Abstract
Serotonergic dysregulation is thought to underlie much of the pathology in bulimia nervosa (BN). The purpose of this review is to expand the serotonergic model by incorporating specific and nonspecific contributions of estrogens to the development and maintenance of bulimic pathology in order to guide research from molecular genetics to novel therapeutics for BN. Special emphasis is given to the organizing theory of general brain arousal which allows for integration of specific and nonspecific effects of these systems on behavioral endpoints such as binge eating or purging as well as arousal states such as fear, novelty seeking, or sex. Regulation of the serotonergic system by estrogens is explored, and genetic, epigenetic, and environmental estrogen effects on bulimic pathology and risk factors are discussed. Genetic and neuroscientific research support this two-system conceptualization of BN with both contributions to the developmental and maintenance of the disorder. Implications of an estrogenic-serotonergic model of BN are discussed as well as guidelines and suggestions for future research and novel therapeutic targets.
Keywords: bulimia nervosa, estrogen, serotonin, brain arousal, sex hormones, epigenetics
1. Introduction
Bulimia nervosa (BN) is a serious mental disorder (Klump et al., 2009) characterized by repetitive episodes of binge eating and compensatory behaviors, such as excessive exercise, self-induced vomiting, diuretic or laxative abuse (American Psychiatric Association, 1994). According to the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV; APA, 1994), an episode of binge eating is characterized by both of the following: (1) eating, in a discrete period of time (e.g. within any 2-hour period), an amount of food that is definitely larger than most people would eat during a similar period of time and under similar circumstances; (2) a sense of lack of control over eating during the episode (e.g. a feeling that one cannot stop eating or control what or how much one is eating). This definition provides an impetus for considering both the objective behavior of overeating and the subjective appraisal of this behavior as ‘out of control’. The corresponding use of extreme compensatory behaviors such as purging is believed to be functional and reinforced by reducing negative affect. Prospective and naturalistic investigations of binge eating and purging suggest these behaviors are used intentionally to reduce negative affect and stress, although this may be due to an increase in positive affect among some individuals as opposed to true reductions in negative emotional states (Crosby et al., 2009; Smyth et al., 2007).
Prevalence rates for BN are approximately 1.0-1.5% of the population, but a significant gender disparity has been noted, with approximately three times more women affected than men (Hoek, 2006; Hudson, Hiripi, Pope, & Kessler, 2007). Identifying biological reasons for this disparity has been difficult. There are a number of proposed biological mechanisms thought to cause and perpetuate BN pathology, both at the trait level and the behavioral level. One of the leading models involves a disruption of neurotransmitters, primarily serotonin, with secondary influences on this system by dietary restriction and hormonal changes (Kaye, 2008; Steiger & Bruce, 2007). Although this model holds promise, there are theoretical challenges to account for heterogeneity in BN. Namely, the nature of the serotonergic dysregulation (hypo vs hyperserotonergic) as an etiological or maintenance factor has been hard to establish and therapeutics targeting serotonin dysregulation have been only moderately successful (Shapiro et al., 2007). Thus, the aim of this review is to formally integrate the role of estrogens into the neurobiological model of BN in efforts to address some of these challenges in theory and treatment. To do this, we will first briefly review the existing models of BN and suggest their relationship to a fundamental problem with general arousal. We will then review evidence supporting the role of estrogens and serotonin in BN symptoms. We then propose an integration of the two systems by reviewing evidence suggesting estrogenic regulation of the serotonin system. Finally, we conclude by providing suggestions for future therapeutic targets and argue that symptoms of BN can be conceptualized as part of a larger problem of generalized arousal.
2. Basic Model of BN Pathology
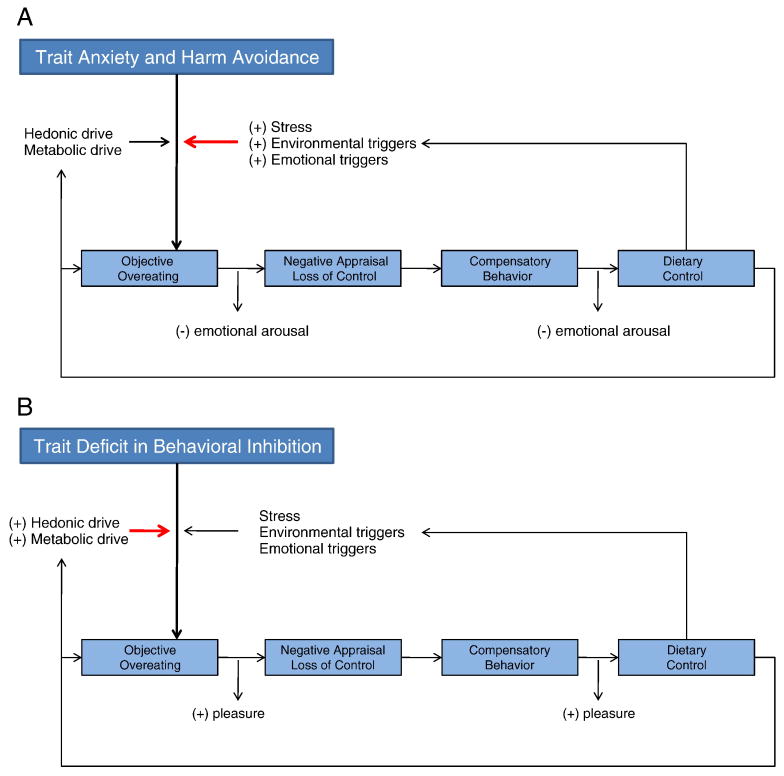
The core behavioral phenomenon of BN is the binge-purge cycle and it is thought to be influenced by hedonic and metabolic processes such as the drive for reward and drive to eat (Lutter & Nestler, 2009) as well as personality traits such as impulsivity and compulsivity (Engel et al., 2005; Lilenfeld, Wonderlich, Riso, Crosby, & Mitchell, 2006). Utilizing this framework, a binge—purge episode can be conceptualized as a consequence of a trait-based deficit in inhibition or as a consequence of a compulsive attempt to reduce fears or worries about weight/shape changes or other negative emotional states. Figure 1 summarizes these basic patterns of the binge—purge cycle. The binge—purge cycle is marked initially by overeating, which by consequence of a negative appraisal of the eating episode, is perceived as ‘out of control.’ Purging, or other compensatory behavior, acts to relieve the distress of this perception and provides a temporary sense of increased control over food intake, shape, or weight status. Resolution of the binge-purge cycle returns the individual to a pattern of strict dietary control. What differentiates the two types of binge-purge cycles is the functional significance of each behavior. For those with trait-level disturbances in inhibition, overeating results from an inability to inhibit the motivational state generated by food or eating cues (hedonic or metabolic). Thus, the pattern can be conceptualized as positively reinforcing. Alternatively, for those with trait-level excess in anxiety or obsessionality overeating results from increased priming for food consumption (i.e., hyperfocus on food and food cues). Overeating and compensatory behavior temporarily reduces anxiety and unwanted autonomic arousal, providing relief from the anxious/obsessional state. Thus, this pattern can be conceptualized as negatively reinforcing. The propensity for either pattern is likely influenced by these trait-level disturbances as well as basic reward properties of food. Although either pattern can be triggered by a range of external factors including stress, environmental triggers, or emotional triggers or a range of internal factors originating from basic drive states for energy balance or reward, the sensitivity to these triggers is potentially differentially influenced by the trait deficit. External influences are more likely to lead to negative emotional states and thus provide a state primed for negative reinforcement. Internal drive states are more likely to lead to increased pressure to seek out and consume food or other positive reinforcers and thus provide a state primed for positive reinforcement. These two types of triggers can interact with each other to influence the binge—purge cycle, but with trait anxiety more sensitive to these external triggers and trait deficits in inhibition more sensitive to natural drive states.
Figure 1.
Panels A and B present a simplified model of the binge—compensatory behavior cycle. In panel A, trait based anxiety and harm avoidance place an individual in a perpetual state of concern about overeating. This concern makes them more alert and sensitive to environmental cues relevant to eating (e.g., diet commercials, food adds, etc.), more susceptible to stress responses, and more emotionally driven. The increased pressure from these triggers leads to an increased likelihood of overeating, which is reinforced through reduction of anxious or stressful state associated with eating. Similarly, compensatory behaviors (e.g., exercise, vomiting, etc.) serve to temporarily reduce the anxious or stressful state associated with having overeaten. In panel B, the paradigm is similar except the individual is particularly vulnerable to hedonic or metabolic motivational states. Overeating and compensatory behaviors serve to be temporarily pleasurable and satiating. We note that these deficits and their basic reinforcement patterns are not mutually exclusive and often co-occur in the same individual. There is also feedback from the dietary control (via deprivation in nutrition or thinking about dieting) to increase stress and the drive for reward and eating.
These impulsive and compulsive traits can be linked to serotonin dysfunction and genetic polymorphisms within the serotonin system (Baca-Garcia et al., 2005). For instance, obsessive-compulsive spectrum disorders share a common dysregulation in serotonin function and genetic polymorphisms of serotonergic genes (Bloch et al., 2008; Saiz et al., 2008). Similarly, impulsivity has been linked to low serotonin in a range of behavioral disturbances including alcohol use disorders, binge eating, drug use, and risky sexual behavior (Cyders & Smith, 2008; Fischer, Smith, & Cyders, 2008). Thus, the serotonin system appears to be a unifying neurobiological system for explaining trait based influences on BN pathology through trait disturbances in response inhibition or anxiety/compulsivity (Kaye, 2008; Steiger & Bruce, 2007).
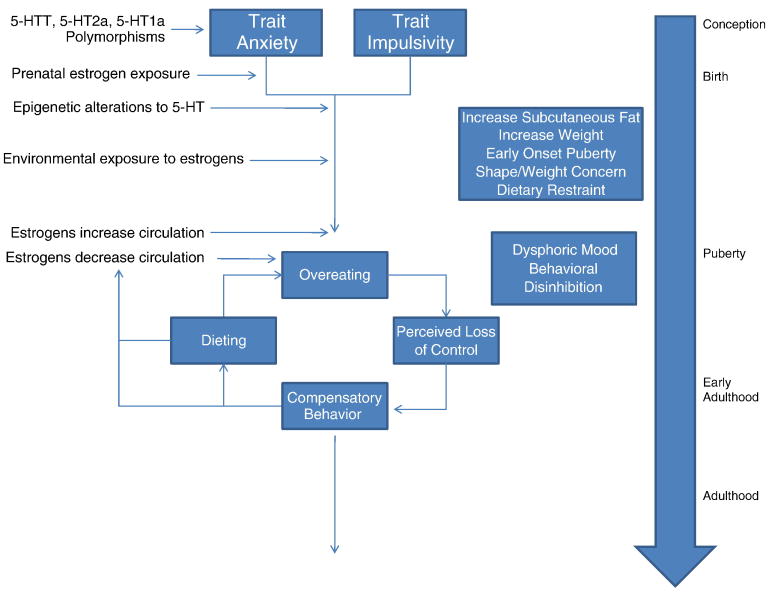
Figure 2 summarizes influences of both estrogens and serotonin on the development and maintenance of BN. Genetic influences, present at conception, predispose individuals to development of trait anxiety/harm avoidance or impulsivity. Prenatal exposure to estrogens has some organizational effects on brain development, perhaps priming the central nervous system (CNS) for sensitivity to later estrogenic effects. After birth, the individual is exposed to environmental influences over the serotonergic and estrogenic system leading to increased propensity for overeating and visceral fat deposits (via estrogenic regulation of female adiposity). In turn, societal pressures contribute to dissatisfaction with weight and the individual develops a pattern of dietary restraint to cope with these pressures. Early puberty accelerates the shape/weight dissatisfaction because bodily changes are inconsistent with the thin ideal. At the same time, estrogen levels rise and phasic changes begin to occur in conjunction with the menstrual cycle. These changes ramp up estrogenic controls of serotonin function, which may have increasingly detrimental effects on mood dysregulation and behavioral disinhibition. Overeating in combination with these effects increases the likelihood of binge eating episodes occurring (i.e., overeating + perceived loss of control). Compensatory measures, such as purging, exercise, or dietary restriction, lead to dysregulation of the menstrual cycle and decrease the level of available estrogens which in turn exacerbate existing serotonergic disturbances. This pattern continues until the individual is able to re-regulate eating patterns and cease purging methods.
