Abstract
Ultraviolet B radiation (UVB) is a potent stimulator of epidermal cytokine production. In addition to cytokines such as tumor necrosis factor-alpha (TNF-α), UVB generates bioactive lipids including platelet-activating factor (PAF). Our previous in vitro studies in keratinocytes or epithelial cell lines have demonstrated that UVB-mediated production of PAF agonists is due primarily to the pro-oxidative effects of this stimulant, resulting in the non-enzymatic production of modified phosphocholines (oxidized glycerophosphocholines). The current studies use human skin to assess whether UVB irradiation generates PAF-receptor (PAF-R) agonists, and the role of oxidative stress in their production. These studies demonstrate that UVB irradiation of human skin results in PAF agonists, which are blocked by the antioxidant vitamin C and the epidermal growth factor receptor inhibitor PD168393. Inasmuch as UVB-generated PAF agonists have been implicated in animal model systems as being involved in photobiologic processes including systemic immunosuppression and cytokine (TNF-α) production, these studies indicate that this novel activity could be involved in human disease.
Introduction
Ultraviolet B radiation (290–320 nm; UVB) has profound effects on human skin. UVB exerts many of its effects through its ability to stimulate the production of bioactive proteins and lipids (1,2). Amongst the mediators produced by UVB is the lipid platelet-activating factor (1-O-alkyl-2-acetyl glycerophosphocholine, PAF). PAF is an inflammatory phospholipid mediator that exerts its effects through a single, specific G-protein coupled receptor, the PAF receptor (reviewed in 3). The PAF receptor is expressed by cells of the innate immune system, but also by keratinocytes (4). The PAF-R is linked to numerous signal transduction pathways including activation of protein kinase C (PKC), nuclear factor kappa B, and mitogen-activated kinases. Moreover, activation of the PAF-R results in the enzymatic synthesis of PAF (5).
PAF is synthesized in response to diverse stimuli including cytokines, endotoxin, and calcium ionophores (5–7). Of note, direct damage to keratinocytes by either heat or cold stimuli result in significant PAF production (8). PAF is produced enzymatically, yet PAF and sn-2 short-chained acyl glycerophosphocholines with PAF-R agonistic activity can also be produced via free radical-mediated damage (reviewed in 9). Through its ability to act as a potent pro-oxidative stressor (10), UVB has been demonstrated to trigger production of PAF and oxidized glycerophosphocholines (ox-GPC) with PAF-R agonistic activity (9, 11,12).
Biological effects of UV irradiation occur as a consequence of absorption of electromagnetic energy by certain molecules within cells. The photochemical activation of molecular oxygen generates reactive oxygen species (ROS) that mediate many UV irradiation-induced responses including apoptosis (10,13–15). Interestingly, activation of the epidermal growth factor receptor (EGF-R) by UVB also results in the formation of ROS that subsequently induces the growth arrest and DNA damage-inducible gene (GADD45) (16). Several studies have indicated that UVB-mediated production of ROS in human keratinocytes involves the EGF-R and NADPH oxidase (10, 16–18).
Several lines of evidence have indicated that keratinocyte-produced PAF species are important in UVB-mediated effects. First, UVB-mediated production of cytokines including TNF-α (19,20) and IL-10 (11) are due in part to subsequent PAF-R activation. Second, UVB-mediated systemic immunosuppression has been demonstrated to be reproduced by systemic treatment with PAF or UVB-irradiated glycerophosphocholine (11,21,22), and is blocked by PAF-R antagonists and the PAF-metabolizing enzyme PAF-acetylhydrolase (11,23) and absent in PAF-R-deficient mice (22). Thus, UVB-generated PAF species appear to play an important role in UVB-mediated effects. Inasmuch as the ability of UVB to generate PAF agonists in human skin has not been examined, these studies sought to assess the ability of UVB irradiation of human skin explants to stimulate PAF agonists and assess the role of ROS in their production.
Materials and Methods
Reagents and UVB irradiation source
All chemicals were obtained from Sigma unless indicated otherwise. PD168393, a specific EGF-R inhibitor (24) was obtained from Calbiochem (La Jolla, CA). PAF-acetylhydrolase and phospholipase A1 were kind gifts of Dr. Thomas McIntyre (Cleveland Clinic, Cleveland OH). The UV source was a Philips F20T12/UV-B lamp (270–390 nm; containing 2.6% UVC, 43.6% UVB, and 53.8% UVA) (19,22). The intensity of the UVB source was measured prior to each experiment using an IL1700 radiometer and a SED240 UVB detector (International Light, Newburyport, MA) at a distance of 8 cm from the UVB source to the skin tissue. All chemicals used in the irradiation protocols were first tested to ensure there was no ability to absorb UVB (i.e., sunblock effect) by testing the intensity of UVB (as measured by detector) irradiation underneath a Kodacel membrane with/without application of the dose used in the protocols.
KBPAF-R model system
The human epidermoid cell line KB cells were grown in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc.; Grand Island, NY) supplemented with 10% fetal bovine serum (Intergen; Purchase, NY), 2 mM L-glutamine, and 100 µg/ml penicillin and streptomycin. A KB PAF-R model system was created by transduction of PAF-R-negative KB cells with the MSCV 2.1 retrovirus encoding the human leukocyte PAF receptor as described previously (25). KB cells stably transduced with the PAF receptor (designated as KBP cells) or with the control MSCV2.1 retrovirus (defined as KBM cells) were characterized by Southern, Northern, radioligand binding and by calcium transient studies that demonstrate the presence of a functional PAF-R signaling system in these cells.
