after Image J analysis of the dot blots. Pictures of representative dot blots are shown. (mAβ1–42= monomeric Aβ1–42 peptide; pF Aβ1–42= pre-fibrillar Aβ1–42 peptide; F Aβ1–42= fibrillar Aβ1–42 peptide; pF α-Syn= pre-fibrillar α-synuclein protein; pF iAPP= pre-fibrillar islet amyloid polypeptide). Bars represent means, the error bars represent SEM.
We hypothesized that the differences in the types of conformation-sensitive antibodies produced in individual dogs may induce differential clearance of Aβ from the central nervous system. Due to the limited number of dogs in the study, we addressed this question qualitatively and used previously published brain Aβ data (Head et al., 2008). We noted a tendency for dogs with monomeric Aβ-sensitive or Aβ-specific antibodies to show more extensive brain Aβ clearance, while dogs developing monomeric and fibrillar Aβ sensitive antibodies and dogs developing antibodies against aggregated proteins showed less effective clearance (Supplemental results, Fig. 1A, B, C and D).
Conformational-sensitive antibodies have distinct pattern of binding to amyloid deposits in TAPIR assay
Differential clearance of brain Aβ in immunized dogs (Head et al., 2008), as a function of the antibody response (i.e. linear vs conformational), may be attributed to the differences in binding to Aβ plaques. To test this hypothesis, we characterized the ability of dog antibodies to bind plaques in the Tg2576 mouse model of AD (Hsiao et al., 1996) using a tissue amyloid plaque immunoreactivity (TAPIR) assay (Hock et al., 2002; Hock et al., 2003). Serum samples from the final vaccination at T25 were selected to represent each antibody group as described above, and were used for immunohistochemistry either with or without prior incubation with short linear peptides. Thioflavin S was used to define the dense core Aβ deposits in this animal model. Immunostaining with serum from a dog that produced monomeric Aβ-selective antibodies was co-localized with Thioflavin S staining, and was completely inhibited with the competing short linear peptides (Fig. 6A). Serum from animals producing Aβ-specific antibodies (monomeric, pre-fibrillar and fibrillar) had a wider range of plaque staining that included not only Thioflavin S positive deposits but also areas around these plaques, which was also significantly inhibited with the short linear peptides (Fig. 6B). Interestingly, serum from dogs that produced monomeric-fibrillar Aβ-selective or aggregated proteins sensitive antibodies have a distinctive pattern of immunostaining as shown in Fig. 6C and 6D. Antibodies from these animals preferred labeling the region surrounding the plaque core, leaving the core itself unlabeled, thus creating a halo of positive immunoreactivity surrounding a Thioflavin S positive nucleus. Furthermore, short linear peptides were ineffective at inhibiting the immunostaining by serum from these dogs.
Fig. 6.
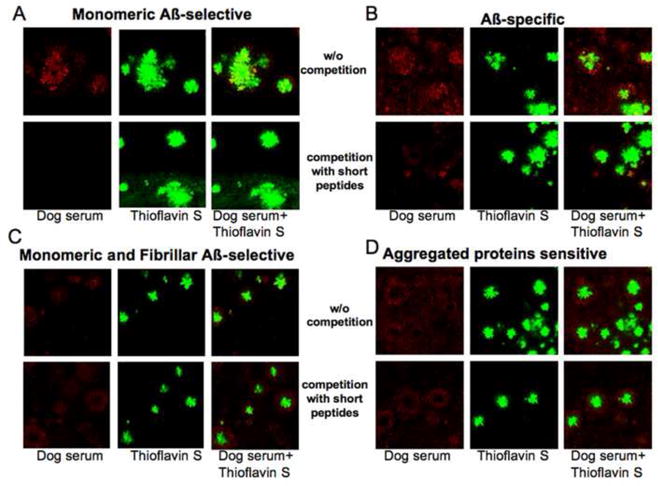
Immune canine serum detects amyloid plaques from a mouse model of AD with differential patterns of immunostaining, depending on the type of antibodies produced. (A) Monomeric Aβ-selective antibodies. (B) Aβ-specific antibodies independent of conformation. (C) monomeric and fibrillar Aβ-selective antibodies. (D) Aggregated proteins sensitive antibodies. Representative serum from the defined groups was pre-incubated with competing short peptides or left without competition and then used for the immunodetection of amyloid plaques in 18 months old APP Tg2576 mouse brains. Thioflavin S was used to identify dense core plaques. 40× original magnification. Red – dogs immunoglobulins, green – Thioflavin S, yellow – merged.
