Abstract
Age-related thymic involution is characterized by reduction in T cell production together with ectopic adipocyte development within the hematopoietic and thymic niches. PPARγ is required for adipocyte development, glucose homeostasis and is a target for several insulin-sensitizing drugs. Our prior studies showed that age-related elevation of PPARγ expression in thymic stromal cells is associated with thymic involution. Here, using clinically relevant pharmacological and genetic manipulations in mouse models, we provide evidence that activation of PPARγ leads to reduction in thymopoiesis. Treatment of aged mice with anti-hyperglycemic PPARγ-ligand class of Thiazolidinedione drug, Rosiglitazone caused robust thymic expression of classical pro-adipogenic transcripts. Rosiglitazone reduced thymic cellularity, lowered the naïve T cell number and T cell receptor excision circles (TRECs) indicative of compromised thymopoiesis. To directly investigate whether PPARγ activation induces thymic involution, we created transgenic mice with constitutive-active PPARγ (CA-PPARg) fusion protein in cells of adipogenic lineage. Importantly, CA-PPARγ transgene was expressed in thymus and in Fibroblast Specific Protein-1/S100A4 (FSP1+) cells, a marker of secondary mesenchymal cells. The CAPPARγ fusion protein mimicked the liganded PPARγ receptor and the transgenic mice displayed increased ectopic thymic adipogenesis and reduced thymopoiesis. Furthermore, the reduction in thymopoiesis in CA-PPARγ mice was associated with higher bone marrow adiposity and lower hematopoietic stem cell progenitor pool. Consistent with lower thymic output, CAPPARγ transgenic mice had restricted T cell receptor (TCR) repertoire diversity. Collectively, our data suggest that activation of PPARγ accelerates thymic aging and thymus-specific PPARγ antagonist may forestall age-related decline in T cell diversity.
Introduction
According to current predictions, the aging population will steadily increase and by year 2030, approximately 1 in 8 people will be over the age of 65 (Sierra et al. 2009; NIA-NIH#07-6134. 2007). Aging is known to be associated with T cell defects, reduced immune-surveillance and higher risk of infections (Miller 1996). Strikingly, approximately 20% patients over the age of 65 years have frank diabetes (Morley 2008; Poulsen et al 2009; Lu et al. 2009). In addition, nearly 40% of Americans age 50 years or older are overweight (BMI 25-29.9 kg/m2). Such forecasts translate into 9.3 million more obese (BMI >30 kg/m2) adults between 20 to 74 years of age in 2011 than in year 2000. Importantly, 8.3 million of these additional obese subjects will be older than 50 years of age (Fontaine et al. 2003; Wang et al. 2007). Obesity is known to promote development of several age-related diseases and also accelerates thymic involution process and immunosenescence (Dixit et al 2007; Yang et al 2009a). Furthermore, considering that adiposity and aging are known risk factors for insulin resistance, the use of antihyperglycemic agents is predicted to rise. The peroxisome proliferator activated receptor gamma (PPARγ) is a member of the nuclear receptor superfamily of ligand-activated transcription factors that plays a key role in insulin-sensitivity (Evans et al. 2004; Tontonoz et al. 2008). Various fatty acids serve as endogenous ligands for PPARs and regulate transcription of several metabolic regulators via well characterized transactivation and transrepression mechanisms (Evans et al. 2004; Tontonoz et al. 2008). Accordingly, the PPARγ represents a direct link between fatty acid concentrations, regulation of gene transcription, adipocyte development and glucose homeostasis. The synthetic PPARγ-ligands, Thiazolidinediones (TZDs) are among the most widely prescribed drugs to promote insulin-sensitivity in type 2 Diabetes (Yki-Järvinen 2004; Staels et al. 2005). Although PPARγ activating TZDs have potent antihyperglycemic actions (Saltiel and Olefsky 1996), they also have several off-target safety issues (Rubenstrunk et al. 2007; Lago et al. 2007; Guan et al. 2005) which includes adipocytic transformation of mesenchymal cells in bone marrow and increased risk of fractures (McDonough et al. 2008).
The age-related deterioration of thymic stromal cell microenvironment and ectopic adipocyte development within thymus is associated with reduction in T cell production, a process recognized as thymic involution (Linton and Dorshkind 2004; Taub and Longo 2005; Aspinall and Mitchell 2008; Dixit 2008; Lynch et al. 2009). Inability of aging thymus to replenish the pool of naïve T cells is linked to restriction of T cell receptor diversity in elderly with increased risk of infections, vaccination failures and reduced immune-surveillance (Nikolich-Zugich et al. 2004; Naylor et al. 2005; Miller 1996; Swain et al. 2005). Magnetic resonance imaging has revealed that by age of 45, majority of thymus in metabolically healthy humans is transformed into adipose tissue (Yang et al 2009b). Although, the ectopic adipocytes are predominant cell types that occupy thymic space, the mechanism of this well documented age-related phenomenon remains to be determined.
At birth the thymic stromal microenvironment is mainly composed of a meshwork of cortical and medullary epithelial cells together with antigen presenting dendritic cells, macrophages, endothelial cells and primary mesenchyme-derived fibroblasts (Gray et al. 2006; Muller et al. 2008, Foster et al 2008; Youm et al 2009). The thymic stromal cell composition as well as organization is severely disrupted post-puberty and with progressive aging (Gray et al. 2006). The reduction in thymic epithelial cells (TECs), increase in fibroblasts derived via type 2 epithelial-mesenchymal transition (EMT) and the emergence of adipocytes are among the most dramatic age-related changes in thymic stroma (Youm et al. 2009; Yang et al 2009c, Flores et al. 1999). Thymopoiesis is dependent on periodic import of hematopoietic stem cells (HSC) from bone marrow (Bhandoola et al. 2007). Given that the HSC in turn depend on TECs for subsequent development into a naïve self tolerant T cells (Anderson and Jenkinson 2001) the integrity of TEC function is critical to efficient thymopoiesis. Using genetic fate-mapping, we recently demonstrated that TECs can transition into fibroblasts via the process of epithelial-to-mesenchymal transition (EMT) (Youm et al. 2009; Yang et al 2009c). The secondary mesenchymal cells generated through the process of EMT retain multipotency (Mani et al. 2008), however, the FSP1+, type 2 EMT cells (Zeisberg and Neilson 2009) in aging thymus express PPARγ and appear to be committed to adipocyte lineage (Youm et al. 2009).
