Abstract
Deleterious neurochemical, structural, and behavioral alterations are a seemingly unavoidable aspect of brain aging. However, the basis for these alterations, as well as the basis for the tremendous variability in regards to the degree to which these aspects are altered in aging individuals, remains to be elucidated. An increasing number of individuals regularly consume a diet high in fat, with high fat diet consumption known to be sufficient to promote metabolic dysfunction, although the links between high fat diet consumption and aging are only now beginning to be elucidated. In this review we discuss the potential role for age-related metabolic disturbances serving as an important basis for deleterious perturbations in the aging brain. These data not only have important implications for understanding the basis of brain aging, but also may be important to the development of therapeutic interventions which promote successful brain aging.
Keywords: Aging, Brain, Diabetes, Insulin Resistance, Neurodegeneration, Metabolism, Obesity
What causes the brain to age?
Aging is a largely hypothetical construct that has a wide range of working definitions. For example, aging can be defined as the collection of changes that increase over time to promote morbidity and consequently render individuals progressively more likely to die (Medawar PB 1952). Alternatively, aging has been defined as a gradual deterioration of physiological function with age (Partridge and Mangel 1999). With regards to the aging of tissues, aging can be defined as an intrinsic and potentially irreversible set of processes which result in a loss of viability and increased vulnerability (Comfort A 1964). With specific regards to the brain, it is well established that as the result of increased chronological aging the brain undergoes numerous alterations at the molecular, cellular, and structural level. In this review we will discuss the potential roles of metabolic perturbations such as obesity and insulin resistance, serving as modulators for multiple aspects of brain aging. Additionally, the links between high fat diet consumption and metabolic dysfunction are discussed in the context of brain aging.
Molecular and cellular changes in the aging brain
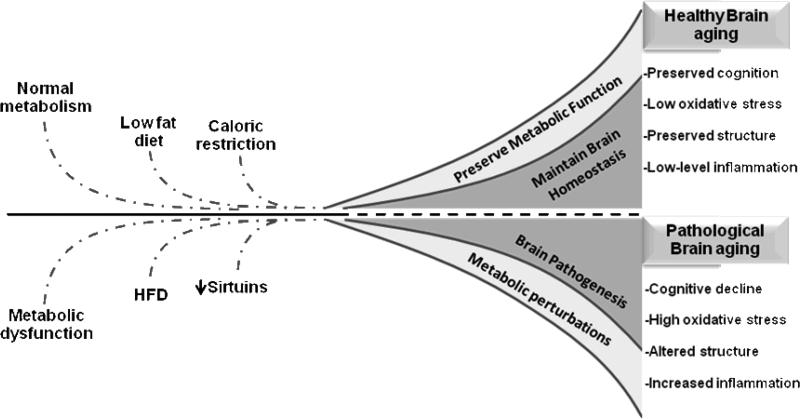
In regards to brain aging some of the most studied molecular and cellular changes can be grouped as being alterations in neurotransmitter signaling, increases in inflammatory signaling, and increases in oxidative stress (Fig. 1). Each of these is discussed below and in later sections discussed in the context of age and diet-induced metabolic dysfunction.
Figure 1. Interplay between metabolic dysfunction, high fat diets and brain aging.
Consumption of a high fat diet (HFD) and the presence of metabolic dysfunction (insulin resistance, adiposity, etc.) are associated with pathological brain aging. Pathological brain aging is linked to excessive cognitive decline, high oxidative stress, alterations in brain structure, and increased inflammatory signaling. In contrast, consumption of a low fat diet, caloric restriction, and maintenance of metabolic function results in successful brain aging. The successful brain aging is defined as preserved cognition, decreased oxidative stress, preserved structure, and reduced inflammatory signaling.
Changes in neurotransmitter signaling within the aging brain
In the aging brain there are well documented changes in multiple aspects of neurotransmitter signaling. For example, declines in the levels of neurotransmitters such as acetylcholine are well documented in the aging brain (Bartus et al. 1982;Gibson and Peterson 1981;Gibson et al. 1981;Ogawa 1989;Terry, Jr. and Buccafusco 2003;Vannucchi and Pepeu 1987;Wu et al. 1988) and age-related neurodegenerative disorders such as Alzheimer's disease (AD) (Bartus et al. 1982;Ogawa 1989;Terry, Jr. and Buccafusco 2003;Jia et al. 2004;Tohgi et al. 1994). Additionally, the levels and density of key neurotransmitters such as dopamine have been demonstrated to be decreased in the aging brain (Bannon and Whitty 1997;Carlsson 1987;Meng et al. 1999;Volkow et al. 1996;Wang et al. 1998). In contrast, studies indicate a role for excessive glutamate signaling occurring in the aging brain, especially in age-related disorders such as AD, culminating in the development of neuron death through excitotoxicity (Coyle et al. 1981;Coyle and Puttfarcken 1993;Hynd et al. 2004;Mattson et al. 1992;Mattson et al. 1995b;Mattson et al. 1995a;Sattler and Tymianski 2000). Together, these data point to gross imbalances in neurotransmitters occuring during brain aging and age-related diseases of the brain.
In addition to having an imbalance in the level of neurotransmitters within the brain, additional studies have documented that there is a deleterious change in the levels of key neurotransmitter receptors during aging. For example, it has been demonstrated that the glutamate receptor subunits (NR1, NR2A, and NR2B), and the AMPA receptor decline with age (Adams et al. 2008;Clark et al. 1992;Gazzaley et al. 1996;Morrison and Hof 1997;Sonntag et al. 2000;Wenk and Barnes 2000;Magnusson 2000;Eckles-Smith et al. 2000;Clayton and Browning 2001;Clayton et al. 2002;Shi et al. 2007;Newton et al. 2008). Similarly, a number of studies have also reported significant age-related decreases in the levels of dopamine receptors D1, D2, and D3 (Wang et al. 1998;Kaasinen et al. 2000;Iyo and Yamasaki 1993;Rinne et al. 1990;Wong et al. 1984). Decreasing levels of different serotonin receptors have also been shown to occur with age. Particularly, humans studied by positron emission tomography have shown that S2 serotonin receptors in the caudate nucleus, putamen, and frontal cerebral cortex decline with age (Wong et al. 1984). Regarding the levels of cholinergic receptors, however, the effects of aging are still unclear. Most studies seem to agree that there is a decrease in muscarinic binding in rodent aging brain, but there is controversy as to which regions show a significant reduction in receptor density (James and Kanungo 1976;Freund 1980;Morin and Wasterlain 1980;Lippa et al. 1981;Pedigo, Jr. et al. 1984). Taken together, these data point to disturbances in both neurotransmitters and neurotransmitter receptors occurring in the aging brain.
Increases in inflammatory signaling within the aging brain
Increased levels of inflammatory signaling are necessary for maintaining the function of all tissues, including the brain. However, in aging the brain appears to undergo at least three negative perturbations in inflammatory signaling and immune function. The first of these negative alterations is the presence of a chronic elevation in the levels of inflammatory signaling. Increases in multiple aspects of inflammatory signaling have been reported with aging including increased levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 (Spulber and Schultzberg 2010;Terao et al. 2002;Tha et al. 2000;Ye and Johnson 1999). Furthermore, studies have linked increases in each of these factors to the development of neuropathology and neuronal dysfunction in the aging brain (Griffin et al. 1995;Patterson 1995;Strauss et al. 1992). Additionally, the levels of cyclooxygenase and lipoxygenase, and their corresponding prostanoids and eicosanoids are known to be chronically increased during brain aging (Leone et al. 2007;Manev et al. 2000;Qu et al. 2000;Uz et al. 1998).
In addition to having a sustained increase in the basal levels of inflammatory signaling, aging is also known to impair the coordinated nature of the immune response (Desai et al. 2010;Giunta 2008). In this model, multiple aspects of an immune response (time required to mount a response, the extent to which the response is activated, and the rapidity to which the response is downregulated following the triggering event) are adversely affected by age. This promotes a more inefficient and ineffective inflammatory signaling within aging tissues such as the brain. Lastly, aging appears to alter the magnitude of peripheral immune cell infiltration into the brain following injury (Bolton and Perry 1998). Presumably, these cells contribute to the modulation of resident immune signaling within the aging brain, although the potential role of this observation to age-related brain inflammation remains to be fully elucidated.
Increases in oxidative stress within the aging brain
Oxidative stress occurs when the levels of oxidative damage reach a point that they promote the development of cellular and/or tissue dysfunction (Ding et al. 2006;Calabrese et al. 2010). An increase in oxidative damage to proteins, lipids, nucleic acids, and sugars in the aging and AD brain is well documented (Adelman et al. 1988;Berlett and Stadtman 1997;Cai et al. 1996;Finkel and Holbrook 2000;Gabbita et al. 1998;Good et al. 1996;Nunomura et al. 1999;Perry et al. 1998;Sayre et al. 1997;Smith et al. 1996;Sohal et al. 1995;Stadtman and Levine 2000;Stadtman and Oliver 1991). Furthermore, studies have demonstrated that each of these forms of oxidative damage is sufficient to result in decreased function of essential proteins and organelles, and thereby contributing to the development of brain aging. Studies on the levels and activity of antioxidants and antioxidant enzymes in the aging and AD brain have demonstrated a mixed bag of results (Calabrese et al. 2000;Calabrese et al. 2003;Calabrese et al. 2004;Calabrese et al. 2006;Droge and Schipper 2007;Kitani et al. 2001;Maier and Chan 2002;Massaad et al. 2009;Navarro and Boveris 2008;Schmitt-Schillig et al. 2005) and it remains unclear to what extent decreased levels of antioxidants may drive age-related increases in oxidative stress in the brain. Perhaps most importantly for the purposes of this review, the oxidative stress has been implicated in mediating the effects of neurotransmitter dysregulation and increased inflammatory signaling within the aging brain. Together, these data highlight a model for interplay between each of these cellular processes and the modulation of brain aging.
