Abstract
Motivated by the need for bioactive materials that can accelerate dermal wound healing, this work describes the response of keratinocytes to covalently immobilized epidermal growth factor (EGF) and how differences in the physical presentation of this growth factor impact cell function. Specifically, human keratinocytes were cultured with EGF delivered in soluble form, immobilized in a homogeneous pattern, or immobilized in a gradient pattern, followed by analysis of cellular signaling, proliferation, and migration. By changing the manner in which EGF was presented, keratinocyte behavior was dramatically altered. Keratinocytes responded to immobilized EGF patterns with high EGF receptor (EGFR) but low ERK 1/2 and Akt phosphorylation, accompanied by low proliferation, high migratory activity, and coordinated cell alignment. In contrast, keratinocytes treated with soluble EGF experienced lower EGFR but higher ERK 1/2 and Akt phosphorylation, and displayed a highly proliferative, rather than migratory, phenotype. Keratinocytes also responded to differences in immobilized EGF patterns, as migration was fastest upon immobilized gradients of EGF. Better understanding the interaction of cells with soluble vs. immobilized growth factors can help elucidate native healing events and achieve greater control over cell function, which may be useful in the development of wound repair treatments for many types of tissues.
Keywords: wound healing, epidermal growth factor, keratinocyte, intracellular signaling, cell migration
Introduction
Growth factors and other mitogens often provide the molecular cues that induce many cellular events, including differentiation, proliferation, and migration [1]. Unfortunately, growth factors can degrade within minutes under physiological conditions, and biomaterial systems designed to deliver soluble growth factors to sites of tissue repair are therefore often ineffective and costly [2, 3]. Thus, synthetic immobilization of biomolecules has become a common technique to prolong bioactivity and control biomolecule presentation and concentration [4-8]. Immobilization not only ameliorates degradation and spatial issues associated with soluble biomolecules, but may also mimic naturally-occurring biomolecule tethering, such as that which occurs during sequestration of growth factors by the extracellular matrix (ECM) [9-14]. For these reasons, immobilization of biomolecules such as growth factors has emerged as a promising method to reduce the cost and increase the efficacy of various bioactive materials or engineered tissues [15-24].
Epidermal growth factor (EGF) participates in the growth and repair of many types of tissues throughout the body. In dermal wound healing, EGF is a critical component that is released in abundance by platelets at the wound site, stimulating epidermal cell growth and migration [25, 26]. A deficiency in EGF is a characteristic common in chronic (non-healing) wounds, and EGF is also capable of reducing scarring by preventing excessive wound contraction [27]. However, despite the importance of EGF in dermal wound healing, the challenges associated with efficiently delivering bioactive growth factors to wound sites have hindered the development of clinical wound healing treatments that incorporate this molecule. Thus, in a previous publication, our group took a step toward addressing these issues of EGF bioavailability in dermal wound dressings by creating covalently immobilized gradients of EGF which induced accelerated and directed keratinocyte migration in vitro [28, 29]. These findings illustrated the potential for immobilized EGF to be used as an effective stimulus for wound re-epithelialization.
The growth factor-induced cell growth and migration events that are crucial in wound healing are ultimately regulated via complex intracellular signaling cascades [30]. In the case of EGF, this signaling is initiated by the recognition of EGF by EGF receptors (EGFR) located in the cell membrane [25]. The EGFR is then internalized, a cascade of phosphorylation events occurs, and the EGFR is eventually recycled back out to the cell surface [30, 31]. EGFR-mediated activation of several intracellular signaling kinases is responsible for the regulation of the pro-survival, proliferative, and migratory actions of keratinocytes during dermal wound healing [30]. Extracellular signal-regulated kinase (ERK) 1/2 and Akt are two important downstream effectors of the EGFR which participate in the regulation of keratinocyte proliferation, differentiation, apoptosis, and migration [30, 32, 33].
Culture of cells upon immobilized growth factors can significantly impact cell proliferation [16, 23], migration [34], morphology [24, 35], ECM production [36], and differentiation [37, 38], although these effects vary with the identity of the growth factor and the cell type used [16]. However, much remains to be learned regarding differences in cellular response to immobilized vs. soluble biomolecules. There exists a growing body of evidence that presentation of a growth factor in a tethered form can elicit distinctly different responses than growth factor delivered in solution. For instance, when human umbilical vein endothelial cells were treated with soluble vascular endothelial growth factor (VEGF), they experienced an increase in proliferation and different phosphorylation kinetics of the VEGF receptor when compared against cells treated with surface-bound VEGF [39]. Meanwhile, covalently tethered EGF has been shown to alter ERK phosphorylation levels and promote increased survival and osteogenic differentiation of mesenchymal stem cells (MSCs) relative to conditions that received soluble EGF [5, 37, 38]. Ito et al. documented that EGF presentation can affect cell proliferation and MAPK pathway signaling in several different cell types [16, 19, 40].
Despite the importance of EGF in dermal wound healing events, the role of immobilized EGF in this context has not yet been examined. Herein, we explore how the manner of growth factor presentation – specifically, EGF presented to keratinocytes in either immobilized patterns or soluble form – impacts not only cellular signaling and morphology, but also functional cellular outcomes that directly impact wound repair, such as keratinocyte proliferation and migration. Our findings reveal that the behavior of keratinocytes can be dramatically altered by changing the manner in which the EGF is presented, which may ultimately aid in the creation and optimization of biomaterial environments that facilitate wound healing.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Cell culture
Immortalized human keratinocytes (HaCaTs, courtesy of N. Fusenig, DKFZ, Heidelberg, Germany) were cultured and maintained in Dulbecco's Modified Eagle Medium, 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C, 5% CO2.
Total EGFR Quantification
In order to determine the EGF concentration necessary to saturate the EGF receptors present on HaCaTs, a quantitative ELISA for total EGFR concentration (EGFR STAR ELISA kit, Millipore, Inc., Danvers, MA) was performed. Briefly, cells were seeded onto unmodified tissue culture polystyrene (TCPS) at a density of 62,500 cells/cm2 and then refed after 4 hours with serum-free medium, complete growth medium, or serum-free medium containing 100 ng/ml soluble EGF. After an additional 24 hours of culture, the cells were lysed in radioimmunoprecipitation assay buffer (RIPA; 1 % sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1 mM iodoacetamide, 140 mM NaCl, 10 mM TrisHCl, (pH 8.0)) as instructed by the manufacturer's protocol. A fraction of the lysate was applied to the kit-provided microplate wells containing a monoclonal mouse anti-EGFR capture antibody; a range of known concentrations of EGFR was applied to separate capture wells to create a standard curve. The sandwich-style ELISA assay was executed as prescribed by the manufacturer, and the amount of total EGFR in the samples was calculated from the sample absorbance readings (450 nm, Synergy HT plate reader, Bio-Tek Instruments, Inc., Winooski, VT) in combination with the EGFR standard curve. The number of cells in each condition was quantified by performing a PicoGreen DNA assay (Invitrogen, Carlsbad, CA) on the aforementioned cell lysate.
