Summary of Recent Advances
The small GTPase Sar1 resides at the core of a regulatory cycle that controls protein export from the ER in COPII vesicles. Recent advances in minimally reconstituted systems indicate continual flux of Sar1 through GTPase cycles facilitates cargo concentration into forming vesicles that ultimately bud from membranes. During export from ER membranes, this GTPase cycle is harnessed through the combinatorial power of multiple coat subunits and cargo adaptors to sort an expanding array of proteins into ER-derived vesicles. The COPII budding machinery is further organized into higher-order structures at transitional zones on the ER surface where the large multi-domain Sec16 protein appears to perform a central function.
Introduction
From the first remarkably intuitive descriptions of the interconnected membrane compartments of the secretory pathway [1], through the elegant use of yeast genetics to define key components [2], to the in vitro reconstitution of individual transport events [3–5], our understanding of the mechanisms of intracellular delivery of proteins has become ever more detailed. In recent years, the structural analysis of individual coat proteins has provided a molecular blueprint of how transport vesicles are sculpted from the endoplasmic reticulum (ER), illuminating how the components of the COPII coat interact with each other and with their client cargo proteins [6•–10]. A current challenge in the field is to place these static structures back in the context of cellular complexity in order to understand more fully how the process of vesicle biogenesis is regulated and adapted for distinct cellular conditions. In this review, we aim to cover recent advances on the coordination of coat assembly with cargo sorting at the ER and the organization of this export machinery at transitional ER (tER) sites.
The GTPase cycle
Biogenesis of COPII vesicles is regulated at the most basic level by a GTPase cycle under direction of the small GTPase Sar1 (Figure 1). Like most small G-proteins, Sar1 is a relatively poor GTPase and requires additional factors to efficiently complete the transition between GDP and GTP states (and back again). In the first layer of regulation of COPII vesicle formation, Sar1 is specifically activated on the surface of ER membranes by its guanine nucleotide exchange factor (GEF), Sec12 [11]. Activation to Sar1•GTP exposes an amphipathic α-helix that inserts into the lipid bilayer and likely initiates membrane curvature [12,13]. Sar1•GTP also recruits the cargo adaptor complex, Sec23/Sec24, which contains the Sar1-specific GTPase activating protein (GAP) Sec23 [14]. The outer layer of the COPII coat, comprising Sec13/Sec31, binds to the assembled Sar1•GTP/Sec23/Sec24 complex and contributes yet another layer of control to the GTP cycle by stimulating the GTPase activity of Sar1/Sec23 approximately 10-fold [15]. A small, unstructured fragment of Sec31 performs this activity by helping to optimally position the catalytic residues of Sar1 and Sec23 [8••]. One conundrum posed by this layered arrangement of incremental contributions to GTPase activity is that once the coat is assembled in it entirety, GTP hydrolysis by Sar1 is maximal and thereby drives immediate coat disassembly [15]. Clearly, additional levels of regulation must occur to prevent premature coat dissociation from the membrane in order to allow productive vesicle budding.
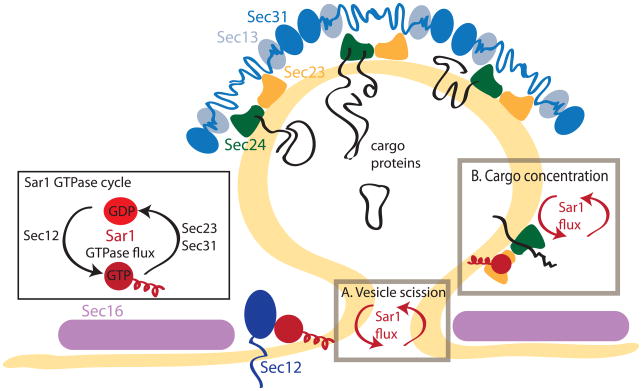
Fig. 1. COPII assembly and the Sar1 GTPase cycle.