Lipid extraction and PAF-R activity measurement
Human Caucasian foreskin tissue were collected and used in these studies within 48 h. The explants were warmed to 37 °C before treatment with various doses of UVB. In some experiments the tissue was left on the bench top for a similar amount of time to serve as a sham control. Following UVB treatment, epidermal part of the skin explant was scraped off using a 5 mm curette after having the skin frozen with liquid nitrogen. In some experiments the residual dermal tissue was used. Scraped epidermal or dermal tissue specimens were weighed and lipids extracted, the reactions were quenched with ice-cold methanol and total lipids extracted by the method of Bligh and Dyer (26). In some experiments, the lipid extract was treated with PAF-acetylhydrolase (10 mg), phospholipase A1 (5 mg) or PBS overnight at 37 °C, and then lipids re-extracted (23). The effectiveness of phospholipase A1 was confirmed by treatment of the PAF-R agonist 1-palmitoyl-2-acetyl-glycerophosphocholine. We have examined residual scraped skin fixed in formalin, embedded in paraffin and stained with hematoxylin and eosin to histologically verify removal of the epidermis. In some experiments, skin explants were treated with topical application of 50 µl of 10 mM vitamin C, 4 mM PD168393, or vehicle alone (10% DMSO in ethanol) for 30 min before UVB irradiation.
The presence of PAF-R agonists in the lipid extracts were measured by their ability to induce an intracellular Ca2+ mobilization response in KBP cells as previously described (23). In brief, PAF-R-expressing KBP or control PAF-R-negative KBM cells were preloaded with the Ca2+-sensitive indicator, FURA-2-AM (2 µM in HBSS) at 37 °C for 90 min, followed by washing, re-suspending and maintained in HBSS at room temperature before use. Lipid extracts dissolved in ethanol (adjusted to 10 mg wet tissue weight/20 µl) were added to an aliquot of KBP cells (1.0–1.5 × 106 cells/2 ml) in a cuvette at 37 °C with constant stirring. FURA-2-AM fluorescence was monitored in a Hitachi F-4010 spectrophotometer with excitation and emission wavelengths of 331 and 410 nm, respectively. The Ca2+ influx was calculated as described (23) and shown as percentage of maximal peak calcium flux induced by 1 µM CPAF.
Data analysis
