Abstract
Background
Maternal infection during pregnancy has been repeatedly associated with increased risk for schizophrenia. Nevertheless, most viruses do not cross the placenta; therefore, the damaging effects to the fetus appear to be related to maternal antiviral responses to infection (e.g. proinflammatory cytokines). Fetal exposure to the proinflammatory cytokine interleukin-8 (IL-8) has been significantly associated with risk of schizophrenia in offspring. This study sought to determine the association between fetal exposure to IL-8 and structural brain changes among schizophrenia cases and controls.
Methods
Subjects were 17 cases diagnosed with schizophrenia from the Developmental Insult and Brain Anomaly in Schizophrenia (DIBS) study. Psychiatric diagnoses were determined among offspring with semi-structured interviews and medical records review. IL-8 was determined from assays in archived prenatal sera and volumetric analyses of neuroanatomical regions were obtained from T1-weighted magnetic resonance imaging in adulthood. Eight controls were included for exploratory purposes.
Results
Among cases, fetal exposure to increases in IL-8 was associated with significant increases in ventricular cerebrospinal fluid, significant decreases in left entorhinal cortex volumes and significant decreases in right posterior cingulate volumes. Decreases that approached significance also were found in volumes of the right caudate, the putamen (bilaterally), and the right superior temporal gyrus. No significant associations were observed among controls.
Conclusion
Fetal exposure to elevations in maternal IL-8 led to structural neuroanatomic alterations among cases in regions of the brain consistently implicated in schizophrenia research. In utero exposure to elevations in IL-8 may partially account for brain disturbances commonly found in schizophrenia.
Keywords: obstetric complications, schizophrenia, proinflammatory cytokines, structural MRI, pregnancy, infection
Introduction
Previous studies have found structural changes throughout the brain in schizophrenia patients compared to non-psychiatric controls. Among these studies, increased ventricular size is the most well-replicated structural anomaly found in schizophrenia research(Gur, Keshavan, & Lawrie, 2007; Wright et al., 2000). Decreases in whole brain volumes and temporolimbic regions also have been repeatedly found among schizophrenia patients (Gur et al., 2007; Wright et al., 2000). Further, studies of individuals who are in the prodrome of schizophrenia also have identified multiple structural brain changes, suggesting that brain disturbances associated with the disorder may have neurodevelopmental origins (Job, Whalley, Johnstone, & Lawrie, 2005; Pantelis et al., 2003). Despite these findings, few investigations have sought to determine the contributions of environmental risk factors to neuroanatomical changes found in schizophrenia.
Although genetic factors are believed to substantially contribute to the etiology of schizophrenia, concordance rates approximating 50% between monozygotic twins indicates the presence of substantial environmental influences (Cannon et al., 1998). Among the possible environmental contributors, maternal infections during pregnancy have been repeatedly linked to an increased risk for schizophrenia (Brown & Derkits, 2010). Nevertheless, many infections do not appear to cross the placenta; therefore the damaging influences to the fetal brain seem related to maternal antiviral responses to infection, such as increases in proinflammatory cytokines (Patterson, 2008). In a previous study using the birth cohort of the current investigation, increases in maternal levels of interleukin-8 (IL-8) during the second/third trimesters of pregnancy were associated with increased risk for schizophrenia among offspring (Brown et al., 2004).
Hence, we sought to examine whether fetal exposure to increases in maternal IL-8 during the second/third trimesters results in more pronounced structural brain alterations among individuals diagnosed with schizophrenia and other spectrum disorders (herein referred to as schizophrenia). Our primary hypothesis was that fetal exposure to IL-8 would result in increases in ventricular cerebrospinal fluid (CSF) volume among cases. In addition to the well-replicated association between increases in ventricular CSF and schizophrenia, this hypothesis was derived from animal studies indicating increased ventricular volumes following fetal exposure to immune activation (Patterson, 2008; Wright et al., 2000). Based on previous findings from studies of schizophrenia patients, prodromal research and animal studies, we also predicted that fetal exposure to increased maternal IL-8 would be associated with reduced volumes of temporal lobe regions, particularly in the hippocampus, parahippocampus, and the superior temporal gyrus, and reductions in basal ganglia volumes (Pantelis et al., 2003; Patterson, 2008; Wright et al., 2000). Exploratory analyses were conducted with control participants and on additional regions of interest (ROIs). Analyses of controls were conducted to obtain preliminary findings on whether there are differential vulnerabilities to fetal exposure to IL-8 among cases versus controls, as has been found in other studies (Cannon, Yolken, Buka, & Torrey, 2008; Ellman, Yolken, Buka, Torrey, & Cannon, 2009).
Materials and Methods
All subjects provided written informed consent and the study was approved by the Institutional Review Boards of the New York State Psychiatric Institute, the Kaiser Foundation Research Institute, and the University of California San Francisco VA Medical Center.
Description of the cohort
The subjects were derived from the Developmental Insult and Brain Anomaly in Schizophrenia (DIBS) study that was based on participants from the Prenatal Determinants of Schizophrenia (PDS) study, which ascertained cases of schizophrenia from a large birth cohort described in detail previously (Susser, Schaefer, Brown, Begg, & Wyatt, 2000). Briefly, the PDS study included pregnant women (n=12,094 live births) receiving obstetric care from the Kaiser Permanente Medical Care Plan (KPMCP) in Alameda County, California, as part of the Child Health and Development Study (CHDS). Maternal serum samples were collected during the pregnancies and were frozen and stored at -20° C.