Figure 2.
The figure above highlights an escalating series of pressures on the estrogenic and serotonergic systems in a developing female. Genetic polymorphisms from birth can limit the efficacy of the serotonergic system. Prenatal exposure to estrogens further organizes the brain for later estrogenic effects. After birth, specific forms of stress brought about by parenting style my further compromise serotonergic genes. Simultaneously, the individual is exposed to environmental estrogens which further predispose the individual for weight gain and increased subcutaneous fat deposition. The consequence of these pressures may include increased attempts at dieting. Puberty brings phasic shifts in estrogens and subsequent regulatory power over the serotonergic system. Social pressures to be thin increase and a challenged estrogen-serotonin system leaves the individual vulnerable to dysphoric mood, behavioral disinhibition, and ultimately a pattern of dieting, binging, and purging. These symptoms further reduce estrogens, exacerbating serotonergic dysfunction. The pattern persists until the individual is able to stabilize their pattern of eating and exercise.
2.1 Generalized Brain Arousal and BN Pathology
Serotonin effects are widespread throughout the brain and contribute to specific behaviors, such as eating (Gruninger, LeBoeuf, Liu, & Garcia, 2007) and sex (Uphouse, Hiegel, Perez, & Guptarak, 2007), but also to different states of arousal such as sleep (Gottesmann, 2004; Monti & Jantos, 2006). The specific and nonspecific roles of this neurotransmitter system implicate its importance in the larger phenomenon of central nervous system (CNS) or brain arousal.
The concept of general brain arousal, its importance to a range or behavioral endpoints, and genetic influences has been introduced (Garey et al., 2003) and subsequent theory developed (Pfaff, 2005a). The general form of brain arousal has been operationalized as follows:
“Generalized arousal” is higher in an animal or human being who is: (S) more alert to sensory stimuli of all sorts, (M) more motorically active, and (E) more reactive emotionally (Pfaff, 2005, pg. 5).
The theory of general brain arousal suggests an activational dependency on arousal for any number of diverse behavioral endpoints, but influence of this arousal is the coordination of the transition from a chaotic (i.e., not aroused) state to an ordered (i.e., aroused state) where systems of neuronal firing facilitate the desired behavior (Pfaff & Banavar, 2007). The arousal system works through both ascending and descending neuronal pathways. Ascending pathways deliver sensory information to the brain for the purpose of brain activation, whereas descending pathways prepare the organism for action by influencing autonomic and musculoskeletal systems (Pfaff & Banavar, 2007). The 5-HT system contains ascending projections to the basal forbrain, frontal cortex, limbic cortex, and hypothalamus (Jacobs & Azmitia, 1992) allowing for 5-HT to activate emotions, autonomic controls, and motor acts based on sensory information. Thus, the serotonin system provides an important neuronal system capable of contributing to a general state of arousal, both through ascending sensory information as well as descending activation through neurons for example in the hypothalamus.
A key feature of the arousal system is its redundancy, activating arousal through a number of mechanisms, which allow for its conservation when specific systems are damaged. A wide range of genes and hormones contribute to regulation of the arousal system (Pfaff, 2005a, 2005b) and thus provide important targets for integrating these broader influences into models of specific behavioral disturbances. For example, estrogens are known to be hormonal regulators of fear, in terms of general arousal as well as coordination of specific behaviors (Morgan, Schulkin, & Pfaff, 2004). Similar evidence for the estrogens' contribution to general arousal originates from detailed analysis of female sexual behavior (Mong & Pfaff, 2004), aggressive behavior (Ogawa, Choleris, & Pfaff, 2004), and locomotor activity (Ogawa, Chan, Gustafsson, Korach, & Pfaff, 2003) in mice.
Building upon this theory, eating behavior (seeking, consumption, and cessation) is dependent upon both general brain arousal and specific system coordination (see Figure 3). Because both estrogens and serotonin contribute to general brain arousal, disturbances in either or both systems may contribute to dysregulation in eating behavior by over-preparing the individual to seek and consume food or under-preparing the individual to stop eating. One aspect where feeding is clearly linked to general arousal is the neurotransmission of stomach sensation through 5-HT mediated mechanisms in the vagus nerve. Disruption in this vago-vagal reflex is thought to underlie BN (Faris et al., 2008) while also increasing the likelihood of affective disturbances (Faris et al., 2006). Overstimulation of this ascending circuit not only alters the negative feedback involved in cessation, but it also alters firing rates of serotonergic and norepinephren neurons (Manta, Dong, Debonnel, & Blier, 2009). Thus, the role of general brain arousal may also help explain other behavioral disturbances likely to co-occur with BN symptoms such as depression, impulsive drug and alcohol use, or compulsive anxiety reducing behaviors and harm avoidance.
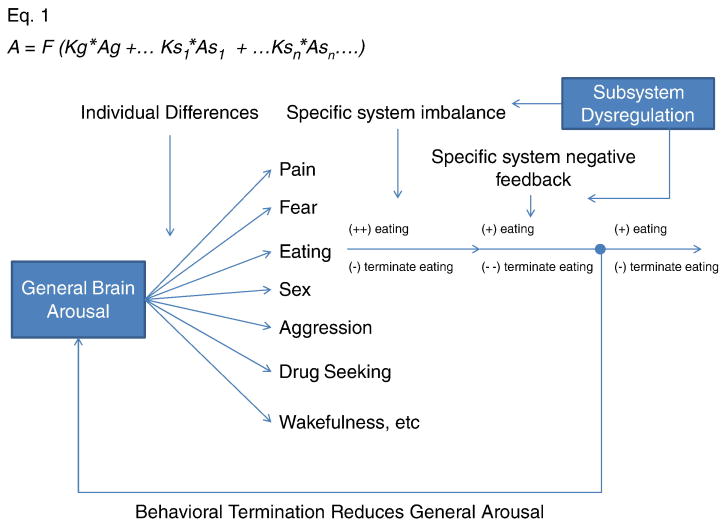
Figure 3.
Simplified schematic of general arousal and its relationship to specific behaviors. Equation 1 (adopted from Garey, et al., 2003) at the top, puts mathematical form to the concept where general arousal contributes to system specific behavioral states. A = arousal. Ag = generalized arousal. K = constant. s = specific system such as fear, eating, etc. This mathematical form also allows for K and A to vary by individual, suggesting different thresholds and dependency on general arousal for each person and for each behavior. As general arousal increases, the specific system transitions into a coordinated increase in behavior followed by a lagged inhibition of this behavior. Both processes occur simultaneously, but the balance between the specific behavioral system dictates which behavior is expressed. When the behavior terminates, it decreases general arousal, temporarily reducing the likelihood for the wide range of behaviors and relevant arousal states. The above model also allows for specific system dysregulation. For instance, 5-HT1a receptor dysregulation in the hypothalamus may reduce the eating specific system's negative feedback loop making it particularly difficult to stop eating once initiated.
2.2 Genetics of Bulimia Nervosa
Much of the genetic research on bulimia nervosa (BN) suggests a global contribution for the disorder (Bulik, Sullivan, & Kendler, 1998; Bulik, Sullivan, Wade, & Kendler, 2000) and for specific behaviors like disordered eating (Klump, McGue, & Iacono, 2002) and bingeing and purging (Sullivan, Bulik, & Kendler, 1998). Heritability estimates for BN range from 28-83% with an average of 50% (Gorwood, 2004; Hettema et al., 1995; Jacobi et al., 2004). Covariate linkage analyses suggest evidence for loci associated with age of menarche, body mass index (BMI), anxiety, concern over mistakes, and food obsessions in women with BN (Bacanu et al., 2005). However, the genetics data for BN is far from robust and the evidence for eating disorder linkage in families has been modest. Among the candidate genes identified, 5-HT gene polymorphisms are most clearly linked to disease states (Grice et al., 2002; Monteleone & Maj, 2008).
Epigenetic models provide some promise for elucidating the role of genes in BN pathology. Epigenetics effects include changes to the ways in which DNA are processed within the cell. Such changes to gene processing can alter the function of gene products (e.g., 5-HT receptors) transcribed from the DNA without the presence of polymorphisms in the original gene sequence. These types of changes are likely to be an important source of individual variability in genetically regulated aspects of eating disorder pathology. For instance, one such epigenetic change involves alteration of certain proteins (i.e., histones) which are attached to DNA and involved in the regulation of gene expression (Szyf, Weaver, & Meaney, 2007). There is some evidence that epigenetic changes alter both memory formation and fear conditioning (Miller & Sweatt, 2007), which are implicated in the pathogenesis and maintenance of eating disorders (Lascelles, Field, & Davey, 2003). Recent data also implicate epigenetic changes in the decreased mRNA transcription of the 5-HT transporter (5-HTTT; Philibert et al., 2008), which is broadly implicated in psychiatric illness and certain aspects of eating disorder pathology. With respect to BN, there is some evidence of these types of epigenetic effects on genes important for regulating serotonergic and dompamonergic function (Frieling et al., 2008; Frieling et al., 2009), although the functional impact of this elevation needs further investigation. An exciting aspect of the epigenetic model is that it also provides mechanisms for environmental influence over gene transcription and function. For example, one model suggests that maternal behavior alters gene function in the hippocampus (Weaver, Meaney, & Szyf, 2006) and ultimately the hypothalamic-pituitary adrenal (HPA) stress response of individuals into adulthood (Liu et al., 1997). If epigenetic effects are responsible for changing some aspects of gene function in the 5-HT system, then individuals exposed to epigenetic influences may be further primed for increased feeding and weight, response disinhibition, or trait anxiety.
In addition to epigenetic mechanisms, environmental exposure to certain chemicals may contribute to difficulty with weight regulation and traits such as impulsivity. Several chemicals that influence an individual's ability to regulate lipids, promote adipogenesis and rate or volume of fat deposition have been identified (Grun & Blumberg, 2009a, 2009b). It is believed that these chemicals (e.g., phytoestrogens, synthetic estrogens, environmental estrogens) act to regulate metabolic processes early in development and alter one's ability to naturally keep weight or body fat regulated. For example, exposure to low doses of bisphenol A (BPA), a substance found in plastics, leads to decreased fetal baby weight and subsequent estrous cycle dysregulation in offspring of rats (Rubin, Murray, Damassa, King, & Soto, 2001). Exposure to BPA also yields increased novelty seeking and approach behavior in rats, via dysregulation of the estrogen system (Gioiosa, Fissore, Ghirardelli, Parmigiani, & Palanza, 2007) suggesting that early dysregulation of the estrogen system may lead to trait based deficits in inhibition as well as changes to basic metabolic processes. These types of chemical effects appear to be regulated through estrogenic influence over enzymes that regulate glycolysis (Kostanyan & Nazaryan, 1992), insulin sensitivity (Kumagai, Holmang, & Bjorntorp, 1993), mitochondrial structure and function (Justo et al., 2005) and the tricarboxylic acid cycle (Yan et al., 2004). Taken together, the exposure to different exogenous estrogenic agents can have a robust impact on energy metabolism and homeostasis (Chen, Brown, & Russo, 2009) or impulsivity, perhaps predisposing individuals to weight change that either promotes early attempts at dieting or the tendency for binge eating.