The COPII coat assembles on the ER membrane through the coordinated action of a number of components. The small G-protein, Sar1, is recruited to the ER membrane by its guanine nucleotide exchange factor (GEF), Sec12, exposing an amphipathic α-helix upon GTP binding. Sar1•GTP in turn recruits the Sec23/Sec24 dimer and the Sec13/Sec31 tetramer to create the coat polymer. The GTPase cycle of Sar1 (inset) is controlled by the GEF, Sec12, and the GTPase activating protein (GAP), Sec23. Sec31 further stimulates the GAP activity of Sec23. Sec16 stably marks ER exit sites and is required for their integrity. Recent evidence suggests that instead of a rigid stepwise assembly of the coat, flux of Sar1 through the GTP cycle is required for specific events in the biogenesis of a COPII vesicle. A. Vesicle release from the donor membrane is impaired when the N-terminal helix of Sar1 is removed [12] or when GTP hydrolysis is prevented [13]. B. Concentration of cargo proteins in a minimally reconstituted system is reduced when Sar1 cannot hydrolyse GTP [19••]. Repeated Sar1 GTPase cycles could promote higher affinity associations between Sec23/24 and ER export signals on cargo.
Recent experiments making use of minimally reconstituted systems have started to shed some light on how the GTP cycle on Sar1 is modulated to permit cargo loading and vesicle release. The continual presence of Sec12 is able to prolong Sar1 loading onto synthetic liposomes to sufficient levels to maintain association of the COPII coat even in the presence of GTP [16•,17•]. However, this likely involves constant turnover of the coat as individual coat components dissociate from the membrane and are replaced by newly associating molecules. This may be important in sustaining coat recruitment to the ER membrane to permit encounters with cargo proteins, but is difficult to reconcile with a stable coat assemblage that is capable of the coordinated action likely responsible for budding a vesicle. Conversely, when cargo proteins are present, Sec23/24 shows a prolonged association with liposomes even after Sar1 has hydrolyzed GTP [17•]. The GTPase activity on Sar1 remained unchanged under these conditions, suggesting that cargo is unlikely to directly delay GTP hydrolysis but instead stabilizes the coat by providing increased affinity for Sec23/24 to bind the membrane. These observations are consistent with previous data that demonstrated the prolonged association of Sec23/24 and Sec13/31 on gently purified vesicles generated with GTP [4].
The link between cargo capture and the GTPase cycle remains to be fully explored. In other vesicle budding systems (eg. the COPI coat), signal-containing cargo peptides have been observed to inhibit the GTPase activity of the regulatory G-protein, Arf1 [18]. Such activity has not been demonstrated for the COPII coat, but an intriguing recent study using single molecule fluorescence microscopy raises the possibility that concentration of cargo into nascent vesicles depends on continual flux through the Sar1 GTPase cycle: in the presence of the non-hydrolysable GMP-PNP, fluorescent cargo proteins failed to accumulate in punctate structures observed by TIRF microscopy [19••]. Furthermore, segregation of a bona fide cargo from an ER resident protein was also dependent on the presence of GTP suggesting a proof-reading function for GTPase activity of Sar1. Although the mechanism by which GTP hydrolysis controls cargo selection remains to be determined, one possibility is that turnover of the Sar1•GTP/Sec23/Sec24 complex permits more efficient sampling of the ER membrane such that coat components bound to incorrect cargo, or recruited to the membrane absent any cargo, are recycled for another chance at productive assembly. Repeated GTPase cycles would then lead to concentration of appropriate cargo into vesicles. It is unclear how to reconcile these ideas with previous observations that transport vesicles bearing a full complement of cargo proteins (and lacking ER residents) bud from microsomal membranes in the presence of GMP-PNP, but further exploration of the nature of the cargo-containing puncta observed in vitro may help elucidate how cargo capture is influenced by the GTP cycle (and vice versa).