Structural changes in the aging brain
In the context of brain aging the best studied structural changes include synaptic changes, white matter changes, and vascular changes. Each of these changes is outlined below, and then later in the review is discussed in the context of aging and metabolic perturbations below.
Synaptic changes
Studies have documented an aging-related decrease in synaptic number occurring in specific domains within both experimental animal studies as well as studies of human brains (Bertoni-Freddari et al. 2006). However, the size of these synapses has been found to be increased in these experimental settings (Bertoni-Freddari et al. 1996;Bertoni-Freddari et al. 1992;Bertoni-Freddari et al. 1990). In both normal aging and AD brains, a negative correlation has been found between synapse number and synapse size. This appears to potentially be a compensatory response to minimize the effects of decreased synapse number, specifically to attempt to maintain the flux of information at the junctional zones, and to stabilize the synaptic contact per unit area (Scheff et al. 1990). However, ultimately the ability of the cortex to compensate seems to be exceeded and promote a decline in function (DeKosky and Scheff 1990).
The mechanisms responsible for synaptic changes during aging and AD are not clearly established. However, it has been demonstrated that oxidative stress may contribute to the pathophysiology of aging. However, the contribution of oxidative stress to the regulation of synaptic function is clearly complex. For example, it has been shown that superoxide is necessary for synaptic plasticity and memory in young animals (Klann et al. 1998;Klann 1998;Knapp and Klann 2002;Thiels and Klann 2002;Thiels et al. 2000), while chronically elevated levels of superoxide likely contribute to synaptic dysfunction during aging (Hu et al. 2006).
White matter changes
Through the use of neuroimaging technologies considerable advances have been made in our understanding of how white matter is altered in the aging brain. For example, regional differences in regards to age-related susceptibilities in white matter loss are known to occur (Guttmann et al. 1998;Hsu et al. 2008;Kennedy and Raz 2009;Lee et al. 2009), with prefrontal white matter observed to be most sensitive to the effects of aging (Gunning-Dixon et al. 2009). In addition to changes in white matter density, it is known that aging is associated with an increase in white matter lesions (Adami et al. 2008;Barber et al. 1999;Bugalho et al. 2007;Launer 2003;Roman 2004). These deleterious perturbations in connectivity throughout the brain are presumed to contribute to impairments in brain function. Despite our progress in being able to detect and measure white matter changes, there are two questions that need to be answered in this research area. Firstly, the role of white matter structural alterations as mediators of cognitive disturbances in the aging brain remain to be clarified beyond the point of correlation studies. Secondly, the mediators of white matter changes remain to be elucidated, although a role for metabolic disturbances as mediators of white matter pathology is clearly emerging.
Vascular changes
Aging, as well as many neurodegenerative disorders, are associated with structural and functional alterations of the cerebral vascularization (Aguero-Torres et al. 2006;Behrendt and Ganz 2002). Aging is characterized by a loss of elongation in endothelial cells, decreased number of endothelial mitochondria, and progressive impairment in endothelium-dependent vasodilation (Brandes et al. 2005;d'Alessio 2004;Finch 2005). Additionally, aging is associated with cerebral blood flow dysregulation and hypoperfusion, abnormal angiogenesis and remodeling, all of which may promote neuronal injury and neuron death (de la Torre 2004;Xiao et al. 2004). It is thought that an augmented production of pro-inflammatory cytokines and vasoactive molecules could be the responsible of the cognitive decline, potentially through a mechanism of suppression of cerebral blood flow and amplification of cellular stress (Zlokovic 2005).
Another feature of senescent endothelial cells is the increased generation of endothelial adhesion molecules (d'Alessio 2004), markers of endothelial inflammation. Increased levels of blood adhesion molecules have been reported in several brain disorders, namely atherosclerosis, ischemic stroke, cerebrovascular disease and AD (Fassbender et al. 1999;Frohman et al. 1991;Roher et al. 2004). In addition, soluble intracellular adhesion molecule-1 (sICAM-1) has been shown to be present in high levels in cerebrospinal fluid of individuals with inflammatory diseases of the central nervous system, and it has been suggested that cerebral endothelial cells are the primary source of sICAM-1 (Roher et al. 2004). This raises the possibility for molecules such as sICAM-1 contributing to vascular reactivity in the aging brain.
An increase in blood brain barrier (BBB) permeability has been documented to occur during aging in healthy individuals. Morphological changes of BBB have been also shown in rodent models of aging (Hosokawa and Ueno 1999). Since some of these models exhibit increased oxidative stress at an early age (Yasui et al. 2003) prior to the development of other pathological features, it has been suggested that alterations in BBB could be promoted via oxidative stress-mediated mechanisms. On the other hand, it has been demonstrated that hypertension, a common feature of older people, can induce BBB alterations (Nag and Kilty 1997;Baumbach and Heistad 1988). Taken together, these findings highlight the complexity in understanding the relationship between oxidative stress and morbidities such as hypertension, in regulating the biological aging of the brain.
Variability in rates and extent of brain aging
While it is clear that chronological aging promotes a generalized aging phenotype in the brain, it is equally clear that there is dramatic and significant variability in both the rate and severity of such alterations. For example, studies in humans document that there is considerable variability in regards to deterioration in cognitive performance with advancing age (Williams et al. 2005;Hultsch et al. 2002). Since variability at the performance level could be reflecting variations and alterations at a cellular and system level in the brain, understanding the basis for this observation is likely important to understanding the basis of brain aging, and developing interventions for promoting successful brain aging.
Our laboratory, as well as numerous other laboratories, believes there is an important role for age-related metabolic perturbations in mediating not only brain aging, but also in explaining the basis for the variability in the rates of brain aging. There is mounting evidence for metabolic dysfunction, in particular conditions such as obesity and insulin resistance, as significant contributors to the aging of modern westernized societies.
It is well known that aging is associated with the progressive development of generalized insulin resistance (DeFronzo 1981;Elahi et al. 2002), inhibition of lipolysis (Kumar et al. 1999;Miller and Allen 1973;Nyberg et al. 1976) and a loss in hepatic control of glucose production (Gupta et al. 2000) and triglyceride secretion (Bravo et al. 1996). Cumulatively, each of these manifestations is sufficient to promote an increase in a number of morbidity factors including cardiovascular and cerebrovascular diseases, gastrointestinal and respiratory alterations, cancer, and cognitive decline and dementia (Haslam and James 2005;Perlmuter et al. 1988;Qiu et al. 2007). Moreover, people from countries with traditionally higher mean lifespan are known to undergo an increase in a number of morbidities after changing their nutritional intake towards energy-rich diets. However, the availability of pharmacological treatments for these morbidities has allowed for these deleterious morbidities to not significantly decrease lifespan in the population (Picard and Guarante, 2005). Despite such progress, it remains unclear whether pharmacological management of metabolic disorders (obesity, insulin resistance, etc.) is able to also preserve brain function as these same individuals age.
Age-related neurologic manifestations include sensory deficits (taste, smell, vibration, vision, and hearing), disturbances in sensing pain, motor dysfunction (altered gait and posture), sleep disturbances, and impaired memory and cognition. For example, aging promotes the slowing of central processing and therefore may prolong the time it takes to complete tasks. Declines in motor strength, reflex responses, and reaction time with aging are related to sensori-motor changes. Changes associated with aging also include mild forgetfulness, a decrease in vocabulary, and learning difficulties (Braun and Anderson 2006). These changes typically occur by the seventh decade of life, but have a great deal of heterogeneity and variability in regards to the severity of dysfunction which is observed in elderly individuals (McClearn 1997). While the basis for the variability in brain aging is not known, one possible mediator is metabolic dysfunction, with increased levels of dietary fat intake serving as a potential mediator of metabolic dysfunction. For example, high-fat diets are known to be sufficient to promote insulin resistance and obesity in both humans and rodents (Buettner et al. 2007;Damjanovic and Barton 2008;Oakes et al. 1997;Tschop and Heiman 2001). What is less known is what effect high-fat diets have on aging, particularly brain aging. With the intersection of an increasing aging and obese society, that increasingly regularly consumes a high fat diet, it is clear that understanding the impact of diet-induced metabolic perturbations on the aging brain is of mounting clinical importance. The possible contribution of metabolic dysfunction as a modulator of brain aging raises the possibility that factors such as diet may be centrally linked to the regulation of brain aging. In particular, diet-induced metabolic dysfunction likely serves as a pro-aging stimulus for multiple aspects of brain biochemistry and physiology. Interestingly, several clinical and epidemiological studies, as well as experimental evidence in rodents, suggest that the presence of Western diet-induced metabolic dysfunction is sufficient to promote cognitive decline and even dementia, thus accelerating the normal process of aging.