Discussion
The purpose of the current study was to analyze the maturation of antibody isotypes, Aβ B-cell epitope specificity, and the anti-Aβ antibody conformational selectivity, induced in aged canines in response to long-term (2.4 years) immunization with aggregated Aβ1–42 peptide formulated in alum adjuvant. In our previous study, we observed a limited cognitive benefit with vaccination on measures of learning and memory, some evidence for maintenance of executive function, and significant Aβ clearance from the central nervous system in aged beagles (Head et al., 2008). We found that antibody titers, elicited in dogs, were higher than those observed in the AN1792 human clinical trial, but lower than these reported in nonhuman primates (Lemere et al., 2004). Given the prolonged treatment time of our animals with 25 vaccinations, we were able to establish changes in the antibody isotypes and B-cell epitopes, which shifted over time. Furthermore, we detected a sequential shift towards enhanced production of conformation-sensitive antibodies in response to repeated immunizations. We also confirmed that dog antibodies do not cross-react with native APP. Interestingly, the Aβ linear B-cell epitopes recognized by the canine antibodies were very similar to those generated in nonhuman primates and in humans in response to Aβ immunization, which differ significantly from the antibody response in mouse models (Gevorkian et al., 2004; Lee et al., 2005; Lemere et al., 2004). Taken together, these results suggest that the aged canine model has predictive validity for efficacy of reducing brain Aβ levels, as well as for testing vaccine candidates prior to translation to human clinical trials.
Aged canines rapidly and robustly produced anti-Aβ antibodies in response to the same antigen (aggregated Aβ1–42) used in the AN1792 human clinical trial, and the antibody response was maintained at high levels for the duration of the study by monthly booster vaccinations. The canine anti-Aβ antibody titers were higher and showed significantly less individual variability than the reported titers in the AN1792 clinical trial, where only 23.6% of patients had titers >1:1,000 after 4 injections (Bayer et al., 2005). There are several possible explanations for this difference including dose, route of administration and adjuvant. In the canine study, we used 0.5 mg of aggregated Aβ1–42 peptide, whereas in humans 0.225 mg was injected. This resulted in a 15-fold higher dose in the canines compared to the human clinical trial, as calculated based on the average beagle weight of 10 kg. In addition, the vaccine was delivered subcutaneously in canines but by intramuscular injection in humans. Lastly, the dog study used alum as an adjuvant whereas the human studies used QS-21, each targeting a different T helper-type of response, which may also have contributed to the observed differences in the immune responses between the two species (Cribbs et al., 2003). These data are consistent with the hypothesis that dose and adjuvant are important considerations for immunotherapy and were also confirmed in studies of nonhuman primates. Cynthia Lemere’s group reported uniform and rapid anti-Aβ antibody responses after 3 injections with Aβ formulated in Freund’s adjuvant (CFA/IFA) in older vervet monkeys (Lemere et al., 2004). The concentration of anti-Aβ antibodies peaked at of 1.6 mg/ml of plasma after 5 injections, and dropped to steady levels of 0.45 mg/ml of plasma after 8 injections. In dogs we observed significantly lower concentrations of anti-Aβ antibodies, which peaked after 4 injections at 33.1 μg/ml of serum, but remained stable for two years until the end of experiment at 37.3μg/ml. The significantly higher levels of anti-Aβ antibodies in vervet monkeys was likely related to the higher dose of Aβ used for injections (1mg per injection, which is approximately 4 times higher per kg of body weight compared with dogs). Also, the concentration of antibodies is consistent with the use of the more potent adjuvant, CFA, which initially boosted the immune response, while subsequent injections with much weaker IFA were not able to maintain high antibody titers. However, it is important to note that the CFA is prohibited for use in humans and in dogs due to a high incidence of adverse events.