Cargo regulation
The molecular characterization of cargo-coat interactions has defined the Sec24 subunit as the main component that drives efficient uptake of specific cargoes into nascent vesicles in yeast [9,20,21]. Recent studies have expanded this analysis to mammalian cells and highlighted both the complexity of cargo interactions in the context of multiple isoforms of each COPII subunit and the combinatorial importance of cargo-coat interactions in human development and disease. Goldberg and colleagues [22] identified a novel interaction between human Sec24a and Sec24b isoforms and a folded epitope on the SNARE protein, Sec22, which is consistent with a previous mutagenesis study of yeast Sec24 [21]. Conversely, Sec24c and Sec24d have specificity for two other SNAREs, membrin and syntaxin5, at a novel site on the membrane-proximal surface [23]. The importance of such isoform-specific interactions was highlighted by a recent study that identified loss-of-function mutations in Sec24b in a forward genetic screen for neural tube closure defects in mice [24•]. Additional genetic interactions with mutations in Vangl2, a component of the Wnt signaling pathway, allowed the dissection of the molecular basis for the developmental defect: Vangl2 is packaged into COPII vesicles exclusively by Sec24b and in the absence of this interaction Vangl2 secretion is abrogated, thereby perturbing signaling events that lead to normal neural tube closure. A similarly detailed analysis of the basis for the hereditary developmental disease, CLSD, further cements our understanding of the combinatorial power of the expanded repertoire of COPII coat proteins in metazoans. A point mutation in SEC23A renders the mutant protein incapable of recruiting Sec13/31 to the ER membrane [25•]. However, this is only true of Sec23a complexed with the Sar1b isoform – complex assembly is normal with the Sar1a protein. How these impaired interactions translate to the disease state is not entirely clear, but likely stems from tissue specificity in expression of the different paralogs.
Despite these detailed descriptions of cargo-coat interactions, it remains to be determined whether there are additional levels of regulation in the cargo-selection process: is cargo capture by Sec24 a stochastic event that relies on simple diffusion and affinity to capitalize on chance encounters between cargo and coat, or is there a hierarchy of cargo recruitment such that the coat is “primed” by occupation of select cargo proteins (eg. SNAREs) to more avidly bind additional cargo? Certainly the available crystal structures are not suggestive of allosteric effects of cargo binding on Sec24, but a more precise measurement of affinities of Sec24 for distinct cargoes under different conditions may yet reveal cooperativity in cargo capture.
A corollary to the question of how the coat senses cargo proteins is how the process of ER export manages to accommodate cargoes of widely disparate sizes. In principle, the geometry of the COPII coat should be permissive for generating a variety of structures that would be capable of accommodating cargo of many different sizes. Single particle cryo-EM reconstruction of the spherical “cage” formed by purified Sec13/Sec31 revealed a basic architecture that in principle is capable of coming together in a variety of ways to create structures of increasing dimensions [10], although alternative configurations that correspond to tubular structures have not yet been directly observed. Certainly, the composition of the COPII coat can directly influence the size of the vesicle; in the presence of the yeast Sec24 paralog, Lst1, vesicles generated in vitro are significantly larger than those generated with Sec24 alone [26]. Whether this is a direct consequence of the structure of Lst1 or results from the enhanced incorporation of Lst1-specific cargoes remains to be determined, although Lst1-containing vesicles retained their size advantage even when the abundant oligomeric cargo, Pma1, was rendered monomeric [27].
Understanding how outsized cargo proteins are handled by the ER export machinery is a major challenge for the field. Oligomeric assemblies of pro-collagen form 300 nm filaments within the ER that are too large for the canonical 80–100 nm COPII vesicle, yet COPII components are clearly required for their transport. Morphometric analysis raised the controversial model that the function of COPII in procollagen export was at the organizational level: the outer coat component, Sec31, was localized adjacent to (but not co-incident with) tubular regions of the ER that seemed to be engaged in traffic of pro-collagen [28]. However, quantitative EM analysis following the fates of the smaller cargo, VSVG, and of pro-collagen after release of an ER retention block revealed a significant cumulative co-localization of pro-collagen with the inner coat component, Sec23 [28]. This suggests an intimate coupling between pro-collagen and the cargo adaptor component of the COPII coat, albeit with slower kinetics than observed for the smaller cargo protein, VSVG. Furthermore, Sec13 depletion from either human cells or zebrafish led to a specific defect in collagen transport, further solidifying a link between the COPII coat and efficient traffic of this unusually large cargo client [29•]. More recent results indicate there may be a physical link between pro-collagen and the COPII coat: the ER membrane protein TANGO1 co-immunoprecipitates with procollagen and interacts with Sec23/Sec24 in a yeast two-hybrid assay [30••]. Yet TANGO1 itself is not incorporated into COPII vesicles generated in vitro, thus does not behave as expected for a canonical cargo receptor. However, it is not clear whether this in vitro reaction actually recapitulates pro-collagen export, so TANGO1 may yet prove to be a standard cargo adaptor that simply links a lumenal cargo protein to the cytoplasmic coat. Conversely, TANGO1 may play a broader role in the organization of ER exit sites, perhaps by creating favorable regions of high membrane curvature through its distinct topology.