Several abnormalities of carbohydrate and lipid metabolism have been commonly observed to increase in Western societies with age, namely insulin resistance, hyperglycemia, hyperinsulinemia, dyslipemia, triglyceridemia, and free fatty acid alterations (Astrup et al. 2008;Bullo et al. 2007;Matia et al. 2007;Pasinetti and Eberstein 2008;Steemburgo et al. 2007). However, it is not yet completely understood whether these alterations become manifest as a consequence of the molecular mechanisms operating in the aging process or as a result of the influence of many cultural/environmental factors such as diet and a decrease in physical activity (Paolisso et al. 1995).
In the context of brain aging metabolic dysfunction must be understood not only in terms of diabetes but also in terms of pre-diabetic states where individuals exhibit hyperinsulemia but remain euglycemic. A high concentration of circulating insulin (hyperinsulinemia) may represent a failure in insulin metabolism as well as a compensatory response to insulin resistance. In either case, hyperinsulinemia is correlated with insulin resistance in pre-diabetic individuals (Howard et al. 1998;Laakso 1993). At this point it is worth to mention that many studies have reported that the elderly are more insulin resistant that young individuals (Chen et al. 1985;Coon et al. 1992;DeFronzo 1979;Ferrannini et al. 1996;O'Shaughnessy et al. 1992;Rowe et al. 1983), although this point remains controversial (Ahren and Pacini 1998;Boden et al. 1993;Broughton et al. 1991;Dechenes et al. 1998;Pacini et al. 1988). Important to take into account is that several factors may influence insulin action and secretion, including the degree and type of obesity (Peiris et al. 1986), level of fitness (Kirwan et al. 1993;Miller et al. 1994), and circulation hormone profiles (Nielsen et al. 1997;Rizza et al. 1982a;Rizza et al. 1982b). Additionally, some studies have shown that central obesity (increased waist-to-hip ratio) is associated to insulin resistance and aging. Furthermore, the intra-abdominal fat depot in central-obesity has been postulated to contribute most to the development of insulin resistance, and therefore the insulin resistance observed in aging may be related more to changes in body composition during aging than to aging per se. Moreover, intra-abdominal fat has been postulated to be the most metabolically active of the adipose depots and correlates with insulin resistance and age in healthy nondiabetic individuals regardless of gender and after controlling for obesity (Cefalu 2001;Cefalu et al. 1995).
Adiposity
Adipose tissue has long been considered as a mere energy-store but is increasingly appreciated for its roles in participating in several physiological events such as inflammation, angiogenesis, hypertension, and vascular homeostasis (Fruhbeck 2008). Moreover, the adipose tissue synthesizes and secretes a large number of factors collectively termed “adipokines” (Fruhbeck 2008). More than 50 different adipokines have been identified and characterized for their role in influencing energy homeostasis and feeding behaviour (Ahima and Osei 2008). However, an increasing number of studies have identified that adipokines may play a role in mediating long-term potentiation, neuroprotection, and neuroinflammation.
A major role for adipose tissue (adipocytes) is to sequester and store circulating lipid, which protects other cells and tissues in the body form the cytotoxic effects of free fatty acids in the circulation. Failure of adipocytes to effectively remove free fatty acids from the circulation is believed to contribute to a variety of health complications including hypertension, atherosclerosis, and ultimately the development of metabolic syndrome (Fagot-Campagna et al. 1998;Moller and Kaufman 2005;Smith and Wilson 2006;Wang et al. 2008). Conversely, the deposition of adipose/lipid into muscle, liver, or bone marrow is known to promote not only metabolic syndrome but localized tissue dysfunction (Kuk et al. 2009). Therefore, it is clear that adipocytes perform essential functions for the overall well being of individuals, but it is equally clear that adipose in the incorrect places or in excessive quantity also negatively impacts the health of an individual.
Aging is known to impair the ability of adipocytes to sequester free fatty acids, and promote the release of pro-inflammatory signals from adipose tissue. Many studies have suggested that the decline in specific adipose depots is due to a decrease in adipocyte size and not a reduction in number. Additional studies have demonstrated that aging impairs the process of adipocyte differentiation resulting in an increase in more highly reactive preadipocytes. Preadipocytes do not have the capacity to sequester free fatty acids from the circulation to the same degree as mature adipocytes, and an age-related increase in preadipocytes may therefore result in promotion of adverse events in regards to the regulation of circulating lipids. Taken together, these studies strongly suggest that aging has direct and complex effects on adipose tissue including a redistribution of adipose depots as well as fundamental alterations in the cell biology of adipocytes, which together may promote metabolic dysfunction and inflammatory signaling throughout the body.
An increase in visceral adiposity is a common feature of aging, and epidemiological evidence supports its role as a prominent risk factor for insulin resistance, diabetes, and mortality from atherosclerotic cardiovascular disease (Ferrannini et al. 1997;Fujimoto et al. 1999;Lamarche 1998;Shimokata et al. 1989;Cefalu et al. 1995). Typically, elderly people have a lighter body weight as compared to younger individuals but increased waist circumference, consistent with elevated levels of abdominal adipose tissue. It has been observed that a complex relationship exists between aging, obesity and increased visceral fat which is not always sufficient to promote insulin resistance (Carr et al. 2004). It has been reported that caloric restriction extends life in a variety of species and decreases the development of most age-related diseases, and it is believed that these effects may be due to the ability of dietary restriction to reduce fat stores, especially visceral fat. In addition, the removal of visceral fat in rats has demonstrated to improve insulin action, delay the onset of age-dependent insulin resistance, glucose intolerance and diabetes, as well as increase in mean and maximum lifespan in rodent models of obesity and diabetes (Barzilai et al. 1999;Gabriely et al. 2002). Visceral fat removal has shown to improve metabolism even without any changes in body weight, fat mass, and lean body mass (Gabriely et al. 2002). It is thought that surgical removal of visceral fat depots disrupts a cross-talk between fat depots and multiple sites within the body (Wang et al. 1999).
The issue of adiposity itself in aging is a complex biological problem to study. For example, total fat mass is known to peak at middle age in both humans and rodents, which is followed by a precipitous decline with advancing age. However, the decline in fat mass with age does not coincide with a decrease in percent body fat because lean muscle mass is also decreased with age. Therefore, body mass index and other such measures do not do an accurate identification of the dramatic increase in ectopic fat and visceral fat deposition that occurs in aging. Additionally, it is poorly understood how such changes in adiposity contribute to the development of chronic disease in the elderly, or the development of metabolic syndrome which occurs in 40-70% of the elderly.
Impact of high-fat diets on aspects of aging
High-fat diets (HFD) have been used for many years to study the effects of increased adiposity, dyslipidemia and insulin resistance in rodents. It has been shown that the disturbances originated by high-fat feeding closely resemble the metabolic disturbances observed in humans (Woods et al. 2003). Several different types of HFDs are commonly utilized in the study of diet-induced obesity and insulin resistance. One problem with this literature is that all of the effects of these various HFDs (which vary in the amount and source of dietary fat) have all been summarized as being “HFD effects”. This has inevitably been source of great variability in the findings reported. Additionally, many studies have contrasted the effects of HFD to animals fed a standard chow diet, which dramatically affects the interpretation of findings as compared to using appropriate low fat control diets. This is because standard chow diets differ in the amount and source of dietary fat, as well as other dietary components, and therefore do not allow controlled studies on the specific effect elevated dietary fat has on individual parameters.
Despite the tremendous progress which has been made in our understanding of dietary fat and obesity/diabetes with using these different HFDs, it is important to point out that relatively little is currently available in regards to the study of HFDs and brain aging. For example, studies have not firmly established the ability of HFDs to promote or accelerate different aspects of brain aging. Additionally, studies have not examined in a comprehensive manner what specific aspects of perturbed metabolism (high fasting glucose, hyperinsulemia, elevated adiposity, cholesterol level, fatty acid levels, etc.) may be most involved in the promotion of brain aging.
In addition to the use of diets, many rodent strains have been utilized as models of obesity, and the use of these models has contributed strongly to the understanding of obesity (Kanoski et al. 2007;Wu et al. 2004). In particular, the ob/ob mouse, which is characterized by an increase in both number and size of adipocytes, and also by its perturbations in leptin treatment, has been used to test the effects of obesity on the brain (Itateyama et al. 2003;Terao et al. 2008). However, due to the polygenic character of this disorder and the numerous congenic perturbations observed in these rodent models (ob/ob mice, fa/fa Zucker rats) they have an obvious limitation in the study of brain aging. Thus, HFD-induced models of metabolic dysfunction should be considered the best option for studying the effects of metabolic dysfunction on human brain aging.
HFD can accelerate age-related adiposity
As pointed out above, aging leads to a redistribution of body fat, with a stabilization or enhancement of visceral fat and fat deposition into tissues such as liver, muscle, and bone. Similarly, HFD consumption has been also found to increase visceral fat mass (Park et al. 2001). This increased amount of visceral adipose tissue usually results in elevated concentrations and flux of circulating free fatty acids into the portal circulation (Bjorntorp 1990), since the abdominal fat has a high lipolytic activity and is very sensitive to adrenergic stimulation due to the peculiarities of the lipolytic cascade (Wahrenberg et al. 1989). This elevation of circulating free fatty acids is associated with increased hepatic glucose production (Ferrannini et al. 1983), reduced hepatic insulin clearance (Svedberg et al. 1990), decreased peripheral glucose uptake (Ferrannini et al. 1983), and increased very-low-density lipoprotein-triglyceride synthesis (Kissebah et al. 1976). Thus a higher rate of lipolysis is able to cause the disturbances in glucose and lipoprotein metabolism associated with diet-induced obesity. HFD consumption is therefore capable of promoting novel metabolic perturbations as well as accelerate individual aspects of age-related metabolic disturbances.