We next characterized the linear B-cell epitopes recognized by antibodies produced in vaccinated aged canines for comparison with the anti-Aβ antibody response in immunized humans, nonhuman primates and mice. Initially, aged canines produced antibodies that bound to the N-terminus of Aβ1–42 as well as in the middle of the peptide at aa 11–25/16–30 (see also (Head et al., 2006)). Subsequent vaccinations shifted this response to primarily an N-terminus epitope. A similar N-terminus epitope localization has been reported in vervet monkeys (Lemere et al., 2004) and humans (Lee et al., 2005). Consistent with these observations, dog antibodies did not recognize full-length canine or human APP similar to the reports in nonhuman primates (Lemere et al., 2004) and humans (Lee et al., 2005), as opposed to mice (McLaurin et al., 2002). This is an important finding because potential cross-reactivity of Aβ antibodies to APP could potentially lead to antibody-mediated adverse events. However, the adverse events, meningoencephalitis, reported in the Elan AN1792 clinical trial was most likely caused by autoreactive T cells rather than the anti-Aβ antibodies (Pride et al., 2008).
A second novel finding in the current study was the development of conformation sensitive antibodies in aged dogs in response to repeated vaccination. In the initial reports on antibody responses in AN1792 human clinical trial Hock and colleagues described a tissue amyloid plaque immunoreactivity (TAPIR) assay (Hock et al., 2002; Hock et al., 2003), and demonstrated that patients with the immunoreactive sera against dense core plaques in APP/Tg mouse brains showed significantly slower rates of decline of cognitive function and activities of daily living (Hock et al., 2003). However, the presence of conformational specific antibodies in human immune serum was later discounted after complete analyses of the full cohort of AN1792 participants (Lee et al., 2005). Nevertheless, one reason for the lack of development of conformation dependent antibodies in humans in response to vaccination with aggregated Aβ may have been due to the fewer number of vaccinations in the AN1792 clinical trial. Furthermore, the current study shows that there can be significant individual variability in the extent and type of conformation sensitive anti-Aβ antibodies produced. We extended the analysis of the immune dog serum using the TAPIR assay (Hock et al., 2003) and found that there are two types of dense core plaque staining. Some of the dog serum immunostained the center of Thioflavin S positive plaques (Fig. 6A and B), while others preferentially immunolabeled the area surrounding plaques creating a halo appearance (Fig. 6C and D). Importantly, the type of conformation dependent antibodies may predict how effectively Aβ will be cleared from the brain (Supplemental Fig. 1A–D), and association with cognitive benefits of immunization (Supplemental Fig. 1F). Interestingly, in the original paper by Dr. C. Hock (Hock et al., 2003), he found a strong correlation between high TAPIR scores of human AN-1792 immunized patients (in our case these are the groups with monomeric Aβ selective and Aβ-specific antibody responses) and prevention from disease progression (these two groups had less pathological features and better behavior outcome in our restricted analysis as well). However, our sample size was relatively small and larger studies would be needed to clearly establish this association. Given that most animals produced antibodies against monomeric Aβ initially, but that the timing of Aβ clearance in the brains of these animals is as yet unknown, it may be that monomeric Aβ-selective as well as Aβ-specific antibodies are both likely responsible for Aβ reduction and cognitive benefits.
We were surprised to see the staining opposite of what one would expect – binding of monomeric Aβ-selective and Aβ-specific serum to the core of the plaque, and staining of the plaque halo by the aggregated proteins sensitive antibodies. One of the possible explanations is that the vaccine formulation (aggregated Aβ42) in vitro may not correspond to the actual Aβ conformation deposited in dense core plaque in vivo, and antibodies, raised against synthetic Aβ, may recognize only specific regions of amyloid plaques. Strong evidence for the conformational differences between plague Aβ and synthetic Aβ were presented by Dr. M. Jucker’s group (Meyer-Luehmann et al., 2006) in a series of seeding experiments with intracranial injections of 10% brain extracts from AD patients or APP/Tg mice into the mice, which showed the absence of seeding by intracranial injections of synthetic aged Aβ. Besides, the formal structure of the amyloid dense core plaque is complex and not fully understood, and our results may bring some additional information about amyloid plaque architecture.