Ascertainment and diagnosis
Case ascertainment and screening were accomplished following computerized record linkage between the CHDS and KPMCP identifiers from inpatient, outpatient, and pharmacy registries of CHDS participants who belonged to KPMCP from 1981-1997 (corresponding to the initiation of KPMCP computerized psychiatric registries and to the end of follow-up). Potential cases were diagnosed using DSM-IV criteria following assessment with the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994), chart review, and consensus of 3 experienced research psychiatrists.
All cases and controls were targeted for neuroimaging assessments in adulthood. DIBS study participants consisted of 23 cases of schizophrenia and 25 controls for MRI acquisition and analysis. Two cases were excluded due to unusable images from movement artifacts. The final sample was comprised of 17 cases and 8 controls with available MRI and available second/third trimester cytokine data. Among the 17 cases, 7 were diagnosed with schizophrenia, 4 with schizoaffective disorder, and 6 with other schizophrenia spectrum disorders. Demographic characteristics of the cases and controls are provided in Table 1. There were no significant differences in any demographic characteristics between cases and controls. There also were no significant differences in any demographic variables between DIBS cases and cases from the PDS study with available cytokine data who were not ascertained as part of the DIBS study (Table 2).
Table 1.
Demographic Characteristics of Sample
| Characteristic | SSD (n= 17) | No Diagnosis (n=8) | p-value |
|---|---|---|---|
| % Female | 5 (29.41%) | 2 (25%) | 0.819 |
| Age at MRI Scan | 39.96 (1.78) | 41.17 (1.69) | 0.140 |
| Birth Weight | 3508.73 (522.13) | 3378.38 (572.20) | 0.323 |
| Gestational Length (Weeks) | 40.39 (1.58) | 40.14 (0.71) | 0.722 |
| Maternal Age | 28.12 (6.54) | 26.88 (6.58) | 0.662 |
| Maternal Earlier Deliveries | 1.88 (2.18) | 0.75 (0.89) | 0.173 |
| Maternal Education <=HS graduate | 12 (70.59) | 4 (66.67) | 0.858 |
| Maternal Race | |||
| White | 12 (70.59%) | 7(87.50%) | 0.096 |
| Black | 5 (29.41%) | 0 | |
| Asian | 0 | 1 (12.50%) | |
| IL-8 (pg/ml) | 952.04 (1083.45) | 315.24 (248.60) | 0.118 |
| Medication Use | |||
| Atypical Antipsychotics | 4 (23.53) | 0 | |
| Typical Antipsychotics | 3 (17.65) | 0 | |
| On any antipsychotic | 7 (18.92) | 0 | |
| Anti-pyramidal drugs | 3 (17.65) | 0 | |
| Mood Stabilizers | 2 (11.76) | 0 | |
| SSRIs | 2 (11.76) | 0 | |
| Other Anti-depressant (Trazadone) | 1 (5.88) | 0 | |
| Ritalin | 1 (5.88) | 0 | |
| Methadone | 1 (5.88) | 0 |
**Information was incomplete for 2 DIBS controls on maternal education and for 3 DIBS gravidasmaternal race was incomplete; therefore this variable was extrapolated from child's race for these gravidas.
Table 2.
Demographic characteristics of DIBS and Non-DIBS cases
| Characteristic | DIBS cases (n=17) | Non-DIBS cases* (n=42) | p-value |
|---|---|---|---|
| % Female | 5 (29.41%) | 15 (35.71%) | 0.643 |
| Birth Weight (grams) | 3508.73 (522.13) | 3332.48(562.67) | 0.245 |
| Gestational Length (Weeks) | 40.39 (1.58) | 40.59 (2.26) | 0.738 |
| Maternal Age | 28.12 (6.54) | 27.91 (5.59) | 0.90 |
| Maternal Earlier Deliveries | 1.88 (2.18) | 2.05 (1.90) | 0.77 |
| Maternal Education <=HS graduate | 12 (70.59) | 24 (66.67) | 0.775 |
| Maternal Race | |||
| White | 12 (70.59%) | 17 (41.46%) | 0.775 |
| Black | 5 (29.41%) | 23 (56.10%) | |
| Asian | 0 | 1 (2.443%) | |
| IL-8 (pg/ml) | 952.04 (1083.45) | 1020.81(1644.09) | 0.875 |
Non-DIBS cases were defined as any cases of schizophrenia with available IL-8 data who were part of the larger PDS cohort, but not ascertained in the present study.
**Information was incomplete for 8 non-DIBS cases on maternal education, for 1 non-DIBS case on gestational length, and for 1 non-DIBS case on maternal and child race. For 3 DIBS gravidas maternal race was incomplete; therefore this variable was extrapolated from child's race for these gravidas.
Interleukin-8 assay
Analyses were restricted to second/third trimester IL-8 values, because 1) maternal IL-8 during the second/third trimester was previously associated with schizophrenia in this birth cohort, whereas other proinflammatory cytokines (IL-6, TNF-α, IL-1β) did not (Brown et al., 2004) and 2) we sought to reduce the probability of Type I errors by limiting the number of analyses.