In contrast to signal-mediated export of proteins (either through direct interaction with Sec24 in the case of membrane proteins, or indirect recruitment through receptors in the case of soluble lumenal cargo), some secretory proteins seem to enter transport vesicles absent any positive selection. Known as “bulk flow”, this signal-independent export likely stems from stochastic sampling of the membrane and fluid phases of the ER during vesicle formation. Recent detailed measurement of the rate of bulk flow transport in mammalian cells revealed surprisingly efficient secretion: a rapidly folding domain of a viral capsid protein was detected in the extracellular medium within 16 minutes of synthesis, before any other cellular proteins were detectable [31•]. Precisely how many native secretory proteins rely on this non-selective process remains to be seen, however the abundant pancreatic enzymes, amylase and chymotrypsinogen, seem to employ bulk flow transport, in that they are not enriched in COPII vesicles over their prevailing concentration in the ER [32]. The reliance of such proteins on efficient production of COPII vesicles without being active players in their biogenesis raises questions as to how cells would regulate vesicle formation to ensure adequate levels of secretion. This issue is particularly pertinent in light of experiments demonstrating that cargo load in the ER can dramatically influence the abundance of ER exit sites [33–35••], presumably at least in part through the direct recruitment of COPII proteins. The unfolded protein response (UPR) is required for this increase in COPII-positive structures [35••], and it is tempting to speculate that the UPR, which has the capacity to upregulate COPII proteins and fine-tune lipid synthesis for expansion of the ER membrane [36,37], will turn out to play a central role in the cross-talk between cargo abundance in the ER and the ability of the ER to manage the efficient folding and export of its client load under a variety of conditions.
3. Higher order structural organization and the role of Sec16
The ability of cargo to influence the number of transitional ER exit sites raises important questions regarding the fundamental nature of these domains: what are the molecular mechanisms by which they are generated, maintained, remodeled and disassembled in response to changing cellular conditions? Unlike the simple self-assembly of individual COPII coat proteins on a planar membrane [38], sites of vesicle biogenesis in vivo are more complex, undergoing dynamic fusion and fission while segregating folded cargo and coat components from the bulk ER [39]. Sec16 is emerging as a key player in maintaining the integrity of ER exit sites. Originally isolated as a classic secretion mutant in Saccharomyces cerevisiae, an organizational role for Sec16 was first demonstrated by Glick and colleagues, who isolated a Pichia pastoris sec16 mutant in a screen for fragmented tER exit sites [40]. More recently, a number of groups have characterized Sec16 orthologs from metazoans and find a number of common features. Knockdown of Sec16 causes fragmentation of ER exit sites and blocks secretion, similar to the effect of mutating Sec16 in both yeast systems [41•–44•]. Sec16 localizes to ER exit sites independently of the Sec23/Sec24 and Sec13/Sec31 coat components and thus seems to act upstream in recruiting these components to sites of vesicle formation [43•,44•]. The relationship between Sec16 and Sar1 is a little less clear; in mammalian cells, Sar1-GTP was required for recruitment of Sec16 to ER exit sites [41•], whereas in Drosophila S2 cells, the opposite is true – Sec16 recruits Sar1 [44•]. It is worth noting, however, that in the S2 dSar1 knockdown experiment and in cells expressing the GTP-locked form of Sar1, Sec16-positive punctae were larger and fewer in number, suggesting dynamic remodeling occurs at these sites during the GTP cycle [44•]. Interestingly, in both Drosophila S2 and human cells, Sec16 localizes to distinct cup-shaped membrane structures [43•,44•], although whether Sec16 generates these domains or simply has a preference for these curved membranes remains to be seen.