Data from the tissue studies are presented as mean ± standard error of the mean (SEM). Student's t-tests were used to assess statistical significance for differences in means. Significance was set at p<0.05.
Results
UVB irradiation of human skin generates PAF-R agonistic activity
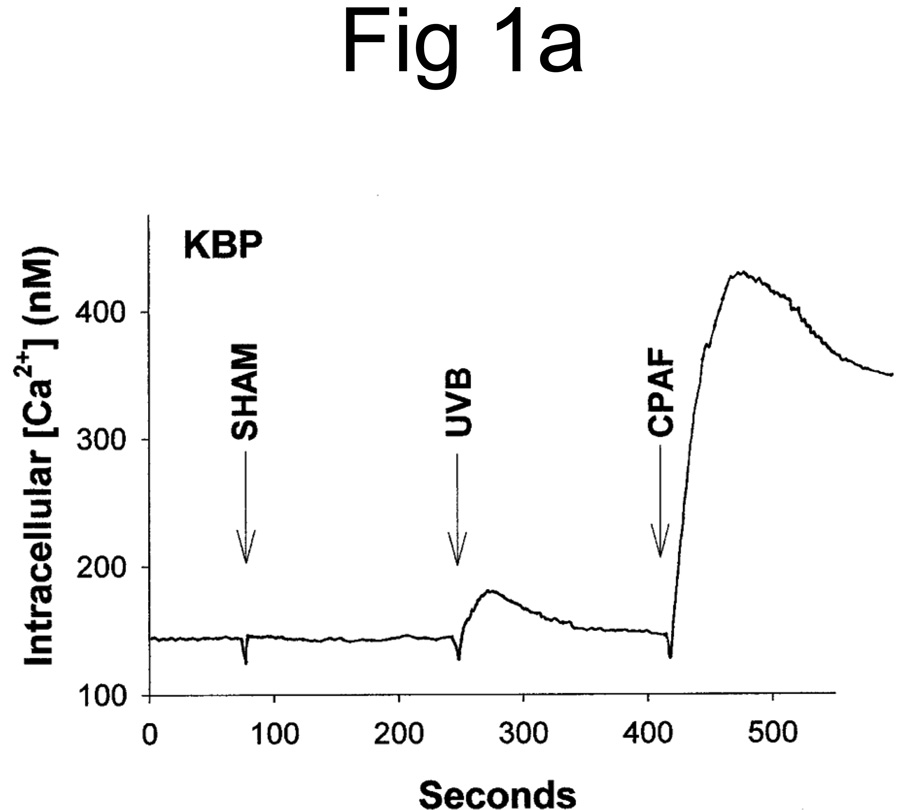
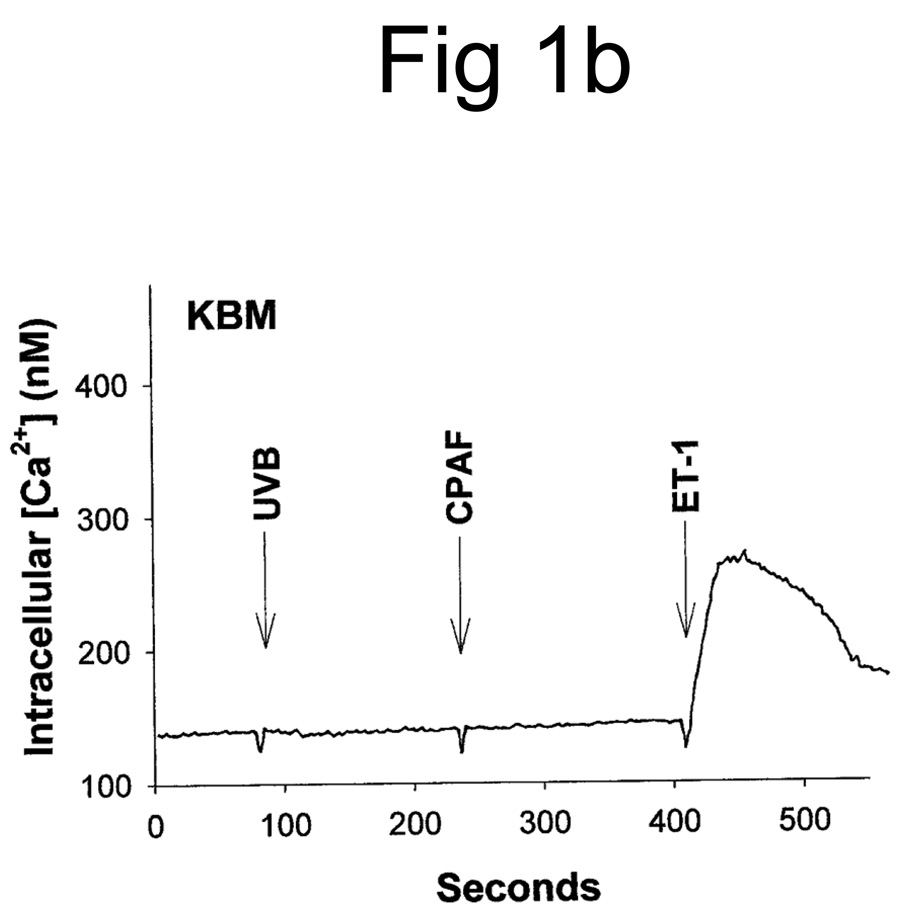
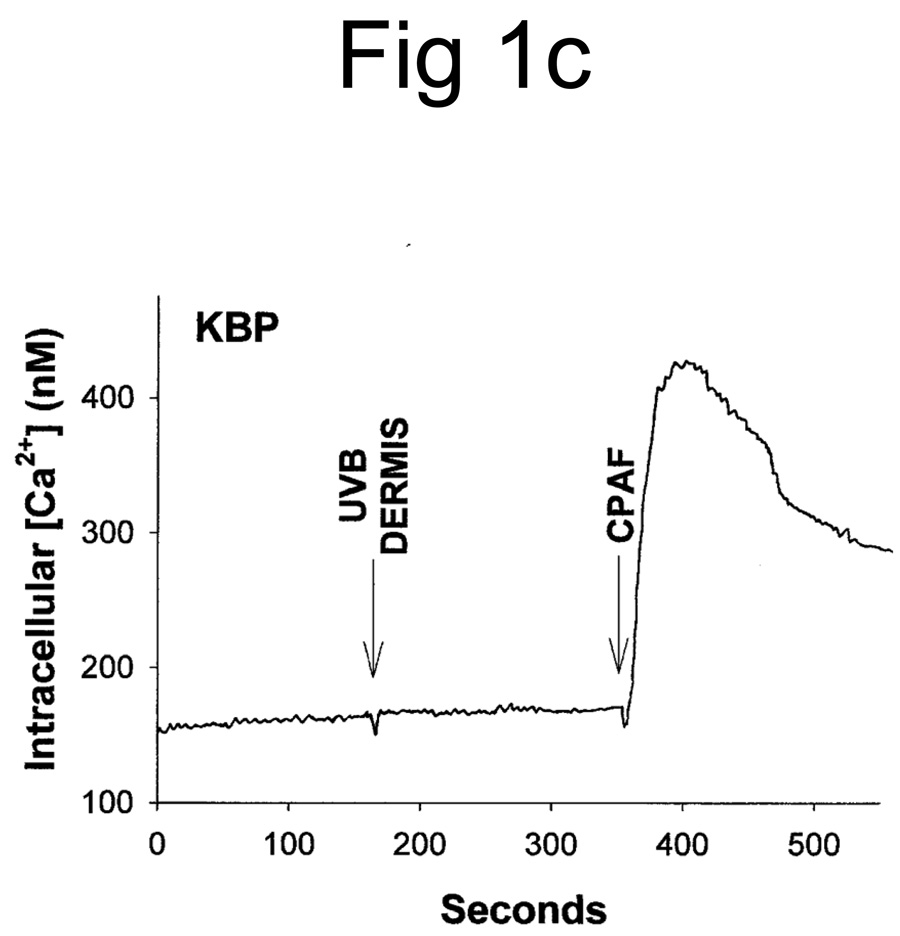
UVB irradiation of human epithelial cells in vitro (11,12,27) or murine skin in vivo (23) has been shown to stimulate the production of PAF agonists. Thus, our first studies assessed whether UVB irradiation of human skin resulted in the production of PAF-R agonistic activity. Inasmuch as PAF-R agonists consist of both native PAF as well as ox-GPCs with PAF-R agonistic activity, we measured total PAF-R agonistic activity using the biochemical assay of intracellular calcium mobilization response in PAF-R-expressing KBP cells (23,25). Discarded human foreskins were treated with UVB or sham-irradiated. At various times, the epidermis was removed from the irradiated area via a curette, and the tissue was weighed, then lipids extracted and tested for the ability to trigger a calcium mobilization response in PAF-R-expressing KBP versus control PAF-R-negative KBM cells. As shown in Fig 1A, lipid extracts derived from UVB-irradiated human epidermis triggered an intracellular calcium mobilization response in PAF-R-expressing KBP cells. Lipid extracts derived from sham-treated skin did not contain appreciable PAF-R agonistic activity (Fig 1A). Treatment of PAF-R-negative KB cells transduced with the MSCV2.1 vector (KBM) with lipid extracts derived from UVB-irradiated human epidermis did not result in an intracellular calcium mobilization response (Fig. 1B). It should be noted that no appreciable PAF-R agonistic activity was found in the dermis following UVB irradiation of skin (Fig 1C).
Figure 1. Detection of PAF-R agonistic activity in UVB-irradiated human skin.
Stimulation with lipid extracts from epidermis of UVB-irradiated (2,000 J/m2, 1 h) but not unirradiated (SHAM) human skin results in calcium mobilization response in PAF-R-positive KBP cells (A) but not in control PAF-R-negative KBM cells (B). Lipid extracts from dermis of UVB-irradiated (5,000 J/m2, 1 h) skin does not result in a significant calcium mobilization response in KBP Cells (C). Stimulation with 1 µM of CPAF or endothelin-1 (ET-1) were used as positive controls.