Assays were conducted blind to diagnosis of the offspring and were carried out under Level II biohazard containment conditions. Each serum specimen was thawed and made 2mM with respect to phenylmethylsulfonyl fluoride, a protease inhibitor, to prevent degradation of cytokines by serum proteases. Measurement of IL-8 was conducted using the sandwich enzyme-linked immunosorbent assay described in detail in a previous study from this cohort (Brown et al., 2004).
Image acquisition and analysis
MR images were acquired using a 1.5-Tesla Siemens system. Coronal T1-weighted images were obtained from 3D MP-RAGE sequences (TR/TI/TE = 10/250/4 ms, resolution 1 × 1 mm2, 1.4 mm slice thickness). MRI tissue segmentation and regional voluming in the Talairach coordinate system were used based on the cited methods (Collins et al., 1998; Kwan, Evans, & Pike, 1999). These methods have been shown to be reliable (Manji, Moore, & Chen, 2000) and valid (Fein et al., 2000) in previous studies. In-house software was used to: 1) remove the skull and meninges from the images; 2) coregister each of the interleaves of the spin-echo images to T1 images reformatted to the axial plane; 3) perform RF inhomogeneity correction in 3D; 4) define seeds (based on peaks in the 3D histogram of T1 pixel intensities) for the K-means cluster analysis; and 5) transfer the data to statistical software which performs the actual cluster analysis. The initial process was followed by computer-assisted editing of the data to separate cortical from subcortical gray matter, ventricular CSF from sulcal CSF, and to reclassify white matter incorrectly classified in the first pass into a category of white matter with an abnormal MRI signal or white matter signal hyperintensity (WMSH). This was followed by manual delineation of the boundaries of cortical regions, subcortical structures, the cerebellum, and the hippocampi. The transformation to the Talairach coordinate system involved piecewise linear transformations of 12 compartments for each subject's brain. Each subject's tissue contribution to the commonly defined ROI was then computed by superimposing the subject-specific ROI on the subject's segmented image, and counting the (segmented) pixels contained in the ROI. Using this approach, reliability studies for 20 normal subjects on two separate occasions revealed inter- and intraoperator correlations of 0.91 to 0.99 for ventricular CSF, sulcal CSF, cortical gray matter, white matter, and % white matter signal hyperintensities. All cortical volumes, including superior temporal gyrus (Brodmann area 22), entorhinal cortex (Brodmann area 28), inferior frontal cortex (Broadmann areas 44 and 45), dorsolateral prefrontal cortex (DLPFC, Brodmann areas 9 and 46), anterior cingulate (Brodmann areas 24, 25, and 32), and posterior cingulate (Brodmann area 31) were obtained based on the methods above.
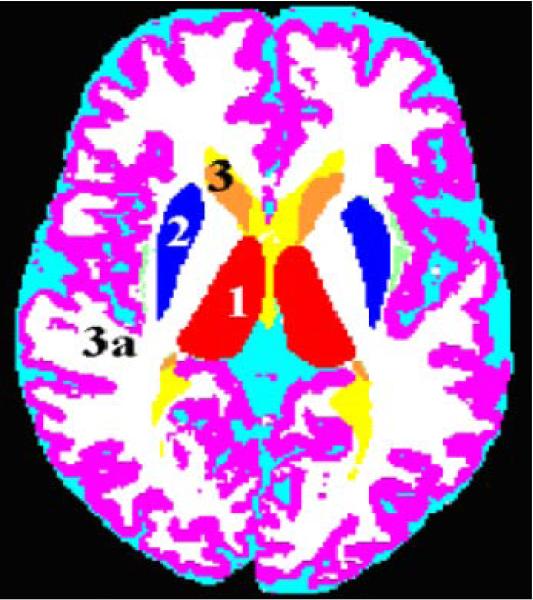
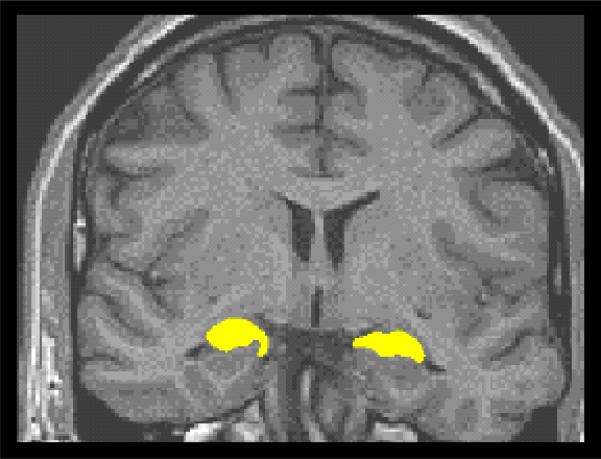
The thalamus (See Figure 1; Deicken, Eliaz, Chosiad, Feiwell, & Rogers, 2002), and hippocampus (See Figure 2; Deicken, Pegues, & Amend, 1999; Talairach & Tournoux, 1988) were manually traced using the cited methods. Reliability studies of hippocampal and thalamic volumes (n=10) of normal controls revealed interoperator correlations of 0.92 to 0.96.