The classical dogma that adipocytes are ‘passive filler cells’ that ‘infiltrate’ the vacant thymic and bone marrow niches has been challenged by recent lineage-tracing studies that demonstrate that adipocytes transition from thymic stromal cells and inhibition of thymic and BM adipogenesis is coupled with elevated thymopoiesis and hematopoiesis (Youm et al. 2009; Yang et al. 2009c; Naveiras et al 2009). Interestingly, we have recently found that increase in PPARγ expression in thymus (Yang et al. 2009c) is associated with increased thymic involution and reduction of PPARγ by caloric restriction can forestall thymic adipogenesis and involution. In further support of these data, we have shown that ghrelin and ghrelin-receptor deficient mice, which develop accelerated thymic involution, also express higher PPARγ in thymic stromal cells suggesting that PPARγ may be functionally linked to the loss of thymopoiesis in aging (Youm et al. 2009). Furthermore, the activation of PPARγ signaling in an in vitro cell culture system lead to direct inhibition of T cell development from bone marrow derived HSCs. (Yang et al. 2009c). However, whether activation of PPARγ signaling in vivo promotes thymic aging is not known. Based on our primary findings, we hypothesized that constitutive-activation and TZD mediated ligand-activation of PPARγ induces thymoadipogenesis and reduces thymopoiesis. To this end, we created adipocyte lineage-specific constitutive active PPARγ transgenic (CA-PPARγ) animals and also utilized Rosiglitazone treatments in aging mice to investigate the impact of PPARγ activation on thymic function. Using defined genetic and clinically relevant pharmacological manipulation of PPARγ activity in vivo we provide evidence that activation of PPARγ receptor compromises generation of naïve T cell from thymus.
Results
PPAR-γ ligand, Rosiglitazone promotes thymic adipogenesis and thymic involution in aging mice
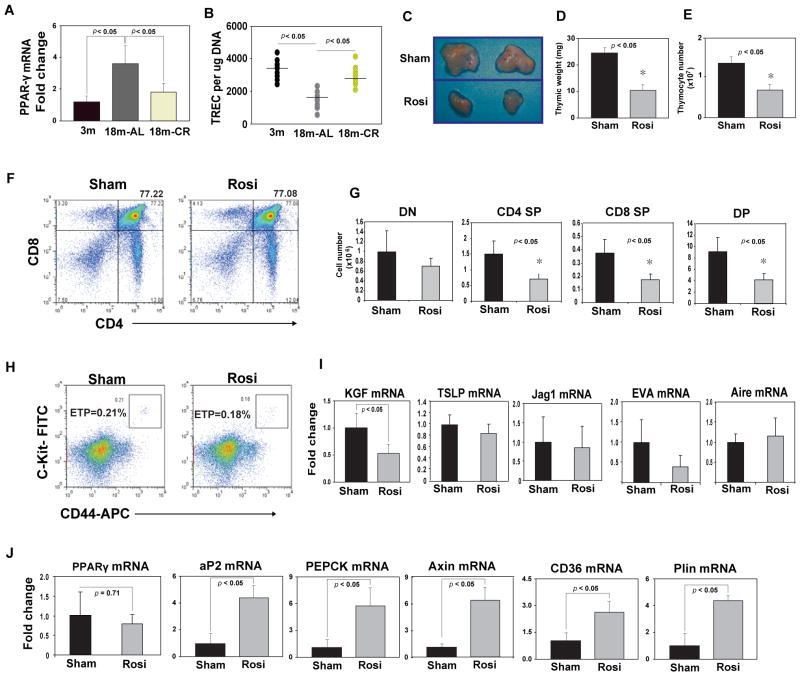
We have recently demonstrated that thymic PPARγ expression increases in middle-aged (12mo) mice and pro-longevity metabolic intervention, caloric restriction can prevent thymic-adipogenesis (Yang et al. 2009c). In further support of our previous findings, analysis of 18 month old mice revealed that PPARγ mRNA expression is significantly elevated by age and CR is highly effective in forestalling age-related increase in thymic PPARγ expression (Figure 1A). The quantitation of episomal DNA circles, termed TCR rearrangement excision circles (TRECs), is used as an index of thymic output (Sempowski et al 2002; Dixit et al 2007; Yang et al 2009a). Consistent with our hypothesis (Yang et al 2009c), age-related increase in PPARγ expression was correlated with reduction in thymic output (Figure 1B). The quantitative real-time PCR of splenic CD4+ T cells revealed that inhibition of PPARγ by CR significantly increased the TREC numbers in 18m old animals (Figure 1 B), suggesting increased thymopoiesis.
Figure 1.
PPAR-γ ligand, Rosiglitazone promotes thymoadipogenesis and increases thymic involution during aging. The thymi from young, old and age-matched CR mice were used to quantitate PPARγ by real time PCR analysis. (B) The T cell receptor excision circles (TRECs) in splenic CD4+ T cells were analyzed using quantitative PCR. (C, D, E) Rosiglitazone (Rosi) infusion (10mg/kg/day) for 2 weeks via s/c osmotic minipumps reduces the thymic size, thymic weight and total thymocyte counts in 18m old ad libitum fed C57/B6 mice. (F, G) The representative FACS plots of thymocytes stained with CD4 and CD8, from sham and rosiglitazone treated 18m old mice are shown. Rosi infusion reduces number of developing T cells without inducing defects in thymocyte development. (H) The earliest thymocyte progenitors (ETP; LinloCD25-CD44+Kithi) were gated on lineage low/negative cells (including CD25 in lin cocktail; Yang et al 2009c). The ETP cells (percent gated) did not show any significant difference between sham (0.23±0.02) and Rosiglitazone (0.19 ±0.03) treated mice. (I) Rosiglitazone treatment reduces the thymic mRNA expression of FGF7/KGF without significantly affecting, TSLP, Jag1, EVA and Aire. (J) Compared to vehicle infused mice, Rosiglitazone treatment led to significant upregulation of aP2, CD36, Perilipin, PEPCK and axin with no change in PPARγ mRNA expression. All data is expressed as mean SEM (n=8).
An important question emerging from our findings is whether the ligand activation of PPARγ receptor via anti-diabetic Thiazolidinediones (TZDs) in aging mice adversely impacts thymopoiesis. To this end, the 18 month old C57/BL6 mice, which have elevated thymic PPARγ (Figure. 1A), were treated with synthetic PPARγ-ligand, Rosiglitazone at a dose known to improve insulin-sensitivity (Purushotham et al 2007). Compared to vehicle (DMSO) infused control animals, 2 week rosiglitazone treatment in 18m old mice reduced the thymic size, weight and thymocyte numbers (Figure 1C, D, E). Notably, after the Rosiglitazone treatment there was also a reduction in CD4+CD8- (CD4SP), CD8+CD4- (CD8SP) and CD4+CD8+ (DP) cells without qualitative change or alteration in thymocyte development stages or CD4-CD8- DN cells (Figure 1F, G). Considering that earliest thymocyte progenitors (ETP, Lin-CD44+CD25-Kithi) are present within DN1 (LinloCD44+CD25-Kithi) population and are precursor of DN2 (LinloCD44+CD25+ Kithi) stage of thymocytes (Bhandoola et al 2007), we also analyzed ETPs in thymus. Compared to vehicle infused mice, no significant differences in ETP frequencies in thymi of rosiglitazone treated mice were found (Figure 1H). Rosiglitazone reduced the expression of primary mesenchymal cell derived thymopoietic growth factor, fibroblast growth factor 7 (FGF7 or KGF) (Figure 1I) without affecting the expression of Thymic Stromal Lymphopoietin (TSLP), Jag1 or epithelial cell specific genes, EVA and medullary TEC expressed Aire gene (Figure 1I). Interestingly, rosiglitazone infusions caused marked induction of pro-adipogenic transcripts, aP2, CD36, perilipin, axin and PEPCK without affecting PPARγ mRNA expression (Figure 1J). These data suggest that Rosiglitazone does not alter the percentages of ETPs in the thymus but that TZDs may quantitatively reduce the ability of ETPs to develop into mature thymocytes because the stromal cells maybe skewed towards adipogenic programming.