Growth Factor Modification and Homogeneous Immobilization
Recombinant human EGF (Peprotech, Inc., Rocky Hill, NJ) was rendered photoactive via conjugation to Sulfo-SANPAH (sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino]hexanoate; Pierce Biotechnology, Inc., Rockford, IL), a heterobifunctional crosslinker containing a photosensitive phenyl azide group on one end and an amine-reactive N-hydroxysuccinimide on the other. Photoreactive EGF was synthesized via reaction of primary amine groups of the growth factor with the N-hydroxysuccinimide functionality of Sulfo-SANPAH (SS). The coupling reaction of EGF with SS was performed in HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffered saline, pH 8.4, for 8 hours at room temperature with gentle shaking and a 50-fold molar excess of SS in order to ensure that a maximal amount of growth factor was rendered photoactive. All synthesis and purification steps were performed in the dark to preserve the photoactive moiety on SS.
To prepare homogeneously immobilized EGF conditions, 30-110 μl (depending upon substrate area) of SS-epidermal growth factor (SS-EGF) solution (0.015 or 0.15 μg/μl) was pipetted into TCPS wells and allowed to dry in an oven at 40°C. All samples, including TCPS controls with no SS-EGF, were exposed to ultraviolet light at 365 nm wavelength and 90 mW/cm2 for 120 seconds (Novacure 2001, EXFO UV Curing, Mississauga, Ontario, Canada). Upon UV exposure, the phenyl azide group enables immobilization of the SS-EGF to the dish as described previously [28]. All plates were rinsed twice with deionized water (diH2O) and then filled with diH2O to rinse overnight on an orbital shaker (30 rpm). To prepare for cell seeding, the plates were UV-sterilized in a laminar flow hood for 1 hour.
Quantification of Total and Phosphorylated EGFR, ERK 1/2, and Akt
Keratinocytes were seeded in a 96-well TCPS plate at 62,500 cells/cm2 in culture medium containing 0.5% FBS to allow for cell attachment, with 6 wells per condition. These wells contained either homogeneously immobilized EGF, soluble EGF, or no EGF. The immobilized EGF condition was created by homogeneously immobilizing EGF onto the well surface at two concentrations (1 or 10 ng/cm2) prior to cell seeding, as described above [28]. Treatment with soluble EGF was performed by adding EGF in solution (10 or 100 ng/ml) to the culture medium upon cell seeding. The negative control consisted of keratinocytes seeded into unmodified wells with no EGF treatment. Quantitative ELISAs for total and phosphorylated EGF receptor (EGFR; Dual Detect CELISA EGFR Assay Kit, Millipore), ERK1/2 (Dual Detect CELISA ERK Assay Kit, Millipore), and Akt (Dual Detect CELISA Akt Assay Kit, Millipore) were used according to manufacturer's instructions to assay cultured cells at 30 minutes, 1 hour, and 24 hours following exposure to either soluble or immobilized EGF.
Briefly, following fixation and permeabilization, samples were incubated overnight at 4°C with primary antibodies against total EGFR (monoclonal mouse anti-EGFR, clone LA22) and phosphorylated EGFR (monoclonal rabbit anti-phospho-EGFR (Tyr1173)) diluted in blocking buffer. Samples were then washed and incubated for 1 hour with the corresponding secondary antibodies for total EGFR and phospho-EGFR 1/2 – HRP-labeled goat anti-mouse IgG and AP-labeled goat anti-rabbit IgG, respectively – then developed in HRP and AP fluorogenic substrate solutions for 30 minutes. Fluorescence was read at 550/590 nm (ex/em) to detect the HRP signal, and 360/460 nm to detect the AP signal (Synergy HT plate reader, Bio-Tek Instruments, Inc.). The HRP readings represented the amount of total EGFR in the cells, whereas the AP readings represented the amount of phosphorylated EGFR (Tyr1173) in the cells.
Measurement of total and phosphorylated ERK1/2 and Akt followed a similar procedure. Namely, samples were incubated with primary antibodies against total ERK or Akt (monoclonal mouse anti-ERK, clone MK12; monoclonal mouse anti-Akt) and phosphorylated ERK1/2 or Akt (monoclonal rabbit anti-phospho-ERK1/2 (Thr202/Tyr204)/(Thr185/Tyr187), clone AW39R; monoclonal rabbit anti-phospho-Akt (Ser473)), followed by incubation with the secondary antibodies noted above (HRP-labeled goat anti-mouse IgG and AP-labeled goat anti-rabbit IgG) and subsequent detection of fluorescence. The HRP signal represented the amount of total ERK or Akt in the cells, whereas the AP signal represented the amount of phosphorylated ERK 1/2 or Akt in the cells. Data for these assays are presented as the fluorescence of the AP (phosphorylated) signal divided by the fluorescence of the HRP (total) signal. Because HRP and AP fluorescence are measured separately in arbitrary fluorescence intensity units, these ratios may be greater than one. ERK and Akt phosphorylation were also measured at 30 minutes post-seeding in the presence of an EGFR tyrosine kinase inhibitor, PD168393 (1 μM, EMD Chemicals, Gibbstown, NJ).
Keratinocyte Proliferation
Keratinocytes were seeded into wells of a 24-well TCPS plate at a density of 62,500 cells/cm2 in medium containing 0.5% FBS. These wells contained either homogeneously immobilized EGF (10 ng/cm2), soluble EGF (100 ng/ml) or no EGF. The culture medium was replaced with serum-free medium 24 hours following seeding, with replenishment of soluble EGF for the soluble treatment condition. After 3 days of culture, keratinocytes were fixed in 10% formalin for 5 min and rinsed twice with phosphate buffered saline (PBS). The cells were then permeabilized in 0.1% Triton-X-100 for 5 min followed by two rinses with PBS. Cells were blocked with 5% bovine serum albumin (BSA) for 1 hour, followed by incubation with rabbit anti-human Ki67 (Abcam, Cambridge, MA; 100 μg/ml in 3% BSA in PBS) for 1 hour. After four rinses with PBS, samples were incubated for 40 minutes in the goat anti-rabbit Alexa Fluor 488 secondary antibody (Invitrogen; 5 μg/ml 3% BSA in PBS), followed by four rinses with PBS. DAPI (4′,6-diamidino-2-phenylindole, 1 μg/ml) was then added to the samples as a nuclear counterstain and incubated for 5 minutes, followed by two rinses in PBS. Images of three randomly-selected fields of view (100× magnification) were captured in each well of each condition (n = 9). Positive staining for proliferation was indicated by the appearance of green fluorescence, and total cell count was obtained from the number of cells stained with DAPI in each photomicrograph. The proliferation index was then calculated as the number of proliferating cells as a percentage of the total cell count in each field of view.