Figure 1.
Axial MRI tissue segmented image
Figure 2.
ROI tracing of hippocampus
The head of the caudate (See Figure 1) was defined on the transaxial plane as the mass of gray matter comprising the lateral walls of the lateral ventricles bounded inferiorly and laterally by the anterior limb of the internal capsule and superiorly and laterally by the external capsule. Region placement for the caudate and the putamen began inferiorly when the operator could see clearly at least on one side the anterior limb of the internal capsule dividing the caudate head and the putamen. The boundary for the head of the caudate continued superiorly until the thalamus could no longer be visualized and the anterior horn of the lateral ventricles became confluent with the posterior horn. The body of the caudate was defined as the portion above the thalamus after the confluence of the anterior and posterior horns of the lateral ventricles. The caudate tail was defined as a structure of gray matter closely adjacent anterolaterally to the posterior horns of the lateral ventricles and posterolaterally to the thalamus until it became confluent with the body of the caudate. Reliability studies of caudate volumes (n=10) of normal controls revealed interoperator correlations of 0.92 to 0.93.
The putamen (See Figure 1) is bounded laterally by the external capsule, and posteroand antero-medially by the internal capsule. The boundary between the putamen and the globus pallidus is noted by the difference between the two structures and when possible a strip of white matter was identified between the gray matter masses of the two structures. Regional tracing for the putamen extended superiorly until no more gray matter pixels could be detected medially to the claustrum band. Reliability studies of putamen volumes (n=10) of normal controls revealed interoperator correlations of 0.93 to 0.94.
All ROIs were divided by intracranial volume to correct for head size. Ratios were chosen instead of controlling for intracranial volumes, in order to minimize degrees of freedom given the modest sample size.
Statistical analyses
As is often the case with cytokine distributions, there was a wide range of IL-8 values (3.63 pg/ml to 3717.7 pg/ml) and this distribution was skewed. IL-8 values were log-transformed due to this non-normal distribution, as well as ease of interpretation. Multiple regressions were conducted in SAS version 9.1 (SAS, Inc., Cary, N.C.). Standardized parameter estimates were calculated in addition to traditional unstandardized estimates to allow for interpretation of the magnitude of the relationship between IL-8 and volumetric measures, as represented in standardized units. Standardized estimates represent the standard deviation changes in volumetric ratios that result from a change of one standard deviation in log IL-8 values. Maternal education at birth (HS graduate or below vs. higher education) was added to the models to control for the potential influences of postnatal adversity. Maternal education has been highly correlated with other measures of socioeconomic status in the PDS study and is often used as a proxy variable for postnatal adversity in studies of prenatal risk factors to control for the potential contributions of postnatal disadvantages to brain alterations (Schlotz & Phillips, 2009). Age of participants, participants’ sex, and medication status were explored as potential control variables and/or confounders by examining the relationship between these variables and IL-8 levels and volumetric measures. Statistical significance was based on p < .05; all tests were two-tailed. Primary analyses included all analyses with a priori, directional hypotheses; whereas secondary analyses were used for exploratory purposes.
Results
There were no significant differences in age or sex between cases and controls (See Table 1). In addition, among several demographic variables examined, age (r=0.0575, p= .785), (F=0.35, df=1, 24, p=0.559), and medication status of cases (on antipsychotics or not; F=0.01, df=1, 24, p=0.924) were not significantly related to IL-8 levels. Further, there were no differences in brain volumes between cases who were and were not taking antipsychotics at the time of the assessment (results available upon request).
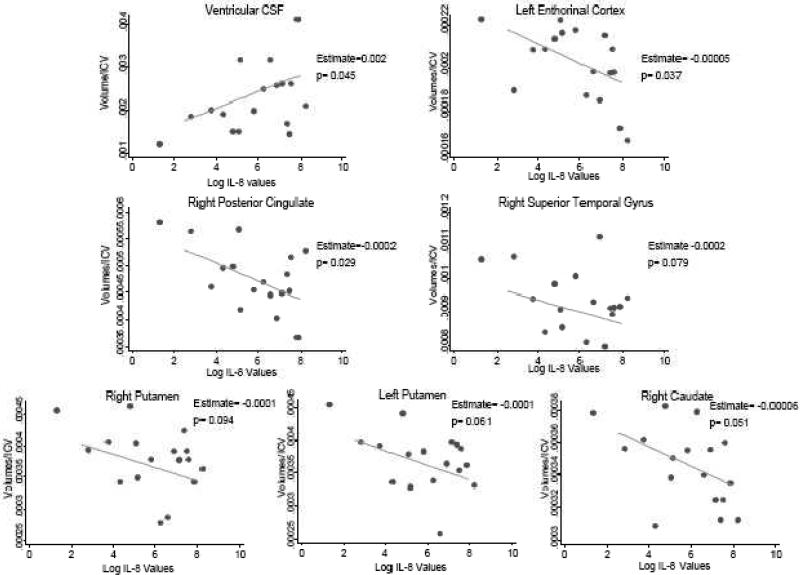
In primary analyses among cases (see Table 3 & Figure 3), fetal exposure to increases in maternal IL-8 levels was significantly associated with increases in ventricular CSF volume among cases (p=0.045). Fetal exposure to IL-8 also was related to significant decreases in left entorhinal cortex volumes (p=0.037) and decreases that approached significance in the right superior temporal gyrus (p=0.079), the right caudate (p=0.051), and the putamen bilaterally (p=0.061 and p=0.094, right and left, respectively). There were no associations between fetal exposure to IL-8 and hippocampal volumes.