Biochemical characterization of Sec16 has been somewhat difficult and its precise molecular function remains unclear [45]. However, the large size and multi-domain structure of Sec16 coupled with the ability to interact with all of the COPII coat subunits suggests a scaffolding role in promoting or organizing coat assembly [43•,46,47]. Consistent with this model, Sec16 is required for the cargo-dependent increase in ER export [35••], functioning to increase the size of ER exit sites (as defined by the COPII coat proteins themselves) to cope with an additional cargo load. Whether a fraction of Sec16 is itself incorporated into COPII vesicles, or more stably resides at ER exit sites remains unclear. Although Sec16 was detected on COPII vesicles generated in vitro [48], recent high resolution microscopy suggests that at least a population of Sec16 is juxtaposed with, but segregated from, the majority of Sec23/24 and Sec13/31 [43•]. However, interpretation of each of these experiments is problematic: the in vitro incorporation of Sec16 into vesicles was very low (~1% of the donor population was found on vesicles), which may represent non-specific association, and conversely, the segregation of Sec16 from the COPII coat comes from a steady-state observation that may not accurately reflect significant co-localization in a highly dynamic system.
Although it is clear that Sec16 is a key player in the organization of ER exit sites, we still lack a detailed understanding of exactly what this large multi-domain protein is doing. Does Sec16 function in the context of cargo proteins themselves to regulate access to ER exit sites, or is it simply a placeholder for COPII recruitment? How is Sec16 itself regulated in response to changing cellular conditions? What acts upstream of Sec16 to recruit it to ER exit sites, or is it the foundation for these sites and defines these domains where it self-assembles? While several questions remain regarding the mechanisms of Sec16 action at tER sites, directed experiments to dissect Sec16 function should be informative.
Membrane lipids in ER export and tER site assembly
While substantial progress has been made in defining the core protein machinery that catalyzes ER export, the role of specific membrane lipids in vesicle budding from the ER is relatively unexplored. Minimal budding reactions reconstituted with purified COPII proteins and synthetic liposomes indicated a requirement for acidic phospholipids (e.g. phosphatidic acid or PI4P) to recruit Sec23/24 and produce budded vesicles [49]. Additional in vivo and in vitro studies suggest that lipid-modifying activities including PLD [50] and LPAT [51] are required for COPII vesicle formation. Moreover synthesis of PI4P [52] and fatty acids [53] are required for normal tER site structure and function. The stages in COPII-dependent ER export from coat recruitment to vesicle scission could depend on general membrane properties wherein a range of different lipid compositions could satisfy these biophysical requirements. Alternatively, COPII subunits, Sec16 and other uncharacterized components could contain specific lipid binding domains that are required for coat assembly and for higher order organization of tER sites. Finally, localized lipid modification(s) during vesicle formation could act in promoting membrane curvature or vesicle scission. Testing these potential models will require new lines of investigation but seem critical to fully elucidate ER export mechanisms.
Conclusions
Over the past several years major advances have been realized in identifying and structurally characterizing core components of the ER export machinery, establishing molecular models for cargo sorting and coat assembly that are controlled by the Sar1 GTPase, and connecting components of the COPII machinery to cellular structures that can be monitored in live cells. The challenges that lie ahead are to further integrate our knowledge on this core machinery into a cellular context where dynamic cargo and lipid concentrations are coupled to cellular status and together regulate ER export. Manipulation of minimally reconstituted in vitro assays has been extremely powerful in defining molecular mechanisms therefore including additional layers of control in these refined systems (e.g. addition of functional Sec16, distinct cargo molecules and lipids) should continue to be informative. However it will be equally important to combine these in vitro assays with high-resolution real-time imaging approaches to test regulatory models and to directly measure coat assembly and budding rates in live cells. Both approaches appear to be well underway and we look forward to future developments.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (GM085089 and GM078186 to EAM and GM052549 to CB) and the Cystic Fibrosis Foundation (EAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 2.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 3.Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- 4.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 5.Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 6•.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. This paper defines the structure of several elements of the COPII outer coat: Sec13 in complex with two fragments of Sec31. Intriguingly, Sec13 forms a 6-bladed βpropeller that is transformed into a 7-bladed structure with an extra blade contributed by Sec31. This region of Sec31 links an α-solenoid domain to an independent 7-bladed β-propeller that likely makes the critical protein-protein contacts required for assembly of the Sec13/31 tetramer into a cage. [DOI] [PubMed] [Google Scholar]