Creation of Growth Factor Gradients
To create 2-D surfaces patterned with gradients of growth factor, standard photo-immobilization techniques were used in combination with a gradient-patterned photomask film. Several 1 cm diameter radial gradient images were created in Adobe Illustrator 10, and were then used to print photomask transparency films (Imagesetter, Madison, WI). The slope of the gradient was controlled via alterations in photomask pattern design, and the gradient profile was designed to present a concentration range of approximately 0.2-10 ng/cm2 immobilized EGF, with the highest EGF density occurring at the center of the circular area.
Silicone isolators (1 cm diameter circles, Grace Bio-Labs, Inc., Bend, OR) were placed onto TCPS dishes, and 110 μl of SS-EGF solution (0.15 μg/μl) was pipetted into one isolator on each dish and allowed to dry in an oven at 40°C. The dried SS-EGF was then covered with film photomasks patterned with the rg1 radial gradient pattern (Table 1) and immobilized using the photo-patterning conditions described earlier for homogeneous EGF immobilization.
Table 1.
Characteristics of the gradient pattern used in photo-immobilization of EGF gradients
| Pattern | Photomask | Description | Equation | R2 Value |
|---|---|---|---|---|
| rg1 |  |
Quadratic | y = 0.104x2 - 1.7443x + 6.5076 | 0.9976 |
Keratinocyte Migration in Immobilized vs. Soluble EGF Environments
A modified fence method [41] was used to examine radial migration of keratinocytes. To construct this system, 1 cm diameter silicone elastomer cylinders were placed in the center of each well of a 6 well tissue culture polystyrene plate (total well diameter = 3.5 mm); these removable cylinders were used to temporarily cover the area containing the radial gradient pattern of immobilized EGF, the homogeneously immobilized EGF, or an unpatterned area of the same dimensions for the soluble EGF and untreated control. Because keratinocytes prefer to migrate as a sheet, they were seeded as a confluent monolayer, at 5 × 105 cells/ml, in each well surrounding the cylinders in reduced-serum medium (0.5% FBS). After allowing 24 hours for cell attachment, the reduced-serum medium was replaced with serum-free medium and the cylinders were removed, thus allowing the cells to migrate in the uncovered circular area. For the soluble EGF condition, 100 ng/ml EGF was added daily to the cultures.
Cells were fixed in 10% formalin for five minutes, rinsed with PBS four times, stained with hematoxylin, and photomicrographed at days 0, 3, and 7. Using ImageJ software (NIH; http://rsb.info.nih.gov/ij/download.html), the marked outline of the 1 cm diameter circle and the leading edge of keratinocytes on days 0, 3, and 7 were carefully traced. The cell-free area was subtracted from the total area of the circular migration field to yield total cell-populated area, which was then divided by total area to yield percent closure of the circular “wound” area at each time-point.
Keratinocyte Alignment
After 3 days of migration on immobilized radial gradient patterns of EGF, homogeneously immobilized EGF, soluble EGF, or no EGF, keratinocytes were fixed in 10% formalin for 5 minutes and rinsed twice with PBS. The cells were then permeabilized in 0.1% Triton-X-100 for 5 minutes followed by two rinses with PBS. Phalloidin (5 units/ml), to visualize cytoskeletal f-actin, and DAPI (1 μg/ml), a nuclear counterstain, were both added to the samples and incubated at 37° C for 20 minutes in the dark, followed by four rinses with PBS. Photomicrographs (100×) were taken of the cells that were located one field of view behind the leading edge of migration in three random locations on each of three wells for each condition (Olympus IX71 microscope with 3i spherical aberration correction (Intelligent Imaging Innovations), Hamamatsu ORCA-ER monochromatic camera, and SlideBook 4.2 image acquisition software). Photomicrographs were taken such that the center of the well was always at a 90 degree orientation.
A minimum of 100 cells in each photomicrograph (n = 9) were measured, using DAPI images and ImageJ software. Individual DAPI-stained nuclei were manually circled, and then, using ImageJ software, the nuclei were fit to an ellipse, and the orientation angle measured. Orientation angles were grouped into bins of +/- 15 degrees at 0, 30, 60, 90, 120, and 150 degrees relative to the center of the well. An orientation angle of 90 degrees indicates that the long axis of the cell is aligned with the radii of the circular area the cells migrated into, and thus oriented to be “facing” toward the center of the well.
Statistics
All experiments were performed a minimum of three separate times, with n ≥ 3. Data were compared using ANOVA with Tukey's HSD post-hoc test. P values less than or equal to 0.05 were considered statistically significant. Data are presented as mean ± standard deviation.
Results
Quantification of Total EGF Receptors per Cell
The total number of EGFR molecules per cell was calculated to determine the amount of EGF needed to theoretically saturate these receptors in the immobilized and soluble EGF treatment conditions. HaCaT keratinocytes cultured in serum-free medium displayed an average of 8.6 ± 1.4 × 105 EGFR molecules per cell, which is within the range previously reported for this cell type [42]. The use of serum-containing medium or the addition of soluble EGF lowered this value, but not significantly. Thus, this highest value was used to determine that a treatment of 10 ng/ml soluble EGF or 1 ng/cm2 immobilized EGF would theoretically occupy approximately 60-70% of the EGFR molecules present in the keratinocyte cultures, while treatment with 100 ng/ml soluble EGF or 10 ng/cm2 immobilized EGF would theoretically exceed the amount needed to saturate the receptors by 6-7 fold.
Immobilized vs. Soluble EGF: EGFR Phosphorylation
Total and phosphorylated EGF receptor (EGFR) were quantified following exposure to either soluble or homogeneously-immobilized EGF to investigate whether the manner in which EGF is presented can impact the cellular recognition and subsequent signaling initiated by this growth factor. At 30 minutes post-seeding (Figure 1a), culture of keratinocytes on homogeneously immobilized EGF (1 or 10 ng/cm2) stimulated up to a 3-fold increase in EGFR phosphorylation relative to soluble EGF (p<0.0001) and up to a 6-fold increase relative to the untreated control (p<0.02). EGFR phosphorylation also demonstrated a significant dose-dependent response to the immobilized EGF conditions, as the higher concentration of immobilized EGF induced higher phospho-EGFR levels than the lower concentration (p<0.005). At 1 hour post-seeding (Figure 1b), both concentrations of immobilized EGF continued to elicit significantly greater EGFR phosphorylation than the untreated control (p<0.02) and both soluble EGF treatments (p<0.0001). At this time point, the phosphorylation of EGFR upon keratinocyte treatment with soluble EGF was not significantly different from that found in the untreated control, while EGFR phosphorylation in keratinocytes cultured on immobilized EGF continued to demonstrate a dose-dependent response (p<0.005). By 24 hours post-seeding (Figure 1c), levels of EGFR phosphorylation on homogeneously immobilized EGF conditions were lower in comparison to previous time points, although they still retained their dose-dependent trends (p<0.005). EGFR phosphorylation in keratinocytes cultured with no EGF or treated with soluble EGF remained relatively unchanged from the 1-hour time point.