Table 3.
Results of unadjusted & adjusted regression analyses of maternal IL-8 and brain volumes in cases and controls: Primary analyses
| Control Participants (n=8) Unadjusted Models | Control Participants (n=8) Models Adjusted for Maternal Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Parameter Estimate | 95% CI | β | p-value | Parameter Estimate | 95% CI | β | p-value |
| Ventricular Regions | ||||||||
| Ventricular cerebrospinal fluid | 0.002 | -0.006, 0.011 | 0.249 | 0.552 | -0.003 | -0.023, 0.016 | -0.386 | 0.631 |
| Cortical/Limbic Structures | ||||||||
| entorhinal cortex (right) | 0.000005 | -0.0003, 0.0003 | 0.013 | 0.975 | 0.0002 | -0.0004, 0.0008 | 0.731 | 0.393 |
| entorhinal cortex (left) | -0.00004 | -0.0004, 0.0003 | -0.097 | 0.820 | 0.0002 | -0.0007, 0.001 | 0.481 | 0.562 |
| hippocampus (right) | 0.0002 | -0.00003, 0.0004 | 0.649 | 0.082† | 0.0004 | -0.00003, 0.0008 | 1.281 | 0.061† |
| hippocampus (left) | -0.00001 | -0.0002, 0.0001 | -0.077 | 0.856 | -0.00002 | -0.0004, 0.0004 | -0.166 | 0.858 |
| superior temporal gyrus (right) | 0.000002 | -0.001, 0.001 | 0.002 | 0.996 | 0.0005 | -0.002, 0.002 | 0.544 | 0.518 |
| superior temporal gyrus (left) | -0.0001 | -0.001, 0.001 | -0.104 | 0.806 | 0.0004 | -0.002, 0.003 | 0.377 | 0.661 |
| Subcortical Structures | ||||||||
| caudate (right) | 0.0001 | -0.0005, 0.0007 | 0.184 | 0.662 | 0.0002 | -0.0001, 0.0006 | 0.978 | 0.142 |
| caudate (left) | 0.0002 | -0.0002, 0.0005 | 0.449 | 0.264 | 0.0002 | -0.0005, 0.0008 | 0.471 | 0.508 |
| putamen (right) | 0.0001 | -0.0004, 0.0006 | 0.238 | 0.571 | 0.0002 | -0.0009, 0.001 | 0.508 | 0.573 |
| putamen (left) | 0.0002 | -0.0003, 0.0006 | 0.367 | 0.371 | 0.0003 | -0.0007, 0.001 | 0.759 | 0.381 |
| Schizophrenia Cases (n=17) Unadjusted Models | Schizophrenia Cases (n=17) Models Adjusted for Maternal Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Parameter Estimate | 95% CI | β | p-value | Parameter Estimate | 95% CI | β | p-value |
| Ventricular Regions | ||||||||
| Ventricular cerebrospinal fluid | 0.002 | -0.00005, 0.004 | 0.473 | 0.055† | 0.002 | 0.00005, 0.004 | 0.487 | 0.045* |
| Cortical/Limbic Structures | ||||||||
| entorhinal cortex (right) | -0.00001 | -0.00007, 0.00004 | -0.138 | 0.597 | -0.00001 | -0.00007,0.00004 | -0.124 | 0.630 |
| entorhinal cortex (left) | -0.00005 | -0.00009, -0.000005 | -0.525 | 0.030* | -0.00005 | -0.00009, -0.000003 | -0.519 | 0.037* |
| hippocampus (right) | 0.00002 | -0.00005, 0.00009 | 0.172 | 0.509 | 0.00002 | -0.00005, 0.0001 | 0.169 | 0.531 |
| hippocampus (left) | -0.00002 | -0.0001, 0.00006 | -0.142 | 0.588 | -0.00003 | -0.0001, 0.00006 | -0.160 | 0.523 |
| superior temporal gyrus (right) | -0.0002 | -0.0004, 0.00007 | -0.353 | 0.165 | -0.0002 | -0.0004, 0.00002 | -0.379 | 0.079† |
| superior temporal gyrus (left) | -0.0001 | -0.0003, 0.0001 | -0.223 | 0.390 | -0.0001 | -0.0003, 0.0001 | -0.242 | 0.320 |
| Subcortical Structures | ||||||||
| caudate (right) | -0.00006 | -0.0001, -0.000002 | -0.497 | 0.042* | -0.00006 | -0.0001, 0.0000002 | -0.495 | 0.051† |
| caudate (left) | -0.00003 | -0.0001, 0.00007 | -0.147 | 0.573 | -0.00003 | -0.0001, 0.00008 | -0.144 | 0.594 |
| putamen (right) | -0.0001 | -0.0002, 0.00003 | -0.393 | 0.118 | -0.0001 | -0.0002, 0.00002 | -0.409 | 0.094† |
| putamen (left) | -0.0001 | -0.0002, 0.000008 | -0.456 | 0.066† | -0.0001 | -0.0002, 0.00001 | -0.467 | 0.061† |
Table 3 includes the results from the primary multiple regression analyses with and without controlling for maternal education at birth. Unstandardized parameter estimates, 95% confidence intervals of the unstandardized estimates, standardized parameter estimates (β), and p-values for regression analyses of the relationship between the log of IL-8 and brain volumes/icv are presented.† denotes a results that approached significance.
denotes a result that was significant at p<.05.
denotes a result between p=.05 and p=.10.