HFD can exacerbate peripheral inflammation
HFD-induced metabolic dysfunction has been shown to positively correlate with markers of acute-phase response such as C-reactive protein (CRP) (Koenig et al. 1999;Mendall et al. 1996) and fibrinogen (Juhan-Vague et al. 1993). Elevated levels of CRP have been associated with high fasting glucose, high serum lipids, high body mass index (Koenig et al. 1999;Mendall et al. 1996;Yudkin et al. 1999) . It has been shown that chronic, subclinical inflammation is part of insulin resistance syndrome and that dyslipidemia, abdominal obesity, low insulin sensitivity and hypertension parallel increasing levels of CRP (Festa et al. 2000). It is possible that HFD consumption can provoke cytokine secretion to the point it leads to insulin resistance, and may thereby accelerate age-related processes leading to insulin resistance (Festa et al. 2000).
Decreased insulin sensitivity may be responsible of the increased CRP expression, since the physiological effect of insulin on hepatic protein synthesis includes the stimulation of albumin synthesis and inhibition of fibrinogen synthesis (De Feo et al. 1993). Resistance to insulin would then be expected to lead to increased synthesis of acute-phase proteins, such as fibrinogen and CRP. In addition to these mechanisms, HFD via the acceleration of adipose reactivity may promote a generalized increase in inflammatory signaling as the result of adipose-derived adipokine signaling throughout the body. All these data indicate that subclinical inflammation would be expected to be present early in HFD-induced metabolic dysfunction (such as elevated adiposity and insulin resistance), and thereby link HFD-induced modulation of metabolic dysfunction to age-related changes in inflammatory signaling.
Molecular pathways for HFD effects on metabolic dysfunction
Mitochondrial dysfunction
Several studies have related diet-induced metabolic disturbances with mitochondrial alterations. For example, a decreased expression of genes involved in mitochondria biogenesis has been observed following HFD consumption (Handschin and Spiegelman 2006;Nisoli et al. 2007;Shen et al. 2004). Similarly, HFDs have been demonstrated to decrease the levels of mitochondrial complex 1 activity (Nisoli et al. 2007). Complex I, also known as NADH-ubiquinone oxidoreductase, is a membrane enzyme complex of the mitochondrial electron transport chain that catalyzes electron transfer from NADH to ubiquinone thus generating proton motive force. Decreased levels of this complex will have pronounced deleterious effects on mitochondrial energy-generating efficiency, which together with an altered biogenesis, would be expected to compromise energy availability in the aging brain. HFD effects on mitochondria may therefore serve as a potential mechanism by which metabolic dysfunction induces by HFD consumption modulates the rates of brain aging.
Oxidative stress
Oxidative stress is thought to be one of the earliest steps in HFD-induced pathogenesis (Matsuzawa-Nagata et al. 2008). Reactive oxygen species (ROS) are normal byproducts of biological processes but are known to be capable of reactivity with proteins, nucleic acids, and lipids. Mitochondrial-derived ROS are believed to be a major site of HFD-induced oxidative stress (Cecarini et al. 2007;Lau et al. 2007;Joseph et al. 2005), although studies have also identified a role for NADPH oxidase in HFD induced oxidative stress (Bruce-Keller et al. 2010). Increased levels of oxidative stress have been found in animals and humans following HFD-induced metabolic dysfunction (Matsuzawa-Nagata et al. 2008;Iqbal 2007;Yaffe 2007;Moreira et al. 2008;Furukawa et al. 2004;Urakawa et al. 2003;Diniz et al. 2006;Fridlyand and Philipson 2006;Mantena et al. 2008). Taken together, these data suggest that the HFD consumption may play an important role in regulating age-related increases in oxidative stress.
Sirtuin signaling
Sirtuins are a family of NAD+-dependent histone/protein deacetylases involved in a broad range of physiological functions, including control of gene expression, metabolism, and aging (Bordone and Guarente 2005). Many studies have associated sirtuins with caloric restriction-mediated health benefits including increased longevity (Blander and Guarente 2004;Gray and Ekstrom 2001;Smith et al. 2000;Tanner et al. 2000). Because of the ability of caloric restriction to promote beneficial effects on metabolism and brain aging, these studies identify a potentially important role for sirtuins in promoting the beneficial effects of caloric restriction on the brain. Seven distinct sirtuins (Sirt1-Sirt7) have been described in mammals, being Sirt1 expressed in several tissues and implicated in effects such as stress resistance, reduced apoptosis, and metabolic changes associated with calorie restriction (Blander and Guarente 2004).
Almost all Sirt1-deficient animals die during early postnatal development (Chen et al. 2005), with reduced triglycerides, insulin, and blood glucose observed in these same animals. Such findings further link Sirt1 to metabolic regulation. Sirt1 is also present in white adipose tissue, where has been shown to reduce adipogenesis and triglyceride accumulation in the lipid droplets of a cell model of white adipocytes. These observations have been related to decreased expression of genes involved in fatty acid metabolism, such as peroxisome proliferator-activated receptors gamma (PPARγ). Additionally, in vivo, fasting induces Sirt1 and promotes lipolysis by inhibiting PPARγ-mediated fatty acid trapping. The function of Sirt1 in the release of insulin by pancreatic β cells has also been studied, and it has been found that reduction of Sirt1 expression in β cell lines promotes a reduction in insulin secretion (Bordone et al. 2006).
Studies have shown that a modest overexpression of Sirt1 protects hepatic lipid and glucose metabolism from damage caused by HFDs. Additionally, Sirt1 has been found to be involved in the protection from metabolic syndrome. Sirt1 activators have been found to be beneficial for mitochondrial and metabolic function in obese rodents and are currently being studied as potential treatments for type-2 diabetes (Milne et al. 2007). It has been reported that moderate Sirt1 overexpression in mice prevents HFD-induced glucose intolerance and nonalcoholic fatty liver disease (Pfluger et al. 2008). Together, these data raise the possibility of HFDs mediating their effects on the aging brain in a sirtuin-dependent manner, although more work in this area is clearly needed.
Evidence for HFD exacerbating brain aging
HFD consumption is thought to induce changes not only in energy metabolism but also in brain function. Considerable clinical evidence suggests that HFD consumption and the presence of metabolic dysfunction are sufficient to exacerbate brain aging, promoting the development of cognitive alterations including dementia (Yaffe 2007;Haan et al. 2003;Taylor and MacQueen 2007). Moreover, obese adults have shown more severe brain atrophy than non-obese adults (Ward et al. 2005), further linking HFD consumption to brain perturbations.
Insulin has been suggested to play important roles in regulating memory, so any disturbance in insulin signaling would be expected to have a negative impact on memory (Luchsinger et al. 2004). Some studies have demonstrated that HFDs are able to increase amyloid beta peptide (Aβ) levels and Aβ-neuropathy in brains of mice exhibiting Alzheimer's disease (AD) pathology (Ho et al. 2004). Experimental evidence suggests that alterations in insulin metabolism may influence the onset of AD through their influence on the synthesis and degradation of Aβ peptides. Moreover, high levels of circulating insulin may also promote the accumulation of Aβ peptides by directly competing with Aβ for the insulin-degrading enzyme (IDE), thereby limiting Aβ degradation by IDE. However, whether the risk of AD is increased in human patients with diabetes mellitus is a matter of some debate. Some studies have reported that diabetic patients have elevated risk of developing AD (Leibson et al. 1997;Ott et al. 1999;Arvanitakis et al. 2004;Craft et al. 1998). However, several clinical studies have indicated that diabetes does not seem to be associated to AD (Luchsinger et al. 2004;Tariot et al. 1999;Curb et al. 1999;MacKnight et al. 2002;Akomolafe et al. 2006). Moreover, almost all neuropathological studies so far have failed to detect any associations between AD-related pathology and diabetes (Heitner and Dickson 1997;Petrovitch et al. 2000;Peila et al. 2002;Arvanitakis et al. 2006;Janson et al. 2004). Instead, diabetes has been associated to the presence of cerebral infarcts (Peila et al. 2002;Arvanitakis et al. 2006). Since diabetes present altered capillary blood vessels in the peripheral vasculature, it is not surprising that diabetes promotes changes in brain microvasculature. Interestingly, data from the Framingham study has demonstrated a link between type-2 diabetes and lower cognition scores (Elias et al. 1997), and hyperglycemia has been found to negatively influence cognitive impairment in population-based studies (Franceschi et al. 1984;Langan et al. 1991;Messier et al. 1999;Robertson-Tchabo et al. 1986).