3. The Role of 5-HT in Bulimia Nervosa
Dysregulation of the serotonergic system is perhaps the most robust neurobiological correlate of BN pathology (Monteleone, Brambilla, Bortolotti, & Maj, 2000; Steiger et al., 2004). The effects of 5-HT manipulation on feeding behavior have been demonstrated in both animal and human subjects (e.g., Blundell, 1986; Mancilla-Diaz, Escartin-Perez, Lopez-Alonso, & Cruz-Morales, 2002; Soulairac, 1963) and the general trend is a reduction in 5-HT is associated with increased feeding (Brewerton, 1995). Increased feeding in the context of reduced 5-HT may partially explain the link between increased risk for obesity and the development of BN (Allen, Byrne, Forbes, & Oddy, 2009). For example, the increased feeding and consequential weight gain potentiated by low 5-HT may lead to increased efforts at dietary control. However, the relationship between the 5-HT system and BN is difficult to delineate accurately, as 5-HT dysregulation may reasonably function as a trait, state, and/or a “scar” of BN (Steiger, 2004). The aspects of the 5-HT system that appear abnormal in patients with BN include the 5-HT transporter (5-HTT), 5-HT2a receptor, and 5-HT1a receptor (Guido et al., 2002).
3.1 The Role of the 5-HT Transporter in Bulimia Nervosa
The 5-HTT plays a broad regulatory role in the 5-HT system and alterations to 5-HTT availability or function are most clearly linked to an anxious phenotype that includes anxious arousal and perception (Holmes, Murphy, & Crawley, 2003; Zhao et al., 2006), but also altered sleep (Wisor et al., 2003), altered sensory thresholds for pain (Vogel et al., 2003), and increased gastric motility (Chen et al., 2001). These broad consequences are consistent with 5-HTT's role in general brain arousal, supporting a state of heightened alertness, increased behavioral avoidance, and intense negative affect which occur as part of the trait anxiety found among some women with BN.
The 5-HTT is a membrane receptor that functionally conserves available 5-HT and can increase the specificity of information communicated between neurons by limiting 5-HTT diffusion (Rudnick, 2006). Less available 5-HTT or poor functionality of existing 5-HTT (due to genetic polymorphism or epigenetic changes) leads to too much or too little brain arousal depending on the context. For example, electrophysiological studies suggest that 5-HTT polymorphisms lead to decreased spontaneous firing rates of 5-HT neurons in the dorsal raphe (Gobbi, Murphy, Lesch, & Blier, 2001) and delayed restoration of normal firing rates in hippocampal neurons (Mannoury la Cour et al., 2001). Furthermore, this loss of specificity in neuronal firing can increase levels of anxiety and avoidant behavior via changes to other 5-HT receptors in the serotonergic neurons that populate the cortico-limibic circuits responsible for anxious appraisal, emotions, and associated behavioral responses (Murphy et al., 2008).
BN patients consistently show decreased 5-HTT availability. Tauscher and colleagues (2001) noted a 17% decrease in 5-HTT availability in the hypothalamus and thalamus among women with BN when compared to controls and this deficiency was correlated with duration of BN. This deficency may be in partially related to higher rates of the short-allele polymorphism for 5-HTT in BN patients (Di Bella, Catalano, Cavallini, Riboldi, & Bellodi, 2000), although this finding has yet to be replicated. Ekman, Sundblad-Elverfors, Landen, Eriksson, and Eriksson (2006) noted low density but high affinity of platelet [3H] paroxetine (an SSRI) binding in women with BN relative to controls. Nevertheless, the link between dysregulation of the 5-HTT gene and binge symptoms occurs consistently, including with obese binge eaters (Kuikka et al., 2001). Thus, there is some support for a link between low 5-HTT binding and binge eating, with the exact mechanisms still debated (Frank, Bailer, Henry, Wagner, & Kaye, 2004).
These observations support the phenotypic observations in human genetic studies of increased anxiety, harm avoidance, and negative bias associated with 5-HTT polymorphisms (Anguelova, Benkelfat, & Turecki, 2003; Murphy et al., 2003). Personality disturbances have also been linked to 5-HTT disturbances. For instance, Greenberg and colleagues (2000) found a significant association between the s allele polymorphism for 5-HTT and higher NEO Neuroticism and lower NEO-PI- Agreeableness scores across individuals and within families. Also, Lesch and colleagues (1996) determined that the s allele 5-HTT polymorphism accounts for an estimated 3-4% of the total variation and 7-9% of inherited variance in anxiety-related personality traits in individuals and siblings. Coccaro, Berman, Kavoussi, and Hauger, (1996) found an inverse relation between platelet 5-HTT binding and impulsive aggression, while others have noted a similar relationship between platelet 5-HTT binding and self-mutilation in women with personality disorders (Stein et al., 1996). Some of the differential effects of 5-HTT polymorphism on traits such as anxiety versus impulsivity may be due to interactive affects with other aspects of serotonergic metabolism. For instance, Passamonti and colleagues (2008) found interactions between monomamine-oxidase-A (MAO-A) and 5HTT genes in clearance of serotonin in prefrontal brain areas thought to coordinate inhibition. This interaction yielded lower available 5-HT for neural transmission and functionally weakened serotonergic specificity, reducing the ability of cortical structures to inhibit emotionally driven behaviors.
3.2 The Role of the 5-HT2a Receptor in Bulimia Nervosa
The 5-HT2a receptor is a postsynaptic receptor that increases neuronal firing and is located in a number of cortical and prefrontal areas, but particularly in the caudate nucleus, hippocampus, and nucleus accumbens (Barnes & Sharp, 1999; Pazos, Probst, & Palacios, 1987). The 5-HT2a receptor downregulates in response to prolonged 5-HT exposure (Sanders-Bush, 1990). Genetic polymorphisms of the 5-HT2a receptor have been found to be positively correlated with novelty seeking, suggesting that lower functioning 5-HT2a receptors yield a higher likelihood of an approach response in the context of environmental uncertainty (Heck et al., 2009). Furthermore, increased density of the 5-HT2a receptor in the mPFC correlates with the coupling of mPFC-amygdala activation, implicating 5-HT2a functionality in the integration of emotionally relevant stimuli through cortico-limbic circuits (Fisher et al., 2009). Thus, decreased 5-HT2a functionality or density is likely to lead to increased impulsivity or difficulty regulating affect (Frokjaer et al., 2008; Moresco et al., 2002), for example through functional polymorphisms (Nomura et al., 2006).
Imaging studies of 5-HT2a in women with BN generally suggest decreased receptor binding in cortico-limbic circuits. For example, Bailer et al. (2004) found decreased 5-HT2a receptor binding in patients who recovered from the binge-purge subtype of anorexia (ANBP) relative to control patients. This finding, however, was not statistically significant. Kaye and colleagues (2001) noted reduced 5-HT2a receptor binding in the medial orbital frontal cortex (mOFC) of women who had recovered from BN (REC-BN). The REC- BN group also failed to show the expected age-related decreases in 5-HT2a receptor binding found in healthy controls. Some evidence suggests that 5-HT2a polymorphisms may lead to this observed decrease 5-HT2a binding (Nishiguchi et al., 2001; Ricca et al., 2002; Bruce et al., 2005), but these associations are not always replicated (Enoch et al., 1998; Nacmias et al., 1999) and this may be due to small sample sizes available in the latter studies.
Other studies of 5-HT receptor binding in patients with BN have yielded intriguing results. Steiger, Young, and colleagues (2001) found reduced platelet binding of [3H]-peroxetine and a lower density of [3H]-peroxetine binding sites in women with BN relative to healthy controls. Furthermore, this same group noted blunted responses following meta-chlorophenylpiperazine (m-CPP) in women with BN who reported self-harm behaviors relative to both healthy controls and women with BN who did not engage in self-harm (Steiger, Koerner et al., 2001). These changes to 5-HT function may only exist for the duration of the illness. For instance, researchers have shown that in REC-BN patients do not differ from controls or may be significantly higher in response to a 5-HT agonist (Wolfe et al., 2000). These data suggest the possibility that alterations to the 5-HT system may be related to pre-existing personality, change or increase as function of the bulimic symptoms, and resolve with recovery from BN.
3.3 The 5-HT1a Receptor in Bulimia Nervosa
The 5-HT1a receptor is an inhibitory autoreceptor typically found on the both on the presynaptic and postsynaptic neuron in the hippocampus, lateral septum, cingulated and entorhinal cortex, amygdala and raphe nuclei (Barnes & Sharp, 1999). Electrophysiologic studies confirm this inhibitory effect; 5-HT1a activation reduces cell firing in hippocampus (Kasamo et al., 2001), forbrain (Ashby, Edwards, & Wang, 1994), and raphe nuclei (Haddjeri, Lavoie, & Blier, 2004) neurons. The inhibitory role of 5-HT1a over serotonergic transmission implicates it in anxiety related phenomena. For instance, reduced 5-HT1a binding (yielding difficulty inhibiting serotonergic-dependent activation) is found in most anxiety disorders (Akimova, Lanzenberger, & Kasper, 2009). As such, most models of 5-HT1a dysregulation suggest increases in harm avoidance (Hansenne & Ansseau, 1999), although anxiety behaviors can occur with both under or overexpression of the receptor (Overstreet et al., 2003).
The 5-HT1a receptor is likewise implicated in BN pathology. Tiihonen and colleagues (2004) found elevated levels of brain 5-HT1a receptor binding in patients with BN. Bendotti and Samanin (1987) found that the activation of 5-HT1a receptors is associated with increased feeding in rats permitted to feed ad libitum. The 5HT1a effects are partially mediated by raphe nuclei projections of these neurons to the amygdala (Parker & Coscina, 2001), suggesting a role in the hedonic regulation of feeding or regulation of preferences for palatable food (Moreau, et al., 1992). The role of 5HT1a is also implicated in trait disturbances found in BN. For instance, there is evidence that 5-HT1a and 5-HT2a are co-expressed in mPFC neurons (Amargos-Bosch et al., 2004) suggesting a possible synergistic effect on trait-level disturbances in compulsivity and impulsivity (Carli, Baviera, Invernizzi, & Balducci, 2006). Administration of 5-HT1a agonist increases commission errors in impulsivity paradigms (Carli & Samanin, 2000). Additional effects of the 5-HT1a neurons are found in the consolidation and retrieval of emotional memory which is likely to play an important role in anxiety and mood disturbances (Drevets et al., 2007; Ogren et al., 2008).
4. The Role of the Estrogens in Bulimia Nervosa
Gonadal hormones, such as estrogen, have been implicated in the organization of neuronal systems involved in eating and activation of patterns of disordered eating. Several epidemiologic features suggest this role, such as the observation that BN is significantly more common in women (Hoek & van Hoeken, 2003; Hudson et al., 2007), these symptoms usually begin at puberty (Hayward et al., 1997), and their frequency often diminish by mid-life and the menopausal years (Leon, Keel, Klump, & Fulkerson, 1997; Strober, Freeman, & Morrell, 1997). In particular, estrogen is well positioned to affect general brain arousal found in traits such as impulsivity and compulsivity as well as specific behavioral endpoints such as feeding, which are implicated in BN pathology.