Figure 1.
Phosphorylation of EGFR (epidermal growth factor receptor) in keratinocytes cultured with homogeneously immobilized EGF (1 or 10 ng/cm2), soluble EGF (10 or 100 ng/ml), or no EGF at (a) 30 minutes, (b) 1 hour, and (c) 24 hours post-seeding. Data are expressed as the ratio of phosphorylated EGFR (Tyr1173) fluorescence to total EGFR fluorescence. * p<0.02 compared to no EGF (‘None’) control, # p<0.0001 compared to both soluble conditions, ˆ p<0.005 compared to lower concentration of same condition; n=6 per condition.
Immobilized vs. Soluble EGF: ERK1/2 and Akt Phosphorylation
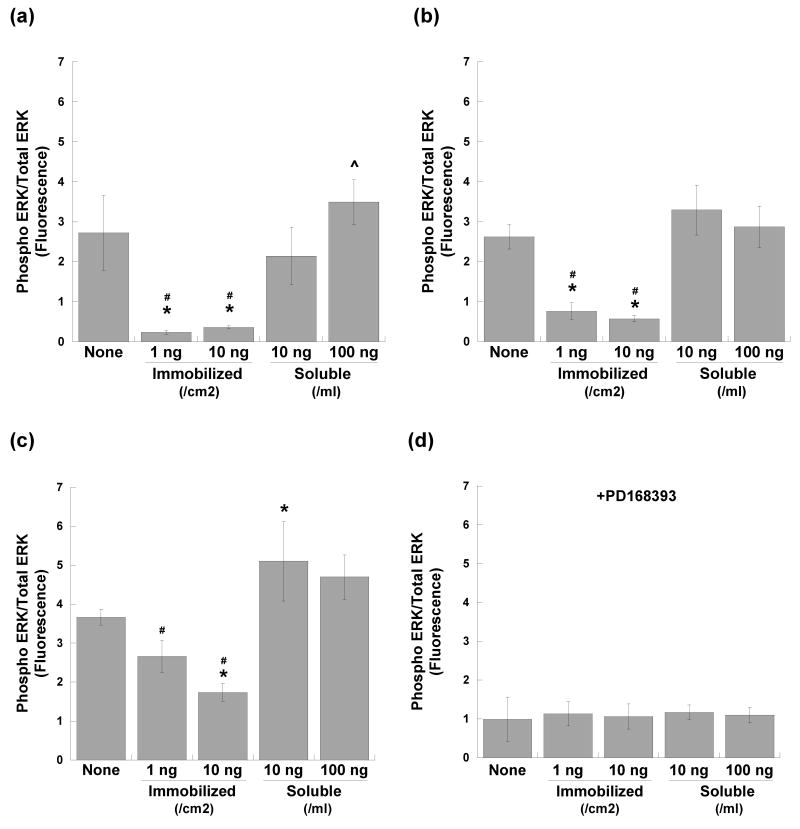
Cell signaling downstream of EGFR was examined via quantification of ERK1/2 and Akt phosphorylation. At 30 minutes post-seeding (Figure 2a), phosphorylation of ERK1/2 was significantly decreased in keratinocytes cultured on homogeneously immobilized EGF in comparison to those treated with soluble EGF (p<0.005). In keratinocytes treated with soluble EGF, ERK1/2 phosphorylation was related to EGF concentration in a dose-dependent manner (p<0.03 for low vs. high soluble EGF). Similar trends continued at 1 hour (Figure 2b), with both concentrations of immobilized EGF eliciting lower levels of ERK1/2 phosphorylation than the untreated control (p<0.01) and both soluble EGF conditions (p<0.005). By 24 hours (Figure 2c), ERK1/2 phosphorylation had increased in all conditions except the untreated control, although ERK1/2 phosphorylation in keratinocytes cultured on immobilized EGF still remained significantly lower than that found in keratinocytes treated with soluble EGF (p<0.005). Inhibition of EGFR tyrosine kinase activity through addition of PD168393 resulted in a significant decrease in ERK 1/2 phosphorylation across all conditions at 30 minutes (Figure 2d), verifying that the observed trends in ERK signaling could be attributed to the EGF delivered to the cultures.
Figure 2.
Phosphorylation of ERK1/2 in keratinocytes cultured with homogeneously immobilized EGF (1 or 10 ng/cm2), soluble EGF (10 or 100 ng/ml), or no EGF at (a) 30 minutes, (b) 1 hour, and (c) 24 hours post-seeding. Data are expressed as the ratio of phosphorylated ERK1/2 (Thr202/Tyr204,Thr185/Tyr187) fluorescence to total ERK1/2 fluorescence. Addition of an EGFR tyrosine kinase inhibitor (PD168393) at 30 minutes post-seeding resulted in reduced ERK 1/2 phosphorylation (d). * p<0.01 compared to no EGF (‘None’) control, # p<0.005 compared to corresponding soluble condition, ˆ p<0.03 compared to lower concentration of same condition; n=6 per condition.