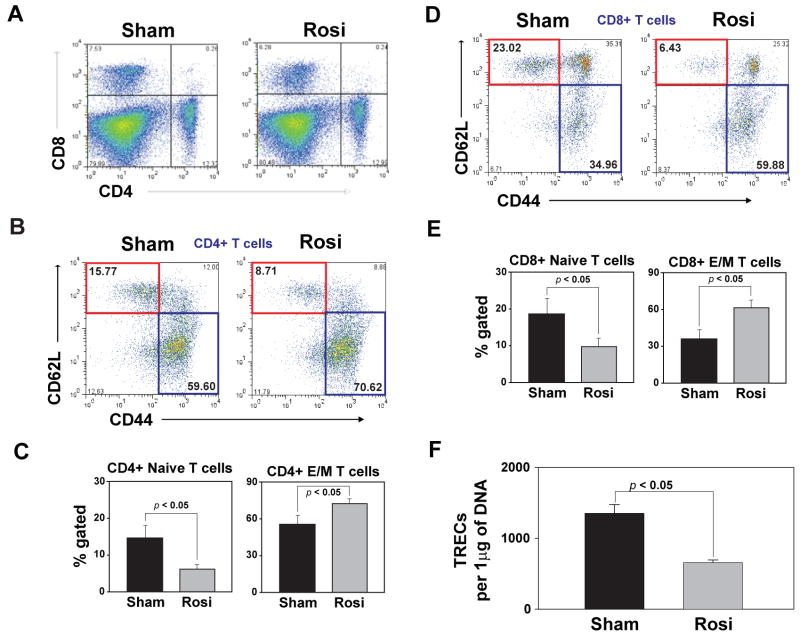
Considering that thymic involution is associated with reduction in naïve T cells and homeostatic expansion of effector-memory (E/M) T cells (Miller 1996; Swain et al 2005), we investigated thymic output by TREC analysis and naïve, E/M T cell numbers. We found that Rosiglitazone treatment did not affect total splenocyte count (data not shown) and did alter CD4:CD8 ratio in spleen (Figure 2A). Interestingly, we found that rosiglitazone markedly lowered the number of naïve CD4 cells (Figure 2B, C). Furthermore, increase in thymic adipogenesis via PPARγ-ligand dependent activation led to reduction in naïve CD8 cells (CD44-CD62L+) (Figure 2D) and significantly expanded the effector/memory T cells in spleen (Figure 2C, E), typically seen as a consequence of age-related thymic involution (Swain et al. 2005). Concurrent with decreased thymic cellularity, Rosiglitazone treatment also reduced the number of TREC+ CD4 cells in the spleen (Figure 2F) indicative of lower thymic export. Together, these data reveal that anti-hyperglycemic drugs like TZDs may accelerate thymic involution and compromise naive T cell production during aging.
Figure 2.
Rosiglitazone reduces naïve T cells and expands effector-memory cells. (A) Rosi infusion doesnot alter CD4 and CD8 frequency in spleen. (B). Rosiglitazone infusion causes significant decrease in naïve (CD62L+CD44- , red box) and increases the expansion of E/M (CD62L-CD44+ , blue box) CD4 (B and C) and CD8 (D and E) T cells. (F) Quantitative - PCR analysis of TRECs in splenic CD4+ T cells post Rosiglitazone infusion. All data is expressed as mean SEM (n=6-8).
Constitutive-active PPARγ transgenic mice exhibit increased thymoadipogenesis and bone marrow adiposity
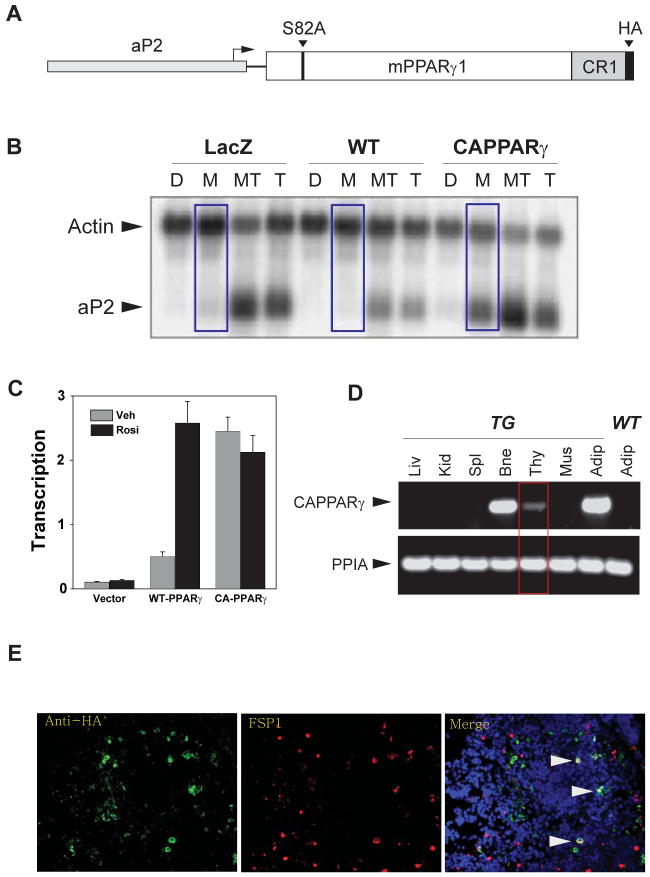
To directly assess the role of PPARγ in age-related thymic involution; we created a transgenic mouse line expressing a constitutive-active PPARγ (CAPPARγ) gene driven by aP2 promoter which is known to be highly expressed in adipocyte-lineage cells. The CAPPARγ transgenic mice were generated by mutating a negative-acting MAP kinase phosphorylation site at serine 82 residue of mouse PPARγ1 gene, and fusing the P300 binding domain of the adenovirus E1a gene to the C-terminus of the gene (Figure 3A). The rationale for utilizing the E1a CR1 region was based on its well characterized ability to bind to the transcriptional coactivator P300 (Goodman and Smolik 2000). P300 normally functions as a potent transcriptional coactivator of PPAR upon ligand binding (Dowell et al 1997). We reasoned that the presence of the E1a domain would mediate P300 binding to the CA-PPARγ protein in the absence of ligand binding and faithfully mimic the coactivator recruitment function of wild-type PPARγ that occurs in the presence of a ligand. Therefore, we next determined whether the CAPPARγ fusion protein mimics the liganded PPARγ receptor.
Figure 3.