4.1 Estrogenic Effects on Feeding and Weight
Body weight is known to be a relative robust sexually dimorphic trait, with females being smaller than males, where estrogens play a key role in keeping meals small, weight down, and subcutaneous (as opposed to visceral) fat high (Butera, 2009; Shi & Clegg, 2009). In humans, reliable caloric decreases are found among women during the periovulary phase of the menstrual cycle when estrogen is at its peak (Buffenstein, Poppitt, McDevitt, & Prentice, 1995). In animals, overiectomy of adults leads to increased body weight via increased meal size and overall food intake (Asarian & Geary, 2002; Blaustein & Wade, 1976; Wade, 1975) and treatment with estradiol, a synthetic estrogen, decreases food intake and body weight (Czaja, Butera, & McCaffrey, 1983; Wade, 1975). Estradiol injected into female rats post-ovariectomy normalizes their body weight and feeding patterns (Asarian & Geary, 2002). The centralized effects of estrogen in the hypothalamus and hindbrain are likely where this regulation occurs (Butera, 2009). These centralized effects of estrogens have downstream activational effects on hormonal systems that signal satiety (Butera, Bradway, & Cataldo, 1993), ultimately leading to earlier cessation of feeding behavior. Specifically, available estradiol acting in the parventicular nucleus (PVN) of the hypothalamus increases the sensitivity of the brain to satiety signals of cholecystokinin (CCK; Eckel & Geary, 1999; Thammacharoen, Geary, Lutz, Ogawa, & Asarian, 2009). Thus, estrogenic effects on cessation of feeding appear to be partially dependent upon the brain arousal brought about by available circulating estrogens.
Estrogens also play a significant role in signaling amount and type of adiposity to the brain. Subcutaneous fat is higher among females than males and depends partially on leptin signals sent from this peripheral tissue into the blood stream (Schwartz, Woods, Porte, Seeley, & Baskin, 2000). Estrogens are responsible for sensitizing metabolic systems to leptin signals in the hypothalamus (Clegg, Benoit, Barrera, & Woods, 2003; Clegg, Tamashiro, Strader, & Woods, 2004), thus regulating energy expenditure. Furthermore, subcutaneous fat has a differentially high number of estrogen receptors, implicating its role in regulating these peripheral signals of adiposity (Crandall, Busler, Novak, Weber, & Kral, 1998). As estrogens increase during puberty, subcutaneous fat increases and this may trigger attempts at dieting due to anxiety about these changes in outward appearance.
Very little research has been done specifically with estrogens in the context of binge eating. One notable exception, Yu, Geary, and Corwin (2008) found that under binge-type conditions, female rats that were injected with ovarian hormones after being ovariectomized had smaller binge episodes and consumed less fat than controls. Progesterone, an estrogen receptor antagonist, consistently induces overeating in rats (Roberts, Kenney, & Mook, 1972; Rodier, 1971; Zucker, 1969); so, the interplay of progesterone and estrogen are likely to affect feeding behavior among females and ultimately the overeating aspects of binge eating found among those with BN.
4.2 Evidence of Estrogenic Influence on Symptoms of Bulimia Nervosa
Around 45-62% of women with BN experience some form of menstrual dysregulation, particularly oligomenorrhea, although amenorrhea occurs in 5-40% of women with BN (Crow, Thuras, Keel, & Mitchell, 2002; Gendall, Bulik, Joyce, McIntosh, & Carter, 2000; Poyastro Pinheiro et al., 2007). Austin and colleagues (2008) reported a significant association between vomiting and irregular menses in adolescent females, suggesting that purging may impact menstruation. However, certain weight-loss practices commonly employed by patients with BN (e.g., prolonged dietary restriction) are directly known to dysregulate menstruation (Loucks, 2003), but these observations suggest the possibility of disturbed estrogen function. Table 1 summarizes the studies specifically examining the relationship between estrogen level and binge eating in women with BN. When studied using menstrual status as an indicator of estrogen, the data indicate that binge eating worsens during the premenstrual or mid-luteal phase and consequently when estrogen is low and progesterone is high. These results have been replicated by Klump and colleagues (2008), who examined frequency of binge eating severity across the course of a menstrual cycle in two community samples. The authors noted robust associations between ovarian hormones (decreased estrogen and increased progesterone) and binge eating in clinical and non-clinical women. For women with BN, the converging evidence suggests that decreases in estrogen are associated with overeating and a greater likelihood of feeling “out of control.”
Table 1. Summary of Prospective Studies Examining the Relationship Between Menstrual Status and Binge Eating.
| Study | Sample | Diagnosis | Menstrual Phase | |||
|---|---|---|---|---|---|---|
| Follicular | Ovullatory | Midluteal | Premenstrual | |||
| Edler, Lipson, & Keel (2007) | 17 F | 9 BN; 8 NC | -.30 (.35) | -.37 (0.21) | .61 (.33) | -.08 (.35) |
| Lester, Keel, & Lipson, (2003) | 16 F | 8 BN; 8 NC | -0.13 (.23) | -.29 (.34) | .35 (.70) | .41 (.37) |
| Gladis & Walsh, (1987) | 15 F | 15 BN | N/A | -.43 (.93) | N/A | .61 (.73) |
| Price, Torem, & DiMarzio, (1987)* | 10 F | 10 BN | 4.10 (.66) | 6.65 (1.87) | ||
Note. Z-scores and standard errors of binge episodes reported in each cell. F = adult female. BN = Bulimia Nervosa. NC = Normal Control. N/A = not available.
z-scores not available so raw means (SD) reported.
4.3 Organizational and Activational Effects of Estrogen on Disordered Eating
Some researchers (e.g., Klump et al., 2006) have postulated that gonadal hormones affect both sexes during prenatal (organizational) and post-natal (activational) periods such that these hormone differences account for much of the observed sex differences in behavior (e.g., disordered eating). According to this hypothesis, prenatal exposure to testosterone plays a protective role against the development of an eating disorder by organizing the CNS to be more masculine, while estrogen, which spikes in young women during the onset of puberty, activates disordered eating. Evidence from non-human primates suggests that elevated prenatal circulating androgen yields higher expression of cystolic and nuclear androgen receptors, but that the functional effects of this organization may be partially explained by the aromatase pathway and the ability of estrogen to influence the masculinized or de-masculinized brain (Resko & Roselli, 1997). Thus, organizational vulnerabilities may involve moderating the general brain arousal effects of estrogens by organizing the CNS to be more or less sensitive to circulating gonadal hormones during puberty.
The hypothesized post-natal or activational role of estrogens is based on correlational evidence between estrogen and disordered eating (Klump et al., 2006) as well the moderating role of puberty on symptoms of disordered eating in women (Klump, Perkins, Alexandra Burt, McGue, & Iacono, 2007). Evidence for the role of sex hormones on feeding is also well established and suggests that prenatal exposure to testosterone increases feeding while lowering activity (Madrid, Lopez-Bote, & Martin, 1993) and increases in estrogen are associated with decreased feeding and increased activity (Eckel, 2004). The post-natal estrogen effects may depend on these pre-natal environments to bring about binge eating as well as other disturbances in brain arousal that manifest as increased anxiety or impulsivity.
The generalized organization-activation hypothesis raises a number of important questions. Given that most women do not have an eating disorder despite the universal increase in estrogen levels in women who reach puberty, the possibility exists that the activational influences may be genetically mediated (i.e., variations in the genes encoding for estrogen receptors or systems effected by estrogen) or moderated by environmental effects. In addition, the general effect of estrogen is to reduce feeding behavior (Geary, 2001), but this may be context specific (Shelley, Dwyer, Johnson, Wittkowski, & Pfaff, 2007) and involve estrogen regulation of a neurotransmitter systems such as 5-HT.
5. Mechanisms of Estrogenic Effects on Symptoms of BN
5.1 Estrogenic Effects on 5-HT
The effects of ovarian steroids on the 5-HT neurotransmitter system have been subject to considerable investigation (Bethea, Pecins-Thompson, Schutzer, Gundlah, & Lu, 1999) and effects can broadly be conceptualized as regulatory, although the specific role varies across brain regions and within different neural circuits (Bethea, Lu, Gundlah, & Streicher, 2002). Figure 4 summarizes how estrogens influence serotonergic neuronal firing. These effects include changes to density and function of 5-HTT, 5-HT1a, and 5-HT2a receptors as well as changes to the rate of synthesis and degredation of 5-HT. The overall effect of estrogen differs based on the nature and location of the specific neurons affected; however, estrogen can be conceptualized as increasing the sensitivity of the serotonergic system, rendering it more efficient and prepared to deliver information to relevant brain regions responsible for coordinating specific behaviors.
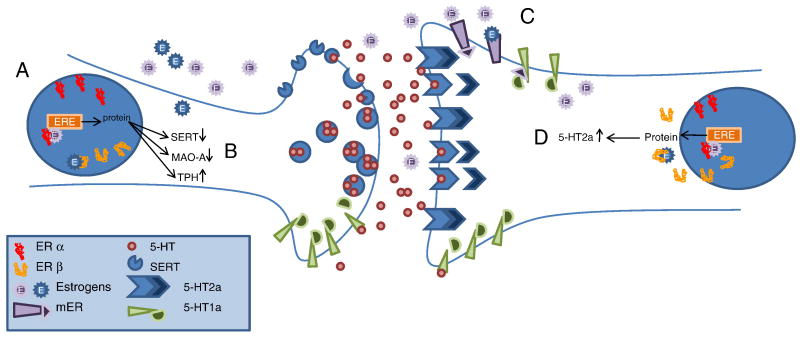
Figure 4.
Estrogens influence serotonergic transmission in a number of specific ways summarized above. (A) Estrogens bind to nuclear ER α or β which activate transcription factors for a number of proteins via EREs. Transcription results in increased SERT and TPH and decreased MAO-A. (B) The net result of these changes is increased intracellular 5-HT and decreased clearance of 5-HT from the synaptic cleft. TPH degrades tryptophan into 5-hydroxytryptophan whereas MAO-A degrades 5-HT into the inactive 5-HTIAA metabolite. SERT recovers synaptic 5-HT from the cleft, conserving it and functionally terminating the neural transmission. (C) Activation of the mERs deactivates 5-HT1a receptors by uncoupling G proteins. The 5-HT1a receptor is an autoreceptor that inhibits neural firing, so deactivating of the receptor functionally reduces the inhibitory effects of 5-HT1a. (D) Post-synaptic 5-HT2a receptors are excitatory neurons which increase neuronal firing. Estrogens working through nuclear receptors increase 5-HT2a mRNA and functionally increase the neuronal sensitivity to presynaptic 5-HT release. ER = estrogen receptor. ERE = estrogen response element. mER = membrane estrogen receptor. E = estrogen. SERT = serotonin reuptake transporter. MAO = monoamine oxidase. TPH = tryptophan hydroxylase.
Estrogen exerts its effects within the brain primarily through estrogen receptors, of which there are two identified subtypes: alpha and beta (Enmark et al., 1997; Kuiper et al., 1997). These subtypes are not true isoforms as they are encoded by different genes on separate chromosomes (Enmark et al., 1997; Tremblay et al., 1997) The beta subtype primarily localizes among serotonergic neurons in primates (Gundlah et al., 2000) although specific regions such as the hypothalamus have evidence of both alpha and beta estrogen receptors (Bethea, Brown, & Kohama, 1996). Serotonin neurons project primarily to the forbrain and have substantial inputs in the hypothalamus, amygdala, hippocampus, and prefrontal cortex (Azmitia, 2007; Hornung, 2003). Some effects of estrogen receptors on 5-HT neurons is dependent upon activation of progesterone receptors and thus progesterone can moderate estrogenic effects on serotonin neurons (Bethea, 1994).