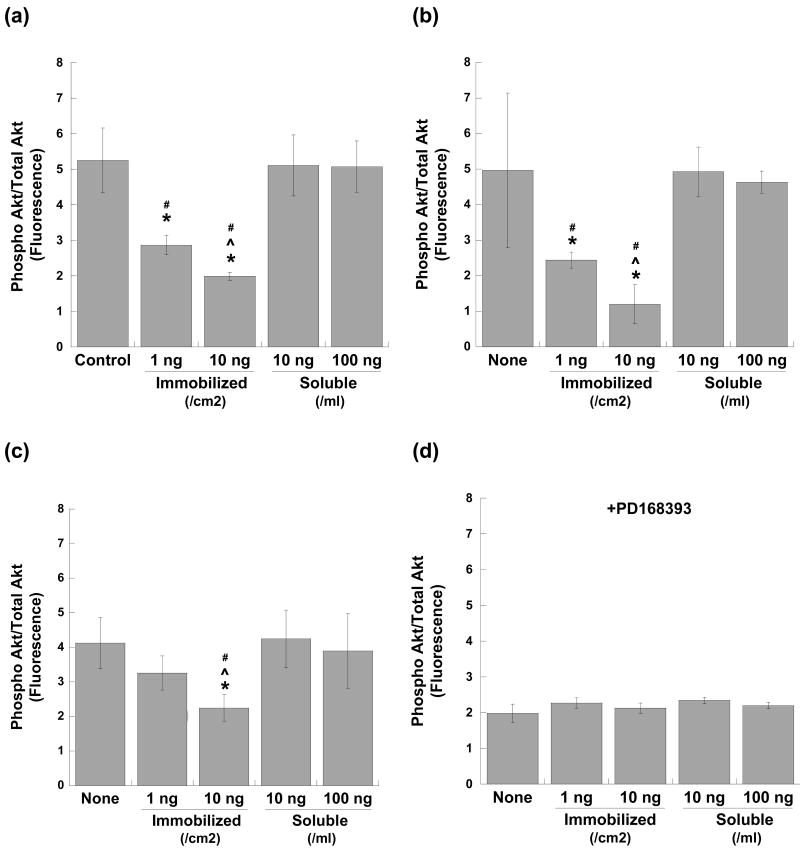
Phosphorylation of Akt was also found to be decreased upon culture of keratinocytes on homogeneously immobilized EGF, as demonstrated in Figure 3. At 30 minutes and 1 hour post-seeding (Figures 3a and 3b), Akt phosphorylation was significantly decreased on both concentrations of immobilized EGF relative to the untreated control and the soluble EGF treatments (p<0.01). The decrease in Akt phosphorylation occurred in a dose-dependent manner, with lower Akt phosphorylation accompanying the higher concentration of immobilized EGF. At 24 hours, only the 10 ng/cm2 concentration of immobilized EGF remained at a decreased level of Akt phosphorylation relative to the untreated control and soluble EGF conditions (Figure 3c). Throughout the time course, Akt phosphorylation in keratinocytes treated with soluble EGF remained at a similar level as that seen in the untreated control, suggesting that this time course may have missed the peak in Akt phosphorylation stimulated by soluble EGF, as discussed later in this paper. Inhibition of EGFR tyrosine kinase activity through addition of PD168393 resulted in a significant decrease in Akt phosphorylation across all conditions at 30 minutes (Figure 3d).
Figure 3.
Phosphorylation of Akt in keratinocytes cultured with homogeneously immobilized EGF (1 or 10 ng/cm2), soluble EGF (10 or 100 ng/ml), or no EGF at (a) 30 minutes, (b) 1 hour, and (c) 24 hours post-seeding. Data are expressed as the ratio of phosphorylated Akt (Ser473) fluorescence to total Akt fluorescence. Addition of an EGFR tyrosine kinase inhibitor (PD168393) at 30 minutes post-seeding resulted in reduced Akt phosphorylation (d). * p<0.01 compared to no EGF (‘None’) control, # p<0.01 compared to corresponding soluble condition, ˆ p<0.05 compared to lower concentration of same condition; n=6 per condition.
Effect of Immobilized vs. Soluble EGF on Keratinocyte Proliferation
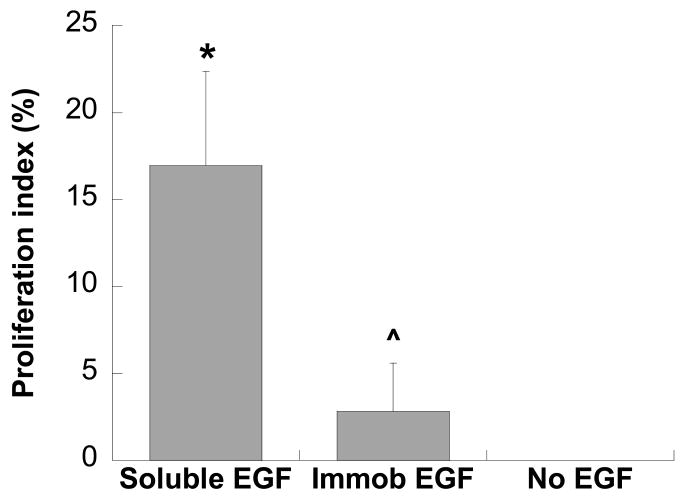
As shown in Figure 4, keratinocytes treated with soluble EGF demonstrated significantly increased proliferation in comparison to those cultured on homogeneously immobilized EGF (p<0.00002). Although the proliferation of keratinocytes cultured on homogeneously immobilized EGF was significantly reduced compared to the soluble condition, keratinocyte proliferation on these substrates was still significantly higher than that found on the untreated control (p<0.02). No proliferation was detected on the untreated controls, which is not unusual given the serum-free and EGF-free nature of this condition.
Figure 4.
Proliferation of keratinocytes when cultured upon immobilized EGF (10 ng/cm2) or in the presence of soluble EGF (10 ng/ml) for 3 days. Proliferation index is expressed as the percent of proliferating cells relative to the total cell count. * p<0.00002 compared to immobilized EGF and no EGF conditions; ˆ p<0.02 compared to no EGF condition. n=9 per condition.
Effect of Immobilized vs. Soluble EGF on Keratinocyte Migration
Keratinocyte migration was examined upon culture in environments that presented gradient-immobilized EGF, homogeneously immobilized EGF, soluble EGF, or no EGF. Stereoscope images of hematoxylin-stained keratinocytes at 0, 3, and 7 days following removal of the migration fence in each condition are presented in Figure 5. As seen in this figure, the trend in cell migration was: gradient-immobilized EGF > homogeneously immobilized EGF > soluble EGF treatment > no EGF, where only keratinocytes on immobilized gradient patterns of EGF migrated to fully close the circular “wound” area within 7 days of culture.
Figure 5.
Stereoscope photomicrographs of radial migration of keratinocytes upon treatment with gradient-immobilized EGF, homogeneously immobilized EGF, soluble EGF, or no EGF at time points of 0, 3, and 7 days following removal of the migration fence. The small black markings were used to indicate the initial boundary of the fence at day 0, and keratinocytes were stained (blue) with hematoxylin at each specified time point. 5× magnification, n=3 per condition.