Another metabolic disturbance associated with HFD-consumption and obesity is hypertension which is considered a great risk factor for cerebral stroke (Rosamond et al. 2008). One possible mechanism by which obesity may increase the risk of stroke is via an alteration in cerebral perfusion. Cerebral perfusion is regulated through active constrictor and dilator mechanisms and by the physical properties of the cerebral vasculature, such as myogenic tone and vessel structure. Increased myogenic tone has been reported to occur in middle and posterior cerebral arteries from spontaneously hypertensive rats, as well as vasopressin-deficient rat (Dunn et al. 1998;Gonzalez et al. 2008). Additionally, a great body of evidence has reported vascular remodeling in the cerebral circulation in different models of hypertension, spontaneously hypertensive rats among them (Dunn et al. 1998;Arribas et al. 1996;Baumbach and Heistad 1989). The most common changes in hypertensive populations are mechanical and architectural properties of the cerebral vasculature, particularly an inward remodeling of the large arteries (Baumbach et al. 1988;Baumbach and Heistad 1989;Baumbach and Heistad 1988). Inward remodeling not only increases the risk of flow obstruction but it is also detrimental in ischemic conditions. After ischemia, brain vessels are near-maximally vasodilated, so reductions in maximum lumen diameter become a constraint on perfusion. In addition, it is well known that alterations of arterial pressure cause deleterious changes in vascular structure, as vessels become rigid, wall thickness can increase and lumen diameter can decrease (Baumbach and Heistad 1989;Heistad et al. 1990), causing an extra impairment of blood flow during ischemia. In addition, studies carried out in adult obese Zucker rats (OZRs) with moderate hypertension and severe insulin resistance have demonstrated an increased cerebral vascular myogenic tone, inward cerebral vascular remodeling, and increased cerebral tissue death after ischemia/reperfusion injury in these rodents (Osmond et al. 2009). Important to note, young OZRs, which are obese and insulin resistant but not yet hypertensive, did not show any of those deficits (Osmond et al. 2009). Additionally, other studies carried out in leptin deficient ob/ob mice and also in diet-induced obese mice have shown that obesity worsens the response to ischemia (Nagai et al. 2007;Terao et al. 2008). All these data together suggest that obesity together with prolonged moderate hypertension serve as risk factors for cerebral vascular dysfunction and stroke.
Dyslipidemia is another common disease associated with HFD consumption. More than 50% of the US population older than 20 years old have cholesterol levels of 200 mg/dl or higher, and more than 18% have levels of 240 mg/dl or higher. The link between dyslipidemia and having a higher risk of cognitive impairment or dementia is controversial (Kuo et al. 1998;Wieringa et al. 1997;Lesser et al. 2001;Scacchi et al. 1998). Reduced levels of high-density lipoprotein (HDL) cholesterol have been reported in some cases of dementia (Kuo et al. 1998;Wieringa et al. 1997;Muckle and Roy 1985;Kuriyama et al. 1994;Kuriyama et al. 1992;Michikawa 2003;van Exel et al. 2002) but not all cases of dementia.
Direct evidence for HFD promoting brain pathogenesis
A large body of evidence from rodent and human studies has demonstrated that HFD-induced metabolic dysfunction promotes cognitive alterations and dementia (Craft 2005;Kumari et al. 2000;Li et al. 2007;Pasinetti et al. 2007;Razay et al. 2007). Different studies have been performed on rats in order to clarify the influence specific diets may have on different aspects of cognition. Interestingly, it has been documented that even maternal HFD consumption sensitizes offspring to the deleterious consequences of HFD (White et al. 2009). Studies carried out in rats have shown that, in agreement with biochemical and physiological evidence, a diet high in saturated fatty acids can impair learning and memory functions (Greenwood and Winocur 1990;Granholm et al. 2008). Moreover, direct injection of triglycerides into the brain has shown to have detrimental consequences for learning and memory (Farr et al. 2008). In addition, the strong relationship between obesity and cognitive impairment found in animals (White et al. 2009;Granholm et al. 2008;Baran et al. 2005;Winocur and Greenwood 2005) supporting an association between obesity and deficits in learning, memory, and executive functioning in human patients (Elias et al. 2003;Elias et al. 2005). At present, oxidative stress and brain inflammation are thought to play key roles in HFD-induced cognitive loss, since brain markers of oxidative stress and inflammation have been found to be increased in mice fed HFD which show clear cognitive impairment (Pistell et al. 2010;Souza et al. 2007;Zhang et al. 2005). Furthermore, HFD consumption has been shown to increase age-related oxidative stress in the brain (Mattson et al. 2003;Perry et al. 2003). For example, rodents fed high-fat or high-calorie diets have shown to have increased levels of oxidative stress (Zhang et al. 2005) and protein oxidation (Souza et al. 2007) in the brain. However, it is still unclear whether oxidative stress in HFD models of obesity is the result of obesity per se, or is more related to the associated metabolic dysfunction. Although there is a clear relation between obesity and brain inflammation, the real mechanisms whereby HFD contributes to brain inflammation and cognitive impairment also remain elusive. Certainly, brain function is sensitive to inflammatory pathways and mediators. For example, IL-1β and IL-6 are able to alter physiologic mechanisms involved in cognition and memory (Bellinger et al. 1995;Jankowsky and Patterson 1999;Gemma and Bickford 2007). Adiponectin and leptin, two well known adipokines, could be considered as important links between obesity, insulin resistance and related inflammatory disorders (Antuna-Puente et al. 2008;Fantuzzi 2005). Adiponectin has been shown to exhibit neuroprotective and anti-inflammatory properties in the brain (Chen et al. 2009) and to have important roles against the development of insulin resistance, dyslipidemia and atherosclerosis (Rasouli and Kern 2008). Leptin has also been reported to have important roles in cognition (Harvey et al. 2005;Oomura et al. 2006) and in the modulation of inflammatory signaling in microglia (Pinteaux et al. 2007). Among the afferent inputs that the brain uses to adjust food intake and energy metabolism, leptin is one of the best understood, supressing food intake and increases energy expenditure (Friedman and Halaas 1998). Studies such as these raise the possibility of roles for leptin in the aging brain that are independent of leptin effects in the hypothalamus and the regulation of feeding behavior.
There is also reasonable evidence for believing that insulin might also play a role in mediating the effects of HFD consumption on the brain, since insulin is known to act within the hippocampus. In vitro studies have shown that insulin can modulate hippocampal synaptic plasticity (Izumi et al. 2003;van der Heide et al. 2005;Zhao et al. 2004). In addition, it is also known that hippocampal cognitive performance strongly depends on glucose supply (Gold 2005;McNay et al. 2000;McNay and Gold 2002), which is regulated in larger part by peripheral insulin levels. It has been reported that hippocampus of AD brains have lower levels of glucose utilization and a corresponding impairment in insulin signaling (Frolich et al. 1998;Hoyer 2004;Steen et al. 2005).
Summary
Considerable evidence suggests a role for metabolic dysfunction as a modulator of brain aging, and consequently raises the specter of stressors such as HFD consumption as mediators of accelerated brain aging. In this model HFD consumption promotes a myriad of metabolic disturbances during aging, which directly and/or indirectly modulate the rate the brain ages. Of particular interest is the possibility that metabolic dysfunction during aging (in response to HFD consumption, genetics, physical activity, etc.) accounts for the considerable variability in the rates of brain aging that are observed in the elderly. Despite the progress made in this research area, several key questions remain to be answered. For example, which component(s) of metabolic dysfunction are responsible for the initiation/propagation of brain aging? How do individual aspects of metabolic dysfunction mediate neurochemical, structural, and/or functional changes in the aging brain? Answering these questions will likely contribute to not only our understanding of brain aging, but also to the development of therapeutic interventions which promote healthy brain aging.
Acknowledgements
This work was supported by grants from the NIA (AG029885, AG025771) and the Hibernia National Bank/Edward G Schlieder Chair (J.N.K.).