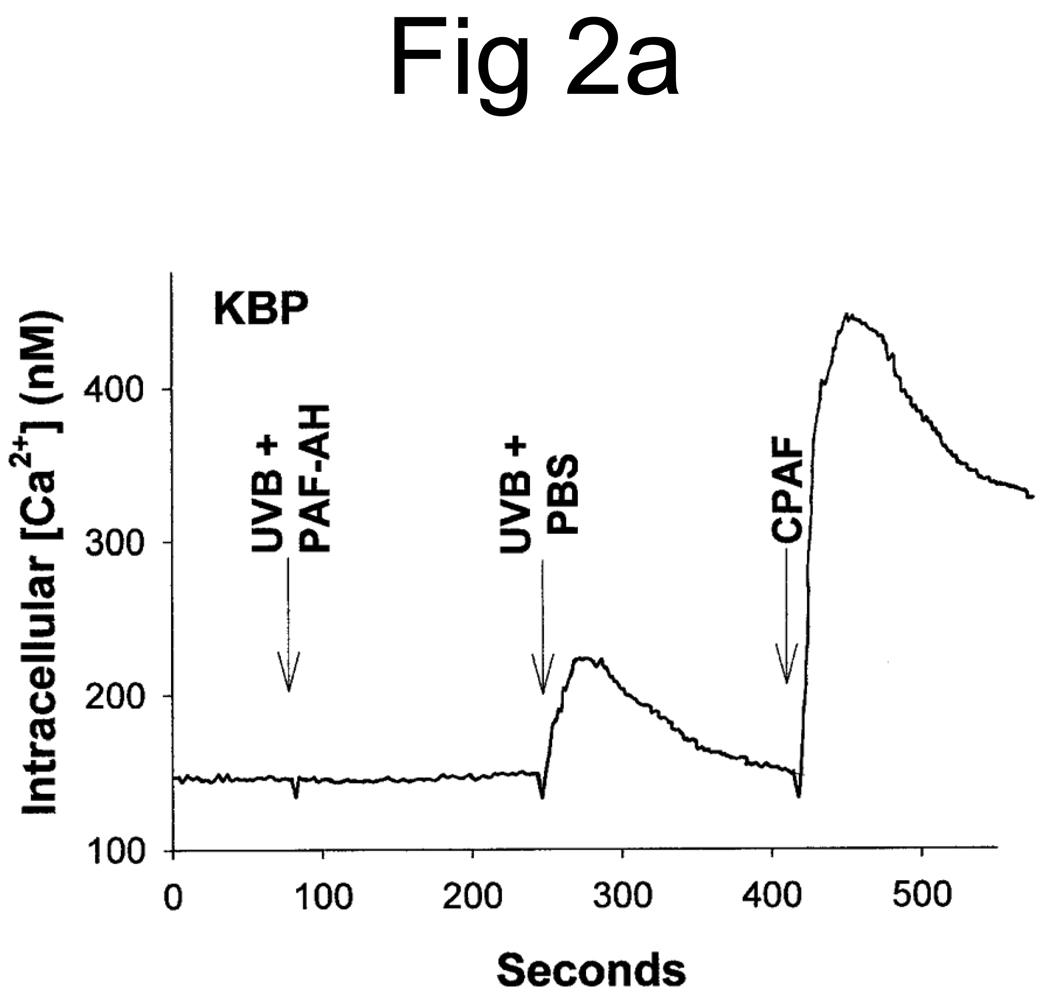
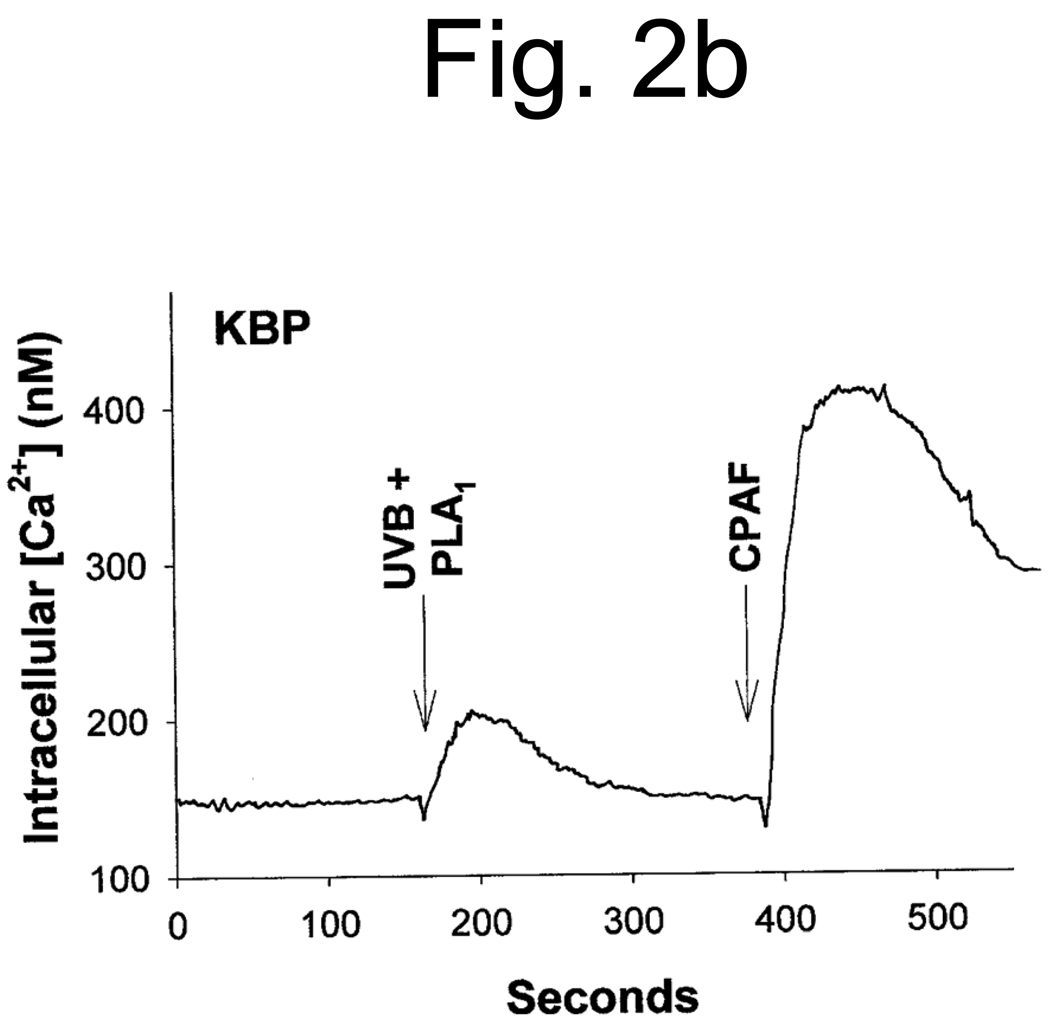
Studies characterizing the PAF-R activity found in oxidized low density lipoproteins (28, 29) and the UVB-irradiated PAF-R-deficient human epithelial cell line KB (12) have demonstrated that the majority of the PAF-R activity is sensitive to the enzyme PAF acetylhydrolase. Moreover, the presence of an sn-1 ether linkage was inferred by the insensitivity of this activity to the enzyme phospholipase A1 (PLA1). The next studies examined the effect of these enzymes on UVB-generated PAF-R agonists derived from human epidermal skin. As shown in Figure 2A, treatment with PAF acetylhydrolase completely ablated the PAF-R activity. However, the lipid extracts derived from UVB-irradiated human epidermal skin were essentially insensitive to PLA1 treatment (Fig. 2B). These studies indicate that the UVB-generated PAF-R activity is an sn-1 ether-linked glycerophosphocholine.
Figure 2. Partial enzymatic characterization of PAF-R agonistic activity from UVB-irradiated human skin.
Lipid extracts from epidermis of UVB-irradiated (5,000 J/m2, 1 h) human skin were pretreated with PAF-acetylhydrolase (UVB + PAF-AH) or PBS as control (UVB + PBS) (A) or phospholipase A1 (UVB + PLA1) (B) before PAF-R agonistic activity was assessed in KBP cells loaded with FURA-2-AM. Stimulation with 1 µM CPAF was used as control.
Dose- and time-dependence of UVB-generated PAF-R agonistic activity
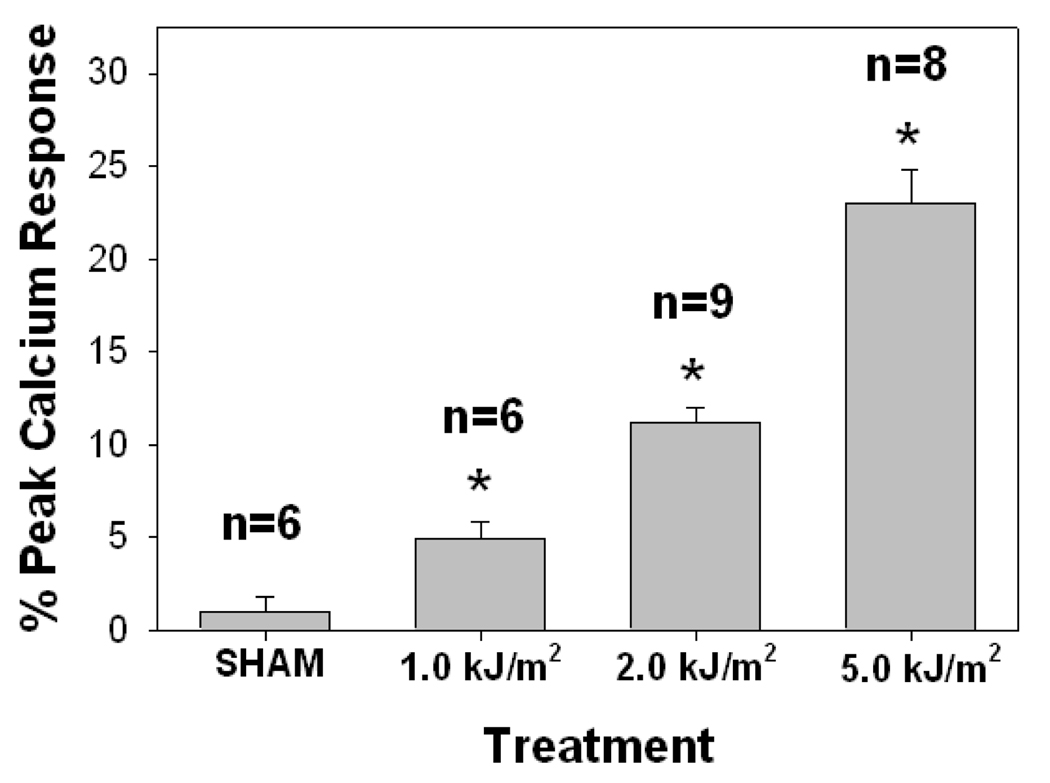