A quantitative analysis of the trends evident in these images is presented in Figure 6, which shows the average cumulative migration distance (as percent of wound area closed) of keratinocytes cultured in the various EGF-modified environments over the 7-day migration period. Figure 6 demonstrates that the general manner of EGF presentation – as either an immobilized or soluble molecule – had the greatest impact on keratinocyte migration, with migration on both immobilized EGF conditions exceeding that achieved with either soluble EGF treatment or the control. The effect of changing the type of EGF immobilization – homogeneous vs. gradient – was still significant, but much more modest than the differences observed in comparing immobilized vs. soluble conditions. Overall, keratinocyte migration on immobilized gradient patterns of EGF was significantly greater than that found on all other conditions on day 3 (p<0.002), and significantly greater than the day 7 migration of keratinocytes that had received soluble EGF or no EGF (p<0.0001). The homogeneously immobilized EGF was also highly effective at stimulating keratinocyte migration, as the extent of wound closure in this condition on days 3 and 7 was approximately 2-fold greater than that obtained upon treatment with soluble EGF.
Figure 6.
Quantitative evaluation of radial keratinocyte migration upon treatment with gradient-immobilized EGF (“immob grad”), homogeneously immobilized EGF (“immob”), soluble EGF, or no EGF as the percentage of each circular “wound” that was closed on days 0, 3, and 7. *p<0.002 for immobilized gradient of EGF compared to all other conditions on day 3; #p<0.0001 for immobilized gradient of EGF compared to soluble EGF and no EGF conditions.
Effect of Immobilized vs. Soluble EGF on Keratinocyte Alignment
Qualitative observations of keratinocyte morphology during radial migration experiments indicated the presence of coordinated cell alignment in some experimental conditions. Thus, keratinocyte morphology and actin organization were further examined following staining of filamentous actin and cell nuclei in migrating keratinocyte cultures. Photomicrographs of keratinocytes located just behind the leading migration edge of cells cultured with gradient-immobilized EGF, homogeneously immobilized EGF, soluble EGF, or no EGF revealed coordinated alignment of keratinocytes cultured on both immobilized presentations of EGF (homogeneous and gradient), but not upon delivery of soluble EGF or no EGF (Figure 7).
Figure 7.
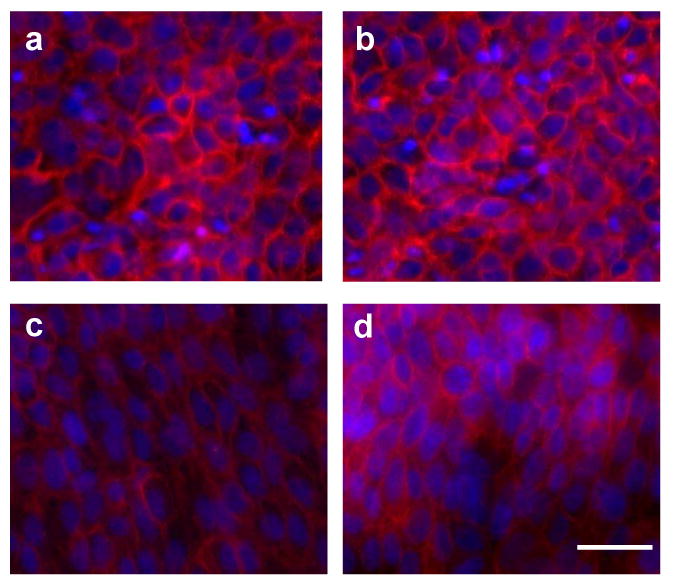
Representative photomicrographs of migrating keratinocytes on day 3 upon treatment with (a) no EGF, (b) soluble EGF, (c) homogeneously immobilized EGF, and (d) gradient patterns of immobilized EGF. In all pictures, the cells are migrating toward the top edge of the photo. (10× magnification, scale bar represents 60 μm)
Quantification of this cell alignment (Figure 8) demonstrates that keratinocytes on both immobilized EGF conditions (homogeneous and gradient patterns) exhibited a Gaussian distribution trend in orientation in which the majority of cells were at 90 ± 30 degrees, where a 90-degree angle of orientation indicates that the long axis of the cell was oriented along the radial “spokes” of the circle. On both the homogeneously immobilized EGF (Figure 8c) and the gradient of immobilized EGF (Figure 8d), significantly more keratinocytes were oriented at 90 degrees than any other orientation angle (p<0.005). No trends in cell alignment were evident in ether keratinocytes treated with soluble EGF (Figure 8b) or those cultured in the absence of EGF (Figure 8a). Cell density is not believed to be an influence in these results, as there was no significant difference in the number of cells per field of view across the conditions analyzed.
Figure 8.

Keratinocyte alignment toward center of circular migration area on day 3 of radial migration experiments upon exposure to (a) no EGF, (b) soluble EGF, (c) homogeneously immobilized EGF, and (d) gradient patterns of immobilized EGF. Each bar represents percent of cells oriented in a particular direction, with 90 degrees being oriented toward the center. *p<0.005 for 90 degree orientation compared to all other orientations. n=3 per condition.
Discussion
The present study is the first to examine differences in EGF presentation in the context of dermal wound healing. In this work, we demonstrate that immobilized and soluble EGF can play very different, yet complementary, roles in regulating keratinocyte function. Specifically, immobilized EGF induced initial hyperstimulation of the EGF receptor, minimal phosphorylation of ERK 1/2 and Akt, and was associated with low proliferation and high migratory activity in keratinocytes. In contrast, keratinocytes treated with soluble EGF experienced lower EGFR but higher ERK 1/2 and Akt phosphorylation, and displayed a highly proliferative, rather than migratory, phenotype. These very distinct effects of a single growth factor that was simply delivered to the cells via different mechanisms provide us with highly valuable information that will help in the design of biomaterial environments and possibly aid in better understanding the role of growth factors in wound healing.
As noted above, the treatment of keratinocytes with either immobilized or soluble EGF yielded significantly different trends in EGFR, ERK 1/2, and Akt phosphorylation. The observation that keratinocytes cultured on immobilized EGF presented with greater levels of phospho-EGFR at both 30 and 60 min post-seeding than cells cultured in soluble EGF suggests a model wherein receptor activation and/or trafficking could exhibit sensitivity to ligand valency and mobility. With the immobilized ligand, it is possible that the ligand is presented in a multivalent fashion that can facilitate receptor oligomerization on the surface and lead to a slower rate of receptor trafficking from the surface to intracellular sites when compared to receptor exposed to soluble ligand [43]. The immobilized ligand thus would be expected to lead to elevated receptor clustering and intermolecular phosphorylation but would also lead to slower receptor internalization and decreased degradation/dephosphorylation [44]. Similarly, the effective concentration of the immobilized EGF is likely to persist at a higher level over time given that the soluble ligand can be more readily internalized and degraded. This effect may provide an explanation for the sustained EGFR phosphorylation when plated on immobilized ligand compared to the more rapid disappearance of EGFR phosphorylation when using soluble EGF.