Based on the results from our long-term immunotherapy study in canines, we have proposed a model whereby there is a gradual progression or drift in the antibody responses, which occurs during prolonged immunizations with aggregated Aβ1–42 peptide (Fig. 7). The initial immunizations induced antibodies selective against monomeric Aβ, however after multiple immunizations development of antibodies recognizing all Aβ forms independent of conformation were detected. Additional vaccinations can lead to two possible responses: anti-Aβ antibodies recognizing preferentially monomeric and fibrillar Aβ forms, or antibodies recognizing mainly aggregated proteins independent of the peptide sequence. These observations suggest that the analysis of conformation-specific antibody response may possibly be useful as a predictive marker for successful immunotherapy. Other important questions also remain regarding active immunization for anti-Aβ immunotherapy such as: 1) the Aβ B-cell epitope to use for inducing therapeutic anti-Aβ antibodies capable of reducing the level of Aβ in the central nervous system; 2) the design of the immunogen and formulation with an appropriate adjuvant; 3) timing of the immunotherapy as initiation of the vaccination, injection intervals and duration of the immunotherapy; 4) the antibody isotype that initiates the best overall therapeutic benefit via the various Fc-mediated effector functions (Bard et al., 2003).
Fig. 7.
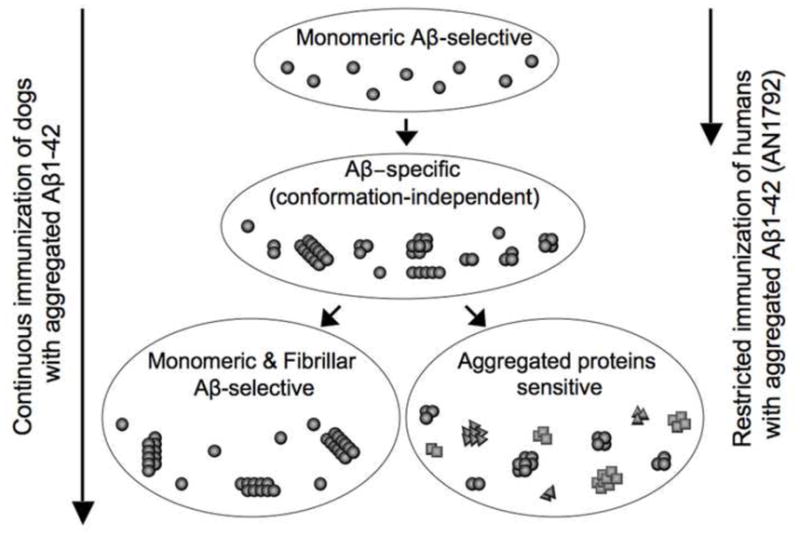
Proposed scheme of the maturation of conformational selective antibodies in dogs after prolonged immunization with aggregated Aβ1–42 peptide. Initial injections induced mainly monomeric Aβ-selective antibodies (human clinical trials were postponed at this stage). Subsequent injections induced Aβ-specific antibodies independently of conformation, additional injections stimulated the development of either monomeric and fibrillar Aβ-selective antibodies or antibodies against aggregated proteins independent of protein sequence.
In summary, this is the first report on the effects of long-term active immunization with Aβ1–42 peptide on the humoral immune response in a canine animal model of Aβ-pathogenesis, and these results provide new insights into changes that occur in response to repeated immunization with the full-length peptide, which may be particularly relevant for the next generation of immunogens that contain an Aβ B cell epitope conjugated to carrier proteins for long-term active immunization in elderly AD patients. Thus, immunotherapy studies in the canine model of amyloidosis may be particular helpful for facilitating translation of anti-Aβ vaccine candidates to human clinical trials.