The constitutive-active PPARγ transgenic mice. (A) The constitutive-active PPAR-γ1 (CA-PPARγ) transgene construct contains the full length 476 aa PPAR-γ1 gene linked 80 aa CR1 region of adenovirus-2 E1a gene (containing a P300 interaction domain) and a mutation in the negative-acting MAP kinase phosphorylation site at serine 82. This construct was placed under the control of mouse aP2 promoter/enhancer region (-5528 to +22 relative to aP2 transcriptional start site. (B) CA-PPARγ induces adipocyte differentiation in the absence of an activating ligand. Swiss3T3 cells expressing wild type or CA-PPARγ were generated by infection with retroviral vectors expressing the PPAR constructs, or control virus expressing LacZ. Infected cultures were treated with DMSO (D), standard MDI adipogenic hormonal cocktail (M), the cocktail plus 10 μM Troglitazone (MT) or Troglitazone alone (T) for six days. Total mRNA was prepared and adipogenesis was estimated by measuring aP2 mRNA by northern blotting. (C) The transient transfection showing ligand independent transcriptional activity of the CA-PPARγ construct. NIH3T3 cells were transfected with wild type or CA-PPARγ constructs (driven by the CMV promoter) together with a PPRE driven luciferase reporter. Cells were treated for 24 hours with 20 μM Rosiglitazone (Rosi) or vehicle (Veh). Luciferase activity was normalized to β-galactosidase activity from a co-transfected reference plasmid and is presented as the mean (± standard deviation) of three separate experiments. (D) CA-PPARγ is selectively expressed at high levels in adipose tissue and bone with modest transgene expression in thymus. (E) Thymic crypsections from 3mo old CA-PPARγ tranegenic mice were stained with anti-HA (to detect CA-PPARγ fusion protein) and FSP1/S100A4 (marker of secondary mesenchynmal cells) and nuclei were stained with DAPI. Arrow heads show co-localization of CA-PPARγ fusion protein with FSP1+ cells in thymus.
We studied adipogenesis in Swiss 3T3 fibroblast cells in response to constitutive-active PPARγ construct. The Swiss 3T3 fibroblasts do not differentiate into adipocytes unless PPARγ protein is provided along with an activating ligand (in this case Troglitazone). As expected, exposure of WT Swiss 3T3 cells with adipogenic cocktail (methylisobutylxanthine, dexamethasone and insulin; MDI) without addition of PPARγ ligand Troglitazone did not induce adipogenesis and aP2 (Figure 3B). Addition of Troglitazone together with MDI in WT cells induced aP2 expression (Figure 3B). Importantly, consistent with our construct design, we found that in CAPPARγ transfected Swiss 3T3 cells, MDI alone without the PPARγ ligand Troglitazone led to robust expression of aP2 (Figure 3B). Further characterization of constitutive-active PPARγ construct in NIH3T3 fibroblast cells revealed that ligation of wild type PPARγ receptor by Rosiglitazone achieved comparable transcriptional activity to the constitutive-active PPARγ fusion protein (Figure. 3C). Collectively, these data indicate that the CAPPARγ fusion protein functions in the fibroblast cells without causing overt toxicity and is able to induce adipogenesis by mimicking the physiologically liganded PPARγ receptor.
The CAPPARγ transgene was expressed under the control of adipocyte-lineage specific aP2 promoter and as expected we detected high CAPPARγ expression in adipose tissue (Figure 3D). In addition, we observed robust expression of CAPPARγ transgene in bone marrow with modest expression in thymus (Figure 3D). Considering that adipocytes are thought to originate from mesenchymal lineage (Gesta et al. 2007) and PPARγ is required for differentiation of fibroblasts to adipocytes (Tontonoz et al. 2008), we investigated the cellular localization of CAPPARγ fusion protein in thymus. Consistent with our recent findings that Fibroblast Specific Protein1 (FSP1+) type 2 mesenchymal cells express adipogenic markers (Youm et al 2009; Yang et al 2009b), we observed that in thymus CAPPARγ fusion protein (identified by anti-HA antibody) displayed partial co-localization with FSP1+ thymic fibroblasts (Figure 3E).
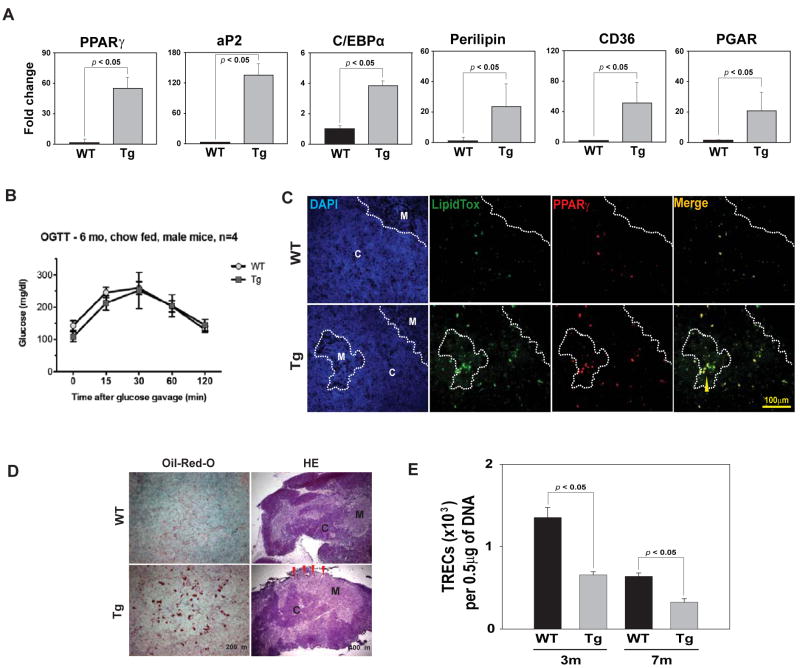
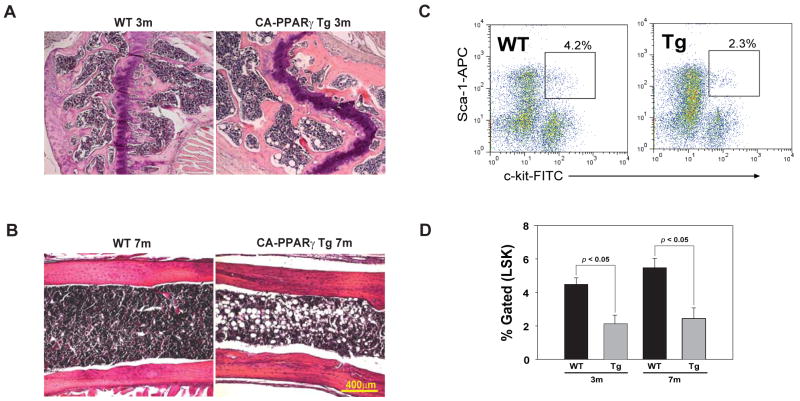
Consistent with our hypothesis, the CAPPARγ transgenic mice displayed increased thymoadipogenesis as evidenced by significantly higher levels of PPARγ, aP2, C/EBPα, perilipin, CD36 and PPAR gamma angiopoietin related (PGAR) mRNA (Figure 4A). Surprisingly, despite high CAPPARγ fusion protein expression in peripheral adipose tissue, oral glucose tolerance test (OGTT) in chow fed 6 month CAPPARγ transgenic mice revealed no significant change in glucose homeostasis (Figure 4B). Furthermore, at this age no significant differences in body weight or fasting plasma glucose levels were detected (data not shown) suggesting that observed thymic effects in CAPPARγ transgenic mice are independent from systemic metabolic alterations. We found that consistent with increase in pro-adipogenic PPARγ target genes; there was an increase in lipid-expressing cells in the thymus which co-localized with PPARγ in thymic medulla as well as cortex (Figure 4C). The thymic architecture of CAPPARγ transgenic mice was compromised as evidenced by increase in lipid droplet accumulation and contraction of thymic cortex and an inversion of cortical and medullary zones (Figure 4D). In addition, compared to WT control mice, the CAPPARγ transgenic animals show a significant reduction in TREC+ CD4 cells in spleen suggestive of reduced thymopoiesis (Figure 4E). These data suggest that induction of thymoadipogenesis by CAPPARγ led to a significant reduction in thymopoiesis (Figure. 4E).