Reference List
- Adami A, Rossato G, Cerini R, Thijs VN, Pozzi-Mucelli R, Anzola GP, Del SM, Finocchi C, Meneghetti G, Zanferrari C. Right-to-left shunt does not increase white matter lesion load in migraine with aura patients. Neurology. 2008;71:101–107. doi: 10.1212/01.wnl.0000316798.25510.f2. [DOI] [PubMed] [Google Scholar]
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp. Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman R, Saul RL, Ames BN. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguero-Torres H, Kivipelto M, von SE. Rethinking the dementia diagnoses in a population-based study: what is Alzheimer's disease and what is vascular dementia?. A study from the kungsholmen project. Dement. Geriatr. Cogn Disord. 2006;22:244–249. doi: 10.1159/000094973. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Osei SY. Adipokines in obesity. Front Horm. Res. 2008;36:182–197. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- Ahren B, Pacini G. Age-related reduction in glucose elimination is accompanied by reduced glucose effectiveness and increased hepatic insulin extraction in man. J Clin. Endocrinol Metab. 1998;83:3350–3356. doi: 10.1210/jcem.83.9.5107. [DOI] [PubMed] [Google Scholar]
- Akomolafe A, Beiser A, Meigs JB, Au R, Green RC, Farrer LA, Wolf PA, Seshadri S. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch. Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Gordon JF, Daly CJ, Dominiczak AF, McGrath JC. Confocal microscopic characterization of a lesion in a cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke. 1996;27:1118–1122. doi: 10.1161/01.str.27.6.1118. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Astrup A, Dyerberg J, Selleck M, Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes. Rev. 2008;9(Suppl 1):48–52. doi: 10.1111/j.1467-789X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48:969–977. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O'Brien J. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer's disease, vascular dementia, and normal aging. J Neurol. Neurosurg. Psychiatry. 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Dobrin PB, Hart MN, Heistad DD. Mechanics of cerebral arterioles in hypertensive rats. Circ. Res. 1988;62:56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am. J Cardiol. 2002;90:40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba SG, Campbell IL, Siggins GR. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci. Lett. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J. Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Ruge W, Ulrich J. Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimer's disease. Brain Res. 1990;517:69–75. doi: 10.1016/0006-8993(90)91009-6. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Paoloni R, Caselli U, Galeazzi L, Meier-Ruge W. Synaptic structural dynamics and aging. Gerontology. 1996;42:170–180. doi: 10.1159/000213789. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Pieroni M, Meier-Ruge W, Ulrich J. Enlargement of synaptic size as a compensative reaction in aging and dementia. Pathol. Res. Pract. 1992;188:612–615. doi: 10.1016/S0344-0338(11)80066-X. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Mocchegiani E, Malavolta M, Casoli T, Di SG, Fattoretti P. Synaptic and mitochondrial physiopathologic changes in the aging nervous system and the role of zinc ion homeostasis. Mech. Ageing Dev. 2006;127:590–596. doi: 10.1016/j.mad.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Desantis RA, Kendrick Z. Effects of age and body fat on insulin resistance in healthy men. Diabetes Care. 1993;16:728–733. doi: 10.2337/diacare.16.5.728. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Perry VH. Differential blood-brain barrier breakdown and leucocyte recruitment following excitotoxic lesions in juvenile and adult rats. Exp. Neurol. 1998;154:231–240. doi: 10.1006/exnr.1998.6927. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS. Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc. Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Braun CA, Anderson CM. Pathophysiology: functional alterations in human health. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- Bravo E, Rivabene R, Bruscalupi G, Calcabrini A, Arancia G, Cantafora A. Age-related changes in lipid secretion of perfused livers from male Wistar rats donors. J Biochem. 1996;119:240–245. doi: 10.1093/oxfordjournals.jbchem.a021229. [DOI] [PubMed] [Google Scholar]
- Broughton DL, James OW, Alberti KG, Taylor R. Peripheral and hepatic insulin sensitivity in healthy elderly human subjects. Eur. J Clin. Invest. 1991;21:13–21. doi: 10.1111/j.1365-2362.1991.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: Exacerbation by high fat diet. Free Radic. Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. (Silver. Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- Bugalho P, Viana-Baptista M, Jordao C, Secca MF, Ferro JM. Age-related white matter lesions are associated with reduction of the apparent diffusion coefficient in the cerebellum. Eur. J Neurol. 2007;14:1063–1066. doi: 10.1111/j.1468-1331.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007;10:1164–1172. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- Cai Q, Tian L, Wei H. Age-dependent increase of indigenous DNA adducts in rat brain is associated with a lipid peroxidation product. Exp. Gerontol. 1996;31:373–385. doi: 10.1016/0531-5565(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Bates TE, Stella AM. NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem. Res. 2000;25:1315–1341. doi: 10.1023/a:1007604414773. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Butterfield DA, Stella AM. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer's disease. Ital. J Biochem. 2003;52:177–181. [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Guagliano E, Sapienza M, Mancuso C, Butterfield DA, Stella AM. Redox regulation of cellular stress response in neurodegenerative disorders. Ital. J Biochem. 2006;55:263–282. [PubMed] [Google Scholar]
- Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid. Redox. Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Brain neurotransmitters in aging and dementia: similar changes across diagnostic dementia groups. Gerontology. 1987;33:159–167. doi: 10.1159/000212870. [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. Biophys. Acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp. Biol Med. (Maywood) 2001;226:13–26. doi: 10.1177/153537020122600103. [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR, III, Hinson WH, Terry JG, Anderson R. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism. 1995;44:954–959. doi: 10.1016/0026-0495(95)90251-1. [DOI] [PubMed] [Google Scholar]
- Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu CA, Liu XY, Li CL, Zhao SM, Campbell W. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009;1273:129–137. doi: 10.1016/j.brainres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen M, Bergman RN, Pacini G, Porte D., Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin. Endocrinol Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behav. Neurosci. 1992;106:324–335. doi: 10.1037//0735-7044.106.2.324. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol. Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort A. Ageing: The Biology of Senescence. London: 1964. [Google Scholar]
- Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin. Endocrinol Metab. 1992;75:1125–1132. doi: 10.1210/jcem.75.4.1400882. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Bird SJ, Evans RH, Gulley RL, Nadler JV, Nicklas WJ, Olney JW. Excitatory amino acid neurotoxins: selectivity, specificity, and mechanisms of action. Based on an NRP one-day conference held June 30, 1980. Neurosci. Res. Program. Bull. 1981;19:1–427. [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging. 2005;26(Suppl 1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- Curb JD, Rodriguez BL, Abbott RD, Petrovitch H, Ross GW, Masaki KH, Foley D, Blanchette PL, Harris T, Chen R, White LR. Longitudinal association of vascular and Alzheimer's dementias, diabetes, and glucose tolerance. Neurology. 1999;52:971–975. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- d'Alessio P. Aging and the endothelium. Exp. Gerontol. 2004;39:165–171. doi: 10.1016/j.exger.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Damjanovic M, Barton M. Fat intake and cardiovascular response. Curr. Hypertens. Rep. 2008;10:25–31. doi: 10.1007/s11906-008-0007-0. [DOI] [PubMed] [Google Scholar]
- De Feo P, Volpi E, Lucidi P, Cruciani G, Reboldi G, Siepi D, Mannarino E, Santeusanio F, Brunetti P, Bolli GB. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- Dechenes CJ, Verchere CB, Andrikopoulos S, Kahn SE. Human aging is associated with parallel reductions in insulin and amylin release. Am. J Physiol. 1998;275:E785–E791. doi: 10.1152/ajpendo.1998.275.5.E785. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J. Leukoc. Biol. 2010 doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid. Redox. Signal. 2006;8:163–172. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- Diniz YS, Rocha KK, Souza GA, Galhardi CM, Ebaid GM, Rodrigues HG, Novelli Filho JL, Cicogna AC, Novelli EL. Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur. J Pharmacol. 2006;543:151–157. doi: 10.1016/j.ejphar.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WR, Wallis SJ, Gardiner SM. Remodelling and enhanced myogenic tone in cerebral resistance arteries isolated from genetically hypertensive Brattleboro rats. J Vasc. Res. 1998;35:18–26. doi: 10.1159/000025561. [DOI] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res. Mol. Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Elahi D, Muller DC, Egan JM, Andres R, Veldhuist J, Meneilly GS. Glucose tolerance, glucose utilization and insulin secretion in ageing. Novartis. Found. Symp. 2002;242:222–242. [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int. J Obes. Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging. 2005;26(Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Cupples LA, Wilson PW, Silbershatz H, Wolf PA. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Fagot-Campagna A, Balkau B, Simon D, Warnet JM, Claude JR, Ducimetiere P, Eschwege E. High free fatty acid concentration: an independent risk factor for hypertension in the Paris Prospective Study. Int. J Epidemiol. 1998;27:808–813. doi: 10.1093/ije/27.5.808. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M. Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large- and small-vessel disease. Stroke. 1999;30:1647–1650. doi: 10.1161/01.str.30.8.1647. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin. Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Jarvinen H. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR). Hypertension. 1997;30:1144–1149. doi: 10.1161/01.hyp.30.5.1144. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- Finch CE. Developmental origins of aging in brain and blood vessels: an overview. Neurobiol. Aging. 2005;26:281–291. doi: 10.1016/j.neurobiolaging.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Cecchetto R, Minicucci F, Smizne S, Baio G, Canal N. Cognitive processes in insulin-dependent diabetes. Diabetes Care. 1984;7:228–231. doi: 10.2337/diacare.7.3.228. [DOI] [PubMed] [Google Scholar]