- 7.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 8••.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. This paper defines the structural basis for Sec31-mediated stimulation of the GTPase activity of Sar1•Sec23. The authors first dissected the different domains of Sec31 to identify the active fragment, which lies in a proline-rich region that was absent from the existing crystal structures. This fragment was then co-crystalized with Sec23 and Sar1 to reveal a relatively unstructured coil that lies across the top of the Sec23/Sar1 interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 10.Stagg SM, Gürkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 11.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 15.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 16•.Futai E, Hamamoto S, Orci L, Schekman R. GTP/GDP exchange by Sec12p enables COPII vesicle bud formation on synthetic liposomes. EMBO J. 2004;23:4146–4155. doi: 10.1038/sj.emboj.7600428. Using real-time assays to monitor coat assembly on synthetic liposomes, the authors find that the presence of the cytosolic domain of Sec12 can prolong coat assembly, providing an explanation of how vesicle production may occur in the presence of GTP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Sato K, Nakano A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol. 2005;12:167–174. doi: 10.1038/nsmb893. Again making use of real-time assays (this time fluorescence resonance energy transfer between cargo and coat), the authors demonstrate that both Sec12 and cargo proteins can prolong the association of coat components with synthetic liposomes even in the presence of GTP. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg J. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell. 2000;100:671–679. doi: 10.1016/s0092-8674(00)80703-5. [DOI] [PubMed] [Google Scholar]
- 19••.Tabata K, Sato K, Ide T, Nishizaka T, Nakano A, Noji H. Visualization of cargo concentration by COPII minimal machinery in a planar lipid membrane. EMBO J. 2009 doi: 10.1038/emboj.2009.269. This study uses single molecule fluorescence microscopy to monitor the behaviour of flourescent cargo proteins during vesicle formation. Addition of Sar1 and Sec23/24 was sufficient to induce dimerization of the SNARE Bet1 prior to addition of Sec13/31, which in turn caused accumulation into larger puncta and vesicle release. Of particular note is the observation that Sar1 GTP cycling was required for the clustering of Bet1, suggesting dynamic interplay between the coat and cargo during the early stages of vesicle production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 22.Mancias JD, Goldberg J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell. 2007;26:403–414. doi: 10.1016/j.molcel.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2009 doi: 10.1038/ncb2002. This tour de force of mouse genetics maps a developmental mutant to the Sec24b locus and defines the Wnt signaling component, Vangl2, as a key cargo that is perturbed in its trafficking. In vitro assays confirm the isoform specificity of Vangl2 capture into COPII vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. Another link between the COPII coat and a developmental disorder is dissected in this study of the molecular effect of a point mutation in Sec23a that causes cranio-facial abnormalities. The authors observe that this mutation renders Sec23a incapable of binding Sec13/31, dependent on the specific isoform of Sar1 used in the in vitro assays. The effect of this uncoupling of the inner and outer coat is the accumulation of cargo-containing tubules that fail to release from the ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J Cell Biol. 2000;151:973–984. doi: 10.1083/jcb.151.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MCS, Hamamoto S, Schekman R. Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J Biol Chem. 2002;277:22395–22401. doi: 10.1074/jbc.M200450200. [DOI] [PubMed] [Google Scholar]
- 28.Mironov AA, Beznoussenko GV, Trucco A, Lupetti P, Smith JD, Geerts WJC, Koster AJ, Burger KNJ, Martone ME, Deerinck TJ, et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell. 2003;5:583–594. doi: 10.1016/s1534-5807(03)00294-6. [DOI] [PubMed] [Google Scholar]
- 29•.Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ. Efficient coupling of Sec23–Sec24 to Sec13–Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci. 2008;121:3025–3034. doi: 10.1242/jcs.031070. The authors show that RNAi knockdown of Sec13 causes a concommittent decrease in Sec31 levels, altering the cellular distribution of ER exit sites marked by both cargo and Sec23/24. Secretion of the small soluble cargo protein, VSVG, is unperturbed under these conditions but collagen traffic is severely impaired. These findings suggest that very low levels of the outer COPII coat suffice to generate small vesicles but a greater quantity of protein is required to generate the larger carriers occupied by collagen. [DOI] [PubMed] [Google Scholar]