The increased extent and duration of EGFR phosphorylation detected after seeding keratinocytes on plates presenting immobilized EGF when compared to soluble ligand was inversely related to ERK1/2 and Akt phosphorylation, e.g., less ERK1/2 and Akt phosphorylation was observed using immobilized EGF vs. soluble EGF. In the case of ERK, this effect may result, at least in part, from decreased access of adapters (such as Shc), which are needed to initiate the Ras-ERK cascade, to the phosphorylated EGFR when the receptor is held in an immobilized oligomeric state on the surface following binding to the polyvalent/immobilized ligand. Because movement of the receptor within membrane microdomains may be required for binding upstream effectors of the Ras-ERK system [45], the immobilization of the receptor upon binding immobilized ligand may attenuate the capacity of the EGFR to promote ERK phosphorylation. Because ERK1/2 phosphorylation can also serve as a feedback mechanism for EGFR regulation, the differential ERK1/2 phosphorylation that occurred when cells were plated on immobilized vs. soluble EGF may allow for an additional level of receptor control based on the mode of ligand presentation. However, it should also be noted that the effects of immobilized EGF on ERK signaling may vary with cell type, as Fan et al. [5] and Ito et al. [19] demonstrated that culture of mesenchymal stem cells (MSCs) and PC12 cells, respectively, on immobilized EGF led to increased ERK phosphorylation, in contrast to the decreased ERK phosphorylation observed in keratinocytes exposed to immobilized EGF.
Phosphorylation of Akt was examined as a potential mechanism for the hypermigratory response observed when keratinocytes were cultured upon immobilized, but not soluble, EGF. Although the primary function of Akt is commonly considered to be cell survival, its activation has also been linked to increased motility of several types of cells, including keratinocytes [46, 47]. Located downstream of EGFR activation, Akt is the main effector of the PI3 kinase pathway, which regulates cell chemotaxis and motility [48]. Others have shown that inhibition of PI3 kinase activity in dermal keratinocytes significantly reduces EGF-mediated chemokinesis and chemotaxis [46]. However, in the current study, a positive correlation between Akt phosphorylation and cell migration was not observed. In fact, for the highly migratory cells cultured on immobilized EGF, Akt phosphorylation was significantly depressed relative to all other conditions. Thus, further studies that intentionally activate or inhibit these and other signaling pathways (i.e., PLCγ [33]) will be needed to directly correlate the observed trends in keratinocyte migration and proliferation with intracellular signaling events.
One significant limitation of the signaling studies presented herein is the absence of time points earlier than 30 minutes, as many kinase phosphorylation events occur within 2-10 minutes of exposure to a stimulus. Unfortunately, studying the cellular response to an immobilized stimulus represents a unique challenge in this respect, as the exposure to this stimulus commences the instant the cells are seeded upon the substrate. In contrast, in traditional signaling studies, the timing of stimulus exposure commences upon addition of that stimulus to a culture of cells which are already adherent to their substrate. Because the t=0 time point occurs the instant that a cell suspension is added to a substrate containing immobilized EGF, the analysis of early time points is complicated by the lack of adherent cells at these times; this is particularly problematic in the case of keratinocytes, whose adhesion occurs very slowly, and the fact that the quantitative phosphorylation ELISAs performed herein required adherent cells. The absence of time points prior to 30 minutes is believed to be a factor in the ERK 1/2 and Akt phosphorylation levels being similar across the untreated control and the soluble EGF treatments, as this time point may occur too late to capture the increase in ERK 1/2 and Akt phosphorylation that usually occurs rapidly upon exposure of cells to soluble EGF [37]. However, as discussed earlier, quantification of signaling even at later time points was still able to highlight significant differences in the cellular response to immobilized vs. soluble EGF.
The impact of differential growth factor presentation on cellular response was not only evident at the intracellular level, but also dramatically affected cellular functions related to wound healing, such as proliferation and migration. Specifically, we found that culture of keratinocytes on immobilized EGF significantly decreased proliferation relative to cells treated with soluble EGF. These findings are consistent with both Ito et al. [19], who found that soluble EGF elicited greater proliferation of PC12 cells than immobilized EGF, as well as Anderson et al., who recently reported increased endothelial cell proliferation upon exposure to soluble VEGF compared to covalently immobilized VEGF [39]. However, this response is known to be dependent upon cell type, as immobilized EGF has also proven to be more mitogenic than soluble EGF for Chinese hamster ovary cells and mouse fibroblast STO cells [16]. Although the immobilized EGF in the present study stimulated significantly less proliferation than soluble EGF, the level of proliferation on immobilized EGF remained higher than in the untreated control, a trend which is consistent with previous reports [24, 40].
Keratinocyte migration was also heavily influenced by the manner in which EGF was presented to the cells. Namely, immobilized EGF tended to support significantly greater cell migration than EGF delivered in solution. Although presentation of the immobilized EGF in a gradient pattern was able to further accelerate migration compared to homogeneously immobilized EGF, the most profound differences in migration were found in comparing either type of immobilized presentation (gradient or homogeneous) against soluble EGF delivery. Because the homogeneous patterns of EGF do not explicitly provide any directional guidance to the cells, these findings suggest that the mere presentation of EGF in an immobilized fashion, independent of the existence of a defined EGF pattern, can stimulate a migratory phenotype in keratinocytes.
Conclusions
Characterization of the mechanisms by which cells interact with immobilized growth factors is a necessary step toward engineering controlled environments that regulate cell function and attempting to translate our extensive body of knowledge on soluble growth factors to create immobilized growth factor systems. Our results demonstrate that keratinocytes interact quite differently with immobilized EGF than they do with soluble EGF, resulting in different functional outcomes for the cells. Revealing such differences between immobilized vs. soluble EGF recognition and signaling may be useful in both designing bioactive materials and understanding natural physiological phenomena. Ultimately, as both proliferation and migration are critical components of normal wound healing, these findings motivate future work to explore the development of wound healing environments that present growth factor cues in both soluble and immobilized form.
In biomaterial applications, many types of tissue repair involve the need to implant materials into a wound site and coerce surrounding cells to infiltrate and populate the material (e.g., polymeric nerve conduits, vascular grafts). In the realm of basic science, these materials not only provide a means to control a specific cell function (which has many implications in itself), but they also enable us to characterize how cells recognize and interact with tethered biomolecules, which is both a natural phenomenon that is highly understudied and a synthetic technique that is gaining much use in tissue engineering. The work described herein provides a launching point for the development of more complex wound healing systems that will include the incorporation and synergism of multiple immobilized and soluble growth factors, and may help to further our understanding of the cellular response to immobilized vs. soluble biomolecules.