Intracelllular estrogen receptors function by binding to estrogen molecules in the nucleus of the cell and acting as transcription factors, interacting with estrogen response elements, to alter the expression of a number of genes that influence 5-HT cell functioning (Bagchi, Tsai, Omalley, & Tsai, 1992; Baniahmad & Tsai, 1993). Ligands with different binding affinities have been identified for the alpha and beta subtypes (Katzenellenbogen, Muthyala, & Katzenellenbogen, 2003) although both receptors display equivalent affinity for the naturally occurring estrogen, 17β-estrodiol (Kuiper et al., 1997). These types of estrogen mediated cellular changes are considered genomic effects and alter cell firing less rapidly than nongenomic estrogen effects.
Nongenomic estrogen effects on cellular excitability are also of interest (McEwen, 2002; Spencer et al., 2008). It has long been observed that estradiol could rapidly alter neuronal firing in the hypothalamus (Kelly, Moss, & Dudley, 1976; Kelly, Moss, Dudley, & Fawcett, 1977) suggesting the possibility of rapid actions mediated through membrane receptors. There is a number of membrane estrogen receptors (mERs) including ER-X, Gq-mER, and GPR30 (Kelly & Ronnekleiv, 2008). These receptors are thought to coordinate with nuclear estrogen receptors' activation of gene products on cellular excitability (Kelly & Ronnekleiv, 2009). One interesting area of mER activity is the regulation of feeding and energy homeostasis. For instance, there is some evidence that mERs regulate proopiomelanocortin (POMC) and neuropeptide-Y neurons responsible for weight regulation and possibly the rewarding properties of food (Qiu et al., 2006; Roepke, Qiu, Bosch, Ronnekleiv, & Kelly, 2009; Roepke et al., 2008).
There are several potential points of influence for estrogenic effects over the 5-HT system including release, degredation, and receptor activation which function as mechanisms of neural firing. In addition, overall rate of 5-HT firing is regulated by 5-HT synthesis and reuptake. Among these points of influence, estrogen may exert direct and indirect effects at each of them (Bethea, Lu et al., 2002). Because there is some evidence of genetic contributions to BN through both 5-HT receptor polymorphisms and 5-HTT gene polymorphisms, we will focus on estrogenic influences over these aspects of the 5-HT system.
5.2 Estrogenic Effects via Estrogen Receptors
There are at least 14 subtypes of serotonin receptor (Hannon & Hoyer, 2008; Uphouse, 1997) and the 5-HT1a and 5-HT2a are the two subtypes that have the largest associations with BN symptoms. The location of these receptor subtypes is well documented in human and animal models of the 5-HT system. Primarily, 5-HT1a receptors are found in limibic regions, raphe nuclei, hippocampus, lateral septum, cingulated, and entorhinal cortex. The colocalization of 5-HT1a receptors and ER alpha and beta occurs in many of these regions (Shughrue & Merchenthaler, 2001). For instance, the lateral and dorsal hypothalamus and preoptic hypothalamic area all project to median raphe and are densely populated by ER alpha and beta. Similarly, the median raphe also receives projections from the mPFC which contains ER beta neurons (Shughrue, Scrimo, & Merchenthaler, 1998, 2000). Evidence from animal data suggests that estrogens do not alter 5-HT1a mRNA expression (Bethea, Mirkes, Su, & Michelson, 2002; Gundlah, Pecins-Thompson, Schutzer, & Bethea, 1999), but replacement of estrogen in ovariectomized rats yields increased 5-HT1a binding (Le Saux & Di Paolo, 2005). However, mER activations have been found to decrease 5-HT1a function through mER mechanisms (Mize, Poisner, & Alper, 2001). This effect is likely due to increased uncoupling of G-proteins to 5-HT1a receptors (Mize & Alper, 2000, 2002), which effectively reduces their effects on inhibiting neuronal firing. In contrast, 5-HT2a receptors are located primarily in cortical areas, hippocampus, hypothalamus, and thalamus (Burnet, Eastwood, Lacey, & Harrison, 1995; Gundlah et al., 1999) and exhibit classic features of G-protein coupled receptors. Estrogen replacement has been found to increase 5-HT2a receptor density in the dorsal raphe nuclei and prefrontal cortex via increases in mRNA expression (Sumner et al., 1999) and this is mediated through nuclear receptors (Sumner et al., 2007).
Functional investigations of the ERs support their independent contribution as well as 5-HT mediated contributions to BN relevant states and behavior. The role of the estrogen in anxiety and fear condition is perhaps the most relevant to women with BN. Specific investigations of estrogenic effects on 5-HT suggest increased 5-HT system activation. For instance, estrogen down-regulates 5-HT1b autoreceptor mRNA in the raphe nuclei supporting an estrogenic role in increasing 5-HT system activation (Hiroi, McDevitt, & Neumaier, 2006; Hiroi & Neumaier, 2009). This increase is likely to contribute to observable reductions in anxiety associated with estrogen. However, these effects may be receptor specific; the ER beta is believed to mediate this anxiolytic effect of estrogen (Weiser, Foradori, & Handa, 2008). Cumulating evidence from gene knockout and selective estrogen modulator studies suggests the effects of estrogen in the hippocampus via ER beta are responsible for some of this anxiolytic effect (Walf & Frye, 2008a, 2008b). This relationship, however, is complex; ER alpha agonists potentiate fear learning whereas ER beta agonists disrupt the same conditioning (Toufexis, Myers, Bowser, & Davis, 2007). This differential effect may be in part due to the differences in colocalization patterns of ER alpha and beta, but also because ER beta receptor activation decreases in stress response (Lund, Rovis, Chung, & Handa, 2005). Thus, estrogen is likely to affect relevant anxiety states and/or traits among women with BN through direct mechanisms as well as indirectly through regulation of 5-HT.
5.3 Estrogenic Effects on 5-HTT Mechanisms
The 5-HT system's overall function is in part related to 5-HTT that bind to 5-HT in the synaptic cleft and transport these molecules back into the serotonergic neuron. The 5-HTT protein is found primarily at 5-HT neuron terminals (Hoffman, Hansson, Mezey, & Palkovits, 1998). There is some evidence that estrogen increases 5-HTT mRNA expression in the amygdala, lateral septum, hypothalamus, and dorsal raphe nuclei, but decreases binding in the hippocampus (McQueen, Wilson, Dow, & Fink, 1996; McQueen, Wilson, & Fink, 1997). This reduction may occur because estrogen increases the amount of synaptic 5-HT by reducing the presence of 5-HTT proteins (Pecins-Thompson & Bethea, 1999). However, results of similar tests of estrogen on 5-HTT are not consistent (Attali, Weizman, GilAd, & Rehavi, 1997; Krajnak, Rosewell, Duncan, & Wise, 2003; Zhou et al., 2002) and the effect of estrogen on 5-HTT appears to differ based on brain region and involve more than just transcription mechanisms (Lu, Eshleman, Janowsky, & Bethea, 2003). In the context of SSRI treatment, estrogen appears to interfere with the rate of 5-HT clearance from the synapse. Specifically, 5-HTT proteins clear more 5-HT in the presence of estrogen and the SSRI, fluoxamine in the hippocampus (Benmansour, Piotrowski, Altamirano, & Frazer, 2009). The direction and degree to which 5-HTT mechanisms regulate specific 5-HT firing in specific brain regions or neuronal pathways remains a complicated issue. It is clear that estrogen affects this regulatory mechanism, but a comprehensive model of estrogen's influence on 5-HTT has yet to be developed.
5.4 Estrogenic Effects on 5-HT Synthesis and Degradation
The synthesis of 5-HT begins with the breakdown of tryptophan by the enzyme tryptophan hydroxylase (TPH) into 5-hydroxytryptophan, which is then converted to 5-HT. Evidence suggests that mRNA and protein mass for TPH increase in nonhuman primates with estrogen replacement (Bethea, Mirkes, Shively, & Adams, 2000; PecinsThompson, Brown, Kohama, & Bethea, 1996). Thus, estrogen is believed to increase synthesis of 5-HT broadly through increasing TPH. Degradation of 5-HT is also affected by estrogen. Monoamine oxidases (MAOs) are the enzymes responsible for metabolizing 5-HT into the inactive metabolite 5-HIAA. The MAO-A subtype which selectively degrades 5-HT and MAO-B which degrades dopamine have been localized to 5-HT neurons (Saura, Kettler, Daprada, & Richards, 1992; Westlund, Denney, Kochersperger, Rose, & Abell, 1985; Westlund, Denney, Rose, & Abell, 1988). Experimental evidence suggests that estrogen decreases gene transcription for both MAO-A and MAO-B in the dorsal raphe and hypothalamus (Gundlah, Lu, & Bethea, 2002). Furthermore, selective estrogen receptor modulators for ER alpha have also proven to decrease MAO-A as well 5-HTT (Smith, Henderson, Abell, & Bethea, 2004). Thus, the impact of estrogen on 5-HT pathways appears to be an increase in neuronal transmission of serotonergic neurons via increased intracellular 5-HT.
6. Inferences and Outlook
The neurobiological evidence suggests significant contributions to trait and behavioral disturbances associated with BN by both 5-HT and estrogen dysregulation. The neural networks affected by both neurochemicals significantly overlap and share properties by affecting both general brain arousal and by coordinating specific behaviors. Circulating estrogens (like many hormones) and 5-HT are well positioned to alter an organism's sensitivity to sensory information, locomotor activity, and emotional reactivity. Changes in either neurochemical system affect general arousal state, and when functioning, work together to regulate mood as well as the specific behaviors involved in eating. However, BN is marked by dysregulation in both estrogenic and serotonergic functioning. The nature of this dysregulation can be variable, with contributions originating from genetic polymorphisims, prenatal environment, epigenetic changes to gene function, environment exposure to chemicals that alter theses systems, or through bulimic symptoms. Although, both estrogen and 5-HT system contributions to BN are robust, it is almost certain that these systems do not tell the entire story of BN symptomology. Other neurotransmitters, hormones, and peptides are likely to play important roles in this pathology. The link to general arousal offers a number of clear and testable links between dysregulation in eating and a number of other behavioral endpoints or arousal states such as fear, sex, locomotor activity, aggression, or drug seeking.
Overlap of these two systems opens up the possibility for a number of therapeutic targets. Clinical trials beginning in the 1980s have consistently demonstrated the utility of antidepressants such SSRIs in treating symptoms of BN (Fichter et al., 1991; Goldstein, Wilson, Thompson, Potvin, & Rampey, 1995; Pope, Hudson, Jonas, & Yurgelun-Todd, 1983; Sabine, Yonace, Farrington, Barratt, & Wakeling, 1983), and that this remission in BN symptoms occurs independently of the SSRI's effects on depressive symptoms (Goldstein, Wilson, Ascroft, & al-Banna, 1999). Treatment with SSRIs is also indicated for BN patients who do not initially respond to psychotherapy (Walsh et al., 2000). Their effectiveness, although variable, suggests 5-HT is a useful therapeutic target for women with BN. Usefulness of estrogens in treatment of BN has not been evaluated and there is no published research on whether menstrual status moderates SSRI outcomes. Recent animal data suggest that estradiol supplementation in rats with sertraline increases 5-HT2a receptor mRNA (Sell et al., 2008). This finding is consistent with other animal research using fluoxetine (Estrada-Camarena, Fernandez-Guasti, & Lopez-Rubalcava, 2006; Estrada-Camarena, Lopez-Rubalcava, & Fernandez-Guasti, 2006) suggesting an overall synergistic effect between serotonergic and estrogenic therapeutics. In postmenapuasal women, hormone replacement therapy appears to enhance the effects of SSRI on depression (Schneider, Small, & Clary, 2001; Zanardi et al., 2007). An important question to answer is whether estrogens moderate existing treatments for women with BN and whether this hormonal indicator should be a clinical indicator of note for treatment providers.