Figure 4.
Activation of PPARγ induces thymic adipogenesis and thymic involution. (A) Real-time PCR analysis of PPARγ, aP2, C/EBPα, perilipin, CD36 and PGAR mRNA in thymi of 7 month old WT and CA-PPARγTg mice. (B) The oral glucose tolerance test (OGTT) in 6 mo old chow fed WT and CA-PPARg transgenic mice revealed no significant change (P >0.05) in glucose homeostasis. (C) The immunoflouorescence analysis of thymic cryosections from 7m old WT and CA- PPARγ were labelled with anti- PPARγ and neutral lipid. Thymic denoted cortex is denoted by C and medulla by M. Arrow heads show co-localization of lipid expressing cells and PPARγ. (D) Oil-red-O and methyl green staining shows greater lipid accumulation in thymic medulla of 7m old CA- PPARγ mice. The thymic cryosections were also stained with hematoxylin and eosin, thymic involution is evident by cortical thinning (red arrows). (E) Quantitative - PCR analysis of TRECs in splenic CD4+ T cells of 3m and 7m old WT and CA-PPARγTg mice. All data is expressed as mean SEM (n=8).
Considering that HSCs such as Lin-Sca1+Kit+ (LSK) are required for thymopoiesis, high expression of CAPPARγ transgene in bone marrow prompted us to analyze the frequency of HSCs. Interestingly, we found that genetic gain of function of PPARγ in BM was coupled with increase in ectopic BM-adipocytes (Figure 5A, B) and reduction in LSK cells (Figure 5C, D). Interestingly, recent report suggests that pharmacological inhibition of PPARγ can reduce BM adiposity and enhance the ability of BM derived Hematopoietic Stem Cells (HSCs) to reconstitute immune system (Naveiras et al. 2009). Taken together, our findings indicate that reduced thymopoiesis in CAPPARγ transgenic animals may be linked to alterations in stromal microenvironment in primary lymphoid organs and an overall reduction in lymphoid progenitor pool in BM.
Figure 5.
CA-PPARγ promotes bone marrow adiposity and reduces hematopoietic stem cells. (A) Hematoxylin and eosin stain in decalcified femoral head of 3m and (B) femur of 7m old WT and CA-PPARγ transgenic mice. (C) Representative FACS plot gated on lineage negative bone marrow cells and stained with Sca1-APC and C-kit-FITC. (D) The percent gated Lin-Sca1+Kit+ (LSK) cells from 3m and 7m old WT and CA-PPARγ mice. All data is expressed as mean SEM (n = 4 per group).
Constitutive-active PPARγ transgenic mice display reduced T cell repertoire diversity
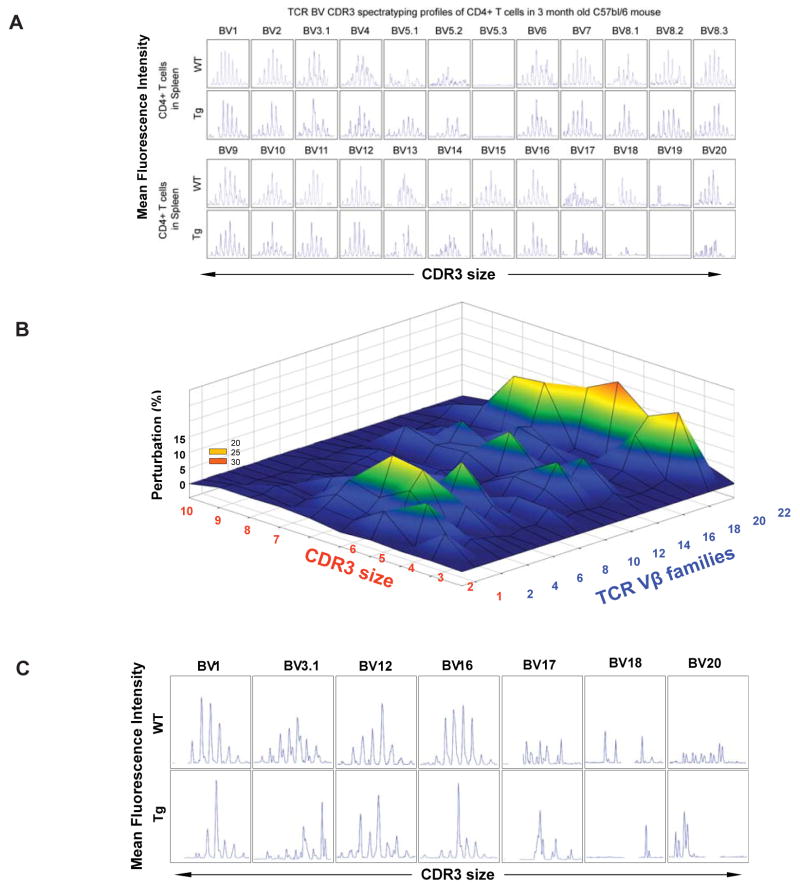
It is well recognized that reduction in naïve T cell output with aging is associated with reduced TCR diversity (Nikolich-Zugich 2004; Naylor et al 2005). Considering that diverse repertoire of naive T cells is essential for efficient adaptive immune response we next investigate the impact of PPARγ driven adipogenesis on TCR diversity. The diversity of the TCR repertoire is generated in the thymus through imprecise assembly of V, D, J, and C gene segments to form T cells expressing αβTCR (von Boehmer et al 1998). The TCR diversity is mainly conferred through third hypervariable region of the receptor chains, called complementarity determining region -3 (CDR3) (Pannetier et al. 1993). Given VDJ recombination involves imprecise joining, in a sample of T cells, a range of CDR3 sizes can exist which typically vary in length by 8-10 amino acids (Pannetier et al. 1993). The CDR3 polymorphism analysis or TCR spectratyping allows for global analysis of the TCRs of the sampled T cell population. Also, CDR3 length spectratyping allows analysis of TCR repertoire at a higher-resolution as each Vβ-Jβ combination shows a Gaussian distribution of 6-10 CDR3 lengths with consecutive additions of 3 base pair that represent in-frame rearrangement. Using TCR spectratyping, we analyzed the sizes of the CDR3s in pool of T cells derived from control and CAPPARγ Tg mice by analyzing Vβ-Cβ PCR products which have been labeled with a runoff reaction on a sequencing gel (Pannetier et al. 1993; Gorochov et al. 1998). Compared to WT mice, in the 3 month old CAPPARγ Tg animals, the TCR repertoire diversity was maintained (Figure. 6A). To understand if progressive aging and reduced thymopoiesis affects TCR diversity we also examined 7 month old CAPPARγ Tg animals (Figure 6B, C). A polyclonal diverse TCR Vβ family is characterized by Gaussian distribution of peaks, while a skewed profile is distinguished by deviations from Gaussian distribution (37, 38) and aberrant amplification of peaks (Figure. 6C). The Gaussian distribution profiles were translated into probability distributions as functions of the area under the curve for each CDR3 length as described previously (31, 34). By these methods we demonstrate that T cells of CAPPARγ transgenic mice exhibited significant perturbations in the TCR repertoire diversity (Figure. 6B, C) in an age-dependent manner. Collectively, these findings provide direct evidence that thymic gain of function of PPARγ causes increased thymoadipogenesis and reduces T cell repertoire diversity.