- Freund G. Cholinergic receptor loss in brains of aging mice. Life Sci. 1980;26:371–375. doi: 10.1016/0024-3205(80)90153-8. [DOI] [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH. Reactive species, cellular repair and risk factors in the onset of type 2 diabetes mellitus: review and hypothesis. Curr Diabetes Rev. 2006;2:241–259. doi: 10.2174/157339906776818541. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Frohman TC, Gupta S, de FA, van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer's disease. J Neurol. Sci. 1991;106:105–111. doi: 10.1016/0022-510x(91)90202-i. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J. Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007;18:137–148. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J. Neurochem. 1981;37:978–984. doi: 10.1111/j.1471-4159.1981.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Peterson C, Jenden DJ. Brain acetylcholine synthesis declines with senescence. Science. 1981;213:674–676. doi: 10.1126/science.7256270. [DOI] [PubMed] [Google Scholar]
- Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm. Res. 2008;57:558–563. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol. Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Somoza B, Conde MV, Fernandez-Alfonso MS, Gonzalez MC, Arribas SM. Hypertension increases middle cerebral artery resting tone in spontaneously hypertensive rats: role of tonic vasoactive factor availability. Clin. Sci. (Lond) 2008;114:651–659. doi: 10.1042/CS20070361. [DOI] [PubMed] [Google Scholar]
- Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer's disease. Am. J. Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SG, Ekstrom TJ. The human histone deacetylase family. Exp. Cell Res. 2001;262:75–83. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav. Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int. J Geriatr. Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Cases JA, She L, Ma XH, Yang XM, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am. J Physiol Endocrinol. Metab. 2000;278:E985–E991. doi: 10.1152/ajpendo.2000.278.6.E985. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am. Geriatr. Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Harvey J, Shanley LJ, O'Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem. Soc. Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Mayhan WG, Coyle P, Baumbach GL. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels. 1990;27:258–262. doi: 10.1159/000158817. [DOI] [PubMed] [Google Scholar]
- Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Ueno M. Aging of blood-brain barrier and neuronal cells of eye and ear in SAM mice. Neurobiol. Aging. 1999;20:117–123. doi: 10.1016/s0197-4580(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Howard G, Bergman R, Wagenknecht LE, Haffner SM, Savage PJ, Saad MF, Laws A, D'Agostino RB., Jr. Ability of alternative indices of insulin sensitivity to predict cardiovascular risk: comparison with the “minimal model”. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Ann. Epidemiol. 1998;8:358–369. doi: 10.1016/s1047-2797(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur. J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Macdonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol. B Psychol. Sci. Soc. Sci. 2002;57:101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem. Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Iqbal N. The burden of type 2 diabetes: strategies to prevent or delay onset. Vasc. Health Risk Manag. 2007;3:511–520. [PMC free article] [PubMed] [Google Scholar]
- Itateyama E, Chiba S, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine in genetically obese animals: its implication of leptin action in the brain. Exp. Biol Med. (Maywood.) 2003;228:1132–1137. doi: 10.1177/153537020322801006. [DOI] [PubMed] [Google Scholar]
- Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415–421. doi: 10.1016/0278-5846(93)90075-4. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Yamada KA, Matsukawa M, Zorumski CF. Effects of insulin on long-term potentiation in hippocampal slices from diabetic rats. Diabetologia. 2003;46:1007–1012. doi: 10.1007/s00125-003-1144-2. [DOI] [PubMed] [Google Scholar]
- James TC, Kanungo MS. Alterations in atropine sites of the brain of rats as a function of age. Biochem. Biophys. Res. Commun. 1976;72:170–175. doi: 10.1016/0006-291x(76)90975-x. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Patterson PH. Cytokine and growth factor involvement in long-term potentiation. Mol Cell Neurosci. 1999;14:273–286. [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Jia JP, Jia JM, Zhou WD, Xu M, Chu CB, Yan X, Sun YX. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer's disease and vascular dementia. Chin Med. J. (Engl.) 2004;117:1161–1164. [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: nutritional considerations. Neurochem. Res. 2005;30:927–935. doi: 10.1007/s11064-005-6967-4. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I, Thompson SG, Jespersen J. Involvement of the hemostatic system in the insulin resistance syndrome. A study of 1500 patients with angina pectoris. The ECAT Angina Pectoris Study Group. Arterioscler. Thromb. 1993;13:1865–1873. doi: 10.1161/01.atv.13.12.1865. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol. Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol. 1993;48:M84–M90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Alfarsi S, Adams PW, Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia. 1976;12:563–571. doi: 10.1007/BF01220632. [DOI] [PubMed] [Google Scholar]
- Kitani K, Minami C, Yamamoto T, Maruyama W, Kanai S, Ivy GO, Carrillo MC. Do antioxidant strategies work against aging and age-associated disorders? Propargylamines: a possible antioxidant strategy. Ann. N. Y. Acad. Sci. 2001;928:248–260. doi: 10.1111/j.1749-6632.2001.tb05654.x. [DOI] [PubMed] [Google Scholar]
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273:4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci. Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Moore RL, Scarpace PJ. Beta3-adrenergic regulation of leptin, food intake, and adiposity is impaired with age. Pflugers Arch. 1999;438:681–688. [PubMed] [Google Scholar]
- Kumari M, Brunner E, Fuhrer R. Minireview: mechanisms by which the metabolic syndrome and diabetes impair memory. J Gerontol. A Biol Sci. Med. Sci. 2000;55:B228–B232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, Roher AE. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1-42 levels. Biochem. Biophys. Res. Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- Kuriyama M, Hokezu Y, Togo S, Nagata K, Takahashi K, Igakura T, Osame M. [Serum lipids, lipoproteins and apolipoproteins in patients with senile dementia]. Nippon Ronen Igakkai Zasshi. 1992;29:559–564. doi: 10.3143/geriatrics.29.559. [DOI] [PubMed] [Google Scholar]
- Kuriyama M, Takahashi K, Yamano T, Hokezu Y, Togo S, Osame M, Igakura T. Low levels of serum apolipoprotein A I and A II in senile dementia. Jpn. J Psychiatry Neurol. 1994;48:589–593. doi: 10.1111/j.1440-1819.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Laakso M. How good a marker is insulin level for insulin resistance? Am. J Epidemiol. 1993;137:959–965. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron. Artery Dis. 1998;9:473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- Langan SJ, Deary IJ, Hepburn DA, Frier BM. Cumulative cognitive impairment following recurrent severe hypoglycaemia in adult patients with insulin-treated diabetes mellitus. Diabetologia. 1991;34:337–344. doi: 10.1007/BF00405006. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Subcell. Biochem. 2007;42:299–318. [PubMed] [Google Scholar]
- Launer LJ. Epidemiology of white-matter lesions. Int. Psychogeriatr. 2003;15(Suppl 1):99–103. doi: 10.1017/S1041610203009037. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann. N. Y. Acad. Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- Leone S, Ottani A, Bertolini A. Dual acting anti-inflammatory drugs. Curr. Top. Med. Chem. 2007;7:265–275. doi: 10.2174/156802607779941341. [DOI] [PubMed] [Google Scholar]
- Lesser G, Kandiah K, Libow LS, Likourezos A, Breuer B, Marin D, Mohs R, Haroutunian V, Neufeld R. Elevated serum total and LDL cholesterol in very old patients with Alzheimer's disease. Dement. Geriatr. Cogn Disord. 2001;12:138–145. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- Lippa AS, Critchett DJ, Ehlert F, Yamamura HI, Enna SJ, Bartus RT. Age-related alterations in neurotransmitter receptors: an electrophysiological and biochemical analysis. Neurobiol. Aging. 1981;2:3–8. doi: 10.1016/0197-4580(81)90052-x. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement. Geriatr. Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J. Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000;14:1464–1469. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matia M, Lecumberri P, Calle P. [Nutrition and metabolic syndrome]. Rev Esp Salud Publica. 2007;81:489–505. doi: 10.1590/s1135-57272007000500006. [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995a;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995b;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Biomarkers of age and aging. Exp. Gerontol. 1997;32:87–94. doi: 10.1016/s0531-5565(96)00067-8. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav. Cogn Neurosci. Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved Problem of Biology. H.K. Lewis, London: 1952. [Google Scholar]
- Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Messier C, Desrochers A, Gagnon M. Effect of glucose, glucose regulation, and word imagery value on human memory. Behav. Neurosci. 1999;113:431–438. doi: 10.1037//0735-7044.113.3.431. [DOI] [PubMed] [Google Scholar]
- Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer's disease? J Neurosci. Res. 2003;72:141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- Miller EA, Allen DO. Hormone-stimulated lipolysis in isolated fat cells from “young” and “old” rats. J Lipid Res. 1973;14:331–336. [PubMed] [Google Scholar]
- Miller JP, Pratley RE, Goldberg AP, Gordon P, Rubin M, Treuth MS, Ryan AS, Hurley BF. Strength training increases insulin action in healthy 50- to 65-yr-old men. J Appl. Physiol. 1994;77:1122–1127. doi: 10.1152/jappl.1994.77.3.1122. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic. Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Morin AM, Wasterlain CG. Aging and rat brain muscarinic receptors as measured by quinuclidinyl benzilate binding. Neurochem. Res. 1980;5:301–308. doi: 10.1007/BF00964618. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Muckle TJ, Roy JR. High-density lipoprotein cholesterol in differential diagnosis of senile dementia. Lancet. 1985;1:1191–1193. doi: 10.1016/s0140-6736(85)92866-1. [DOI] [PubMed] [Google Scholar]
- Nag S, Kilty DW. Cerebrovascular changes in chronic hypertension. Protective effects of enalapril in rats. Stroke. 1997;28:1028–1034. doi: 10.1161/01.str.28.5.1028. [DOI] [PubMed] [Google Scholar]