Estrogenic targets include nuclear receptors alpha and beta as well as mERs. Although a number of selective estrogen receptor modulators (SERMs) exist (Clarke & Khosla, 2009), there utility in psychiatry is in its infancy. A recent study has reported on five cases of depression treated with raloxifine in women with chronic AN and reported significant decreases in depressive symptoms (Yokoyama, Sugiyama, Sugiyama, & Amano, 2008). There are several important questions about the utility of these agents, either in combination with serotonergic drugs, or as ‘stand alone’ treatments. In particular, do the animal studies suggesting favorable changes in mood and feeding behavior translate to psychiatric conditions such as BN? There may also be unique and yet understudied influences of estrogens on specific brain processes in adolescents and young adults. Recent evidence suggests a neuroprotective role for estrogen on serotonergic neurons in the primate brain (Bethea, Reddy, Tokuyama, Henderson, & Lima, 2009), but whether there are similar protective or degenerative effects that occur during puberty is an important area for future study. If altered estrogen levels contribute to specific structural or functional changes in relevant brain targets in adolescence, then the estrogen system could be positioned as a primary target for biological risk markers or sources for early intervention. Finally, estrogenic influence over other relevant neurotransmitter and hormonal systems will be important for expanding this model of BN. There is clearly some role for estrogenic moderation of dopamanergic systems (Alyea & Watson, 2009b) and this moderation may involve environmental estrogen exposure (Alyea & Watson, 2009a). In addition, estrogens may help differentiate stem cells into dopamine neurons (Diaz, Diaz-Martinez, Camacho-Arroyo, & Velasco, 2009), the implications of which are yet to be understood. The role dopamine and other neurotransmitters play in the estrogen—serotonin model of BN will be an important target for future research.
Acknowledgments
Tom Hildebrandt's research on this project was supported by a grant from the National Institute on Drug Abuse (NIDA): K23 024034-01A1. We would like to acknowledge Maggie Zellner, PhD for her extensive feedback on a revision to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biological Psychiatry. 2009;66(7):627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Allen KL, Byrne SM, Forbes D, Oddy WH. Risk factors for full- and partial-syndrome early adolescent eating disorders: a population-based pregnancy cohort study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(8):800–809. doi: 10.1097/CHI.0b013e3181a8136d. [DOI] [PubMed] [Google Scholar]
- Alyea RA, Watson CS. Differential Regulation of Dopamine Transporter Function and Location by Low Concentrations of Environmental Estrogens and 17 beta-Estradiol. Environmental Health Perspectives. 2009a;117(5):778–783. doi: 10.1289/ehp.0800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyea RA, Watson CS. Nongenomic mechanisms of physiological estrogen-mediated dopamine efflux. BMC Neuroscience. 2009b;10:9. doi: 10.1186/1471-2202-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin(1A) and serotonin(2A) receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14(3):281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Molecular Psychiatry. 2003;8(6):574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones and Behavior. 2002;42(4):461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17(3):173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Attali G, Weizman A, GilAd I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Research. 1997;756(1-2):153–159. doi: 10.1016/s0006-8993(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Austin SB, Ziyadeh NJ, Vohra S, Forman S, Gordon CM, Prokop LA, et al. Irregular menses linked to vomiting in a nonclinical sample: findings from the National Eating Disorders Screening Program in high schools. Journal of Adolescent Health. 2008;42(5):450–457. doi: 10.1016/j.jadohealth.2007.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Pharmacology of Neurogenesis and Neuroenhancement. 2007;77:31–49. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Salgado BR, Segal HD, Lorenzo CV, Acosta MN, Romero MA, et al. A pilot genetic study of the continuum between compulsivity and impulsivity in females: the serotonin transporter promoter polymorphism. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29(5):713–717. doi: 10.1016/j.pnpbp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Bulik CM, Klump K, Fichter MM, Halmi KA, Keel P, et al. Linkage analysis of anorexia and bulimia nervosa cohorts using selected behavioral phenotypes as quantitative traits or covariates. [Article] American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2005;139B(1):61–68. doi: 10.1002/ajmg.b.30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi MK, Tsai MJ, Omalley BW, Tsai SY. Analysis of the mechanisms of steroid-hormone receptor-dependent gene activation in cell free systems. Endocrine Reviews. 1992;13(3):525–535. doi: 10.1210/edrv-13-3-525. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, et al. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29(6):1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A, Tsai MJ. Mechanisms of transcriptional activation by steroid-hormone receptors. Journal of Cellular Biochemistry. 1993;51(2):151–156. doi: 10.1002/jcb.240510206. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Samanin R. The Role of putative 5-HT1a and 5-HT1B receptors in the control of feeding in rats. Life Sciences. 1987;41(5):635–642. doi: 10.1016/0024-3205(87)90418-8. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34(3):555–564. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60(1):50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology. 1996;137(10):4372–4383. doi: 10.1210/endo.137.10.8828498. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biological Psychiatry. 2000;47(6):562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Su A, Michelson D. Effects of oral estrogen, raloxifene and arzoxifene on gene expression in serotonin neurons of macaques. Psychoneuroendocrinology. 2002;27(4):431–445. doi: 10.1016/s0306-4530(01)00054-3. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu NZ. Ovarian steroids and neuronal function. Molecular Neurobiology. 1999;18(2):87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Frontiers in Neuroendocrinology. 2009;30(2):212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on meal patterns of female rats. Physiology & Behavior. 1976;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, et al. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: Systematic review. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2008;147B(6):850–858. doi: 10.1002/ajmg.b.30699. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Serotonin manipulations and the structure of feeding-behavior. Appetite. 1986;7:39–56. doi: 10.1016/s0195-6663(86)80051-4. [DOI] [PubMed] [Google Scholar]
- Brewerton TD. Toward a unified theory of serotonin dysregulation in eating and related disorders. [Review] Psychoneuroendocrinology. 1995;20(6):561–590. doi: 10.1016/0306-4530(95)00001-5. [DOI] [PubMed] [Google Scholar]
- Bruce KR, Steiger H, Joober R, Ng Ying Kin NM, Israel M, Young SN. Association of the promoter polymorphism -1438G/A of the 5-HT2A receptor gene with behavioral impulsiveness and serotonin function in women with bulimia nervosa. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2005;137B(1):40–44. doi: 10.1002/ajmg.b.30205. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food-intake and menstrual-cycle-A retrospective analysis, with implication for appetite research. Physiology & Behavior. 1995;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44(12):1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: a review. International Journal of Eating Disorders. 2000;27(1):1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor messenger-RNA in human brain. Brain Research. 1995;676(1):157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- Butera PC. Estradiol and the control of food intake. Physiology and Behavior. 2009 doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiology & Behavior. 1993;53(6):1235–1238. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31(4):757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats' accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 2000;149(3):259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. Journal of Neuroscience. 2001;21(16):6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JQ, Brown TR, Russo J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim Biophys Acta. 2009;1793(7):1128–1143. doi: 10.1016/j.bbamcr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BL, Khosla S. New selective estrogen and androgen receptor modulators. Current Opinion in Rheumatology. 2009;21(4):374–379. doi: 10.1097/BOR.0b013e32832ca447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg D, Benoit S, Barrera J, Woods S. Estrogen mediates body fat distribution and brain sensitivity to adiposity signals. Diabetes. 2003;52(supplement 1):A24–A24. [Google Scholar]
- Clegg D, Tamashiro K, Strader A, Woods S. Gonadal hormones determine body fat distribution. Obesity Research. 2004;12(Supplement S):A126–A126. [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ, Hauger RL. Relationship of prolactin response to d-fenfluramine to behavioral and questionnaire assessments of aggression in personality-disordered men. Biological Psychiatry. 1996;40(3):157–164. doi: 10.1016/0006-3223(95)00398-3. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Busler DE, Novak TJ, Weber RV, Kral JG. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochemical and Biophysical Research Communications. 1998;248(3):523–526. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- Crosby RD, Wonderlich SA, Engel SG, Simonich H, Smyth J, Mitchell JE. Daily mood patterns and bulimic behaviors in the natural environment. Behaviour Research and Therapy. 2009;47(3):181–188. doi: 10.1016/j.brat.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow SJ, Thuras P, Keel PK, Mitchell JE. Long-term menstrual and reproductive function in patients with bulimia nervosa. American Journal of Psychiatry. 2002;159(6):1048–1050. doi: 10.1176/appi.ajp.159.6.1048. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dspositions to rash action: positive and negative urgency. [Review] Psychological Bulletin. 2008;134(6):807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja JA, Butera PC, McCaffrey TA. Independent effects of estradiol on water and food intake. Behavioral Neuroscience. 1983;97(2):210–220. doi: 10.1037//0735-7044.97.2.210. [DOI] [PubMed] [Google Scholar]
- Di Bella D, Catalano M, Cavallini MC, Riboldi C, Bellodi L. Serotonin transporter linked polymorphic region in anorexia nervosa and bulimia nervosa. Molecular Psychiatry. 2000;5(3):233–234. doi: 10.1038/sj.mp.4000689. [DOI] [PubMed] [Google Scholar]
- Diaz NF, Diaz-Martinez NE, Camacho-Arroyo I, Velasco I. Estradiol promotes proliferation of dopaminergic precursors resulting in a higher proportion of dopamine neurons derived from mouse embryonic stem cells. International Journal of Developmental Neuroscience. 2009;27(5):493–500. doi: 10.1016/j.ijdevneu.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiology and Behavior. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20(4):451–456. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Ekman A, Sundblad-Elverfors C, Landen M, Eriksson T, Eriksson E. Low density and high affinity of platelet [H-3]paroxetine binding in women with bulimia nervosa. Psychiatry Research. 2006;142(2-3):219–223. doi: 10.1016/j.psychres.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Engel SG, Corneliussen SJ, Wonderlich SA, Crosby RD, le Grange D, Crow S, et al. Impulsivity and compulsivity in bulimia nervosa. International Journal of Eating Disorders. 2005;38(3):244–251. doi: 10.1002/eat.20169. [DOI] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. Journal of Clinical Endocrinology and Metabolism. 1997;82(12):4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Kaye WH, Rotondo A, Greenberg BD, Murphy DL, Goldman D. 5-HT2A promoter polymorphism -1438G/A, anorexia nervosa, and obsessive-compulsive disorder. Lancet. 1998;351(9118):1785–1786. doi: 10.1016/S0140-6736(05)78746-8. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Participation of the 5-HT1A receptor in the antidepressant-like effect of estrogens in the forced swimming test. Neuropsychopharmacology. 2006;31(2):247–255. doi: 10.1038/sj.npp.1300821. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006;31(8):905–914. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Faris PL, Eckert ED, Kim SW, Meller WH, Pardo JV, Goodale RL, et al. Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. Journal of Affective Disorders. 2006;92(1):79–90. doi: 10.1016/j.jad.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Faris PL, Hofbauer RD, Daughters R, Vandenlangenberg E, Iversen L, Goodale RL, et al. De-stabilization of the positive vago-vagal reflex in bulimia nervosa. Physiology and Behavior. 2008;94(1):136–153. doi: 10.1016/j.physbeh.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter MM, Leibl K, Rief W, Brunner E, Schmidt-Auberger S, Engel RR. Fluoxetine versus placebo: a double-blind study with bulimic inpatients undergoing intensive psychotherapy. Pharmacopsychiatry. 1991;24(1):1–7. doi: 10.1055/s-2007-1014424. [DOI] [PubMed] [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: A meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review. 2008;28(8):1413–1425. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry S, Wagner A, Kaye WH. Neuroimaging studies in eating disorders. CNS Spectrums. 2004;9(7):539–548. doi: 10.1017/s1092852900009639. [DOI] [PubMed] [Google Scholar]