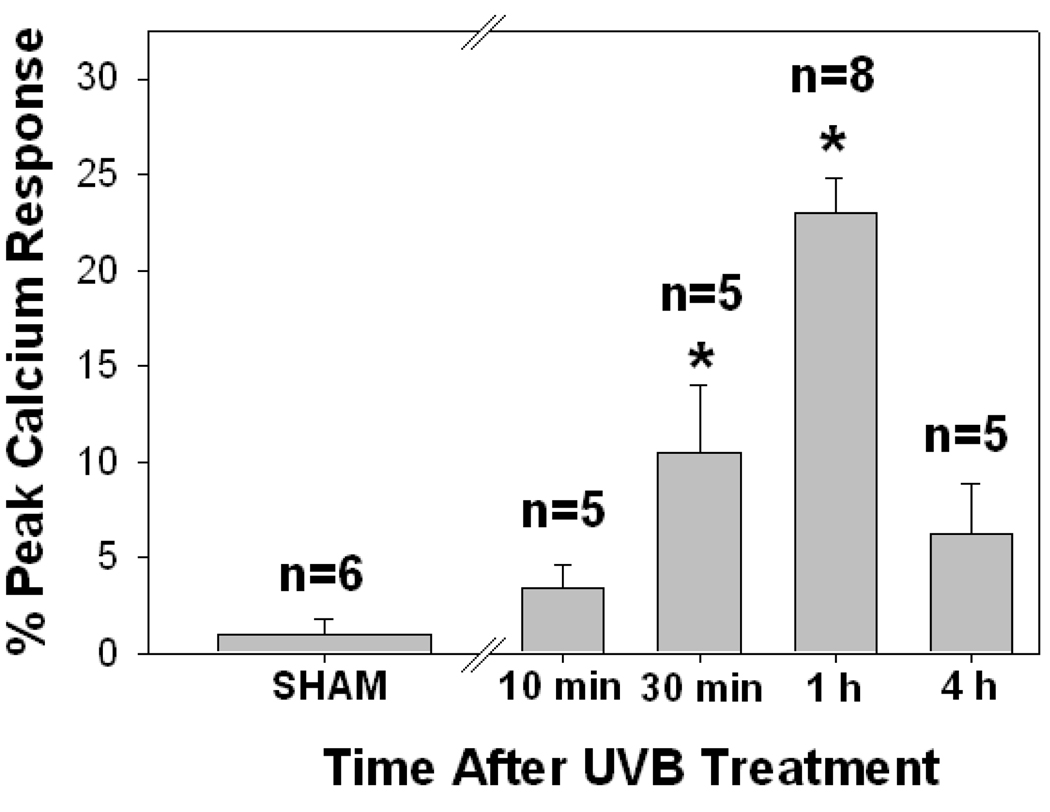
UVB irradiation of human skin resulted in epidermal PAF-R activity in a dose-dependent manner with significant responses measured at 1000 J/m2 and above (Fig 3). UVB-generated PAF-R agonistic activity was measured by 10 minutes and was maximal at 1 hour following UVB treatment (Fig 4). By 4h the levels of PAF-R agonists were still elevated above baseline, but were greatly decreased in comparison to 1 h post-UVB. In two explants tested, the levels of PAF species 24 h post UVB were similar to sham-irradiated tissues (data not shown). These findings fit closely with our previous studies examining the time course of UVB-generated PAF-R agonists in epithelial cells (23). The present studies indicate that UVB irradiation of human skin ex vivo at physiologically relevant doses (eg, 1000 J/m2) results in the production of PAF-R agonistic activity which is contained in the epidermis.
Figure 3. Dose-dependency of UVB-mediated PAF-R agonist production in human skin.
Human skin explants were sham or UVB-irradiated with various doses of UVB. The epidermis was removed at t = 1 h and lipids extracted and tested for PAF-R agonist activity in FURA-2-AM-loaded KBP cells. Data are the mean ± SE percentage of maximal peak calcium flux induced by 1 µM CPAF and numbers of explants tested from each treatment noted in parentheses. * Denotes statistically significant (p < 0.05) differences in comparison to sham control.
Figure 4. Time course of UVB-mediated PAF-R agonist production in human skin.