Supplementary Material
Acknowledgments
This work was funded in part by the following National Institutes of Health R01 Grants; NIA-AG20242 (CWC), NIA-AG20241 & NIA-AG00538 (DHC), NINDS-NS50895 (DHC), NIA-AG031764 (EH). Funding for the UCI-ADRC was provided by NIH/NIA Grant P50 AG16573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anguiano M, et al. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–43. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- Asuni AA, et al. Vaccination of Alzheimer’s model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur J Neurosci. 2006;24:2530–42. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Britschgi M, et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:12145–50. doi: 10.1073/pnas.0904866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–14. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, et al. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17:259–68. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Durham SR, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- German AJ, et al. Measurement of IgG, IgM and IgA concentrations in canine serum, saliva, tears and bile. Vet Immunol Immunopathol. 1998;64:107–21. doi: 10.1016/s0165-2427(98)00132-9. [DOI] [PubMed] [Google Scholar]
- Gevorkian G, et al. Mimotopes of conformational epitopes in fibrillar beta-amyloid. J Neuroimmunol. 2004;156:10–20. doi: 10.1016/j.jneuroim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Gilman S, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Golde TE, et al. Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Curr Alzheimer Res. 2006;3:421–30. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- Gordon DM. Use of novel adjuvants and delivery systems to improve the humoral and cellular immune response to malaria vaccine candidate antigens. Vaccine. 1993;11:591–3. doi: 10.1016/0264-410x(93)90239-t. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Head E, et al. Immunization with fibrillar Abeta(1–42) in young and aged canines: Antibody generation and characteristics, and effects on CSF and brain Abeta. Vaccine. 2006;24:2824–34. doi: 10.1016/j.vaccine.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Head E, et al. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–25. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, et al. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- Head E, et al. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28:3555–66. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8:1270–5. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- Hock C, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–54. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Janus C, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kayed R, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness EF, et al. The dog genome: survey sequencing and comparative analysis. Science. 2003;301:1898–903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol. 2005;58:430–5. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- Lemere CA, et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–97. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lin CY, et al. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes. 2007;56:1324–32. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–9. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Milgram NW, et al. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neurosci Biobehav Rev. 2002;26:679–95. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, et al. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Mucke L, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necula M, et al. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry. 2007;46:8850–60. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Patel M, et al. Sequence of the dog immunoglobulin alpha and epsilon constant region genes. Immunogenetics. 1995;41:282–6. doi: 10.1007/BF00172152. [DOI] [PubMed] [Google Scholar]
- Peters IR, et al. Measurement of immunoglobulin concentrations in the feces of healthy dogs. Clin Diagn Lab Immunol. 2004;11:841–8. doi: 10.1128/CDLI.11.5.841-848.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrushina I, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci. 2007;27:12721–31. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride M, et al. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener Dis. 2008;5:194–6. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- Purkerson J, Isakson P. A two-signal model for regulation of immunoglobulin isotype switching. Faseb J. 1992;6:3245–52. doi: 10.1096/fasebj.6.14.1385241. [DOI] [PubMed] [Google Scholar]
- Rodriguez V, et al. The IgG isotypes of specific antibodies in patients with American cutaneous leishmaniasis; relationship to the cell-mediated immune response. Parasite Immunol. 1996;18:341–5. doi: 10.1046/j.1365-3024.1996.d01-113.x. [DOI] [PubMed] [Google Scholar]
- Rogers KA, et al. Molecular characterization of immunoglobulin D in mammals: immunoglobulin heavy constant delta genes in dogs, chimpanzees and four old world monkey species. Immunology. 2006;118:88–100. doi: 10.1111/j.1365-2567.2006.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–8. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, et al. Dendrimeric Abeta1–15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007;28:813–23. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–8. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Developing preventive therapies for chronic diseases: lessons learned from Alzheimer’s disease. Nutr Rev. 2007;65:S239–43. doi: 10.1111/j.1753-4887.2007.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Tang L, et al. Cloning and characterization of cDNAs encoding four different canine immunoglobulin gamma chains. Vet Immunol Immunopathol. 2001;80:259–70. doi: 10.1016/s0165-2427(01)00318-x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002a;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, et al. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002b;30:552–7. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