Figure 6.
CA-PPARγ reduces thymopoiesis and restrict TCR diversity. (A) The representative TCR spectratyping analysis of peripheral CD4+ cells of 3m old WT and CA-PPARγTg mice (n = 4) revealed no change in CDR3 lengths of TCR-Vβ families. (B) The TCR spectratyping analysis of peripheral CD4+ cells of 7m old WT and CA-PPARγTg mice. The perturbations in TCR diversity are represented as landscape surfaces, in which smooth (blue) landscapes represent an unchanged TCR repertoire (diversity). The Mountain (in green, yellow and orange) depicts area under the curve and diversion from Gaussian amplified peaks of CDR3 lengths. Each line crossing on the y axis of the landscape denotes change from WT specific CDR3 length or size (x-axis) of a particular Vβ family (z-axis). (C) The spectratype analysis with representative Vβ families from 7m WT and CA-PPARγ Tg mice are shown. The CDR3 size is shown on the x-axis and relative peak area as fluorescence intensity is shown on the y-axis. The peak perturbation corresponding to a CDR3 length of 8-10 amino acids are shown for Vβ family 1, 3.1, 12, 16, 17, 18 and 20.
Discussion
The synthetic TZDs serve as PPARγ ligands and are widely used antihyperglycemic therapeutic agents. Emerging evidence suggests that prolonged use of TZDs in elderly increases the risk of cardiovascular events, fluid retentions, edema and also cause osteoporosis and fractures (Rubenstrunk et al. 2007; McDonough et al. 2008; Guan et al. 2005). Therefore selective and tissue specific PPARγ modulators (SPARMs) are under development with the goal to safely harness the insulin-sensitizing actions of PPARγ-ligands without systemic adverse effects (Sporn et al. 2001). Herein we present data that demonstrates that PPARγ is expressed in thymus and increases upon physiological aging and ligand-activation of PPARγ reduces naïve T cell production and restricts TCR repertoire diversity.
Given the purpose of thymus is to establish and maintain T cell arm of immunity and not energy homeostasis, the thymic adiposity during healthy aging process is a puzzling phenomenon and it remains unclear why this maladaptive process is initiated early in life. Recent studies demonstrate that removal of thymus in children undergoing cardiac surgery leads to immunosenescence and premature onset of age-related decline in immune function (Sauce et al. 2009). These data further underscores that thymopoiesis in adult life is required to maintain T cell repertoire diversity. However, the regulators responsible for inducing ectopic adipocyte development within the thymic space during middle-age are incompletely understood. Based upon a variety of experimental approaches, we demonstrate that PPARγ driven thymic-adipogenesis plays a key role in reducing thymopoiesis and causing involution of thymus with increasing age. Although, it is well known that thymic space is replaced with adipose tissue in elderly (Flores et al 2004; Yang et al 2009), the thymus specific adipogenic mechanisms that generate ectopic intrathymic adipocytes have not been considered as major contributors toward thymic loss. We have recently demonstrated that loss of ghrelin-receptor mediated signaling leads to ectopic adipocyte development in thymus with greater expression of PPARγ in thymic stomal cells (Youm et al. 2009). Furthermore, inhibition of PPARγ signaling and thymoadipogenesis by CR is coupled with maintenance of thymic function that prevents erosion of T cell diversity seen during aging (Yang et al 2009). Our findings also suggest that inhibition of adipogenic cellular differentiation within aging thymus by CR due to reduction in PPARγ maybe important to maintenance of thymic function (Yang et al. 2009). Interestingly, despite well known broad metabolic effects of CR, the insulin sensitizing effects of CR are not associated with any significant difference in white adipose tissue expression of PPARγ (Masternak and Bartke 2006). Consistent with greater insulin-sensitivity in CR mice, the effects of CR on pro-adipogenic gene expression were selective and not associated with reduction in PPARγ in adipose depots (data not shown).
Interestingly, the constitutive-active PPARγ expression in bone marrow and thymus lead to greater ectopic adipocyte development. It has recently been shown that pharmacological inhibition of PPARγ reduces bone marrow adiposity and increases hematopoiesis post cytoreductive conditioning regimens of irradiation (Naveiras et al. 2009). Our data from constitutive-active PPARγ tranagenic mice provide further evidence that excessive adipocyte development in BM microenvironment lowers the pool of LSK hematopoietic stem cells. Given thymopoiesis is dependent on periodic homing and seeding of thymus with BM-derived progenitors, we observed reduced thymopoiesis and naïve T cell production in both CA- PPARγ tg as well as rosiglitazone treated mice. Consistent with reduced thymic output, constitutive-activation of PPARγ lead to marked restriction of TCR repertoire diversity. The generation and expression of a broad TCR repertoire on the surface of lymphocytes are critical for development and function of an effective adaptive immune system. Accordingly, restriction of TCR diversity is associated with greater risk of infections and reduced immune-surveillance (Nikolich-Zugich et al. 2004; Yager et al. 2008). Increasing the thymopoiesis is considered as an important means to maintain and expand TCR repertoire. Therefore the activation of PPARγ induced thymic involution and reduction in TCR diversity may lower immune-surveillance.