- Frieling H, Bleich S, Otten J, Romer KD, Kornhuber J, de Zwaan M, et al. Epigenetic downregulation of atrial natriuretic peptide but not vasopressin mRNA expression in females with eating disorders is related to impulsivity. Neuropsychopharmacology. 2008;33(11):2605–2609. doi: 10.1038/sj.npp.1301662. [DOI] [PubMed] [Google Scholar]
- Frieling H, Romer KD, Scholz S, Mittelbach F, Wilhelm J, De Zwaan M, et al. Epigenetic dysregulation of dopaminergic genes in eating disorders. International Journal of Eating Disorders. 2009 doi: 10.1002/eat.20745. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry. 2008;63(6):569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, et al. Genetic contributions to generalized arousal of brain and behavior. Proceedings of the National Academy of Science. 2003;100(19):11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22(8):1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Gendall KA, Bulik CM, Joyce PR, McIntosh VV, Carter FA. Menstrual cycle irregularity in bulimia nervosa. Associated factors and changes with treatment. Journal of Psychosomatic Research. 2000;49(6):409–415. doi: 10.1016/s0022-3999(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Hormones & Behavior. 2007;52(3):307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. Journal of Pharmacology and Experimental Therapeutics. 2001;296(3):987–995. [PubMed] [Google Scholar]
- Goldstein DJ, Wilson MG, Ascroft RC, al-Banna M. Effectiveness of fluoxetine therapy in bulimia nervosa regardless of comorbid depression. International Journal of Eating Disorders. 1999;25(1):19–27. doi: 10.1002/(sici)1098-108x(199901)25:1<19::aid-eat3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wilson MG, Thompson VL, Potvin JH, Rampey AH., Jr Long-term fluoxetine treatment of bulimia nervosa. Fluoxetine Bulimia Nervosa Research Group. British Journal of Psychiatry. 1995;166(5):660–666. doi: 10.1192/bjp.166.5.660. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. Brain inhibitory mechanisms involved in basic and higher integrated sleep processes. Brain Research Reviews. 2004;45(3):230–249. doi: 10.1016/j.brainresrev.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, et al. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. American Journal of Medical Genetics. 2000;96(2):202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology. 2009a;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Minireview: the case for obesogens. Molecular Endocrinology. 2009b;23(8):1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger TR, LeBoeuf B, Liu YS, Garcia LR. Molecular signaling involved in regulating feeding and other motivated behaviors. Molecular Neurobiology. 2007;35(1):1–19. doi: 10.1007/BF02700621. [DOI] [PubMed] [Google Scholar]
- Guido GKW, Meltzer CC, Price J, Drevets WC, Greer P, Mathis C, et al. Alterations of 5HT1A and 2A receptors persist after recovery from anorexia and bulimia nervosa. Biological Psychiatry. 2002;51(8):33. [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERP) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Molecular Brain Research. 2000;76(2):191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2002;160(3):271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Molecular Brain Research. 1999;63(2):325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 2004;29(10):1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioural Brain Research. 2008;195(1):198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hansenne M, Ansseau M. Harm avoidance and serotonin. Biological Psychology. 1999;51(1):77–81. doi: 10.1016/s0301-0511(99)00018-6. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Wilson DM, Hammer LD, Litt IF, Kraemer HC, et al. Psychiatric risk associated with early puberty in adolescent girls. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):255–262. [PubMed] [Google Scholar]
- Heck A, Lieb R, Ellgas A, Pfister H, Lucae S, Roeske D, et al. Investigation of 17 candidate genes for personality traits confirms effects of the HTR2A gene on novelty seeking. Genes, Brain, & Behavior. 2009;8(4):464–472. doi: 10.1111/j.1601-183X.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: Association between gene expression and anxiety behavior in the open field. Biological Psychiatry. 2006;60(3):288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behaviof in the open field. Neuroscience. 2009;158(2):456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Current Opinion in Psychiatry. 2006;19(4):389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. International Journal of Eating Disorders. 2003;34(4):383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Frontiers in Neuroendocrinology. 1998;19(3):187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biological Psychiatry. 2003;54(10):953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. Journal of Chemical Neuroanatomy. 2003;26(4):331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain-serotonin system. Physiological Reviews. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Justo R, Frontera M, Pujol E, Rodriguez-Cuenca S, Llado I, Garcia-Palmer FJ, et al. Gender-related differences in morphology and thermogenic capacity of brown adipose tissue mitochondrial subpopulations. Life Sciences. 2005;76(10):1147–1158. doi: 10.1016/j.lfs.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Kasamo K, Suzuki T, Tada K, Ueda N, Matsuda E, Ishikawa K, et al. Endogenous 5-HT tonically inhibits spontaneous firing activity of dorsal hippocampus CA1 pyramidal neurons through stimulation of 5-HT(1A) receptors in quiet awake rats: in vivo electrophysiological evidence. Neuropsychopharmacology. 2001;24(2):141–151. doi: 10.1016/S0893-133X(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen JA, Muthyala R, Katzenellenbogen BS. Nature of the ligand-binding pocket of estrogen receptor alpha and beta: The search for subtype-selective ligands and implications for the prediction of estrogenic activity. Pure and Applied Chemistry. 2003;75(11-12):2397–2403. [Google Scholar]
- Kaye WH. Neurobiology of anorexia and bulimia nervosa. Physiology & Behavior. 2008:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha CW, Crossan PJ, et al. Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. American Journal of Psychiatry. 2001;158(7):1152–1155. doi: 10.1176/appi.ajp.158.7.1152. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Research. 1976;114(1):152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17alpha-estradiol versus 17beta-estradiol and the response of extrahypothalamic neurons. Experimental Brain Research. 1977;30(1):43–52. doi: 10.1007/BF00237857. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Molecular and Cellular Endocrinology. 2008;290(1-2):14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Molecular and Cellular Endocrinology. 2009;308(1-2):17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Bulik CM, Kaye WH, Treasure J, Tyson E. Academy of Eating Disorders Position Paper: eating disorders are serious mental illnesses. International Journal of Eating Disorders. 2009;42(2):97–103. doi: 10.1002/eat.20589. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Marc Breedlove S. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2006;36(4):539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychological Medicine. 2008;38(12):1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Genetic relationships between personality and eating attitudes and behaviors. Journal of Abnormal Psychology. 2002;111(2):380–389. doi: 10.1037//0021-843x.111.2.380. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Alexandra Burt S, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37(5):627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochim Biophys Acta. 1992;1133(3):301–306. doi: 10.1016/0167-4889(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Duncan MJ, Wise PM. Aging, estradiol and time of day differentially affect serotonin transporter binding in the central nervous system of female rats. Brain Research. 2003;990(1-2):87–94. doi: 10.1016/s0006-8993(03)03441-3. [DOI] [PubMed] [Google Scholar]
- Kuikka JT, Tammela L, Karhunen L, Rissanen A, Bergstrom KA, Naukkarinen H, et al. Reduced serotonin transporter binding in binge eating women. Psychopharmacology. 2001;155(3):310–314. doi: 10.1007/s002130100716. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. [Article] Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kumagai S, Holmang A, Bjorntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiologica Scandinavia. 1993;149(1):91–97. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Lascelles KRR, Field AP, Davey GCL. Using foods as CSs and body shapes as UCSs: a putative role for associative learning in the development of eating disorders. Behavior Therapy. 2003;34(2):213–235. [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. Journal of Psychiatry and Neuroscience. 2005;30(2):110–117. [PMC free article] [PubMed] [Google Scholar]
- Leon GR, Keel PK, Klump KL, Fulkerson JA. The future of risk factor research in understanding the etiology of eating disorders. Psychopharmacology Bulletin. 1997;33(3):405–411. [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LRR, Wonderlich S, Riso LP, Crosby R, Mitchell J. Eating disorders and personality: A methodological and empirical review. Clinical Psychology Review. 2006;26(3):299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Loucks AB. Energy availability, not body fatness, regulates reproductive function in women. Exercise and Sport Sciences Reviews. 2003;31(3):144–148. doi: 10.1097/00003677-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular Psychiatry. 2003;8(3):353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. Journal of Nutrition. 2009;139(3):629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid JA, Lopez-Bote C, Martin E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiology & Behavior. 1993;53(2):329–335. doi: 10.1016/0031-9384(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Mancilla-Diaz JM, Escartin-Perez RE, Lopez-Alonso VE, Cruz-Morales SE. Effect of 5-HT in mianserin-pretreated rats on the structure of feeding behavior. European Neuropsychopharmacology. 2002;12(5):445–451. doi: 10.1016/s0924-977x(02)00059-7. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. Journal of Neuroscience. 2001;21(6):2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. [Article] Journal of Psychiatry & Neuroscience. 2009;34(4):9. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Progress in Hormone Research. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Dow RC, Fink G. Oestradiol-17 beta increases serotonin transporter (SERT) binding sites and SERT mRNA expression in discrete regions of female rat brain. Journal of Physiology-London. 1996;495P:P114–P114. [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Molecular Brain Research. 1997;45(1):13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53(6):857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Research. 2000;859(2):326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17beta-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. 2002;76(6):339–347. doi: 10.1159/000067583. [DOI] [PubMed] [Google Scholar]
- Mize AL, Poisner AM, Alper RH. Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT(1A) receptor function. Neuroendocrinology. 2001;73(3):166–174. doi: 10.1159/000054633. [DOI] [PubMed] [Google Scholar]
- Mong JA, Pfaff DW. Hormonal symphony: steroid orchestration of gene modules for sociosexual behaviors. Molecular Psychiatry. 2004;9(6):550–556. doi: 10.1038/sj.mp.4001493. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Brambilla F, Bortolotti F, Maj M. Serotonergic dysfunction across the eating disorders: relationship to eating behaviour, purging behaviour, nutritional status and general psychopathology. Psychological Medicine. 2000;30(5):1099–1110. doi: 10.1017/s0033291799002330. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Maj M. Genetic susceptibility to eating disorders: associated polymorphisms and pharmacogenetic suggestions. Pharmacogenomics. 2008;9(10):1487–1520. doi: 10.2217/14622416.9.10.1487. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. Effects of activation and blockade of 5-HT2A/2C receptors in the dorsal raphe nucleus on sleep and waking in the rat. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(7):1189–1195. doi: 10.1016/j.pnpbp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Griebel G, Jenck F, Martin JR, Widmer U, Haefely WE. Behavioral profile of the 5HT1A receptor antagonist (S)-UH-301 in rodents and monkeys. [Article] Brain Research Bulletin. 1992;29(6):901–904. doi: 10.1016/0361-9230(92)90163-r. [DOI] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, et al. In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. Neuroimage. 2002;17(3):1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and Biobehavioral Reviews. 2004;28(1):55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Uhl GR, Holmes A, Ren-Patterson R, Hall FS, Sora I, et al. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain and Behavior. 2003;2(6):350–364. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Nacmias B, Ricca V, Tedde A, Mezzani B, Rotella CM, Sorbi S. 5-HT2A receptor gene polymorphisms in anorexia nervosa and bulimia nervosa. Neuroscience Letters. 1999;277(2):134–136. doi: 10.1016/s0304-3940(99)00859-9. [DOI] [PubMed] [Google Scholar]