Figure 3.
Relationship between IL-8 and volumetric changes among schizophrenia cases controlling for maternal education
In secondary analyses among cases (Table 4 & Figure 3), fetal exposure to increases in maternal IL-8 levels was related to volumetric decreases in the right posterior cingulate (p=0.029). No significant relationships were observed between maternal IL-8 levels and any of the additional brain volumes examined among cases or in exploratory analyses among controls, although non-significant increases were observed for the right hippocampus (p=0.061), right anterior cingulate (p=0.097), and the right posterior cingulate (p=0.088) among controls.
Table 4.
Results of unadjusted and adjusted regression analyses of maternal IL-8 and brain volumes in cases and controls: Secondary analyses
| Control Participants (n=8) Unadjusted Models | Control Participants (n=8) Models Adjusted for Maternal Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter Estimate | 95% CI | β | p-value | Parameter Estimate | 95% CI | β | p-value | |
| Ventricular Measures | ||||||||
| sulcal cerebrospinal fluid | 0.008 | -0.012, 0.028 | 0.386 | 0.345 | 0.016 | -0.015, 0.046 | 1.023 | 0.202 |
| Cortical/Limbic Regions | ||||||||
| DLPFC (right) | 0.0002 | -0.0005, 0.0008 | 0.214 | 0.612 | -0.0002 | -0.002, 0.001 | -0.324 | 0.674 |
| DLPFC (left) | -0.0002 | -0.0008, 0.0005 | -0.232 | 0.580 | -0.00007 | -0.002, 0.002 | -0.111 | 0.905 |
| inferior frontal cortex (right) | 0.0003 | -0.0005, 0.001 | 0.361 | 0.379 | 0.0002 | -0.002, 0.002 | 0.221 | 0.806 |
| inferior frontal cortex (left) | 0.0001 | -0.0005, 0.0007 | 0.211 | 0.616 | 0.0003 | -0.001, 0.002 | 0.582 | 0.512 |
| anterior cingulate (right) | 0.0008 | -0.0006, 0.002 | 0.500 | 0.207 | 0.0008 | -0.0003, 0.002 | 0.779 | 0.097† |
| anterior cingulate (left) | -0.002 | -0.004, 0.0009 | -0.527 | 0.180 | -0.001 | -0.007, 0.004 | -0.423 | 0.547 |
| posterior cingulate (right) | 0.0003 | -0.0003, 0.001 | 0.460 | 0.252 | 0.0005 | -0.0001, 0.001 | 0.930 | 0.088† |
| posterior cingulate (left) | -0.0005 | -0.001, 0.0004 | -0.473 | 0.237 | 0.00005 | -0.002, 0.002 | 0.047 | 0.947 |
| Subcortical Regions | ||||||||
| thalamus (right) | 0.0001 | -0.0005, 0.0008 | 0.182 | 0.665 | 0.0006 | -0.0008, 0.002 | 0.855 | 0.279 |
| thalamus (left) | 0.0001 | -0.0004, 0.0006 | 0.219 | 0.602 | 0.0003 | -0.0006, 0.001 | 0.730 | 0.376 |
| White/Gray Matter | ||||||||
| white matter | 0.006 | -0.018, 0.030 | 0.233 | 0.578 | -0.012 | -0.042, 0.017 | -0.625 | 0.265 |
| cortical gray matter | -0.017 | -0.041, 0.006 | -0.594 | 0.120 | -0.002 | -0.046, 0.042 | -0.077 | 0.901 |
| subcortical gray matter | 0.0002 | -0.001, 0.001 | 0.163 | 0.700 | 0.0002 | -0.002, 0.003 | 0.187 | 0.842 |
| Schizophrenia Cases (n=17) Unadjusted Models | Schizophrenia Cases (n=17) Models Adjusted for Maternal Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Parameter Estimate | 95% CI | β | p-value | Parameter Estimate | 95% CI | β | p-value |
| Ventricular Measures | ||||||||
| sulcal cerebrospinal fluid | 0.0004 | -0.006, 0.007 | 0.039 | 0.882 | 0.0006 | -0.005, 0.007 | 0.056 | 0.825 |
| Cortical/Limbic Regions | ||||||||
| DLPFC (right) | -0.000004 | -0.0004, 0.0004 | -0.006 | 0.983 | -0.00002 | -0.0004, 0.0003 | -0.025 | 0.919 |
| DLPFC (left) | 0.000004 | -0.0004, 0.0004 | 0.005 | 0.985 | -0.00001 | -0.0004, 0.0004 | -0.015 | 0.952 |
| inferior frontal cortex (right) | 0.0000004 | -0.0003, 0.0003 | 0.001 | 0.998 | -0.00001 | -0.0003, 0.0003 | -0.014 | 0.956 |
| inferior frontal cortex (left) | -0.00001 | -0.0003, 0.0003 | -0.026 | 0.922 | -0.00002 | -0.0003, 0.0002 | -0.043 | 0.866 |
| anterior cingulate (right) | -0.0002 | -0.0006, 0.00008 | -0.381 | 0.131 | -0.0002 | -0.0006, 0.00007 | -0.392 | 0.123 |
| anterior cingulate (left) | -0.0001 | -0.0006, 0.0004 | -0.148 | 0.571 | -0.0001 | -0.0007, 0.0004 | -0.157 | 0.556 |
| posterior cingulate (right) | -0.0002 | -0.0003, -0.00002 | -0.537 | 0.026* | -0.0002 | -0.0003, -0.00002 | -0.543 | 0.029* |
| posterior cingulate (left) | -0.0001 | -0.0003, 0.0001 | -0.271 | 0.292 | -0.0001 | -0.0003, 0.0001 | -0.285 | 0.266 |
| Subcortical Regions | ||||||||
| thalamus (right) | 0.00001 | -0.0001, 0.0001 | 0.048 | 0.854 | 0.00001 | -0.0001, 0.0001 | 0.035 | 0.892 |