TZDs are also known to promote generation of new adipocytes from resident preadipocyte via the PPARγ pathway. Mounting clinical evidence suggests that sustained TZD use causes higher bone marrow adipogenesis and increased bone loss in aging diabetic patients (McDonough et al. 2008). New evidence suggests that PPARγ expression also promotes osteoclastogenesis and bone resorption (Wan et al. 2007). The 2 week rosiglitazone treatment in 18m old mice caused dramatic thymic loss as evident by lower thymic mass and total thymocyte numbers. Rosiglitazone treatment increased thymic adipogenesis and reduced KGF expression, a key mesenchymal cell derived prothymic growth factor. Reduction in thymocyte counts, thymocyte subsets and TRECs post Rosiglitzone suggests reduction in thymic output. The analysis of splenic naïve and E/M subsets also revealed significant alterations. Although the reduction of splenic naïve cells is reflective of reduced thymic export, expansion of E/M cells post rosiglitazone can also be attributed to direct effects on peripheral T cell proliferation and homeostasis. Overall, these findings suggest that Rosiglitazone accelerates age-related thymic involution and may promote immunosenescence.
In light of our present findings, further work is necessary to determine whether prolonged TZD use in middle aged and elderly insulin-resistant patients presents an added risk of accelerated thymic involution and immunosenescence. Furthermore, several clinical studies have shown improvement that TZDs can lower inflammation and enhance cognition and memory in elderly Alzheimer Disease patients (Landreth et al 2008). Based on our data, the prolonged use of Rosiglitazone in AD and potential adverse impact on adaptive immunity deserves greater scrutiny. Interestingly, similar to TZDs, the synthetic aP2 inhibitors can also reduce inflammation and type 2 diabetes (Furuhashi et al. 2007). Thus taken together with our current findings, it is possible that such aP2 inhibitors, in addition to being effective insulin sensitizing agents, could potentially reduce thymic involution and immunosenescence process. It is important to note that despite massive fatty degeneration of thymus in elderly, naïve T cells produced from thymic remnants can be detected in blood (Hale et al. 2006; Nasi et al. 2006). In addition, several studies in mice and humans suggest that naïve T cell production from aging thymus can be partially restored (Dixit et al. 2007; Napolitano et al 2008; Chidgey et al. 2007) suggesting that thymic rejuvenation to promote T cell immunity is an achievable therapeutic goal in several clinical immunodeficiency disorders. In conclusion, our data provides evidence that activation of PPARγ accelerates age-related thymic involution and reduces T cell diversity. The future development of safer alternatives such as selective and tissue specific PPARγ modulators (SPARMs) drugs for treatment of insulin-resistance require careful evaluation for potential adverse effects on mechanism regulating T cell generation from thymus. Based on our findings, it is likely that cell specific inhibition of PPARγ signaling in thymus may be an additional strategy to prevent deterioration of thymic stromal environment and to enhance naïve T cell production.
Experimental procedures
Mice
The Constitutive-active PPARγ was generated from the mouse PPARγ1 gene by mutating a negative-acting MAP kinase phosphorylation site {Camp, 1997, PMID 9099735; Camp, 1999, PMID 9886850} at serine 82 to alanine, and by attaching the adenovirus E1a CR1 region to the C-terminus of the PPARγ coding sequences. An HA-tag antigenic epitope was attached to the C-terminus for recombinant protein detection. This construct produces a constitutive-active PPARγ protein that activates transcription from a PPARγ-dependent reporter in the absence of ligand, to the same level as fully liganded wild-type PPARγ. The CA-PPARγ construct was designed using PPARγ1 instead of PPARγ2 because of its slightly lower transcriptional activity relative to PPARγ2 in many cell types (personal observations) and its lower adipogenic potential. In vitro analysis of the transcriptional activity of the CA-PPARγ protein supports this contention, showing that it activates transcription from a PPARγ-dependent reporter in the absence of ligand, to the same level as fully liganded wildtype PPARγ (Fig 3C). The CA-PPARγ construction was placed under control of the mouse aP2 gene regulatory sequences on a 5 kb fragment containing both the aP2 enhancer and promoter. The aP2-CA-PPARγ transgenic mouse line was produced by standard procedures at the University of Michigan Transgenic Animal Model core (http://www.med.umich.edu/tamc/) by injection into C57BL/6 × SJL F2 eggs. Four transgenic founder lines where identified, one of which showed detectable expression of the transgene in adipose tissue. This line was bred into C57BL/6J for 8 generations. The animals used in this study were maintained on a standard chow diet.
The female chronically calorie restricted (CR) (n = 12) mice and ad libitum (AL) fed (n = 12) 18 month old C57BL/6 mice were purchased from NIA-aging rodent colony (Harlan Sprague-Dawley, Indianapolis, IN). The 18m old female C57BL/6 mice were treated with rosiglitazone or vehicle at the previously reported (Purushotham et al 2007) insulin-sensitizing dose of (10mg/kg/day) for 2 weeks via s/c osmotic minipumps as described previously (Dixit et al 2007). All protocols were approved by the Institutional Animal Care and Use Committee of Wayne State University and Pennington Biomedical Research Center.
Immunofluorscence microscopy and Histology
The immunofluorescent and histological staining was performed as described previously (Dixit et al 2007; Yang et al 2009c), briefly thymi obtained from mice were fixed in 20% sucrose and prepared for cryosectioning. Immunostained sections were observed with a Zeiss Axioplan 2 and Zeiss confocal microscope. The anti-PPARγ antibody recognizes both γ1 and γ2 isoforms of PPAR protein and was purchased from Santa Cruz Biotechnology. The anti-HA and anti-FSP1/S100A4 rabbit polyclonal antibody were purchased from Abcam.
Transfections and assays
For the transient transfection experiment shown in Fig. 2, NIH3T3 cells were transfected with CMV driven PPARγ constructs (wild type or constitutive active) together with and a PPRE (ap2) driven luciferase reporter as described (PMID 17227883). Rosiglitazone (Ros) 20 micro-molar was added 16 hours after transfection and the cells harvested 24 hours later. For the adipocyte differentiation wild type and CA-PPARγ genes were introduced in the pBMN retroviral vectors and high titer viral stocks were prepared. Swiss 3T3 fibroblasts were infected with pBMN-LacZ, pBMN-CA-PPARγ or pBMN-WT-PPARγ virus. Equal expression levels of wild type and CAPPARγ were confirmed by western blot. The adipogenic cocktail used for differentiation contained dexamethasone (1 μM), 3-isobutyl-1-methylxanthine (MIX, 0.5 mM), and insulin (1.67 μM). Total RNA was prepared using Trizol according to the manufacturers instructions and northern blotting was performed to analyze aP2 mRNA expression.
FACS analysis
The thymocytes, splenocytes and bone marrow single cell suspensions were prepared and stained using anti - CD4-PerCP, CD8-APC, CD44-FITC, and CD4-PE, CD4-FITC, CD4-PerCP, CD11b, Gr-1-PE, CD45R-PE, CD3-PE, CD8-PE, αβTCR-PE, γδTCR-PE, pan-NK-PE, NK1.1-PE, CD11c-PE, , CD19-PE, Ter119-PE and CD127-PE and, , CD25-APC and C-kit-FITC, Sca1-PE (ebiosciences) as described previously (Dixit et al 2007; Yang et al 2009c). The ETP were defined as LinloCD25-CD44+Kithi (Bhandoola et al 2007). A total of 5 million thymocytes were stained using a panel of lineage specific antibodies, CD44 and C-kit as described previously (Dixit et al 2007; Yang et al2009a; Yang et al 2009c). All FACS analyses were performed on BD FACS Calibur and data was analyzed using post collection compensation and single color isotype IgG control using FLOWJO software.