Human skin explants were sham or UVB-irradiated with 5000 J/m2 UVB. The epidermis was removed at various times and lipids extracted and tested for PAF-R agonist activity in FURA-2-AM-loaded KBP cells. Data are mean ± SE percentage of maximal peak calcium flux induced by 1 µM CPAF and numbers of explants tested from each treatment noted in parentheses. * Denotes statistically significant (p < 0.05) differences in comparison to sham control.
UVB-generated PAF-R agonistic activity in human skin involves ROS
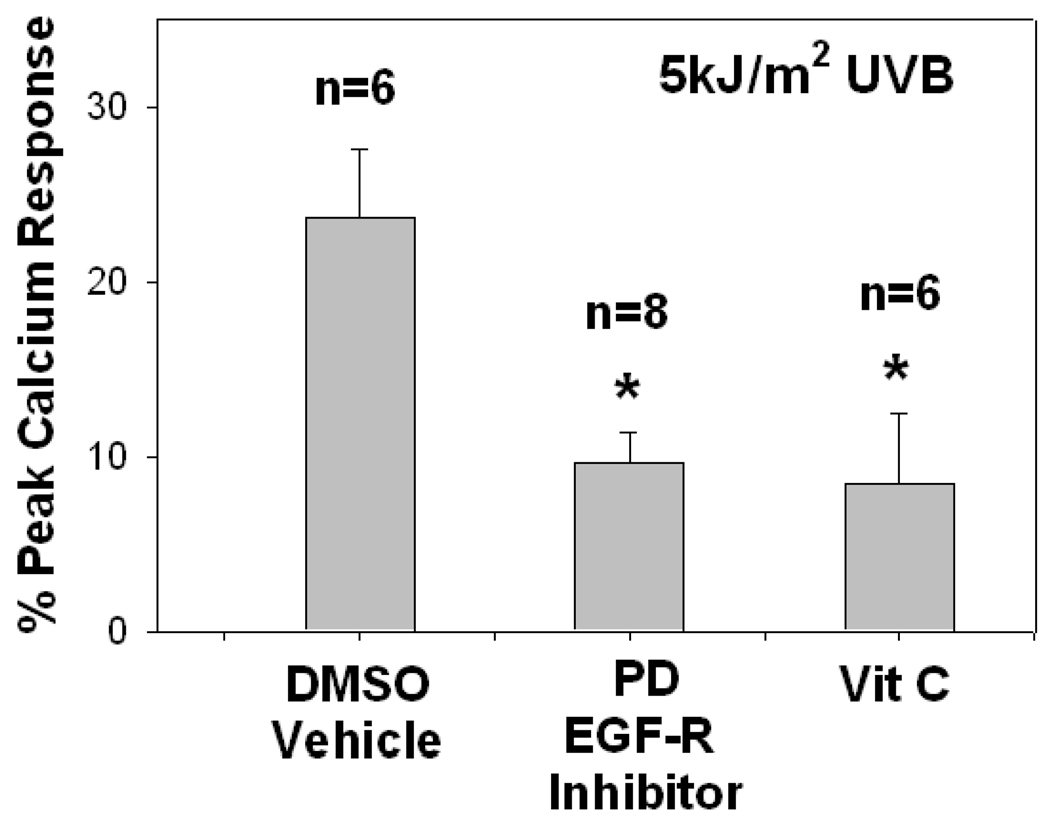
UVB irradiation is a potent inducer of ROS including superoxide radical, hydrogen peroxide and hydroxyl radical (10). Previous studies have provided evidence that UV-mediated ROS in keratinocytes can involve the EGF-R and subsequent NADPH oxidase activation (16–18). Our next studies assessed whether this pathway is involved in UVB-mediated PAF-R agonist production in human epidermal skin. Skin explants were pre-incubated with the antioxidant vitamin C or EGF-R inhibitor PD168393 or DMSO vehicle applied topically 30 min prior to UVB irradiation (5,000 J/m2). As shown in Fig. 5, both Vitamin C and PD168393 pre-treatment inhibited UVB-mediated PAF agonist formation in response to UVB at 1 h. It should be noted that pre-treatment with DMSO did not significantly affect the ability of UVB to generate PAF-R agonists (compare Figure 5 and Figure 4). These studies indicate that UVB mediates PAF-R agonists in human epidermal skin in part through ROS.