| thalamus (left) | 0.00005 | -0.00008, 0.0002 | 0.193 | 0.459 | 0.00005 | -0.00009, 0.0002 | 0.189 | 0.482 |
| White/Gray Matter | ||||||||
| white matter | -0.0005 | -0.006, 0.005 | -0.049 | 0.853 | -0.0004 | -0.006, 0.005 | -0.046 | 0.866 |
| cortical gray matter | -0.002 | -0.008, 0.005 | -0.138 | 0.598 | -0.002 | -0.007, 0.004 | -0.161 | 0.491 |
| subcortical gray matter | -0.00005 | -0.0003, 0.0002 | -0.090 | 0.732 | -0.00004 | -0.0003, 0.0003 | -0.082 | 0.759 |
Table 4 includes the results from the secondary multiple regression analyses with and without controlling for maternal education at birth. Unstandardized parameter estimates, 95% confidence intervals of the unstandardized estimates, standardized parameter estimates (β), and p-values for regression analyses of the relationship between the log of IL-8 and brain volumes/icv are presented.† denotes a results that approached significance.
denotes a result that was significant at p<.05.
denotes a result between p=.05 and p=.10.
Discussion
These results provide the first evidence that fetal exposure to increases in a maternal cytokine is associated with structural neuroanatomic alterations that have been consistently linked to schizophrenia (Wright et al., 2000). Among schizophrenia cases, increases in maternal IL-8 during the second/third trimesters of pregnancy were related to increases in ventricular CSF. In addition, we observed significant associations between maternal IL-8 and decreases in left entorhinal cortex and right posterior cingulate volumes, and volumetric decreases that approached significance in the right caudate, the putamen bilaterally, and the right superior temporal gyrus among cases.
Ventricular enlargement is arguably the most well-replicated neuromorphologic anomaly in schizophrenia (Wright et al., 2000). Further, ventricular enlargement has been previously associated with hypoxia-associated OCs, suggesting that this structural change may have risk factors that are neurodevelopmental in origin (Cannon et al., 2002). Lastly, fetal exposure to activation of proinflammatory cytokines in rodents has been linked to increased ventricular CSF, which is the only known neuromorphologic volumetric finding among animal studies to date (Patterson, 2008). Cumulatively, these findings suggest that fetal exposure to IL-8 may contribute to the known increases in ventricular volumes in schizophrenia.
Many of the other volumetric changes found in the present study also have been observed in schizophrenia and prodromal populations (Borgwardt et al., 2007; Pantelis et al., 2003; Wright et al., 2000). Specifically, parahippocampal and superior temporal gyrus (STG) volume reductions have been repeatedly found among patients with schizophrenia and these regions have been shown to be decreased in the available prodromal studies, as well (Pantelis et al., 2003; Wright et al., 2000). Similar to the present study, in first-episode and prodromal patients basal ganglia volumes were decreased (Corson, Nopoulos, Andreasen, Heckel, & Arndt, 1999; Pantelis et al., 2003).
Interestingly, no significant associations were observed among controls. This pattern of null findings may support the assertion that a genetic or environmental factor associated with schizophrenia is necessary for IL-8 to exert damaging influences on the fetal brain, although studies with larger samples are necessary to confirm this possibility.
IL-8 is a proinflammatory chemokine produced by multiple cells involved in mobilizing, activating and degranulating neutrophils (Janeway, Travers, Walport, & Schlomchik, 2005). Although IL-8 is integral in the initial immune response to infection, elevations in IL-8 also are sometimes associated with other maternal conditions during pregnancy that increase risk for schizophrenia, such as preeclampsia, obesity and anemia (Basso, Gimenez, & Lopez, 2005; Dalman, Allebeck, Cullberg, Grunewald, & Koster, 1999; Fain, 2006; Insel, Schaefer, McKeague, Susser, & Brown, 2008; Laskowska, Laskowska, Leszczynska-Gorzelak, & Oleszczuk, 2007; Schaefer et al., 2000). It is possible that additional obstetric insults may add to, interact with, or precede the effects of IL-8 to cause disruptions in fetal neuronal development among cases, which should be teased apart in future studies.