Real-time RT-PCR and signal joint-T cell receptor excision circle (TREC) analysis
The total RNA was prepared with RNAzol (Isotex Diagnostics). The cDNA synthesis and real-time RT-PCR was performed as described previously (BioRad Real-time RT-PCR analyses were done in duplicate on the ABI PRISM 7900 Sequence Detector TaqMan system with the SYBR Green PCR kit as instructed by the manufacturer (Applied Biosystems). The primer pairs and methods to perform RT-PCR have been described in our prior work (Dixit et al 2007; Youm et al 2009; Yang et al 2009a; Yang et al 2009b; Yang et al 2009c).
The CD4 cells were isolated from splenocytes using mouse positive selection kit (Invitrogen) per manufacturer's instructions. The PCR for performed with mδRec and ψJα specific primers and mδRec-ψJα fluorescent probe as described previously (Dixit et al 2007; Youm et al 2009; Yang et al 2009a; Yang et al 2009b; Yang et al 2009c). The standard curves for murine TRECs were generated by using δRec ψJα TREC PCR product cloned into a pCR-XL-TOPO plasmid, a generous gift from Dr. Gregory D. Sempowski, Duke University Medical Center.
Complementarity Determining Region 3 (CDR3) polymorphism - TCR Spectratyping Analysis
The T cells were used to prepare total RNA and cDNA was prepared as described previously (Dixit et al. 2007, Yang et al. 2009a). A FAM-labeled nested constant β-region primer was used in combination with 24 multiplexed forward murine Vβ-specific primers top measure the CDR3 lengths as described previously (Dixit et al 2007; Yang et al 2009a; Yang et al 2009c). Each peak was analyzed and quantified with ABI PRISM GeneScan analysis software (Applied Biosystems), based on size and density. Data were used to calculate the area under the curve (AUC) for each Vβ family. Each peak, representing a distinct CDR3 of a certain length, was quantified with BioMed Immunotech software.
The oral glucose tolerance test (OGTT)
The OGTT experiments were performed on 6 month WT and CA-PPARg transgenic male mice. All mice were fasted for 2h and given an oral glucose gavage. The glucose was measured in tail blood immediately at 0min, 15, 30, 60 and 120 min using a glucometer (Breeze , Bayer Health Care, USA).
Statistical Analyses
The results are expressed as the mean ± SEM. The differences between means and the effects of treatments were determined by one-way ANOVA using Tukey's test (Sigma Stat), which protects the significance (p < 0.05) of all pair combinations.
Acknowledgments
The research in this laboratory is supported in part by the Coypu Foundation and NIH, National Institute on Aging- Grant AG031797R01. The present work utilized the facilities of the Genomics and CBB Core facilities supported by NIH Grant 1 P20 RR02/1945 and Cell Biology & Bioimaging Core Facility of the Pennington Center of Biomedical Research Excellence (NIH P20 RR- 021945) and Clinical Nutrition Research Unit (NIH P30 DK072476).
Abbreviations
- CDR3
Complementarity determining region 3
- PPARγ
Peroxisome proliferator activated receptor gamma
- CEBPα
CCAAT/enhancer binding protein-alpha
- PGAR
peroxisome proliferator activated receptor gamma angiopoitin related
- PEPCK
phosphoenolpyruvate
- Plin
Perilipin
- FABP4/aP2
Fatty acid binding protein-4
- KGF
Keratinocyte growth factor
- (FSP1)/S100A4
Fibroblast Specific Protein-1
References
- Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol. 2008;43:700–705. doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Chidgey A, Dudakov J, Seach N, Boyd R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin Immunol. 2007;19:331–340. doi: 10.1016/j.smim.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. p300 functions as a coactivator for the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1997;272:33435–33443. doi: 10.1074/jbc.272.52.33435. [DOI] [PubMed] [Google Scholar]
- Evans R, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:1–7. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;8:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debré P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Dargie H, Komajda M, Gubb J, Biswas N, Jones NP. Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocol. Diabetologia. 2005;48:1726–1735. doi: 10.1007/s00125-005-1869-1. [DOI] [PubMed] [Google Scholar]
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–73. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;16:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A. PPARs in Calorie Restricted and Genetically Long-Lived Mice. PPAR Res. 2006;22:284–236. doi: 10.1155/2007/28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4:507–513. doi: 10.1038/ncpendmet0920. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Morley JE. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 2008;24:395–405. doi: 10.1016/j.cger.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, Li K, Killian ML, Bacchetti P, McCune JM. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi M, Troiano L, Lugli E, Pinti M, Ferraresi R, Monterastelli E, Mussi C, Salvioli G, Franceschi C, Cossarizza A. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;5:167–175. doi: 10.1111/j.1474-9726.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. National Institutes of Health. 2007 March; Publication No. 07- 6134. [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- Pannetier C, Cochet M, Darche S, Casrouge A, Zöller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P, Grunnet LG, Pilgaard K, Storgaard H, Alibegovic A, Sonne MP, Carstensen B, Beck-Nielsen H, Vaag A. Increased risk of type 2 diabetes in elderly twins. Diabetes. 2009;58:1350–1355. doi: 10.2337/db08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham A, Wendel AA, Liu LF, Belury MA. Maintenance of adiponectin attenuates insulin resistance induced by dietary conjugated linoleic acid in mice. J Lipid Res. 2007;48:444–452. doi: 10.1194/jlr.M600393-JLR200. [DOI] [PubMed] [Google Scholar]
- Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debré P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;8:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- Sierra F, Hadley E, Suzman R, Hodes R. Prospects for life span extension. Annu Rev Med. 2009;60:457–469. doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Suh N, Mangelsdorf DJ. Prospects for prevention and treatment of cancer with selective PPARgamma modulators (SPARMs) Trends Mol Med. 2001;7:395–400. doi: 10.1016/s1471-4914(01)02100-1. [DOI] [PubMed] [Google Scholar]
- Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- Swain S, Clise-Dwyer K, Haynes L. Homeostasis and the age-associated defect of CD4 T cells. Semin Immunol. 2005;17:370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Aifantis I, Azogui O, Feinberg J, Saint-Ruf C, Zober C, Garcia C, Buer J. Crucial function of the pre-T-cell receptor (TCR) in TCR beta selection, TCR beta allelic exclusion and alpha beta versus gamma delta lineage commitment. Immunol Rev. 1998;165:111–119. doi: 10.1111/j.1600-065x.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity. 2007;15:2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009a;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Rim JS, Galbán CJ, Vandanmagsar B, Dixit VD. Axin expression in thymic stromal cells contributes to age-related increase in thymic adiposity and associated with reduced thymopoiesis independently of ghrelin signaling. J Leukoc Biol. 2009b;85:928–938. doi: 10.1189/jlb.1008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009c;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Youm YH, Yang H, Sun Y, Smith RG, Manley NR, Dixit VD. Deficient Ghrelin receptor mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284:7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]