Figure 5. Effects of antioxidant and EGF-R inhibitor on UVB-mediated PAF-R agonist production in human skin.
Human skin explants were pretreated with 50 µL of a 10 mM solution of the antioxidant vitamin C, 4 mM of the EGF-R inhibitor PD168393, or vehicle alone (10% DMSO in ethanol) for 30 min before UVB (5,000 J/m2) irradiation. The epidermis was removed at one hour post-UVB treatment and lipids extracted and tested for PAF-R agonist activity in FURA-2-AM-loaded KBP cells. Data are the mean ± SE percentage of maximal peak calcium flux induced by 1 µM CPAF and numbers of explants tested from each treatment noted in parentheses. * Denotes statistically significant (p < 0.05) differences in comparison to DMSO vehicle control.
Discussion
The present studies demonstrate that UVB irradiation of human skin results in the production of PAF-R agonists and implicate ROS in their formation. UVB-mediated PAF-R agonists were only found in the epidermal compartment of human skin, which indicates that keratinocytes are the cell type responsible for their generation. That the time course of UVB-generated PAF agonists in human skin (Fig. 4) resembles that seen in epithelial KB cells (23), also suggests keratinocyte involvement.
The synthetic pathway for PAF consists of two enzymes: phospholipase A2 generates the lysolipid backbone by releasing the sn-2 fatty acyl residue from alkyl phosphocholine and PAF acetyltransferase transfers an acetyl residue from acetyl-CoA to this newly generated lysolipid (reviewed in 30). The activities of these two enzymes are tightly regulated, with increased intracellular calcium levels being the premier regulator. The limited amounts of free lyso-phosphocholine and acetyl-CoA in a cell thus limits the production of bioactive PAF. In addition, PAF-R agonists can also be produced through non-enzymatic oxidation, which is not subjected to cellular control (9,31). UVB irradiation generates a variety of ROS that oxidizes phospholipids. Oxidation of esterified fatty acyl residues introduces oxy functions, rearranges bonds and results in fragmentation of carbon-carbon bonds by β-scission that generates a myriad of phospholipid reaction products including PAF-R agonists (28,32). In this regard, cellular membranes serve as the source of oxidized phospholipids and are thus the sources of ROS-mediated PAF-R agonist formation.
Several lines of evidence indicate that the ability of UVB to act as a pro-oxidative stressor is involved in the ability of this agent to generate PAF-R agonists. First, UVB-generated PAF-R agonistic activity is blocked by pre-incubation with the anti-oxidant vitamin C. It should be noted that systemic treatment with vitamin C inhibits PAF-R agonists formed in response to pro-oxidative stressors UVB (23) or cigarette smoke (33) in rodents. Second, the ability of an EGF-R inhibitor to inhibit UVB-generated PAF-R agonists in human skin also fits with involvement of ROS in their formation. Indeed, keratinocyte EGF-R activation has been shown to be critical for UVB-mediated ROS formation (16,18,23). Finally, the time course of UVB-generated PAF agonists in human skin also resembles the time course of UVB-mediated ROS in keratinocytes (23).
Though oxidatively-modified glycerophosphocholines with PAF-R agonistic activity were first described 20 years ago, only several of the structures of these bioactive lipids have been reported (12,21,34). Of interest, our previous studies have shown that PAF (1-hexadecyl-2-acetyl-GPC) itself is formed following UVB irradiation of KB cells or purified 1-hexadecyl-2-arachidonoyl-GPC (12). Other known ox-GPCs with PAF-R activity first described as being associated with oxidized LDL that are GPC with an sn-1 ether lipid and sn-2 of butanoyl (C 4:0) or butenoyl (C 4:1) have also been shown to be formed following UVB (12,28,29). Our ongoing studies are attempting to use mass spectrometry to more fully characterize UVB-generated ox-GPCs and have found more than 10 separate species with PAF-R agonistic activity. Inasmuch as UVB-generated PAF-R agonists appear complex, the use of our intracellular calcium biochemical assay to measure total PAF-R activity using FURA-2-loaded KBP cells is warranted for the present studies.
In summary, these studies document the ability of UVB radiation to stimulate PAF agonists in human skin. Given that the PAF system has been implicated in UVB-mediated processes ranging from optimal cytokine production to systemic immunosuppression, these studies have potential clinical implications.
Acknowledgements
This research was supported in part by grants from the Riley Memorial Association, and the National Institutes of Health grants HL62996, U19 AI070448 and Veteran’s Administration Merit Award (JBT).