Acknowledgments
The authors would like to thank the NIH (NIBIB) for funding support (1R21-EB005440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nimni M. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18:1201–1225. doi: 10.1016/s0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 2.Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115. doi: 10.1186/1472-6963-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol Assess. 2000;4:1–237. [PubMed] [Google Scholar]
- 4.Falconnet D, Csucs G, Grandin HM, Textor M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials. 2006;27:3044–3063. doi: 10.1016/j.biomaterials.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 6.Knerr R, Weiser B, Drotleff S, Steinem C, Gopferich A. Measuring cell adhesion on RGD-modified, self-assembled PEG monolayers using the quartz crystal microbalance technique. Macromol Biosci. 2006;6:827–838. doi: 10.1002/mabi.200600106. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi J, Takarada T, Yamaguchi K, Maeda M. Recent advances in cell micropatterning techniques for bioanalytical and biomedical sciences. Anal Sci. 2008;24:67–72. doi: 10.2116/analsci.24.67. [DOI] [PubMed] [Google Scholar]
- 8.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 9.Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339(Pt 3):481–488. [PMC free article] [PubMed] [Google Scholar]
- 11.Cushing MC, Liao JT, Anseth KS. Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol. 2005;24:428–437. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Sakiyama-Elbert S, Hubbell J. Functional biomaterials: Design of novel biomaterials. Ann Rev Mater Res. 2001;31:183–201. [Google Scholar]
- 13.Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ Res. 2004;94:550–558. doi: 10.1161/01.RES.0000117772.86853.34. [DOI] [PubMed] [Google Scholar]
- 14.Vlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai-Michaeli R, Sasse J, et al. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987;84:2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aizawa Y, Leipzig N, Zahir T, Shoichet M. The effect of immobilized platelet derived growth factor AA on neural stem/progenitor cell differentiation on cell-adhesive hydrogels. Biomaterials. 2008;29:4676–4683. doi: 10.1016/j.biomaterials.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Ito Y, Imanishi Y. Photo-immobilization of epidermal growth factor enhances its mitogenic effect by artificial juxtacrine signaling. Biochim Biophys Acta. 1997;1358:200–208. doi: 10.1016/s0167-4889(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y. Regulation of cell functions by micropattern-immobilized biosignal molecules. Nanotechnology. 1998;9:200. [Google Scholar]
- 18.Ito Y. Surface micropatterning to regulate cell function. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Chen G, Imanishi Y, Morooka T, Nishida E, Okabayashi Y, et al. Differential control of cellular gene expression by diffusible and non-diffusible EGF. J Biochem. 2001;129:733–737. doi: 10.1093/oxfordjournals.jbchem.a002913. [DOI] [PubMed] [Google Scholar]
- 20.Ng L, Hung HH, Sprunt A, Chubinskaya S, Ortiz C, Grodzinsky A. Nanomechanical properties of individual chondrocytes and their developing growth factor-stimulated pericellular matrix. J Biomech. 2007;40:1011–1023. doi: 10.1016/j.jbiomech.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Sharon JL, Puleo DA. Immobilization of glycoproteins, such as VEGF, on biodegradable substrates. Acta Biomater. 2008;4:1016–1023. doi: 10.1016/j.actbio.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Hu X, Bei J, Wang S. The immobilization of basic fibroblast growth factor on plasma-treated poly(lactide-co-glycolide) Biomaterials. 2008;29:2388–2399. doi: 10.1016/j.biomaterials.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Chen G, Imanishi Y. Micropatterned immobilization of epidermal growth factor to regulate cell function. Bioconjug Chem. 1998;9:277–282. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- 25.Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505. [PubMed] [Google Scholar]
- 26.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 27.Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, et al. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 28.Stefonek TJ, Masters KS. Immobilized gradients of epidermal growth factor promote accelerated and directed keratinocyte migration. Wound Repair Regen. 2007;15:847–855. doi: 10.1111/j.1524-475X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Stefonek-Puccinelli TJ, Masters KS. Co-immobilization of gradient-patterned growth factors for directed cell migration. Ann Biomed Eng. 2008;36:2121–2133. doi: 10.1007/s10439-008-9581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 31.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Giorgi V, Sestini S, Massi D, Ghersetich I, Lotti T. Keratinocyte growth factor receptors. Dermatol Clin. 2007;25:477–485. vii. doi: 10.1016/j.det.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J Biomed Mater Res A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 35.Kapur TA, Shoichet MS. Chemically-bound nerve growth factor for neural tissue engineering applications. J Biomater Sci Polym Ed. 2003;14:383–394. doi: 10.1163/156856203321478883. [DOI] [PubMed] [Google Scholar]
- 36.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 37.Platt MO, Roman AJ, Wells A, Lauffenburger DA, Griffith LG. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi-potent marrow stromal cells. J Cell Physiol. 2009;221:306–317. doi: 10.1002/jcp.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt MO, Wilder CL, Wells A, Griffith LG, Lauffenburger DA. Multipathway kinase signatures of multipotent stromal cells are predictive for osteogenic differentiation: tissue-specific stem cells. Stem Cells. 2009;27:2804–2814. doi: 10.1002/stem.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson SM, Chen TT, Iruela-Arispe ML, Segura T. The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials. 2009;30:4618–4628. doi: 10.1016/j.biomaterials.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito Y, Li JS, Takahashi T, Imanishi Y, Okabayashi Y, Kido Y, et al. Enhancement of the mitogenic effect by artificial juxtacrine stimulation using immobilized EGF. J Biochem. 1997;121:514–520. doi: 10.1093/oxfordjournals.jbchem.a021616. [DOI] [PubMed] [Google Scholar]
- 41.Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 42.Game SM, Huelsen A, Patel V, Donnelly M, Yeudall WA, Stone A, et al. Progressive abrogation of TGF-beta 1 and EGF growth control is associated with tumour progression in ras-transfected human keratinocytes. Int J Cancer. 1992;52:461–470. doi: 10.1002/ijc.2910520322. [DOI] [PubMed] [Google Scholar]
- 43.Hyatt DC, Ceresa BP. Cellular localization of the activated EGFR determines its effect on cell growth in MDA-MB-468 cells. Exp Cell Res. 2008;314:3415–3425. doi: 10.1016/j.yexcr.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Offterdinger M, Georget V, Girod A, Bastiaens PI. Imaging phosphorylation dynamics of the epidermal growth factor receptor. J Biol Chem. 2004;279:36972–36981. doi: 10.1074/jbc.M405830200. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Watson A, Morris VL, Chan BM. Coordinated integrin and growth factor regulation of primary keratinocyte migration mediated through extracellular signal regulated kinase and phosphoinositide 3-kinase. Arch Dermatol Res. 2009;301:307–317. doi: 10.1007/s00403-009-0945-7. [DOI] [PubMed] [Google Scholar]
- 47.Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1227–1237. doi: 10.1152/ajpgi.00253.2004. [DOI] [PubMed] [Google Scholar]
- 48.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]