- Nishiguchi N, Matsushita S, Suzuki K, Murayama M, Shirakawa O, Higuchi S. Association between 5HT2A receptor gene promoter region polymorphism and eating disorders in Japanese patients. Biological Psychiatry. 2001;50(2):123–128. doi: 10.1016/s0006-3223(00)01107-0. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kusumi I, Kaneko M, Masui T, Daiguji M, Ueno T, et al. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacology. 2006;187(1):30–35. doi: 10.1007/s00213-006-0398-z. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Choleris E, Pfaff D. Genetic influences on aggressive behaviors and arousability in animals. Annals of the New York Acadamey of Science. 2004;1036:257–266. doi: 10.1196/annals.1330.016. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, et al. The role of 5-HT1A receptors in learning and memory. Behavioural Brain Research. 2008;195(1):54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Commissaris RC, De La Garza R, 2nd, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models. Stress. 2003;6(2):101–110. doi: 10.1080/1025389031000111311. [DOI] [PubMed] [Google Scholar]
- Parker GC, Coscina DV. Lesions of the posterior basolateral amygdala block feeding induced by systemic 8-OH-DPAT. Pharmacology Biochemistry and Behavior. 2001;68(4):729–734. doi: 10.1016/s0091-3057(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, et al. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40(3):1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain--IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21(1):123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89(1):267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- PecinsThompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. Journal of Neuroscience. 1996;16(21):7021–7029. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard University Press; 2005a. [Google Scholar]
- Pfaff DW. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. Journal of Endocrinology. 2005b;184(3):447–453. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Banavar JR. A theoretical framework for CNS arousal. Bioessays. 2007;29(8):803–810. doi: 10.1002/bies.20611. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2008;147B(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Hudson JI, Jonas JM, Yurgelun-Todd D. Bulimia treated with imipramine: a placebo-controlled, double-blind study. American Journal of Psychiatry. 1983;140(5):554–558. doi: 10.1176/ajp.140.5.554. [DOI] [PubMed] [Google Scholar]
- Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. Patterns of menstrual disturbance in eating disorders. International Journal of Eating Disorders. 2007;40(5):424–434. doi: 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. Journal of Neuroscience. 2006;26(21):5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: Observations on guinea pigs and nonhuman primates. Cellular and Molecular Neurobiology. 1997;17(6):627–648. doi: 10.1023/a:1022534019718. [DOI] [PubMed] [Google Scholar]
- Ricca V, Nacmias B, Cellini E, Di Bernardo M, Rotella CM, Sorbi S. 5-HT2A receptor gene polymorphism and eating disorders. Neuroscience Letters. 2002;323(2):105–108. doi: 10.1016/s0304-3940(02)00088-5. [DOI] [PubMed] [Google Scholar]
- Roberts S, Kenney NJ, Mook DG. Overeating induced by progesterone in ovariectomized, adrenalectomized rat. Hormones and Behavior. 1972;3(3):267–276. doi: 10.1016/0018-506x(72)90040-2. [DOI] [PubMed] [Google Scholar]
- Rodier WI. Progesterone-estrogen interaction in control of activity-wheel running in female rat. Journal of Comparative and Physiological Psychology. 1971;74(3):365–373. doi: 10.1037/h0030568. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. Journal of Neuroendocrinology. 2009;21(4):263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Ronnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149(12):6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspectives. 2001;109(7):675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G. Serotonin transporters--structure and function. Journal of Membrane Biology. 2006;213(2):101–110. doi: 10.1007/s00232-006-0878-4. [DOI] [PubMed] [Google Scholar]
- Sabine EJ, Yonace A, Farrington AJ, Barratt KH, Wakeling A. Bulimia nervosa: a placebo controlled double-blind therapeutic trial of mianserin. British Journal of Clinical Pharmacology. 1983;15(Suppl 2):195S–202S. doi: 10.1111/j.1365-2125.1983.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Arango C, Morales B, Bascaran MT, Martinez-Barrondo S, et al. Association study between obsessive-compulsive disorder and serotonergic candidate genes. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(3):765–770. doi: 10.1016/j.pnpbp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E. Adaptive regulation of central serotonin receptors linked to phosphoinositide hydrolysis. Neuropsychopharmacology. 1990;3(5-6):411–416. [PubMed] [Google Scholar]
- Saura J, Kettler R, Daprada M, Richards JG. Quantitative enzyme autoradiography with H-3 RO 41-1049 and H-3 R0 19-6327 in vitro -localization and abuncance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. [Article] Journal of Neuroscience. 1992;12(5):1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. American Journal of Geriatric Psychiatry. 2001;9(4):393–399. [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sell SL, Craft RM, Seitz PK, Stutz SJ, Cunningham KA, Thomas ML. Estradiol-sertraline synergy in ovariectomized rats. Psychoneuroendocrinology. 2008;33(8):1051–1060. doi: 10.1016/j.psyneuen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Shapiro JR, Berkman ND, Brownley KA, Sedway JA, Lohr KN, Bulik CM. Bulimia nervosa treatment: A systematic review of randomized controlled trials. International Journal of Eating Disorders. 2007;40(4):321–336. doi: 10.1002/eat.20372. [DOI] [PubMed] [Google Scholar]
- Shelley DN, Dwyer E, Johnson C, Wittkowski KM, Pfaff DW. Interactions between estrogen effects and hunger effects in ovariectomized female mice. I. Measures of arousal. Hormones and Behavior. 2007;52(4):546–553. doi: 10.1016/j.yhbeh.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiology and Behavior. 2009;97(2):199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139(12):5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ER alpha and ER beta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96(1):41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Heron KE, Sliwinski MJ, Wonderlich SA, Crosby RD, Mitchell JE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Soulairac A. Neurological factors in the control of the appetite. International Review of Neurobiology. 1963;5:303–346. doi: 10.1016/s0074-7742(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in Neuroendocrinology. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger H. Eating disorders and the serotonin connection: state, trait and developmental effects. Journal of Psychiatry & Neuroscience. 2004;29(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- Steiger H, Bruce KR. Phenotypes, endophenotypes, and genotypes in bulimia spectrum eating disorders. Canadian Journal of Psychiatry. 2007;52(4):220–227. doi: 10.1177/070674370705200403. [DOI] [PubMed] [Google Scholar]
- Steiger H, Gauvin L, Engelberg MJ, Bruce KR, Israel M, Kin NY, et al. Serotonin-based moderation of binge antecedents in bulimia nervosa: Does abnormal serotonin function favor restraint- or emotion-based pathways to binge eating? International Journal of Eating Disorders. 2004;35(4):085. [Google Scholar]
- Steiger H, Koerner N, Engelberg MJ, Israel M, Ng Ying Kin NM, Young SN. Self-destructiveness and serotonin function in bulimia nervosa. Psychiatry Research. 2001;103(1):15–26. doi: 10.1016/s0165-1781(01)00264-5. [DOI] [PubMed] [Google Scholar]
- Steiger H, Young SN, Kin NM, Koerner N, Israel M, Lageix P, et al. Implications of impulsive and affective symptoms for serotonin function in bulimia nervosa. Psychological Medicine. 2001;31(1):85–95. doi: 10.1017/s003329179900313x. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Hollander E, DeCaria CM, Simeon D, Cohen L, Aronowitz B. m-Chlorophenylpiperazine challenge in borderline personality disorder: Relationship of neuroendocrine response, behavioral response, and clinical measures. Biological Psychiatry. 1996;40(6):508–513. doi: 10.1016/0006-3223(95)00470-X. [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10-15 years in a prospective study. International Journal of Eating Disorders. 1997;22(4):339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Bulik CM, Kendler KS. Genetic epidemiology of binging and vomiting. British Journal of Psychiatry. 1998;173:75–79. doi: 10.1192/bjp.173.1.75. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Research: Molecular Brain Research. 1999;73(1-2):119–128. doi: 10.1016/s0169-328x(99)00243-0. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Raloxifene blocks estradiol induction of the serotonin transporter and 5-hydroxytryptamine(2A) receptor in female rat brain. Neuroscience Letters. 2007;417(1):95–99. doi: 10.1016/j.neulet.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reproductive Toxicology. 2007;24(1):9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Pirker W, Willeit M, de Zwaan M, Bailer U, Neumeister A, et al. [I-123] beta-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biological Psychiatry. 2001;49(4):326–332. doi: 10.1016/s0006-3223(00)00951-3. [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Research. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Keski-Rahkonen A, Lopponen M, Muhonen M, Kajander J, Allonen T, et al. Brain serotonin 1A receptor binding in bulimia nervosa. Biological Psychiatry. 2004;55(8):871–873. doi: 10.1016/j.biopsych.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ER alpha) and ER beta. Journal of Neuroscience. 2007;27(36):9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Molecular Endocrinology. 1997;11(3):353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Multiple serotonin receptors: Too many, not enough, or just the right number? Neuroscience and Biobehavioral Reviews. 1997;21(5):679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Hiegel C, Perez E, Guptarak J. Serotonin receptor involvement in effects of restraint on female rat lordosis behavior. Pharmacology Biochemistry and Behavior. 2007;86(4):631–636. doi: 10.1016/j.pbb.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Mossner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, et al. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. Journal of Neuroscience. 2003;23(2):708–715. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food-intake and body-weight in female rats. Journal of Comparative and Physiological Psychology. 1975;88(1):183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008a;19(7):789–792. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008b;73(9-10):997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Agras WS, Devlin MJ, Fairburn CG, Wilson GT, Kahn C, et al. Fluoxetine for bulimia nervosa following poor response to psychotherapy. American Journal of Psychiatry. 2000;157(8):1332–1334. doi: 10.1176/appi.ajp.157.8.1332. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Research Reviews. 2008;57(2):309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase-A and oxidase-B populations in primate brain. Science. 1985;230(4722):181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine-oxidase A and monoamine-oxidase B-cell populations in human brain stem. Neuroscience. 1988;25(2):439–456. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, et al. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14(2):233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Metzger ED, Levine JM, Finkelstein DM, Cooper TB, Jimerson DC. Serotonin function following remission from bulimia nervosa. Neuropsychopharmacology. 2000;22(3):257–263. doi: 10.1016/S0893-133X(99)00117-7. [DOI] [PubMed] [Google Scholar]
- Yan L, Ge H, Li H, Lieber SC, Natividad F, Resuello RR, et al. Gender-specific proteomic alterations in glycolytic and mitochondrial pathways in aging monkey hearts. Journal of Molecular Cell Cardiology. 2004;37(5):921–929. doi: 10.1016/j.yjmcc.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Sugiyama N, Sugiyama E, Amano N. Five Female Cases of Prolonged Depression in Chronic Anorexia Nervosa Treated With Selective Estrogen Receptor Modulator Raloxifene-Augmented Therapy. Journal of Clinical Psychopharmacology. 2008;28(6):721–722. doi: 10.1097/JCP.0b013e31818b75c1. [DOI] [PubMed] [Google Scholar]
- Yu ZP, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiology & Behavior. 2008;95(3):501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeraldi E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. [Article] European Neuropsychopharmacology. 2007;17(6-7):400–405. doi: 10.1016/j.euroneuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140(1):321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhou WX, Koldzic-Zivanovic N, Clarke CH, de Beun R, Wassermann K, Bury PS, et al. Selective estrogen receptor modulator effects in the rat brain. Neuroendocrinology. 2002;75(1):24–33. doi: 10.1159/000048218. [DOI] [PubMed] [Google Scholar]
- Zucker I. Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiology & Behavior. 1969;4(4):595–602. [Google Scholar]