- Nagai N, Van HB, Lijnen HR. Plasminogen activator inhibitor-1 contributes to the deleterious effect of obesity on the outcome of thrombotic ischemic stroke in mice. J Thromb. Haemost. 2007;5:1726–1731. doi: 10.1111/j.1538-7836.2007.02631.x. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv. Drug Deliv. Rev. 2008;60:1534–1544. doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol. Aging. 2008;29:1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MF, Wise S, Dinneen SF, Schwenk WF, Basu A, Rizza RA. Assessment of hepatic sensitivity to glucagon in NIDDM: use as a tool to estimate the contribution of the indirect pathway to nocturnal glycogen synthesis. Diabetes. 1997;46:2007–2016. doi: 10.2337/diab.46.12.2007. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007;100:795–806. doi: 10.1161/01.RES.0000259591.97107.6c. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg G, Mellgren G, Smith U. Human adipose tissue in culture. VI. Effect of age on cell size and lipolysis. Acta Paediatr. Scand. 1976;65:313–318. doi: 10.1111/j.1651-2227.1976.tb04891.x. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy IM, Kasdorf GM, Hoffmann RG, Kalkhoff RK. Does aging intensify the insulin resistance of human obesity? J Clin. Endocrinol Metab. 1992;74:1075–1081. doi: 10.1210/jcem.74.5.1569155. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46:1768–1774. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- Ogawa N. [Central acetylcholinergic systems in the normal aged and in the patient with Alzheimer-type dementia (ATD)]. Rinsho Shinkeigaku. 1989;29:1529–1531. [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension. 2009;53:381–386. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van HF, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Pacini G, Valerio A, Beccaro F, Nosadini R, Cobelli C, Crepaldi G. Insulin sensitivity and beta-cell responsivity are not decreased in elderly subjects with normal OGTT. J Am. Geriatr. Soc. 1988;36:317–323. doi: 10.1111/j.1532-5415.1988.tb02358.x. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Scheen A, Lefebvre P. Glucose handling, diabetes and ageing. Horm. Res. 1995;43:52–57. doi: 10.1159/000184237. [DOI] [PubMed] [Google Scholar]
- Park S, Kim YW, Kim JY, Jang EC, Doh KO, Lee SK. Effect of high fat diet on insulin resistance: dietary fat versus visceral fat mass. J Korean Med. Sci. 2001;16:386–390. doi: 10.3346/jkms.2001.16.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Mangel M. Messages from mortality: the evolution of death rates in the old. Trends Ecol. Evol. 1999;14:438–442. doi: 10.1016/s0169-5347(99)01646-8. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Eberstein JA. Metabolic syndrome and the role of dietary lifestyles in Alzheimer's disease. J Neurochem. 2008;106:1503–1514. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Zhao Z, Qin W, Ho L, Shrishailam Y, Macgrogan D, Ressmann W, Humala N, Liu X, Romero C, Stetka B, Chen L, Ksiezak-Reding H, Wang J. Caloric intake and Alzheimer's disease. Experimental approaches and therapeutic implications. Interdiscip. Top. Gerontol. 2007;35:159–175. doi: 10.1159/000096561. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Cytokines in Alzheimer's disease and multiple sclerosis. Curr. Opin. Neurobiol. 1995;5:642–646. doi: 10.1016/0959-4388(95)80070-0. [DOI] [PubMed] [Google Scholar]
- Pedigo NW, Jr., Minor LD, Krumrei TN. Cholinergic drug effects and brain muscarinic receptor binding in aged rats. Neurobiol. Aging. 1984;5:227–233. doi: 10.1016/0197-4580(84)90067-8. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Peiris AN, Mueller RA, Smith GA, Struve MF, Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin. Invest. 1986;78:1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmuter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. J Diabet. Complications. 1988;2:210–213. doi: 10.1016/s0891-6632(88)80011-4. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA. Reactive Oxygen Species Mediate Cellular Damage in Alzheimer Disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Raina AK, Aliev G, Siedlak SL, Harris PL, Casadesus G, Petersen RB, Bligh-Glover W, Balraj E, Petot GJ, Smith MA. A metabolic basis for Alzheimer disease. Neurochem. Res. 2003;28:1549–1552. doi: 10.1023/a:1025678510480. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol. Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–833. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, De RD, Fratiglioni L. The epidemiology of the dementias: an update. Curr. Opin. Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurobiol. Aging. 2000;21:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin. Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch. Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res. 1990;508:349–352. doi: 10.1016/0006-8993(90)90423-9. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin. Endocrinol Metab. 1982a;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes. 1982b;31:663–669. doi: 10.2337/diab.31.8.663. [DOI] [PubMed] [Google Scholar]
- Robertson-Tchabo EA, Arenberg D, Tobin JD, Plotz JB. A longitudinal study of cognitive performance in noninsulin dependent (type II) diabetic men. Exp. Gerontol. 1986;21:459–467. doi: 10.1016/0531-5565(86)90051-3. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35:2623–2627. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- Roman GC. Age-associated white matter lesions and dementia: are these lesions causal or casual? Arch. Neurol. 2004;61:1503–1504. doi: 10.1001/archneur.61.10.1503. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of aging. J Clin. Invest. 1983;71:1581–1587. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J. Mol. Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Scacchi R, De BL, Mantuano E, Vilardo T, Donini LM, Ruggeri M, Gemma AT, Pascone R, Corbo RM. DNA polymorphisms of apolipoprotein B and angiotensin I-converting enzyme genes and relationships with lipid levels in Italian patients with vascular dementia or Alzheimer's disease. Dement. Geriatr. Cogn Disord. 1998;9:186–190. doi: 10.1159/000017045. [DOI] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol. Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Muller WE. Flavonoids and the aging brain. J Physiol Pharmacol. 2005;56(Suppl 1):23–36. [PubMed] [Google Scholar]
- Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr., Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am. J Physiol Endocrinol Metab. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp. Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R. Studies in the distribution of body fat: I. Effects of age, sex, and obesity. J Gerontol. 1989;44:M66–M73. doi: 10.1093/geronj/44.2.m66. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Smith SR, Wilson PW. Free fatty acids and atherosclerosis--guilty or innocent? J Clin. Endocrinol Metab. 2006;91:2506–2508. doi: 10.1210/jc.2006-1018. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Sohal BH. Oxidative stress and aging in the Mongolian gerbil (Meriones unguiculatus). Mech. Ageing Dev. 1995;81:15–25. doi: 10.1016/0047-6374(94)01578-a. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res. Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Spulber S, Schultzberg M. Connection between inflammatory processes and transmittor function-Modulatory effects of interleukin-1. Prog. Neurobiol. 2010;90:256–262. doi: 10.1016/j.pneurobio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Protein oxidation. Ann. N. Y. Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Oliver CN. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Steemburgo T, Dall'Alba V, Gross JL, Azevedo MJ. [Dietary factors and metabolic syndrome]. Arq Bras Endocrinol Metabol. 2007;51:1425–1433. doi: 10.1590/s0004-27302007000900004. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer's disease patients. Lab Invest. 1992;66:223–230. [PubMed] [Google Scholar]
- Svedberg J, Bjorntorp P, Smith U, Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990;39:570–574. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Ogden MA, Cox C, Williams TF. Diabetes and dementia in long-term care. J Am. Geriatr. Soc. 1999;47:423–429. doi: 10.1111/j.1532-5415.1999.tb07234.x. [DOI] [PubMed] [Google Scholar]
- Taylor VH, MacQueen GM. Cognitive dysfunction associated with metabolic syndrome. Obes. Rev. 2007;8:409–418. doi: 10.1111/j.1467-789X.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR. Immune response gene expression increases in the aging murine hippocampus. J. Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- Thiels E, Klann E. Hippocampal memory and plasticity in superoxide dismutase mutant mice. Physiol Behav. 2002;77:601–605. doi: 10.1016/s0031-9384(02)00900-9. [DOI] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Hashiguchi K, Saheki M, Takahashi S. Remarkable reduction in acetylcholine concentration in the cerebrospinal fluid from patients with Alzheimer type dementia. Neurosci. Lett. 1994;177:139–142. doi: 10.1016/0304-3940(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Tschop M, Heiman ML. Rodent obesity models: an overview. Exp. Clin. Endocrinol. Diabetes. 2001;109:307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- van Exel E, de Craen AJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Macfarlane PW, Blauw GJ, Westendorp RG. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann. Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- Vannucchi MG, Pepeu G. Effect of phosphatidylserine on acetylcholine release and content in cortical slices from aging rats. Neurobiol. Aging. 1987;8:403–407. doi: 10.1016/0197-4580(87)90034-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields SD, Gur R. Dopamine transporters decrease with age. J. Nucl. Med. 1996;37:554–559. [PubMed] [Google Scholar]
- Wahrenberg H, Lonnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin. Invest. 1989;84:458–467. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu R, Liu L, Chowdhury R, Barzilai N, Tan J, Rossetti L. The effect of leptin on Lep expression is tissue-specific and nutritionally regulated. Nat. Med. 1999;5:895–899. doi: 10.1038/11335. [DOI] [PubMed] [Google Scholar]
- Wang S, Ma A, Song S, Quan Q, Zhao X, Zheng X. Fasting serum free fatty acid composition, waist/hip ratio and insulin activity in essential hypertensive patients. Hypertens. Res. 2008;31:623–632. doi: 10.1291/hypres.31.623. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC. Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol. Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa GE, Burlinson S, Rafferty JA, Gowland E, Burns A. Apolipoprotein E genotypes and serum lipid levels in Alzheimer's disease and multi-infarct dementia. Int. J Geriatr. Psychiatry. 1997;12:359–362. [PubMed] [Google Scholar]
- Williams BR, Hultsch DF, Strauss EH, Hunter MA, Tannock R. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19:88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wong DF, Wagner HN, Jr., Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Wu CF, Bertorelli R, Sacconi M, Pepeu G, Consolo S. Decrease of brain acetylcholine release in aging freely-moving rats detected by microdialysis. Neurobiol. Aging. 1988;9:357–361. doi: 10.1016/s0197-4580(88)80081-2. [DOI] [PubMed] [Google Scholar]
- Xiao HD, Fuchs S, Frenzel K, Teng L, Bernstein KE. Circulating versus local angiotensin II in blood pressure control: lessons from tissue-specific expression of angiotensin-converting enzyme (ACE). Crit Rev. Eukaryot. Gene Expr. 2004;14:137–145. [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis. Assoc. Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- Yasui F, Ishibashi M, Matsugo S, Kojo S, Oomura Y, Sasaki K. Brain lipid hydroperoxide level increases in senescence-accelerated mice at an early age. Neurosci. Lett. 2003;350:66–68. doi: 10.1016/s0304-3940(03)00827-9. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]