- 30••.Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. Having previously identified TANGO1 as a component necessary for protein secretion using a genome-wide RNAi screen, the authors characterize this putative cargo adaptor using elegant biochemistry. TANGO1 is an ER membrane protein with a distinct topology; it interacts with both collagen and the COPII coat leading the authors to propose a model whereby TANGO1 facilitates collagen capture into COPII vesicles without itself being consumed. [DOI] [PubMed] [Google Scholar]
- 31•.Thor F, Gautschi M, Geiger R, Helenius A. Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic. 2009;10:1819–1830. doi: 10.1111/j.1600-0854.2009.00989.x. This intriguing paper investigates the secretion of a heterologous protein to more quantitatively evaluate the potential contribution of non-specific bulk flow to general secretion. The key advance is in using a cytosolic viral protein that rapidly folds and would thus be unlikely to engage the ER folding machinery. The authors define a surprisingly rapid rate of secretion of this cargo. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Menárguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Linstedt AD. COPII-Golgi protein interactions regulate COPII coat assembly and Golgi size. J Cell Biol. 2006;174:53–63. doi: 10.1083/jcb.200604058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aridor M, Bannykh SI, Rowe T, Balch WE. Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- 35••.Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri H-P. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 2008;27:2043–2054. doi: 10.1038/emboj.2008.136. Using fluorescence microscopy to quantify the behavior of ER exit sites, combined with RNAi to perturb specific cellular components, the authors demonstrate that exposing cells to an increased abundance of cargo in has distinct effects depending on the length of exposure. Acute accumulation of cargo caused tER sites to fuse, becoming larger and fewer in number. Prolonged accumulation caused an increase in abundance of tER sites. Both effects required Sec16, providing a first view of how cells alter their ER organization in response to environmental changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Travers K, Patil C, Wodicka L, Lockhart D, Weissman J, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 37.Schuck S, Prinz W, Thorn K, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009 doi: 10.1083/jcb.200907074. jcb.200907074v200907071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 39.Bevis B, Hammond A, Reinke C, Glick B. De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol. 2002;4:750–756. doi: 10.1038/ncb852. [DOI] [PubMed] [Google Scholar]
- 40.Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 41•.Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–1687. doi: 10.1111/j.1600-0854.2006.00493.x. This study identified a human ortholog of Sec16 that localizes to ER exit sites independently of Sec23/24 and Sec13/31 but required Sar1•GTP to maintain this localization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Bhattacharyya D, Glick BS. Two mammalian Sec16 homologues have nonredundant functions in endoplasmic reticulum (ER) export and transitional ER organization. Mol Biol Cell. 2007;18:839–849. doi: 10.1091/mbc.E06-08-0707. The authors identify two isoforms of human Sec16, a long and a short form, that both localize to ER exit sites in an oligomeric form. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. Using high resolution microscopy, the authors show that human Sec16 is juxtaposed with the canonical COPII proteins rather than sharing an overlapping localization. This association with the membrane is more stable than Sec23/24 or Sec13/31, suggesting a scaffolding function for Sec16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. In characterizing Drosophila Sec16, the authors show that Sec16 localizes to cup-shaped membranes and acts upstream of the COPII coat proteins to organize ER exit sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Supek F, Madden DT, Hamamoto S, Orci L, Schekman R. Sec16p potentiates the action of COPII proteins to bud transport vesicles. J Cell Biol. 2002;158:1029–1038. doi: 10.1083/jcb.200207053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gimeno RE, Espenshade P, Kaiser CA. COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol Biol Cell. 1996;7:1815–1823. doi: 10.1091/mbc.7.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem. 1997;272:25413–25416. doi: 10.1074/jbc.272.41.25413. [DOI] [PubMed] [Google Scholar]
- 48.Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell. 1998;2:703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]
- 50.Pathre P, Shome K, Blumental-Perry A, Bielli A, Haney CJ, Alber S, Watkins SC, Romero G, Aridor M. Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. EMBO J. 2003;22:4059–4069. doi: 10.1093/emboj/cdg390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown WJ, Plutner H, Drecktrah D, Judson BL, Balch WE. The lysophospholipid acyltransferase antagonist CI-976 inhibits a late step in COPII vesicle budding. Traffic. 2008;9:786–797. doi: 10.1111/j.1600-0854.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Shindiapina P, Barlowe C. Requirements for Transitional Endoplasmic Reticulum (tER) Site Structure and Function in Saccharomyces Cerevisiae. Mol Biol Cell. 2010 doi: 10.1091/mbc.E09-07-0605. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]