There were several limitations in the present study that should be noted. Given the multiple tests conducted in this study, it is possible that spurious findings arose from type I error. It appears unlikely, however, that our results are entirely due to chance, as they are consistent with many previous studies in clinical and preclinical studies of schizophrenia. Further, there is the possibility of Type 2 error, given the modest sample sizes. It should considered, however, that the present study is the first of its kind with access to serological evidence of prenatal events and follow-up brain imaging data. Nevertheless, it will be essential to attempt to replicate the results of the present study with larger samples.
In addition, the sample was limited to slightly more than 25% of cases from the overall cohort, raising the potential for selection bias. However, cases who participated in the DIBS study and cases from the original cohort who were not DIBS subjects did not differ with regard to several demographic variables and IL-8 levels; therefore, it is unlikely that case ascertainment bias accounts for our findings. Bias also would be mitigated by the fact that the sample was derived from a population-based study, in contrast to many clinical imaging studies, which draw upon hospital or clinic-based samples.
Lastly, the use of antipsychotics may have influenced our results. Although this is a possibility, IL-8 values were not significantly related to current antipsychotic use, which significantly decreases the likelihood that medication use confounded our findings. Nonetheless, long-term medication use, which is difficult to assess retrospectively, could have influenced the observed brain volumes.
In conclusion, the present study is the first to suggest that brain anomalies previously associated with schizophrenia may be partially attributable to in utero insults, such as fetal exposure to elevated IL-8. These findings support our previous association between fetal exposure to IL-8 and risk of schizophrenia in this birth cohort (Brown et al., 2004). Future research is necessary to replicate this finding and to delineate whether fetal exposure to IL-8 interacts with risk genes for schizophrenia. Our findings underscore the potential importance of prenatal contributions to schizophrenia, with implications for prevention, early intervention, and treatment strategies.
Acknowledgments
This study was supported by research grants to Dr. Brown from the National Institute of Mental Health (R01MH060249, R01MH63264, K02 MH065422-06) and a postdoctoral NIMH schizophrenia research fellowship to Dr. Ellman (5 T32 MH018870-20) and grants N01HD13334 and N01HD63258 from The Eunice Kennedy Shriver Institute of Child Health and Human Development. We also thank Theo van Erp, Ezra Susser, Michaeline Bresnahan, Barbara Cohn, Nashid Chaudhury, Aundrea Cook, Justin Penner, Nicole Stephenson, and the study teams of the CHDS, the PDS, and the DIBS for their contributions to data collection and preparation of this manuscript. The authors also wish to acknowledge Larry Kegeles, M.D. for his helpful comments.
Role of Funding Source
This study was supported by research grants to Dr. Brown from the National Institute of Mental Health (R01MH060249, R01MH63264, K02 MH065422-06) and a postdoctoral NIMH schizophrenia research fellowship to Dr. Ellman (5 T32 MH018870-20) and grants N01HD13334 and N01HD63258 from The Eunice Kennedy Shriver Institute of Child Health and Human Development. These funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no financial disclosures and/or conflicts to report.
References
- Basso B, Gimenez F, Lopez C. IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol. 2005;13(4):207–211. doi: 10.1080/10647440500240664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61(10):1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort: a population-based modeling study. Archives of General Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59(1):35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64(9):797–802. doi: 10.1016/j.biopsych.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry. 1999;46(5):712–720. doi: 10.1016/s0006-3223(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56(3):234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res. 2002;58(2-3):135–144. doi: 10.1016/s0920-9964(01)00330-9. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Pegues M, Amend D. Reduced hippocampal N-acetylaspartate without volume loss in schizophrenia. Schizophrenia Research. 1999;37(3):217–223. doi: 10.1016/s0920-9964(98)00173-x. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65(12):1040–1047. doi: 10.1016/j.biopsych.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55(11):1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Keshavan MS, Lawrie SM. Deconstructing psychosis with human brain imaging. Schizophr Bull. 2007;33(4):921–931. doi: 10.1093/schbul/sbm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry. 2008;65(10):1136–1144. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Schlomchik MJ. 6th ed. Garland Science; New York & London: 2005. Immunobiology: The immune system in health and disease. [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Kwan RK, Evans AC, Pike GB. MRI simulation-based evaluation of image-processing and classification methods. IEEE Trans Med Imaging. 1999;18(11):1085–1097. doi: 10.1109/42.816072. [DOI] [PubMed] [Google Scholar]
- Laskowska M, Laskowska K, Leszczynska-Gorzelak B, Oleszczuk J. Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med. 2007;20(7):527–532. doi: 10.1080/14767050701412719. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biological Psychiatry. 2000;48(8):740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schaefer CA, Brown AS, Wyatt RJ, Kline J, Begg MD, Bresnahan MA, et al. Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophr Bull. 2000;26(2):275–286. doi: 10.1093/oxfordjournals.schbul.a033452. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: Evidence and mechanisms. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26(2):257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]