Nonstandard abbreviations used
- PAF
Platelet-activating factor
- PAF-R
platelet-activating factor receptor
- CPAF
1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine
- GPC
glycerophosphocholine
- ox-GPC
oxidized glycerophosphocholine
- TNF-α
tumor necrosis factor alpha
References
- 1.Garmyn M, Yarosh DB. The molecular and genetic effects of ultraviolet radiation exposure on skin cells. In: Lim HW, Honigsman H, Hawk J, editors. Principles and Practice of Photodermatology. New York, N.Y: Informa Healthcare; 2007. pp. 41–54. [Google Scholar]
- 2.Takashima A, Bergstresser PR. Impact of UVB radiation on the epidermal cytokine network. Photochem. Photobiol. 1996;63:397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x. [DOI] [PubMed] [Google Scholar]
- 3.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prost. & Other Lipid Mediat. 2002;68:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 4.Travers JB, Huff JC, Rola-Pleszczynski M, Gelfand EW, Morelli JG, Murphy RC. Identification of functional platelet-activating factor receptors on human keratinocytes. J. Invest. Dermatol. 1995;105:816–823. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- 5.Travers JB, Harrison KA, Johnson CA, Clay KL, Morelli JG, Murphy RC. Platelet-activating factor biosynthesis induced by various stimuli in human HaCaT keratinocytes. J. Invest. Dermatol. 1996;107:88–94. doi: 10.1111/1523-1747.ep12298295. [DOI] [PubMed] [Google Scholar]
- 6.Albert DH, Snyder F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine by rat alveolar macrophages. J. Biol. Chem. 1983;258:97–105. [PubMed] [Google Scholar]
- 7.Chilton FH, Ellis JM, Olson S, Wykle RL. 1-O-Alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine: a common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J. Biol. Chem. 1984;259:2014–12020. [PubMed] [Google Scholar]
- 8.Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch. Dermatol. Res. 2000;292:256–259. doi: 10.1007/s004030050483. [DOI] [PubMed] [Google Scholar]
- 9.Konger RL, Marathe GK, Yao Y, Zhang Q, Travers JB. Oxidized glycerophosphocholines as biologically active mediators for ultraviolet radiation-mediated effects. Prost. Other Lipid Mediat. 2008;87:1–8. doi: 10.1016/j.prostaglandins.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, Pittelkow MR. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J. Invest. Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 11.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marathe GK, Johnson CA, Billings SD, Southall MD, Pei Y, Spandau D, Murphy RC, Zimmerman GA, McIntyre TM, Travers JB. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol. Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 13.Bertling CJ, Lin F, Girotti AW. Role of hydrogen peroxide in the cytotoxic effects of UVA/B radiation on mammalian cells. Photochem. Photobiol. 1996;64:137–142. doi: 10.1111/j.1751-1097.1996.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrlich P, Bohmer FD. Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 15.Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem. Pharmacol. 2002;64:837–841. doi: 10.1016/s0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 16.Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultures human keratinocytes and in human skin in vivo. Int. J. Mol. Med. 2000;6:683–688. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- 17.Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signaling. Biochem. J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Kochevar IE. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Rad. Biol. Med. 2005;38:890–897. doi: 10.1016/j.freeradbiomed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Dy LC, Pei Y, Travers JB. Augmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptor. J. Biol. Chem. 1999;274:26917–26921. doi: 10.1074/jbc.274.38.26917. [DOI] [PubMed] [Google Scholar]
- 20.Travers JB, Edenberg HJ, Zhang Q, Al-Hassani M, Yi Q, Baskaran S, Konger RL. Augmentation of ultraviolet B radiation-mediated early gene expression by the epidermal platelet-activating factor receptor. J. Invest. Dermatol. 2008;128:455–460. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings SD, Perkins SM, Howard KM, Ishii S, Shimizu T, Travers JB. Staphylococcal liptoechoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J. Clin. Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S, Pollok KE, Travers JB. UVB Radiation-Mediated Inhibition of Contact Hypersensitivity Reactions Is Dependent on the Platelet-Activating Factor System. J. Invest. Derm. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, Wolverton J, Zhang Q, Marathe GK, Al-Hassani M, Konger R, Travers JB. Ultraviolet B Radiation Generated Platelet-activating Factor Receptor Agonist Formation Involves EGF-R-mediated Reactive Oxygen Species. J. Immunol. 2009;182:2842–2848. doi: 10.4049/jimmunol.0802689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascia Fl, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SA, Dy LC, Fertel RH, Nguyen TM, Williams DA, Travers JB. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 26.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.Travers JB. Oxidative stress can activate the epidermal platelet-activating factor receptor. J. Invest. Dermatol. 1999;112:279–283. doi: 10.1046/j.1523-1747.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- 28.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J. Clin. Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman GA, Stephen M, Prescott SM, MacIntyre TM. Oxidatively fragmented phospholipids as inflammatory mediators: the dark side of polyunsaturated lipids. J. Nutr. 1995;125:1661S–1665S. doi: 10.1093/jn/125.suppl_6.1661S. [DOI] [PubMed] [Google Scholar]
- 30.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Ann. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 31.Marathe GK, Harrison KA, Murphy RC, Prescott SM, Zimmerman GA, McIntyre TM. Bioactive phospholipid oxidation products. Free Rad. Biol. Med. 2000;28:1762–1770. doi: 10.1016/s0891-5849(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 32.Frankel EN. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984;23:197–221. doi: 10.1016/0163-7827(84)90011-0. [DOI] [PubMed] [Google Scholar]
- 33.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Presctott SM, McIntyre TM. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokumura A, Takauchi K, Asai T, Kamiyasu K, Ogawa T, Tsukatani H. Novel molecular analogues of phosphatidylcholines in a lipid extract from bovine brain: 1-long-chain acyl-2-short-chain acyl-sn-glycero-3-phosphocholines. J. Lipid Res. 1989;30:219–224. [PubMed] [Google Scholar]